Abstract
Introduction:
We aimed to evaluate the effectiveness and safety of the newly launched thulium fiber laser (TFL) with holmium laser lithotripsy in the miniaturized percutaneous nephrolithotomy (Miniperc) procedure for renal stones.
Methods:
The prospective study included patients with renal stones of size >1 cm to ≤3 cm. The patients who opted for extracorporeal shock wave lithotripsy, retrograde intrarenal surgeries, and stones >3 cm were excluded from the study. Demographics such as patient's age, sex, stone volume, and hardness were evaluated. The patients were randomized into holmium and TFL group for stone lithotripsy. Both the procedures were evaluated for stone disintegration time, operative time, hospital stay, intra- and postoperative complications, and stone-free rate.
Results:
A total of 125 patients with renal stones who underwent Miniperc were included in this study. The average size of the stone was comparable in both the groups (P = 0.053). The median stone disintegration time with holmium laser was 20 min 45 s and with TFL, it was 11 min 19 s (P < 0.001). The most common complications were Clavien grade I and II complications (P = 0.128). Prolonged postoperative hematuria was observed in the Thulium fiber laser group, which was conservatively managed. The stone-free rate with TFL (94.9%) was better than Holmium lithotripsy (90.9%).
Conclusions:
The TFL has significant less stone disintegration time which effectively reduced the operative time of Miniperc procedure. The stone-free rate is better, but the incidence of self-limiting hematuria is higher with TFL as compared to Holmium laser.
INTRODUCTION
Miniaturized percutaneous nephrolithotomy (Miniperc) is a common procedure for endoscopic management of urinary calculi. This technique remains the most preferred procedure over standard percutaneous nephrolithotomy (PCNL) for the management of renal stones between 1 and 2 cm due to reduced bleeding, shorter hospital stays and similar stone clearance rates.[1,2]
The first use of a minimally invasive technique using a holmium:yttrium aluminum garnet (Ho:YAG) laser was well described almost two decades ago.[3] Ho:YAG laser is universally accepted among urologists since the late 1980s. In <10 years, Ho:YAG laser method rapidly became the gold standard for stone management particularly in ureteroscopy.[4] It has several advantages such as limited energy losses, highly efficient in stone fragmentation and dusting, low risk of tissue damage, and can be used for soft tissue applications.[5]
Regardless of the clinical utility of the holmium laser, it has some limitations. Although Ho:YAG laser can be used with higher energy levels, it has limitations when used with higher frequency. In addition, the rate of retropulsion of stone with the use of Holmium laser is also high.[6]
Recently, the new thulium fiber laser (TFL) has been introduced for urolithiasis management. TFL has been explored as a next-generation fiber laser which is superior to conventional Ho:YAG laser. Rather than a bulk solid-state Ho:YAG laser, in Thulium fiber, the laser is generated and pumped by diode into surgical silica fiber. This property of TFL ultimately results in delivery of high-power output in a small fiber resulting in high intensity which improves the stone ablation rate.[6] In vitro study has demonstrated better stone ablation rate with TFL and four times higher dusting rate as compared to Ho:YAG laser.[7] In an in vitro study, it was demonstrated and the thermal effect of TFL is also safe during TFL lithotripsy with moderate irrigation.[8] In a preclinical study, it was observed that the TFL produces less retropulsion as compared to Ho:YAG laser.[9]
The present study aimed to assess the safety and effectiveness of the new TFL in stone lithotripsy. This is the first clinical study that compares the use of TFL with Ho:YAG laser for stone lithotripsy in the Miniperc procedure.
METHODS
Study design and patient selection
The present study was conducted in a prospective fashion in our institute in the Department of Urology. A total of 125 patients were included who were diagnosed with renal stones of diameter >1 cm to ≤3 cm. The patients who opted for extracorporeal shock wave lithotripsy, retrograde intrarenal surgeries and stones >3 cm were excluded from the study [Figure 1]. The study protocol was approved by the Institutional Ethics Committee, and the study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all the patients.
