Abstract
BACKGROUND
Tricuspid regurgitation (TR) is common in patients with severe primary mitral regurgitation (MR). However, the evidence base is insufficient to inform a decision to perform tricuspid valve repair in the presence of moderate TR or less than moderate TR with annular dilation during mitral valve surgery (MVS).
METHODS
We randomly assigned 401 patients undergoing MVS for primary MR to receive MVS with or without tricuspid valve annuloplasty (TA). The primary 2-year endpoint was a composite of death, re-operation for TR, and progression of TR, either from baseline by 2 grades or presence of severe TR.
RESULTS
Patients undergoing MVS+TA experienced fewer primary endpoint events than MVS patients (3.9% vs 10.2%; RR 0.37; CI 0.16-0.86; p=0.02). Two-year mortality was 3.2% in MVS+TA patients and 4.5% in MVS patients (RR 0.69; CI 0.25-1.88). The 2-year prevalence of severe TR was lower in MVS+TA patients (0.6% vs 5.6%; RR 0.10; CI 0.01-0.77). There were no significant between group differences in major adverse cardiac and cerebrovascular events, functional status or quality of life at 2 years, although the rate of permanent pacemaker implantation was significantly higher in MVS+TA patients (14.1% vs 2.5%;rate-ratio 5.75; CI 2.27-14.60).
CONCLUSION
The addition of concomitant TA at time of MVS reduced the composite primary endpoint event rate at 2 years, driven by less frequent progression to severe TR. Tricuspid repair resulted in more frequent need for permanent pacemaker implantation. Whether a reduced rate of TR progression results in long-term clinical benefit requires longer follow-up.
Tricuspid regurgitation (TR) is common among patients undergoing mitral valve surgery (MVS) for primary mitral regurgitation (MR).1–3 Clinical practice guideline recommendations for management of TR during MVS derive largely from observational data.4,5 There is broad agreement that severe TR may not predictably improve after left-sided valve surgery and should be addressed at the time of the index procedure. Indeed, late re-operation for severe TR in patients with right heart failure is associated with high perioperative mortality rates.6,7
The operative management of lesser degrees of TR, however, is widely debated. Surgical and medical treatment of left-sided cardiac disease commonly results in a progressive reduction in the degree of TR with favorable right ventricular (RV) remodeling and/or a decrease in pulmonary artery pressures.8,9 Mild or moderate TR that is not corrected at the time of index left-sided cardiac surgery, however, may progress in approximately 25% of patients and result in reduced late survival and functional outcomes. Risk factors for TR progression include annular dilation exceeding 40mm (21mm/m2) in diameter on pre-operative transthoracic echocardiography, the magnitude of RV dysfunction, leaflet tethering, pulmonary hypertension, atrial fibrillation, and the presence of transvalvular pacing or defibrillator leads.10–14
Several single center observational studies and a small randomized controlled trial with an unblinded endpoint assessment have suggested that concomitant TV repair for moderate TR or mild or less TR with annular dilation is associated with reduced TR progression and improved event free survival compared with conservative management.11–13,15–19 Enthusiasm for uniform adoption of TV repair under these circumstances is tempered by concerns regarding the excess hazard of post-operative conduction disturbances necessitating permanent pacemaker implantation, the increase in cardiopulmonary bypass times, the small chance that TV replacement, rather than annuloplasty repair, might be needed, and the reality that progressive TR does not occur in all patients.1,9,20–26
These several observations have led to wide practice variations in the management of less than severe TR at the time of left-sided cardiac surgery. Rates of tricuspid valve repair at the time of MVS range from 5% to 75% and vary across surgeons and institutions.1,27 To inform decision-making, we conducted a multi-center randomized trial to assess the benefits and risks of concomitant TV repair at time of MVS for patients with moderate or less TR undergoing surgery for primary MR.
METHODS
STUDY DESIGN AND TRIAL OVERSIGHT
Patients with moderate or less TR scheduled for MVS were randomized (1:1 ratio) to MVS alone or MVS with tricuspid valve annuloplasty (MVS+TA). The randomization was stratified by TR severity and clinical center. The trial was designed to enroll 400 patients; one additional patient was consented and randomized prior to enrollment completion. Investigators were blinded to overall outcome data. Endpoints were assessed at 30 days, 6, 12, 18 and 24 months, and, after 24 months, survival will be evaluated annually up to 60 months (the latter follow-up continues).
