Abstract
Background:
Around the world, an increasing number of people are turning towards nature by using the natural herbal products not only because they are inexpensive but also for better cultural acceptability, better compatibility with the human body and minimal side effects. This study assessed the effectiveness of liquorice (root extract) mouth rinse against dental plaque and gingivitis and compared it with 0.2% chlorhexidine (CHX) gluconate mouth rinse.
Materials and Methods:
A double-blind, concurrent parallel randomized controlled clinical trial (CTRI/2016/09/007311) of four months duration was conducted. Forty-four volunteers who met the inclusion criteria were randomized into two groups through the computer-generated random sequence. Based on in vitro minimum inhibitory and minimum bactericidal concentration evaluations on periodontal pathogens, a concentration of 20% (w/v) of aqueous liquorice root extract mouth rinse was prepared. Both the groups were asked to rinse with their respective mouthwash twice daily for 15 days. Gingivitis was evaluated using gingival index (GI), and dental plaque was evaluated using the Turesky modification of the Quigely Hein Plaque Index (PI). The evaluation was carried out at day zero, 8th and 23rd (15 days after intervention). Intra- and intergroup comparisons of indices for both the arms were done using the paired sample t-test and unpaired t-test, respectively.
Results:
There was a statistically significant (P = 0.000) reduction in mean PI and GI scores for both the groups after a follow up of 15 days. The intergroup comparison of plaque and gingival index scores for both the mouth rinse groups came out to be statistically significant (P = 0.000).
Conclusion:
Both liquorice and CHX gluconate mouth rinse restricted plaque accumulation and gingival inflammation. Considering the established side effects of long-term use of chemical formulations, the herbal mouth rinse preparation can promise to be an effective self-care therapy.
Keywords: Chlorhexidine, gingivitis, herbal, liquorice, plaque index
INTRODUCTION
The periodontal diseases are one of the major reasons for tooth loss in adults.[1] The onset or progression of periodontal diseases can be effectively prevented by mechanical as well as chemical plaque control measures,[2,3,4] out of all antiplaque chemical agents used, chlorhexidine (CHX) has proven its effectiveness beyond dispute.[3,4] Since 1954, CHX has been used clinically for several purposes, but it is burdened by some side effects most notably staining of teeth and oral mucosae[4] others being dysgeusia and ulcerations erosions of oral mucosa, thus decreasing patient compliance.[3,4] Considering the potential side effects incapacitating its long-term use, an alternative formulation with comparable efficacy and lesser side effects needs to be explored.
Medical and dental research has recently made evidenced shift of approach for treating many inflammatory oral diseases by using herbal modalities Acacia catechu, Aloe vera, Azadirachata indica, Ocimum sanctum, and Punica granatum to name a few.[5] The earliest recorded evidence of their use in Indian, Chinese, Egyptian, Greek, Roman, and Syrian texts dates back to about 5000 years. The classical Indian texts include Rigveda, Atharvaveda, Charak Samhita, and Sushruta Samhita.[6] Liquorice is one such herb that has been used since ages in the folk medicine as laxative, emmenagogue, contraceptive, galactogogue, anti-asthmatic, and antiviral agent.[7] It has been affirmed as generally recognized as safe (GRAS) for the use in foods by the United States Food and Drug Administration. Liquorice root, liquorice extract, liquorice extract powder, and glycyrrhizin were included in Flavor and Extract Manufacturers’ Association list of GRAS substances.[8,9]
Recent researches suggest that liquorice and its bioactive ingredients such as glycyrrhizin, glabridin, licochalcone A, licoricidin, and licorisoflavan possess potent beneficial effects in oral diseases such as dental caries, periodontitis, candidiasis, and recurrent aphthous ulcers.[10] Although many studies have been published to claim the anticariogenic potential of liquorice,[8,11] in spite of the evidences provided by in vitro studies, only a few human clinical trials have been carried out to support the therapeutic effects of liquorice and its bio-active ingredients in the treatment and prevention of periodontal diseases. Thus, the present study was designed to compare in vitro assessment against anaerobic periodontal pathogens-Porphyromonas gingivalis, Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans and Mutans streptococci. and in vivo efficacy of liquorice mouth rinse and CHX gluconate mouth rinse in the prevention of plaque formation and gingivitis. It was hypothesized that there is no difference in efficacy of liquorice and CHX mouth rinse.