Figure 1.
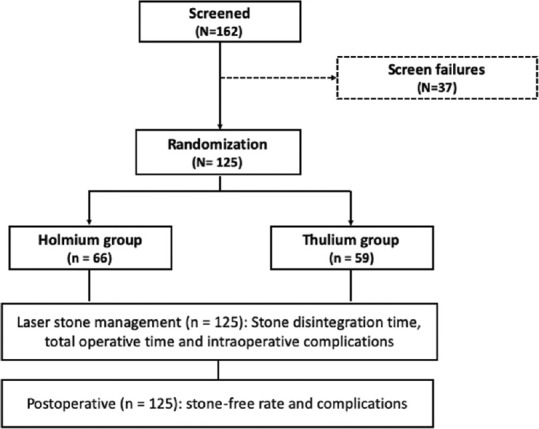
Consort diagram flow chart
Preoperative evaluation
Randomization was done using online research randomizer tool (https://www.randomizer.org/). The patients were assigned to each of the two treatment groups (group I and II). Group I consisted of patients undergoing Miniperc with stone lithotripsy performed with Ho:YAG laser and group II comprised of patients in whom the stone lithotripsy was done using a TFL. Diagnostic imaging such as ultrasonography and contrast-enhanced computerized tomography scan of the kidney ureter bladder (KUB) was performed. Preoperative urine culture was obtained, and appropriate antibiotics were started to make the urine sterile prior to the surgery. The type of stone was categorized according to Guy's stone score. Stone volume was calculated in cubic millimeters by the computed tomography scan software. For multiple stones, all individual stone volumes were added up.
Technique
Miniperc was performed by the standard method by a single urologist. All the procedures were done under general anesthesia, in prone position and the puncture was done by the standard bullseye technique under fluoroscopy guidance. MIP M Miniperc instrument (Karl Storz) was used, and the tract was dilated with the single-shot 16.5/17.5 F dilation system. The stone was fragmented with either Ho:YAG or TFL.
Ho:YAG laser lithotripsy was performed by a 35-watt machine (Litho 35 W, Quanta Systems, Italy) using a 550 μm using fiber. For Ho:YAG laser, the settings ranged from 0.8–1.2 J energy to 10–15 Hz of frequency. The power levels ranged from 8 to 18 watts. The new TFL lithotripsy was performed by a 60-watt machine (Urolase SP, IPG Photonics, Russia) using a 400 μm laser fiber. The TFL settings ranged from 1 to 1.5 J and 6–15 Hz; total power ranged from 6 to 15 watts. Both the lasers were used in fragmentation mode.
The stone disintegration time was noted for TFL and Ho:YAG laser. Fragments were evacuated by vacuum cleaner effect. At the end of the procedure, a thorough visual inspection by the nephroscope was performed of all the calyces to confirm residual fragments. Fluoroscopy was done to further confirm the absence of residual fragments and a double J stent was placed in all the patients. Nephrostomy was placed only in select patients in whom there was bleeding or infection. The operative time for both the procedures was noted. The operative time was calculated from the initial puncture till the exit policy, either placement of nephrostomy or removal of Miniperc outer sheath in tubeless procedures.
The stone disintegration time was assessed for both groups. The stone disintegration time was calculated from the start of laser energy on the stone and till the complete stone was disintegrated.
Postoperative care
Postoperatively after 48 h an X-ray KUB was done to confirm the position of the DJ stent and for the presence of residual fragments. Nephrostomy, if placed, was removed and the patient was discharged after 48 h. On follow-up, after 2-week ultrasonography and KUB was done to re-confirm stone-free status, and then the DJ stent was removed. Stone size of more than 4 mm was considered as significant residual fragments. All patients were followed up every 3 months.