This trial was conducted at 39 clinical centers in the U.S., Canada and Germany with a coordinating center, an echocardiographic core laboratory, an independent event adjudication committee, and an NIH-appointed data and safety monitoring board overseeing trial progress. Participating center institutional review boards approved the protocol, and all patients gave written informed consent.
PATIENTS AND INTERVENTIONS
The target population comprised adults undergoing MVS for primary MR with either moderate TR or none/trace or mild TR with tricuspid annular dilatation (≥ 40 mm or index: ≥21mm/M2 BSA). TR was assessed by transthoracic 2D echocardiography and verified by the central echocardiographic core laboratory. Exclusion criteria included evidence of functional MR, primary tricuspid valve disease, or sub-optimal volume management in the opinion of the site cardiologist (Appendix).
All patients underwent MVS via sternotomy or right mini-thoracotomy. The techniques of reconstructive valve surgery, including suture placement, type of prosthetic annuloplasty ring or valve, were at surgeon discretion. TV repair required an approved rigid, incomplete, nonplanar, and undersized (26, 28, or 30 mm) annuloplasty ring. All patients were to receive guideline-directed medical and/or device therapy as dictated by their clinical disease state, including beta-blockers, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, angiotensin neprilysin inhibitors, antiplatelet agents, anticoagulants, statins, aldosterone antagonists, antiarrhythmic drugs, implantable pacemakers or defibrillators and cardiac resynchronization therapy.
ENDPOINTS
The primary endpoint was treatment failure defined as the composite of death, re-operation for TR, progression of TR from baseline by 2 grades or presence of severe TR at 2 years. Secondary endpoints included death, major adverse cardiac and cerebrovascular events (MACCE; death, stroke, and serious heart failure (HF) events), permanent pacemaker implantation, length of stay, residual TR, echocardiographic indices of right ventricular size and function, NYHA classification, diuretic use, 6-minute walk test, gait-speed test for frailty, quality of life (QoL; 12-Item Short Form Survey (SF-12), Kansas City Cardiomyopathy Questionnaire (KCCQ), and EuroQoL (ED-5D)), serious adverse events, and re-hospitalizations.
STATISTICAL ANALYSIS
The trial used a parallel design with patients randomly assigned to MVS alone or MVS+TA, with 90% power to detect a 52% relative reduction in treatment failure for patients randomized to TA versus no TA using a two-sided, 0.05 level test. We assumed a 25% failure rate for MVS and 12% for MVS+TA patients. One interim analysis was planned, but not conducted, per DSMB recommendation (enrollment complete and primary endpoint assessments nearly done). The primary hypothesis was tested using a log binomial regression model of treatment failure and randomization assignment stratified by baseline TR severity. Patients with missing primary endpoint data had their 2-year status imputed via multiple imputation assuming a missing at random mechanism. The imputation model was stratified by randomization assignment and included age, sex, baseline TR severity, degree of TR at 6 and 12 months.
MACCE and all-cause mortality at 2 years were analyzed using Cox proportional hazards regression models. Thirty-day perioperative mortality, NYHA, diuretic use, and categorical echocardiographic endpoints were compared using chi-squared or Fisher’s exact tests. Six-minute walk, gait-speed, and continuous echocardiographic endpoints were compared using Wilcoxon rank-sum tests. QoL over 2 years was assessed using longitudinal linear mixed-effects models. Length of stay and ICU days during the index hospitalization were compared separately by geographic region using Wilcoxon rank-sum tests. Poisson regression with robust variance estimate was used to test group differences in serious adverse event and readmission rates through 2 years.
All endpoints were evaluated in the intent-to-treat population at the two-sided 0.05 level. There was no correction of the Type I error rate for multiple testing across secondary endpoints, as prespecified. As such, we report 95% CI not adjusted for multiplicity. Analyses were conducted using SAS version 9.4 (SAS Institute, Inc).
RESULTS
PATIENTS
Between 2016 and 2018, 5208 patients were screened; 885 were eligible, and 401 were randomized (E-Figure 1); 203 to MVS alone and 198 to MVS+TA. The two groups had similar pre-operative baseline characteristics (Table 1). The core laboratory confirmed moderate TR in 37.3% (149/399) of patients. Right ventricular systolic function was normal in 90.5% (360/398) of patients, and 30.3% (121/400) of patients had NYHA Class III/IV heart failure.
Table 1.