MATERIALS AND METHODS
The present clinical trial was designed as a concurrent parallel, randomized, double-blind (participant and investigator blind) positive control clinical trial having two intervention groups with allocation ratio of 1:1. The trial was conducted in the department of Public Health Dentistry in a deemed to be university from February 2016 to September 2016 after obtaining ethical clearance (Project no. 748 dated March 28, 2016). The study period included plant material procurement, its certification and authentication, preparation of extracts, in vitro assay, and four months clinical trial period (May 12, 2016–September 5, 2016); encompassing patient enrolment, data collection, and interpretation. It was conducted as per the declaration of Helsinki[12] following the Indian Council of Medical Research Ethical Guidelines for Biomedical Research on human participants[13] and is being reported in accordance with CONSORT 2010 statement.[14] The present trial was registered with Clinical Trial Registry India (CTRI/2016/09/007311). The sample size[15] based on a previous study was estimated to be 40 subjects N = ([4 σ2](Z[1−[α/2]] + Z[1−ß)]]2) ÷ E2 at a power of 80% and confidence interval of 95%. Given an anticipated dropout of 10%, four participants were added to the main sample size inflating it to 44.
The trial was open for participation during the entire study period till the recruitment of the last participant. The recruitment process began with an announcement and display of invitation on the notice boards of institutes and colleges in and around deemed to be university. Students from Engineering, Pharmacy, and Nursing institution voluntarily enrolled in the trial and those who fulfilled following criteria were included in the trial. All the healthy volunteers aged 18–30 years (irrespective of gender) with moderate gingivitis, having at least 20 natural functional teeth without any restorations, orthodontic appliances, and habitual mouth breathing[16] who consented to participate in the trial were included in the study. The criteria for the exclusion were antibiotic and periodontal therapy in past month, allergy to any of test products, habits detrimental to oral health, for example, any form of tobacco use or alcohol or using any other chemotherapeutic antiplaque/antigingivitis products and having severe malalignment of teeth, orthodontic appliances, fully crowned teeth, and removable partial dentures.
At the baseline visit, the objectives of trial and its methodology were explained to volunteers and those fulfilling inclusion criteria were randomized using an online software.[17] As the sample for the trial was 44; unit values in which each integer appears only once option was given to generate the sequence among 44 volunteers. Number set that appeared on the screen was considered study group and the other numbers were assigned to the control group. Prior to randomization, they were directed to draw a token from a box containing 44 numbered tokens and same number was their unique identification code. To address blinding protocol throughout the trial, sequence generation (GMS) and subject allocation (JS) were performed by persons other than principle investigator.
Aqueous and ethanolic preparations of mouthwash were prepared by principle investigator (SS) in M. M. College of Pharmacy, Mullana after taxonomical verification of plant material at Herbarium, Department of Botany, Forest Research Institute, Dehradun. Five ml of both the preparations was sent for in vitro antimicrobial analysis at Department of Microbiology, Maratha Mandal's NGH Institute of Dental Sciences and Research Center, Belgaum, Karnataka, India, to determine the final concentration for mouth rinse preparation.
Evaluation of antimicrobial efficacy of the aqueous and ethanolic root extract against P. gingivalis, F. nucleatum, A. actinomycetemcomitans and Mutans streptococci was determined in vitro by minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) procedure. Then, the results for both the extracts were compared with that of 0.2% CHX as a control. The microbial growth inhibitory potential of both the extracts was determined using the agar disc-diffusion method.[18] As in the in vitro phase, the bacterial strains were found to be sensitive to both aqueous and ethanolic extracts; aqueous liquorice root extract was decided to be prepared as final mouth rinse solution. Based on the results obtained from in vitro microbial testing, 20% concentration (i.e., 2 gm of extract powder/10 ml of distilled water) was decided to be prepared as the final mouth rinse solution. Freshly prepared mouth rinse solution was given to the participant every 4th day throughout his/her trial period in sequentially numbered containers.