Outcomes
The effectiveness of the procedure was assessed based on stone clearance rate, stone disintegration time, operative time, hospital stay, intra-and postoperative complications, and success rate. Intra and postoperative complications were assessed using the Clavien–Dindo Classification system. The stone clearance was assessed after 2 weeks of surgery.
Statistical analysis
Data were analyzed using Statistical Package for the Social Sciences (SPSS) software (version 23.0, Armonk, NY: IBM Corp). The qualitative data were presented as numbers and percentages, while quantitative data were presented as mean (standard deviation) or median (range), depending on the normal or skewed distribution of data. The normal distribution of quantitative data was assessed by an independent sample t-test. Comparison of qualitative variables between the groups was done using the Chi-square test. Comparative analysis of parameters between pre- and postoperative variables was done using the paired sample t-test. A P < 0.05 was considered statistically significant.
RESULTS
Demographic and preoperative characteristics
A total of 125 patients with renal stones were included in this study. Most patients belonged to the age group of >40 to ≤85 years (n = 62) followed by age group >20 to ≤40 years (n = 59) and age ≥2 to ≤18 years (n = 4). The average age was 40.1 years in group I and 41.6 years in group II. In both groups, the numbers of males were higher (69.7% and 67.8%). The average size of the stone was comparable in both the groups (15.6 mm) [group I] and 17 mm [group II] (P = 0.053). The mean stone volume (Group I-3414 cubic mm, Group II– 3710 cubic mm) and the mean stone hardness (Group I-1035 HU, Group II-1160 HU) were comparable in both the groups. A total of 25.8% of patients from group I and 37.3% of patients from group II reported previous surgical history (P = 0.181).
The stones were categorized according to modified Guy's stone score. In Ho:YAG group, grade I stone score was seen in 43 patients, grade II in 19 patients, and four patients were classified into grade III. Similarly, in the TFL group, grade I Guy's stone score was observed in 31 patients, grade II in 26 patients and two patients were having grade III scores. Guy's Grade IV and V stone categories were not observed in any of the patients from both groups [Table 1].
Table 1.
Pre- and intraoperative characteristics of the two patient groups
| Parameter | Group I (Holmium) (n=66)* | Group II (Thulium) (n=59)** | P |
|---|---|---|---|
| Age (years), mean (SD) | 40.1 (12.2) | 41.6 (16.9) | 0.605 |
| Age group (years) | |||
| ≥2–≤18 | - | 4 (6.8) | 0.038 |
| >20–≤40 | 36 (54.5) | 23 (39.0) | |
| >40–≤85 | 30 (45.5) | 32 (54.2) | |
| Sex | |||
| Women | 20 (30.3) | 19 (32.2) | 0.849 |
| Men | 46 (69.7) | 40 (67.8) | |
| Haemoglobin (g/dL), mean (SD) | 13.3 (1.8) | 13.2 (2.0) | 0.828 |
| Serum creatinine (ng/mL), median (range) | 1.1 (0.5-9.7) | (n=58) 1.0 (0.2-2.4) |
0.496 |
| Comorbidities | |||
| Previous surgical history | 17 (25.8) | 22 (37.3) | 0.181 |
| Duration (days) | (n=58) | ||
| ≥2–≤3 | 50 (75.8) | 41 (70.7) | 0.548 |
| >4–≤17 | 16 (24.2) | 17 (29.3) | |
| Type of stone | |||
| Unilateral | 47 (71.2) | 40 (67.8) | 0.701 |
| Bilateral | 19 (28.8) | 19 (32.2) | |
| Stone side | |||
| Right | 35 (53.0) | 34 (57.6) | 0.719 |
| Left | 31 (47.0) | 25 (42.4) | |
| Stone size (mm), mean (SD) | 15.6 (3.8) | 17.0 (4.1) | 0.053 |
| Stone size group (mm) | |||
| ≥10–≤15 | 37 (56.1) | 15 (25.4) | <0.001 |
| >15–≤20 | 22 (33.3) | 14 (23.7) | |
| >20–≤30 | 7 (10.6) | 30 (50.8) | |
| Stone volume (cubic mm), median (range) | 3414.0 (1099.0-7101.0) | 3710.0 (1100.0-6100.0) | 0.737 |
| Stone density (HU) | 1035.0 (550.0-1600.0) | 1160.0 (690.0-1520.0) | 0.309 |
| Guy’s stone score | |||