Baseline and Operative Characteristics
| MVS Alone (N=203) | MVS + TA (N=198) | |
|---|---|---|
| Age (years) | 68.2 ± 9.7 | 66.6 ± 10.7 |
| Male | 153/203 (75.4) | 147/198 (74.2) |
| White | 184/197 (93.4) | 182/189 (96.3) |
| Hispanic or Latino | 0/192 (0.0) | 6/193 (3.1) |
| BMI (kg/m2) | 26.3 ± 4.5 | 26.6 ± 4.5 |
| Medical History | ||
| Atrial Fibrillation | 90/203 (44.3) | 87/198 (43.9) |
| Ventricular Arrhythmia | 14/203 (6.9) | 17/198 (8.6) |
| Myocardial Infarction | 12/203 (5.9) | 7/198 (3.5) |
| Hypertension | 124/203 (61.1) | 111/198 (56.1) |
| Diabetes | 10/203 (4.9) | 16/198 (8.1) |
| Echocardiographic Measures | ||
| LVEF (%) | 64.3 ± 7.4 | 64.1 ± 7.1 |
| LV EDV (ml) | 165.0 ± 48.8 | 160.3 ± 50.4 |
| LV ESV (ml) | 60.7 ± 27.4 | 58.4 ± 25.8 |
| Severe MR | 187/202 (92.6) | 178/193 (92.2) |
| Moderate TR | 76/202 (37.6) | 73/197 (37.1) |
| TV annulus dimension - AP4 view (mm) | 42.2 ± 4.7 | 42.0 ± 4.6 |
| RV D1 basal (mm) | 44.7 ± 5.9 | 43.2 ± 6.2 |
| RV fractional area change (%) | 42.6 ± 7.6 | 43.1 ± 7.4 |
| Normal RV Function | 181/202 (89.6) | 179/196 (91.3) |
| Functional Status and QOL | ||
| NYHA Class III/IV | 68/203 (33.5) | 53/197 (26.9) |
| Six minute walk distance (feet) | 1259.9 ± 396.2 | 1327.9 ± 407.1 |
| SF-12 Physical Health T-Score | 41.9 ± 10.8 | 43.4 ± 11.5 |
| SF-12 Mental Health T-Score | 51.3 ± 9.6 | 51.4 ± 10.3 |
| EQ-5D VAS | 72.8 ± 18.1 | 73.7 ± 18.3 |
| KCCQ Overall Summary Score | 68.0 ± 22.4 | 69.4 ± 23.7 |
| Operative Characteristics | ||
| Cardiopulmonary Bypass Time (minutes) | 132.6 ± 58.8 | 166.1 ± 69.3 |
| Aortic Cross Clamp time (minutes) | 92.5 ± 37.2 | 106.9 ± 37.1 |
| Approach | ||
| Sternotomy | 103/203 (50.7) | 108/198 (54.5) |
| Right Mini-thoracotomy | 100/203 (49.3) | 90/198 (45.5) |
| MV Procedure | ||
| MV Repair | 178/203 (87.7) | 182/198 (91.9) |
| MV Replacement | 25/203 (12.3) | 16/198 (8.1) |
| TV Procedure | ||
| None | 201/203 (99.0) | 2/198 (1.0) |
| TV Repair | 1/203 (0.5) | 196/198 (99.0) |
| TV Replacement | 1/203 (0.5) | 0/198 (0.0) |
| Any Concomitant Procedure | 109/203 (53.7) | 105/198 (53.0) |
| CABG | 22/203 (10.8) | 21/198 (10.6) |
| MAZE | 49/203 (24.1) | 56/198 (28.3) |
| LAA closure | 50/203 (24.6) | 58/198 (29.3) |
| Closure of PFO | 25/203 (12.3) | 29/198 (14.6) |
The majority of patients (89.8%,360/401) underwent MV repair. In tricuspid repair recipients, the average annuloplasty ring size was 29.0±1.9 mm for men and 27.8±1.6 mm for women. Cardiopulmonary bypass time was 33.5 minutes longer in the MVS+TA group (166.1±69.3 vs 132.6±58.8 in MVS alone; 95% CI 20.9, 46.1). Based on surgeon judgment and logistics, four patients were crossed over in the operating room (Table 1). Over 50% of patients underwent concomitant procedures, including coronary bypass grafting, atrial fibrillation ablation, left atrial appendage closure and over-sewing a patent foramen ovale (Table 1).