Participants in the experimental arm received 20% (w/v) liquorice root extract mouth rinse and 0.2% Chlorhexidine gluconate Mouthwash (Hexidine® ICPA; Mumbai, India) was given to participants in control arm. Both the mouth rinse solutions were dispensed in amber-colored sequentially numbered containers with no detectable difference, having equal weight, similar appearance and were tamper proof. The allocation sequence was concealed from principle investigator in sequentially numbered, opaque, sealed, and stapled envelopes. Allocation and concealment were performed by JS. All the study participants were well explained the study protocols and were provided with patient information sheet. Demographic details and oral hygiene practices were recorded on a prestructured format.
All the clinical examinations were carried out by principle investigator (SS). Each participant was assessed for gingival inflammation using gingival index (GI)[19] and Turesky modification of the Quigley-Hein plaque Index (PI)[20] was used for evaluating dental plaque by using G C Triplaque I D gel[21] (GC America). On day zero, clinical assessment was done, and oral prophylaxis was performed to balance out the baseline scores and ensure comparability of follow-up measurements. On the same day, participants were provided a custom-made tooth paste[11] and were advised to adhere to their routine self-performed oral hygiene practices. The gingival inflammation and plaque scores were recorded on day zero, 8th day and 23rd day. On the 8th day, after prerinse assessment, participants were provided mouth rinse solution by JS as per the random-generated sequence and were instructed to rinse with 10 ml of given mouth rinse solution twice daily for 60 s. The participants were asked to brush with given dentifrice at least half an hour before rinsing, refrain from consumption of tea, coffee, and alcoholic beverages immediately after mouth rinse. Under same person's supervision, the participants were asked to rinse with the solution in dental clinic for the first time and were advised to follow the same rinsing pattern for 15 days and were recalled on the 23rd day of the trial. All the participants were regularly reminded of their rinsing schedule through short message services (SMSs) throughout the trial duration. They were also encouraged to report any adverse effect such as oromucosal inflammation, ulceration of oral mucosa, burning sensation in mouth, loss of taste sensation, gastric disturbance, or any other reaction due to usage of mouth rinse during the course of trial, but none of the participants reported any event. The flow of participants and interventions were followed from allocation to the final data analysis on the 23rd day [Figure 1].
Figure 1.
Consort flow diagram for the trial. n – Number of participants
The data collected were entered in the Microsoft Excel sheet version 14.4.7 and analyzed using the SPSS software version 20 (Statistical Package for Social Sciences (SPSS) for Windows, Version 20.0. Armonk, NY: IBM Corp).[22] Descriptive statistics were used for baseline characteristics of participants in both the intervention groups. Intra and intergroup comparisons of indices for both the arms were done using the paired sample t test and unpaired t test, respectively. The level of significance was set at P < 0.05. Drop out analysis was performed using intention-to-treat (imputation method) approach. According to their original group allocation, all of the participants were included in the analysis in either the study or the control group.
RESULTS
In the present trial, 44 volunteers (13 males and 31 females) with age ranging from 18 to 30 years with a mean age of 21.31 ± 2.67 and 20.04 ± 2.17 years in the experimental and control group, respectively, participated. Tables 1 and 2 present MIC and MBC of the liquorice extract used. The intragroup comparison of two groups revealed that there was reduction in PI and GI scores in both arms of the trials [Graph 1]. There was no difference between two groups with respect to outcome measurements at baseline for gingival and plaque indices [Table 3]. The results of the present study demonstrated that both liquorice and CHX mouth rinses reduced PI and GI scores in participants, but the reduction was considerable in CHX group as compared to liquorice group and their differences was statistically significant (P < 0.001) for both the groups after 15 days [Table 3]. Drop-out analysis for study groups is presented in Table 4.
Table 1.