| Grade I | 43 (65.2) | 31 (52.5) | 0.190 |
| Grade II | 19 (28.8) | 26 (44.1) | |
| Grade III | 4 (6.1) | 2 (3.4) | |
| Grade IV | - | - | |
| Grade V | - | - | |
| Puncture site | (n=65) | 0.758 | |
| Lower | 46 (70.8) | 39 (66.1) | |
| Upper | 4 (6.2) | 3 (5.1) | |
| Mid | 15 (23.1) | 17 (28.8) | |
| Postprocedure nephrostomy | 12 (18.2) | 14 (23.7) | 0.451 |
| Blood transfusion | (n=65) 2 (3.1) |
1 (1.7) | 1.000 |
| Stone disintegration time (min s), median (range) | 20.45 (12.35-55.00) | 11.19 (0.52-25.00) | <0.001 |
| Operative time (min), mean | 68 | 55 | 0.001 |
Data shown as n (%), unless otherwise specified. *n=66, **n=59 unless otherwise specified. Group I: Holmium laser, Group II: Thulium laser, SD=Standard deviation
Intra-and postoperative characteristics
The median stone disintegration time was 20 min 45 s for the use of Ho:YAG laser and 11 min 19 s for the use of a TFL (P < 0.001). The mean operative time for thulium fiber laser was significantly less (55 min), as compared to holmium laser lithotripsy (68 min). Nephrostomy was placed in 12 patients from group I and 14 patients from group II. Two patients (3.1%) from group I and one patient (1.7%) from group II required blood transfusions due to significant intraoperative bleeding and a drop in hemoglobin.
The mean drop in hemoglobin after surgery was comparable between the two groups 56 g/dL (group I) and 55 g/dL (group II). Thirteen (22.0%) patients from TFL group experienced persistent hematuria for ≥24 h to 48 h postoperatively and two (3.0%) patients from group I had transient hematuria. The occurrence of postoperative hematuria in group II (22.0%) was significantly higher than group I (3.0%) (P = 0.002). Postoperatively, six patients in group I and 11 patients in group II presented with complications. Of these, the most common complications were Clavien grade I and II complications (P = 0.128), which were conservatively managed [Table 2]. The stone free was obtained in 60 cases (90.9%) in Holmium group I and for 56 patients (94.9%) in the TFL group.
Table 2.
Postoperative characteristics and outcomes of the two patient groups
| Parameter | Holmium laser (n=66)* | Thulium laser (n=59)** | P |
|---|---|---|---|
| Hemoglobin (g/dL) | 12.0 (1.7) | (n=56) 12.1 (2.3) |
0.924 |
| Hematocrit (%) | (n=39) 33.4 (4.9) | (n=39) 34.9 (5.1) | 0.202 |
| Stone-free rate | 60 (90.9) | 56 (94.9) | 0.498 |
| Postoperative complications, n (%) | |||
| Fever | 2 (3.0) | - | 0.005 |
| Haematuria | 2 (3.0) | 13 (22.0) | |
| Clavien–Dindo classification, n (%) | (n=57) | ||
| Grade I | 4 (6.1) | 10 (17.5) | 0.128 |
| Grade II | 2 (3.0) | 1 (1.8) | |
| Grade III | - | - | |
| Grade IV | - | - | |
| Grade V | - | - | |
| Hospital stay (days), n (%) | |||
| 2-3 | 60 (90.9) | 55 (93.2) | 0.748 |
| 4-5 | 6 (9.1) | 4 (6.8) |
Data shown as mean (SD), unless otherwise specified. *n=66, **n=59 unless otherwise specified. Group I=Holmium laser, Group II=Thulium laser, SD=Standard deviation
DISCUSSION
This study evaluated the clinical efficacy and safety of TFL with Ho:YAG laser in stone lithotripsy during the Miniperc technique. All the patients were successfully treated with TFL and Ho:YAG laser. There was no statistically significant difference between the two groups concerning age and pre- and postoperative hemoglobin. The stone lithotripsy with TFL had a shorter stone disintegration time than the Ho:YAG laser technique. This resulted in a significantly lesser mean operative time for TFL as compared to Ho:YAG laser lithotripsy. The stone clearance rate was comparatively higher in the TFL group. Although the incidence of postoperative hematuria was slightly longer with the use of TFL as compared to Ho:YAG laser lithotripsy for stones in Miniperc procedure, it was self-limiting and did not require any additional treatment.