PRIMARY ENDPOINT
Treatment failure at 2 years, defined as a composite of all-cause mortality, reoperation for TR, or TR progression (either by 2 grades from baseline or by the presence of severe TR) with imputation for missing data, was significantly more frequent for MVS patients (10.2%) compared with MVS+TA (3.9%) patients (RR 0.37;95% CI 0.16,0.86;p=0.02;Table 2). Two-year mortality was 4.5% (9/199) in the MVS group and 3.2% (6/190) in the MVS+TA group (RR 0.69;95% CI 0.25,1.88). No patients underwent TV reoperation within 2 years of randomization. The proportion of patients with severe TR at 2 years was higher for the MVS group than the MVS+TA group (5.6% [10/179] vs 0.6% [1/179];RR 0.10;95% CI 0.01,0.77). When stratified by degree of TR at baseline, treatment failure was higher in MVS alone patients compared with MVS+TA patients when moderate TR was present at baseline, but not when TR was less than moderate. This difference in treatment failure rates was driven exclusively by progression to severe TR at 2 years in the MVS alone group (Table 2).
Table 2.
Primary Endpoint by Randomization Group Overall and Stratified by Moderate or less TR at Baseline
| All Patients | MVS Alone (N=203) | MVS + TA (N=198) | Relative Risk (95% CI) | P-value |
|---|---|---|---|---|
| Primary Endpoint | ||||
| Imputed - % (95% CI) | 10.2 (6.0, 14.5) | 3.9 (1.1, 6.7) | 0.37 (0.16, 0.86) | 0.02 |
| Observed | 20/188 (10.6) | 7/185 (3.8) | 0.35 (0.15, 0.81) | - |
| Died within 2 Years | 9/199 (4.5) | 6/190 (3.2) | 0.69 (0.25, 1.88) | - |
| TV Operation within 2 Years | 0/190 (0.0) | 0/184 (0.0) | - | - |
| Progression of TR at 2 Years | 11/179 (6.1) | 1/179 (0.6) | 0.09 (0.01, 0.69) | - |
| <Moderate TR at Baselinea | MVS Alone (N=126) | MVS + TA (N=124) | Relative Risk (95% CI) | |
| Primary Endpoint | ||||
| Observed | 7/115 (6.1) | 4/117 (3.4) | 0.56 (0.17, 1.87) | - |
| Died within 2 Years | 6/123 (4.9) | 3/119 (2.5) | 0.52 (0.13, 2.02) | - |
| TV Operation within 2 Years | 0/117 (0.0) | 0/116 (0.0) | - | - |
| Progression of TR at 2 Years | 1/109 (0.9) | 1/114 (0.9) | 0.96 (0.06, 15.10) | - |
| Moderate TR at Baselinea | MVS Alone (N=76) | MVS + TA (N=73) | Relative Risk (95% CI) | |
| Primary Endpoint | ||||
| Observed | 13/72 (18.1) | 3/67 (4.5) | 0.25 (0.07, 0.83) | - |
| Died within 2 Years | 3/75 (4.0) | 3/70 (4.3) | 1.07 (0.22, 5.13) | - |
| TV Operation within 2 Years | 0/72 (0.0) | 0/67 (0.0) | - | - |
| Progression of TR at 2 Years | 10/69 (14.5) | 0/64 (0.0) | - | - |
- Two patients are excluded from stratified analyses because the echo core lab was unable to read/confirm the degree of TR at baseline
MORTALITY and MACCE
We observed no difference in cumulative 2-year mortality between treatment arms (HR 0.69;95% CI 0.24,1.93;Figure 1A). The perioperative mortality rate was 0.5% (1/203) for the MVS alone and 1.0% (2/197) for the MVS+TA group. The risk of any MACCE endpoint within two years also did not differ between groups as shown in Figure 1B (HR 0.89;95% CI 0.49,1.63).
Figure 1.