Minimum inhibitory concentration of chlorhexidine and liquorice root extract solutions (aqueous and ethanolic) against periodontal pathogens and Streptococcus mutans
| 100% | 50% | 25% | 12.5% | 6.25% | 3.12% | 1.6% | 0.8% | 0.4% | 0.2% | |
|---|---|---|---|---|---|---|---|---|---|---|
| Porphyromonas gingivalis | ||||||||||
| Aqueous | S | S | S | S | R | R | R | R | R | R |
| Ethanolic | S | S | S | S | R | R | R | R | R | R |
| 0.2% CHX | S | S | S | S | S | R | R | R | R | R |
| Fusobacterium nucleatum | ||||||||||
| Aqueous | S | S | S | R | R | R | R | R | R | R |
| Ethonolic | S | S | S | S | S | R | R | R | R | R |
| 0.2% CHX | S | S | S | S | S | S | R | R | R | R |
| Aggregatibacter actinomycetemcomitans | ||||||||||
| Aqueous | S | S | S | S | S | S | S | S | S | R |
| Ethanolic | S | S | S | S | S | S | S | S | R | R |
| 0.2% CHX | S | S | S | S | S | S | R | R | R | R |
| Mutans streptococci | ||||||||||
| Aqueous | R | R | R | R | R | R | R | R | R | R |
| Ethanolic | R | R | R | R | R | R | R | R | R | R |
| 0.2% CHX | S | S | S | S | S | S | R | R | R | R |
S – Sensitive; R – Resistant; CHX – Chlorhexidine
Table 2.
Minimum bactericidal concentration of chlorhexidine and liquorice root extract solutions (aqueous and ethanolic) against periodontal pathogens and Streptococcus mutans
| 100% | 50% | 25% | 12.5% | 6.25% | 3.12% | |
|---|---|---|---|---|---|---|
| Porphyromonas gingivalis | ||||||
| Aqueous | NG | NG | NG | 80 | 120 | 200 |
| Ethonolic | NG | NG | NG | NG | 30 | 60 |
| 0.2% CHX | NG | NG | NG | NG | NG | NG |
| Fusobacterium nucleatum | ||||||
| Aqueous | 250 | 350 | 400 | 500 | 550 | 600 |
| Ethanolic | 300 | 400 | >400 | 450 | 500 | >500 |
| 0.2% CHX | NG | NG | NG | NG | NG | 50 |
| Aggregatibacter actinomycetemcomitans | ||||||
| Aqueous | NG | NG | NG | NG | NG | NG |
| Ethonolic | NG | NG | NG | NG | NG | NG |
| 0.2% CHX | NG | NG | NG | NG | 30 | 80 |
| Streptococci mutans | ||||||
| Aqueous | 250 | >300 | 400 | 450 | 600 | 700 |
| Ethonolic | 200 | 300 | 400 | 500 | 550 | 600 |
| 0.2% CHX | NG | NG | NG | NG | NG | 150 |
NG – No growth; CHX – Chlorhexidine
Graph 1.
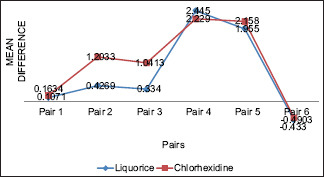
Intragroup comparisons among both mouth rinse groups. G.I – Gingival Index; P.I Plaque Index. Pair 1- G.I. 1 - G.I. 2, Pair 2-G.I. 1 - G.I.3, Pair 3-G.I. 2 - G.I.3. Pair 4-P.I. 1 - P.I. 2, Pair 5- P.I. 1 - P.I.3, Pair 6- P.I. 2 - P.I.3
Table 3.