Ho:YAG laser at present is considered the gold standard in stone lithotripsy. Apart from the stone, it also has application in prostate ablation or enucleation, bladder tumor resection, and urethral stricture disease.[4] Although Ho:YAG laser has effective stone fragmentation rates, it has a few limitations. First, although the Ho:YAG laser lithotripsy is capable of high energy, it has limitations in attaining high frequency. Newer high-power Ho:YAG machines can attain maximum energy of up to 6 J and frequency of only 100 Hz and can reach a total power up to 140 W. Second, the retropulsion rates with Ho:YAG laser is higher.[4]
The limitations of Ho:YAG laser are overcome by the new TFL, in terms of capability to work at a very high frequency with low energy levels. As the TFL wavelength (l = 1908 nm) matches closely the water absorption peak in tissue as compared to Ho:YAG laser (l = 2120 nm), it improves stone ablation.[10] The ablation threshold for Ho:YAG was more than that of TFL (82.6 J/cm2vs. 20.6 J/cm2). The ablation rate of TFL also increased with an increase in laser frequency rates.[10] Enikeev et al.[11] used TFL in high-frequency modes and concluded that higher frequency results in higher efficacy and ablation speed with low complication rates.
Kronenberg et al.[12] suggested that high power levels and frequency are not necessary for faster ablation of stone. However, most of the studies suggest that better stone ablation rates are associated with high frequency and low energy, which is possible with TFL, as it can attain higher frequency of up to 1000 Hz keeping the energy low.[13]
In vitro study by Keller et al.[14] showed that TFL is capable of producing significantly smaller stone particles of size <500 μm for all types of urinary stones composition. It was also observed by Coninck et al.[15] that TFL produces twice the amount of dust as compared to latest Ho:YAG laser Moses (R)technology. Studies have also shown that the ablation thresholds are four times lower for the TFL than for the Ho:YAG laser.[4,10]
Hardy et al.[16] demonstrated that the thulium fiber tends to produce uniform pulse energy similar to that of Moses (R)technology. It produces laser-induced bubbles within a single laser pulse and bubbles dimensions were four times smaller than those produced by Ho:YAG laser. Therefore, the retropulsion threshold was significantly higher for TFL without inducing collateral damage to tissues.
A systematic review of TFL by Kronenberg et al.[17] summarized that the ablation efficiency of TFL was two times faster for fragmentation and four times faster for dusting mode as compared to Ho:YAG laser. In our study also, when we compared the fragmentation mode of TFL with Ho:YAG laser in Miniperc, and we observed that the fragmentation was two times faster with TFL.
Most of the studies have concluded that the stone ablation efficiency of TFL is better than Ho:YAG laser (low power or Moses technology), using the similar pulse energy and frequency settings during stone lithotripsy.[18,19] Enikeev et al.[20] evaluated the efficacy of TFL in PCNL and studied the laser on time, retropulsion rates, and visibility during laser use and found significantly less retropulsion rate with the use of TFL. They also stated that there was no correlation with the laser on time with stone density. The mean laser on time was 6.5 min and resulted in significantly less operative time. In the present study, TFL was highly effective as compared to Ho:YAG laser in terms of stone disintegration time (11 min 19 s vs. 20 min 45 s). The hospital stay was less in the TFL group (2–3 days [n = 55 vs. n = 60], 4–5 days [n = 4 vs. n = 6]), and the stone-free rate was slightly better in the TFL group (n = 60 vs. n = 56), but the difference for both was not statistically significant.