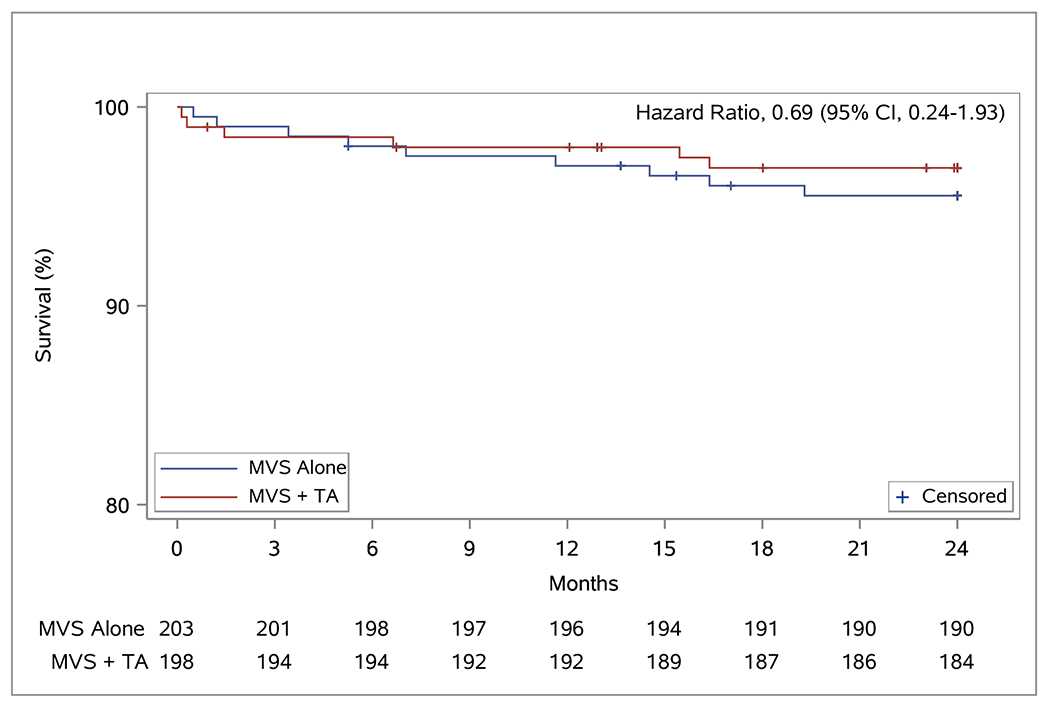
Event-free Survival Curves for Mortality and MACCE. Panel A depicts time to all cause death. Panel B depicts time to first major cerebrovascular or cardiac event (MACCE) defined as the composite event of death, stroke, and serious heart failure events
ECHOCARDIOGRAPHIC ENDPOINTS
The degree of TR over 2 years is depicted in Figure 2A. The likelihood of moderate or severe TR was 25.1% in MVS (45/179) versus 3.4% (6/179) in MVS+TA patients. The median trans-tricuspid valve diastolic peak gradient was 1 mmHg (IQR 1,2) in the MVS alone and 3 (IQR 2,4) in the MVS+TA group. Over 90% of patients in both groups had normal right ventricular systolic function (91.6% (163/178) in MVS versus 91.0% (162/178) in MVS+TA patients). Median LVEF was 60% (IQR 56, 64) in the MVS group and 61% (IQR 56, 64) in the MVS+TA group. At 2 years, 18/178 (10.1%) patients in the MVS group and 15/179 (8.4%) patients in the MVS+TA group experienced recurrent moderate or severe MR (Figure 2B).
Figure 2.
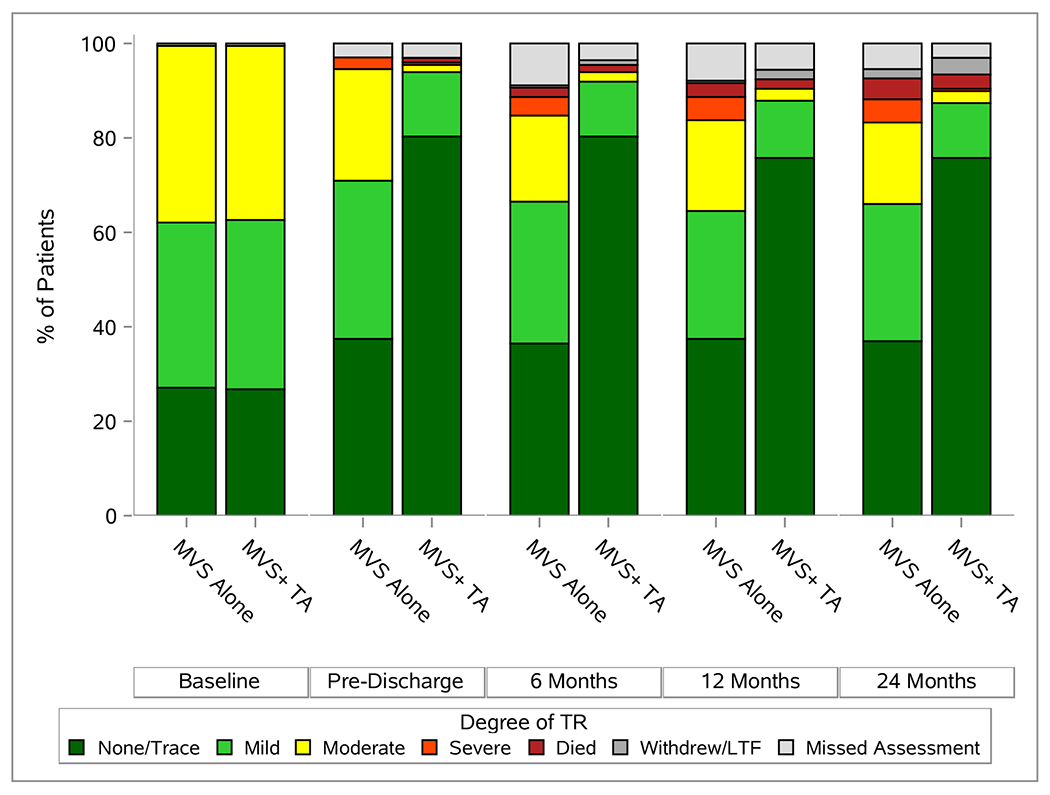
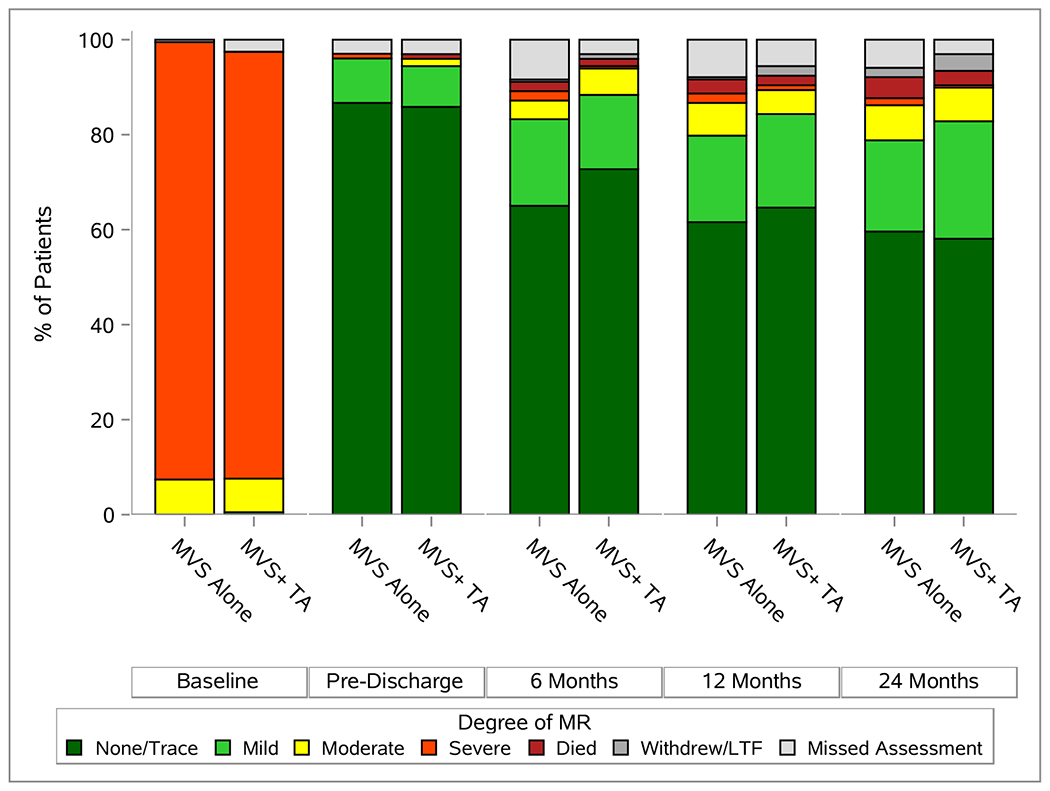
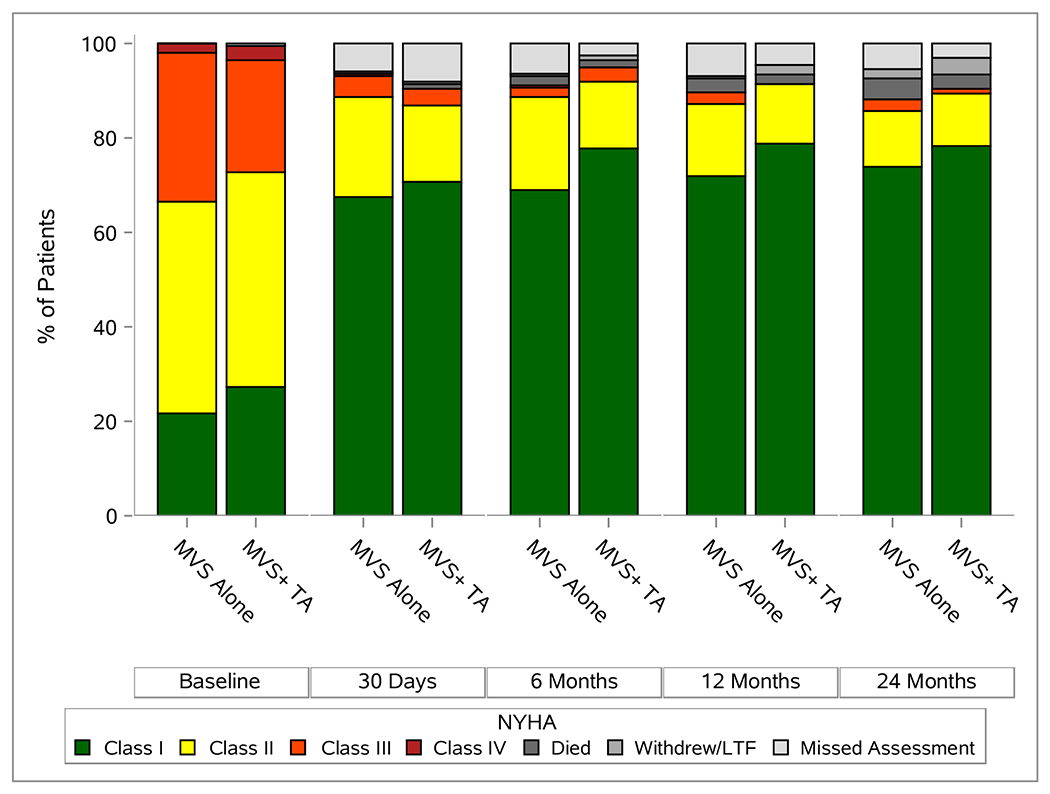
Echocardiographic and Functional Status Overtime. Panel A depicts degree of TR, panel B depicts degree of MR, and panel C gives NYHA over two year post-randomization.
ADVERSE EVENTS AND HOSPITALIZATIONS