Comparison of gingival index and plaque index scores between study groups
| Test group | Control group | t | P | Mean difference | SE difference | |
|---|---|---|---|---|---|---|
| GI 1 | 1.8581±0.22120 | 1.9582±0.13497 | −1.81 | 0.07 | −0.10009 | 0.05525 |
| GI 2 | 1.7509±0.20090 | 1.7945±0.12031 | −0.87 | 0.38 | −0.04364 | 0.04992 |
| GI 3 | 1.4070±0.16727 | 0.7481±0.17397 | 12.35 | 0.000* | 0.65890 | 0.05335 |
| PI 1 | 2.758±0.6884 | 2.655±0.442 | 0.590 | 0.558 | 0.10295 | 0.17448 |
| PI 2 | 0.313±0.0921 | 0.426±0.1783 | −2.641 | 0.01* | −0.11305 | 0.04280 |
| PI 3 | 0.790±0.2051 | 0.471±0.228 | 4.69 | 0.000* | 0.31905 | 0.06798 |
*P<0.05 statistically significant at 95% CI, unpaired t-test. Baseline measurement (1); After 7 days (2); After 15 days (3). GI – Gingival index; PI – Plaque index; SD – Standard deviation; CI – Confidence interval; SE – Standard error; t - t statistic; P – level of statistical significance
Table 4.
Results for drop-out analysis
| Imputation number | R | R2 | Adjusted R2 | SE of the estimate | df 1 | Significant f change |
|---|---|---|---|---|---|---|
| Original data | 0.893 | 0.797 | 0.786 | 0.23391 | 2 | 0.000* |
| 1 | 0.826 | 0.682 | 0.666 | 0.29213 | 2 | 0.000* |
| 2 | 0.677 | 0.458 | 0.431 | 0.38139 | 2 | 0.000* |
| 3 | 0.741 | 0.549 | 0.527 | 0.34801 | 2 | 0.000* |
| 4 | 0.835 | 0.697 | 0.683 | 0.28496 | 2 | 0.000* |
| 5 | 0.801 | 0.642 | 0.624 | 0.31005 | 2 | 0.000* |
*Difference is statistically significant at 95% CI. Predictors PI-1, GI-1, PI-2, GI-2 were constant; 2 Imputation done for PI-3 and GI-3 (missing response). GI – Gingival index; PI – Plaque index; CI – Confidence interval; SE – Standard error; df – degrees of freedom; R – Correlation Coefficient, R2 – Coefficient of Determination, f- f statistic
In the present clinical trial, there were two drop-outs (9.1%) in the liquorice groups and one (4.6%) in the CHX control group. Intention to treat analysis has been carried out for missing responses in both the groups and imputation method was used for estimating the same.
Range of R2 values for various imputation of missing responses was 0.642–0.797, which was statistically significant (f = 0.000).
DISCUSSION
Modern medicine has its roots in ethnobotanical practices that use native flora for curing human diseases and improving certain body conditions.[23] Liquorice or Mulethi is a perennial herb that is being used by various cultures for 1000 of years. The therapeutic properties of liquorice are due to the flavonoids and main saponin present in it, i.e., glycyrrhizin. It possesses adrenocorticoid and anti-inflammatory actions and imparts sweet flavor to liquorice.[24] This sweet nature of liquorice can also aid to its increased acceptability in the form of mouth rinse.