In the first clinical study of super pulse, TFL performed by Traxer et al.[21] The average time required for renal stone fragmentation was 30.2 min. Glybochko et al.[22] in an in vitro study demonstrated that TFL had two times greater ablation rate than the Ho:YAG laser. The overall effect of faster fragmentation with TFL resulted in reduced operative time with TFL, which was observed in most of the in vitro studies.[18,23] Similarly, in our clinical study, we have observed that the fragmentation of the stone was faster with TFL and hence resulted in significant reduction in the operative time as compared to Ho:YAG laser (mean operative time 55 vs. 68 min).
Clavien grade I-II was the most common postoperative complications associated with TFL lithotripsy and we did not come across any case of grades III, IV, and V complications in the present study. A similar rate of complications were observed by Enikeev et al.[15] Another prospective study showed an unexplained exacerbation of pyelonephritis associated with TFL lithotripsy.[24] In our study, we did not observe any infection or pyelonephritis with the use of TFL. Korolev et al.[25] had an overall complication rate of 7.6% with TFL used during retrograde intrarenal surgery procedure. The present study demonstrated that although the incidence of hematuria occurred in 13 patients from the TFL group, it was self-limiting and managed conservatively. However, we are not able to explain the cause of hematuria for a slightly longer duration in the TFL group. The increased heat generation with the use of TFL has been discussed extensively, but there is no confirmed evidence for the same. In a comparative in vitro study by Taratkin et al.,[26] they observed that the temperature rise for TFL was 15.4°C, while for Ho:YAG laser, it was 14.9°C after 60 s of use. Furthermore, with different irrigation flow rates, they found no significant difference in the rise of temperature between TFL and Ho:YAG laser. We attribute the cause of hematuria probably due to the thermal stress injury to the mucosa because of less irrigation and visibility which results temporarily due to stone fragments. Similarly, Hardy et al.[27] showed that the temperature of TFL was 9°C–12°C higher than Ho:YAG laser, especially when the frequency was increased and the irrigation flow was low, leading to thermal stress to surrounding tissue.
Our study is the first prospective study that has compared the effectiveness of TFL with Ho:YAG laser in Miniperc procedure. With almost the same laser settings for both the lasers used for stone lithotripsy (TFL ranging from 6 to 15 W, Holmium laser between 8 and 18 W), we have observed better stone-free rates with TFL than with Ho:YAG laser, probably due to the production of smaller stone fragments and stone dust with TFL, which effectively facilitates drainage of all the stone particles.
Limitations
The present study has a few limitations. First, the stone-free rates were determined on ultrasonography and X-ray KUB. Computed tomography would have been helpful to exactly assess the size of residual fragments. A computed tomography scan was not used for the reasons of cost, as most of our patients were from low socioeconomic backgrounds, and radiation exposure. Second, the sample size is small in this study as it is a single-center study. A multicentric study with larger data will be required in the future to assess the effectivity of TFL in the Miniperc procedure.
CONCLUSIONS
The new TFL rapidly fragments the stone and requires significantly lesser stone disintegration time as compared to Ho:YAG laser; thus, effectively reducing the operative time of the Miniperc procedure. It also produces fine dust, which probably helps in improving the stone-free rates. The incidence of hematuria, though self-limiting and unalarming, is higher with TFL.
Footnotes
Financial support and sponsorship: Nil.
Conflicts of interest: There are no conflicts of interest.