Overall serious adverse event rates were not different between treatment groups at 2 years (Table 3). The rate of HF events was 0.11/24-patient months in the MVS and 0.07/24–patient months in the MVS+TA group (RRt 0.68;CI 0.25,1.85). Sustained supraventricular arrhythmias requiring drug therapy or cardioversion were more frequent in the MVS alone than the MVS+TA (RRt 0.70;CI 0.43,1.12). However, cardiac conduction abnormalities requiring permanent pacemaker implantation were significantly more frequent in the MVS+TA than the MVS group (RRt 5.75;CI 2.27,14.60). The majority of these events occurred during the index hospitalization, with 80% (4/5) of pacemakers implanted in MVS and 78.6% (22/28) in MVS+TA patients pre-discharge. The most common indication for pacemaker implantation was complete or high-grade AV block (57.6%, 19/33).
Table 3.
Serious Adverse Events and Hospitalizations through 2 Years
| MVS Alone (N= 203; Patient Months= 4699.9) | MVS + TA (N= 198; Patient Months= 4576.2) | ||||
|---|---|---|---|---|---|
| No. Patients (%) | No. Events (rate per 24 Pt Months) | No. Patients (%) | No. Events (rate per 24 Pt Months) | Relative Rate (95% CI) | |
| Serious Adverse Events | |||||
| Bleeding | 12 (5.9) | 13 (0.066) | 7 (3.5) | 8 (0.042) | 0.63 (0.24,1.64) |
| Cardiac arrest | 1 (0.5) | 1 (0.005) | 0 | 0 | - |
| Sustained ventricular arrhythmia requiring defibrillation or cardioversion | 2 (1.0) | 2 (0.010) | 2 (1.0) | 2 (0.010) | 1.03 (0.15,7.22) |
| Sustained supraventricular arrhythmia requiring drug treatment/cardioversion | 45 (22.2) | 56 (0.286) | 30 (15.2) | 38 (0.199) | 0.70 (0.43,1.12) |
| New-onset post op atrial fibrillation | 19 (9.4) | 22 (0.112) | 17 (8.6) | 19 (0.100) | 0.89 (0.45,1.74) |