The in vitro assessment of the test preparation demonstrated antibacterial activity against bacterial strains used which is in accordance with earlier studies.[25,26,27] The liquorice and its bioactive ingredients act on primary plaque colonizers and periodontal pathogens that represent the etiological factor in this disease and inhibit their growth. In the present, randomized, concurrent parallel trial, the test and control group did not differ from each other with respect to the plaque and gingival indices scores at baseline. Thus, the groups were compared after the use of the experimental and control mouth rinse. The results of the trial showed that there was statistically significant (P < 0.001) reduction in mean PI and mean GI for both the groups after a follow-up of 15 days. These findings support the evidence from previous studies that state the ability of herbal extracts to reduce inflammation.[28,29,30,31,32,33,34] The anti-inflammatory effect of liquorice can be attributed to glycyrrhizin, one of the main compounds of Glycyrrhiza glabra, exhibits corticosteroids-like action, including inhibitory activity on prostaglandin E2 production by activated macrophages and suppressive action on formation of superoxide and hydroperoxide in macrophages.[7] Glycyrrhizin is also able to inhibit glycosyltransferase activity that in turn inhibits oral bacterial adherence.[35]
There was statistically significant reduction in outcome measurements in both the groups, but magnitude of reduction is greater in CHX group for plaque and gingival indices scores with mean difference of 0.32 and 0.65, respectively (P < 0.001). Considering the chemical properties of both test and control mouth rinses, the superior effect of CHX could be attributed to its property of substantivity. The presence of β cyclodextrin regulates and controls the amounts of CHX released thus, greater the amount of β cyclo dextrin, the more progressive the release of CHX.[4] Whereas this property is not evident in case of liquorice, although it has antimicrobial and anti-inflammatory actions.[7,36] Moreover, the sweet taste of liquorice promoted early swallowing of saliva and its consequent wash out from the oral cavity.[37] However, on delivering liquorice extract as a rinse, the duration might be short to provide optimum contact of drug with the oral cavity. Therefore, the mode in which liquorice is delivered is also a major criterion to effectuate its duration of action. Liquorice preparation has also been tested as a hard candy or chewing gum that has shown an extended release in the mouth which significantly enhanced its sustained action.[38] The present study has revealed that both the herbal and CHX mouth rinse were effective in reducing dental plaque and gingivitis for a period of 15 days. However, there was difference in magnitude of this decrease. Keeping in view, the established side effects of long-term usage of CHX, liquorice mouth rinse can prove to be beneficial as an adjunct to mechanical plaque control. It can promise to be a better preventive home care therapy in developing countries such as India where accessibility, affordability, availability, and sustainability are important issues. In vitro comparison of ethanolic and aqueous base preparations yielded comparable results; however, an in vivo comparison of the preparations was not performed. Although SMS was utilized to ascertain participant's compliance, an unsupervised rinsing practice could be one of the limiting factors in the present study. Further studies can be conducted to explore long-term efficacy of liquorice extract in controlling plaque formation and preventing onset of gingival disease. Microbial evaluation of saliva and other biochemical parameters could be used to substantiate the results of the present study. An in vivo comparisons of alcohol and aqueous-based preparations can also be carried out to ascertain the preventive potential of the two.
CONCLUSION
The results of the present study indicate that liquorice extract reduces plaque formation and thus can alter course of gingival inflammation. However, as compared to CHX, the magnitude of this reduction is less, but keeping in view, the established side effects of long-term usage of CHX, liquorice mouth rinse can be an effective self-care therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors express heartfelt gratitude to all the study participants and the parent institution for providing the necessary resources. We are thankful to Dr Kishore G Bhat; Director, Central Research Lab, Maratha Mandal's NGH Institute of Dental Sciences and Research Centre, Belgaum, Karnataka, India, for generously sharing his expertise.