REFERENCES
- 1.Mishra S, Sharma R, Garg C, Kurien A, Sabnis R, Desai M. Prospective comparative study of miniperc and standard PNL for treatment of 1 to 2 cm size renal stone. BJU Int. 2011;108:896–9. doi: 10.1111/j.1464-410X.2010.09936.x. [DOI] [PubMed] [Google Scholar]
- 2.Shafi H, Moazzami B, Pourghasem M, Kasaeian A. An overview of treatment options for urinary stones. Caspian J Intern Med. 2016;7:1–6. [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson DE, Cromeens DM, Price RE. Use of the Holmium:YAG laser in urology. Lasers Surg Med. 1992;12:353–63. doi: 10.1002/lsm.1900120402. [DOI] [PubMed] [Google Scholar]
- 4.Kronenberg P, Traxer O. The laser of the future: Reality and expectations about the new thulium fiber laser – A systematic review. Transl Androl Urol. 2019;8:S398–417. doi: 10.21037/tau.2019.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Traxer O, Keller EX. Thulium fiber laser: The new player for kidney stone treatment? A comparison with Holmium:YAG laser. World J Urol. 2020;38:1883–94. doi: 10.1007/s00345-019-02654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried NM. Recent advances in infrared laser lithotripsy [Invited] Biomed Opt Express. 2018;9:4552–68. doi: 10.1364/BOE.9.004552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glybochko P, Altshuler G, Yaroslavasky I, Vinarov A, Rapoport L, Enikeev D, et al. Comparative in vitro study of Ho:YAG and tm fiber laser lithotripters in dusting mode of operation. J Urol. 2017;197:E815. [Google Scholar]
- 8.Peng Y, Liu M, Ming S, Yu W, Li L, Lu C, et al. Safety of a novel thulium fiber laser for lithotripsy: An in vitro study on the thermal effect and its impact factor. J Endourol. 2020;34:88–92. doi: 10.1089/end.2019.0426. [DOI] [PubMed] [Google Scholar]
- 9.Andreeva V, Vinarov A, Yaroslavsky I, Kovalenko A, Vybornov A, Rapoport L, et al. Preclinical comparison of superpulse thulium fiber laser and a Holmium:YAG laser for lithotripsy. World J Urol. 2020;38:497–503. doi: 10.1007/s00345-019-02785-9. [DOI] [PubMed] [Google Scholar]
- 10.Blackmon RL, Irby PB, Fried NM. Comparison of Holmium:YAG and thulium fiber laser lithotripsy: Ablation thresholds, ablation rates, and retropulsion effects. J Biomed Opt. 2011;16:071403. doi: 10.1117/1.3564884. [DOI] [PubMed] [Google Scholar]
- 11.Enikeev D, Taratkin M, Klimov R, Inoyatov J, Azilgareeva C, Ali S, et al. Superpulsed thulium fiber laser for stone dusting: In search of a perfect ablation regimen – A prospective single-center study. J Endourol. 2020;34:1175–9. doi: 10.1089/end.2020.0519. [DOI] [PubMed] [Google Scholar]
- 12.Kronenberg P, Traxer O. Update on lasers in urology 2014: Current assessment on Holmium:yttrium-aluminum-garnet (Ho:YAG) laser lithotripter settings and laser fibers. World J Urol. 2015;33:463–9. doi: 10.1007/s00345-014-1395-1. [DOI] [PubMed] [Google Scholar]
- 13.Schembri M, Sahu J, Aboumarzouk O, Pietropaolo A, Somani BK. Thulium fiber laser: The new kid on the block. Turk J Urol. 2020;46:S1–10. doi: 10.5152/tud.2020.20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keller EX, De Coninck V, Doizi S, Daudon M, Traxer O. Thulium fiber laser: Ready to dust all urinary stone composition types? World J Urol. 2021;39:1693–8. doi: 10.1007/s00345-020-03217-9. [DOI] [PubMed] [Google Scholar]