| Cardiac conduction abnormalities or sustained bradycardia requiring PPMs | 5 (2.5) | 5 (0.026) | 28 (14.1) | 28 (0.147) | 5.75 (2.27,14.60) |
| AV Block requiring PPM | 1 (0.5) | 1 (0.005) | 15 (7.6) | 15 (0.079) | 15.41 (2.05,115.52) |
| Pericardial fluid collection | 3 (1.5) | 3 (0.015) | 3 (1.5) | 3 (0.016) | 1.03 (0.21,5.02) |
| Pleural effusion | 16 (7.9) | 20 (0.102) | 8 (4.0) | 10 (0.052) | 0.51 (0.21,1.25) |
| Pneumothorax | 3 (1.5) | 3 (0.015) | 5 (2.5) | 5 (0.026) | 1.71 (0.41,7.07) |
| Hepatic dysfunction | 2 (1.0) | 2 (0.010) | 0 | 0 | - |
| Major Infection - localized | 15 (7.4) | 18 (0.092) | 15 (7.6) | 16 (0.084) | 0.91 (0.44,1.92) |
| Major Infection - endocarditis | 1 (0.5) | 1 (0.005) | 2 (1.0) | 2 (0.010) | 2.05 (0.19,22.50) |
| Major Infection - Sepsis | 11 (5.4) | 12 (0.061) | 5 (2.5) | 5 (0.026) | 0.43 (0.15,1.24) |
| Myocardial infarction (non-procedure related) | 2 (1.0) | 2 (0.010) | 2 (1.0) | 2 (0.010) | 1.03 (0.15,7.26) |
| Myocardial infarction - Peri-CABG | 1 (0.5) | 1 (0.005) | 0 | 0 | - |
| Transient ischemic attack - TIA | 1 (0.5) | 1 (0.005) | 2 (1.0) | 2 (0.010) | 2.05 (0.19,22.46) |
| Ischemic stroke | 3 (1.5) | 3 (0.015) | 9 (4.5) | 9 (0.047) | 3.08 (0.84,11.23) |
| Hemorrhagic stroke | 1 (0.5) | 1 (0.005) | 1 (0.5) | 1 (0.005) | 1.03 (0.06,16.44) |
| Toxic metabolic encephalopathy | 3 (1.5) | 3 (0.015) | 4 (2.0) | 4 (0.021) | 1.37 (0.31,6.06) |
| Seizure | 2 (1.0) | 3 (0.015) | 3 (1.5) | 3 (0.016) | 1.03 (0.16,6.45) |
| Neurological dysfunction - other | 1 (0.5) | 1 (0.005) | 0 | 0 | - |