REFERENCES
- 1.Shaju JP, Zade RM, Das M. Prevalence of periodontitis in the Indian population: A literature review. J Indian Soc Periodontol. 2011;15:29–34. doi: 10.4103/0972-124X.82261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chatterjee A, Saluja M, Singh N, Kandwal A. To evaluate the antigingivitis and antipalque effect of an Azadirachta indica (Neem) mouthrinse on plaque induced gingivitis: A double-blind, randomized, controlled trial. J Indian Soc Periodontol. 2011;15:398–401. doi: 10.4103/0972-124X.92578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaur H, Jain S, Kaur A. Comparative evaluation of the antiplaque effectiveness of green tea catechin mouthwash with chlorhexidine gluconate. J Indian Soc Periodontol. 2014;18:178–82. doi: 10.4103/0972-124X.131320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaur G, Singh A, Patil KP, Gopalakrishnan D, Nayyar AS, Deshmukh S. Chlorhexidine: First to be known, still a gold standard anti-plaque agent. Res J Pharm Biol Chem Sci. 2015;6:1407–24. [Google Scholar]
- 5.Bagade VB, Jadhav VM, Kadam VJ. Study on Antimicrobial activity of herbal formulation. Int J Pharm Life Sci. 2013;4:3099–104. [Google Scholar]
- 6.Pal SK, Shukla Y. Herbal medicine: Current status and the future. Asian Pac J Cancer Prev. 2003;4:281–8. [PubMed] [Google Scholar]
- 7.Sharma V, Agrawal RC. Glycyrrhiza glabra – A plant for the future. Mint J Pharm Med Sci. 2013;2:15–20. [Google Scholar]
- 8.Dietary Supplements: Licorice. Vol. 4. Rockville (MD): United States Pharmacopeial Convention; 2014. [Accessed on 23-09-2015]. The United States Pharmacopeia. National formulary. Available at http://www.pharmacopeia.cn/v29240/usp29nf24s0_m45050.html . [Google Scholar]
- 9.Isbrucker RA, Burdock GA. Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul Toxicol Pharmacol. 2006;46:167–92. doi: 10.1016/j.yrtph.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Ayurvedic Pharmacopoeia of India. Government of India, Ministry of Health and Family Welfare. Part I. III. New Delhi, India: Department of Indian Systems of Medicine and Homoeopathy; 2014. [Google Scholar]
- 11.Fatima G, Darsika C, Sowmya KV, Azra A, Shanmuganathan S. Preparation and evaluation of herbal dentifrice. Int Res J Pharm. 2015;68:509–11. [Google Scholar]
- 12.WMA Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subjects. [Last accessed on 2015 May 21]. Available from: http://www.wma.net/en/30publications/10policies/b3 .
- 13.Indian Council of Medical Research. Ethical Guidelines for Biomedical Research on Human Subjects. New Delhi: Indian Council of Medical Research; 2006. [Google Scholar]
- 14.CONSORT Checklist and Flow Diagram for Randomized Controlled Trials. 2010. [Last accessed on 2015 Jun 20]. Available from: http://www.consort-statement.org .
- 15.Wittes J. Sample size calculations for randomized controlled trials. Epidemiol Rev. 2002;24:39–53. doi: 10.1093/epirev/24.1.39. [DOI] [PubMed] [Google Scholar]
- 16.Wagaiyu EG, Ashley FP. Mouthbreathing, lip seal and upper lip coverage and their relationship with gingival inflammation in 11-14 year-old schoolchildren. J Clin Periodontol. 1991;18:698–702. doi: 10.1111/j.1600-051x.1991.tb00112.x. [DOI] [PubMed] [Google Scholar]
- 17.Random Numbers. [Last accessed on 2015 Mar 15]. Available from: http://www.psychicscience.org/random.aspx .
- 18.Isenberg HD, editor. Clinical Microbiology Procedures Handbook. 2nd ed. Washington, DC: American Society for Microbiology; 2007. [Google Scholar]
- 19.Löe H. The Gingival Index, the plaque index and the retention index systems. J Periodontol. 1967;38:l610–6. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 20.Turesky S, Gilmore ND, Glickman I. Reduced plaque formation by the chloromethyl analogue of victamine C. J Periodontol. 1970;41:41–3. doi: 10.1902/jop.1970.41.41.41. [DOI] [PubMed] [Google Scholar]
- 21. [Last accessed on 2015 Apr 21]. Available from: http://www.gcamerica.com/products/preventive/GC_Tri_Plaque_ID/index.php .