- 15.Coninck V, Keller E, Kovalenko A, Vinnichenko V, Traxer O. MP03-20. Dusting efficiency comparison between Moses technology of Ho:YAG laser and superpulse thulium fiber laser. https://doi.org/10.1097/01.JU.0000554952.38129.59. [Google Scholar]
- 16.Hardy LA, Kennedy JD, Wilson CR, Irby PB, Fried NM. Analysis of thulium fiber laser induced bubble dynamics for ablation of kidney stones. J Biophotonics. 2017;10:1240–9. doi: 10.1002/jbio.201600010. [DOI] [PubMed] [Google Scholar]
- 17.Kronenberg P, Hameed BZ, Somani B. Outcomes of thulium fibre laser for treatment of urinary tract stones: Results of a systematic review. Curr Opin Urol. 2021;31:80–6. doi: 10.1097/MOU.0000000000000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panthier F, Doizi S, Lapouge P, Chaussain C, Kogane N, Berthe L, et al. Comparison of the ablation rates, fissures and fragments produced with 150 μm and 272 μm laser fibers with superpulsed thulium fiber laser: An in vitro study. World J Urol. 2021;39:1683–91. doi: 10.1007/s00345-020-03186-z. [DOI] [PubMed] [Google Scholar]
- 19.Gao B, Bobrowski A, Lee J. A scoping review of the clinical efficacy and safety of the novel thulium fiber laser: The rising star of laser lithotripsy. Can Urol Assoc J. 2021;15:56–66. doi: 10.5489/cuaj.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enikeev D, Taratkin M, Klimov R, Alyaev Y, Rapoport L, Gazimiev M, et al. Thulium-fiber laser for lithotripsy: First clinical experience in percutaneous nephrolithotomy. World J Urol. 2020;38:3069–74. doi: 10.1007/s00345-020-03134-x. [DOI] [PubMed] [Google Scholar]
- 21.Traxer O, Rapoport L, Tsarichenko D, Dymov A, Enikeev D, Proskura A, et al. V03-02 first clinical study on superpulse thulium fiber laser for lithotripsy. J Urol. 2018;199:e321–2. [Google Scholar]
- 22.Glybochko P, Altshuler G, Vinarov A, Rapoport L, Enikeev D, Grigoriev N, et al. 226-comparison between the possibilities of holmium and thulium laser in lithotripsy in vitro. Eur Urol Suppl. 2017;16:e391–2. [Google Scholar]
- 23.Chiron P, Berthe L, Haddad M, Dizi S, Traxer O. PD59-06 in vitro comparison of efficiency between superpulsed thulium fiber laser and HO:YAG laser for endocorporeal lithotripsy. https://doi.org/10.1097/01.JU.0000557239.91246.8b. [Google Scholar]
- 24.Martov AG, Ergakov DV, Guseinov MA, Andronov AS, Dutov SV, Vinnichenko VA, et al. Initial experience in clinical application of thulium laser contact lithotripsy for transurethral treatment of urolithiasis. Urologiia. 2018;1:112–20. [PubMed] [Google Scholar]
- 25.Korolev D, Klimov R, Tsarichenko D, Enikeev N, Dymov A, Ali S, et al. RIRS for kidney stones with novel superpulse thulium (TM) fiber laser: First clinical experience. Eur Urol Open Sci. 2020;19:e14. [Google Scholar]
- 26.Taratkin M, Laukhtina E, Singla N, Kozlov V, Abdusalamov A, Ali S, et al. Temperature changes during laser lithotripsy with Ho:YAG laser and novel Tm-fiber laser: A comparative in-vitro study. World J Urol. 2020;38:3261–6. doi: 10.1007/s00345-020-03122-1. [DOI] [PubMed] [Google Scholar]
- 27.Hardy LA, Wilson CR, Irby PB, Fried NM. Thulium fiber laser lithotripsy in an in vitro ureter model. J Biomed Opt. 2014;19:128001. doi: 10.1117/1.JBO.19.12.128001. [DOI] [PubMed] [Google Scholar]