- 22.IBM Corp. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp; 2011. [Google Scholar]
- 23.Vaish S, Ahuja S, Dodwad V, Parkash H. Comparative evaluation of 0.2% chlorhexidine versus herbal oral rinse on plaque induced gingivitis. J Indian Assoc Public Health Dent. 2012;10:55–62. [Google Scholar]
- 24.Lakshmi T, Geetha RV. Glycyrrhiza glabra Linn commonly known as licorice: A therapeutic review. Int J Pharm Pharm Sci. 2011;3:20–5. [Google Scholar]
- 25.Ajagannanavar SL, Battur H, Shamarao S, Sivakumar V, Patil PU, Shanavas P. Effect of aqueous and alcoholic licorice (Glycyrrhiza glabra) root extract against streptococcus mutans and Lactobacillus acidophilus in comparison to chlorhexidine: An in vitro study. J Int Oral Health. 2014;6:29–34. [PMC free article] [PubMed] [Google Scholar]
- 26.Sedighinia F, Afshar AS, Soleimanpour S, Zarif R, Asili J, Ghazvini K. Antibacterial activity of Glycyrrhiza glabra against oral pathogens: An in vitro study. Avicenna J Phytomed. 2012;2:118–24. [PMC free article] [PubMed] [Google Scholar]
- 27.Moini P, Eslami G, Taheri S, Valaei N, Naji RM. Comparative evaluation of antibacterial activity of Glycyrrhiza glabra (Licorice) aqueous root extract and chlorhexidine against Lactobacillus acidophilus. J Res Dentillofac Sci. 2016;1:7–14. [Google Scholar]
- 28.Ahuja S, Dodwad V, Kukreja BJ, Mehra P, Kukreja P. A comparative evaluation of efficacy of Punica granatum and chlorhexidine on plaque and gingivitis. J Int Clin Dent Res Organ. 2011;3:29–32. [Google Scholar]
- 29.Asiri FY, Alomri OM, Alghmlas AS, Gufran K, Sheehan SA, Shah AH. Evaluation of efficacy of a commercially available herbal mouthwash on dental plaque and gingivitis: A double-blinded, parallel, randomized controlled trial. J Int Oral Health. 2016;8:224–26. [Google Scholar]
- 30.Bhattacharjee R, Nekkanti S, Kumar NG, Kapuria K, Acharya S, Pentapati KC. Efficacy of triphala mouth rinse (aqueous extracts) on dental plaque and gingivitis in children. J Investig Clin Dent. 2015;6:206–10. doi: 10.1111/jicd.12094. [DOI] [PubMed] [Google Scholar]
- 31.Muglikar S, Patil KC, Shivswami S, Hegde R. Efficacy of curcumin in the treatment of chronic gingivitis: A pilot study. Oral Health Prev Dent. 2013;11:81–6. doi: 10.3290/j.ohpd.a29379. [DOI] [PubMed] [Google Scholar]
- 32.Pourabbas R, Delazarb A, Chitsaza MT. The effect of German chamomile mouthwash on dental plaque and gingival inflammation. Iran J Pharm Res. 2005;2:105–9. [Google Scholar]
- 33.Shetty PR, Setty SB, Kamat SS, Aldarti AS, Shetty SN. Comparison of the antigingivitis and antiplaque efficacy of the herboral (herbal extract) mouthwash with chlorhexidine and listerine mouthwashes: A clinical study. Pak Oral Dent J. 2013;33:76–81. [Google Scholar]
- 34.Vangipuram S, Jha A, Bhashyam M. Comparative efficacy of aloe vera mouthwash and chlorhexidine on periodontal health: A randomized controlled trial. J Clin Exp Dent. 2016;8:e442–7. doi: 10.4317/jced.53033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roshan A, Verma NK, Kumar CS, Chandra V, Singh DP, Panday MK. Phytochemical constituent, pharmacological activities and medicinal uses through the millenia of Glycyrrhiza glabra Linn: A review. Int Res J Pharm. 2012;3:45–55. [Google Scholar]
- 36.Racková L, Jancinová V, Petríková M, Drábiková K, Nosál R, Stefek M, et al. Mechanism of anti-inflammatory action of liquorice extract and glycyrrhizin. Nat Prod Res. 2007;21:1234–41. doi: 10.1080/14786410701371280. [DOI] [PubMed] [Google Scholar]
- 37.Jain E, Pandey RK, Khanna R. Liquorice root extracts as potent cariostatic agents in pediatric practice. J Indian Soc Pedod Prev Dent. 2013;31:146–52. doi: 10.4103/0970-4388.117964. [DOI] [PubMed] [Google Scholar]
- 38.Hu CH, He J, Eckert R, Wu XY, Li LN, Tian Y, et al. Development and evaluation of a safe and effective sugar-free herbal lollipop that kills cavity-causing bacteria. Int J Oral Sci. 2011;3:13–20. doi: 10.4248/IJOS11005. [DOI] [PMC free article] [PubMed] [Google Scholar]