| Renal failure | 5 (2.5) | 5 (0.026) | 3 (1.5) | 3 (0.016) | 0.62 (0.15,2.59) |
| Respiratory failure | 5 (2.5) | 7 (0.036) | 5 (2.5) | 7 (0.037) | 1.03 (0.28,3.82) |
| Heart failure | 13 (6.4) | 21 (0.107) | 9 (4.5) | 14 (0.073) | 0.68 (0.25,1.85) |
| Arterial non-CNS thromboembolism | 2 (1.0) | 2 (0.010) | 0 | 0 | - |
| Venous thromboembolic event | 2 (1.0) | 2 (0.010) | 0 | 0 | - |
| Wound dehiscence | 0 | 0 | 2 (1.0) | 2 (0.010) | - |
| Unexpected other serious adverse event | 47 (23.2) | 71 (0.363) | 37 (18.7) | 52 (0.273) | 0.75 (0.47,1.21) |
| All Serious AEs | 109 (53.7) | 259 (1.323) | 109 (55.1) | 216 (1.133) | 0.86 (0.61,1.20) |
| MVS Alone (N=201; patient months=4608.0) | MVS+TA (N=194; patient months=4489.0) | ||||
| No. Patients (%) | No. Events (Rate per 24 Pt Months) | No. Patients (%) | No. Events (Rate per 24 Pt Months) | Relative Rate (95% CI) | |
| Readmission a | |||||
| All-cause Readmissions | 66 (32.8) | 124 (0.646) | 69 (35.6) | 104 (0.556) | 0.86 (0.58,1.27) |
| Cardiovascular Readmissions | 38 (18.9) | 70 (0.365) | 42 (21.6) | 59 (0.315) | 0.87 (0.52,1.43) |
| Heart Failure Readmissions | 7 (3.5) | 16 (0.083) | 5 (2.6) | 11 (0.059) | 0.71 (0.18,2.71) |
- Patient months at risk for readmission were calculated using the patients days alive and free of hospitalization through 2 years post-randomization. Readmission was defined as any emergency department admission or hospitalization >24 hours. 2 patients in the MVS alone and 3 patients in the MVS+TA group died during the index hospitalization, 1 patient in the MVS+TA group withdrew prior to discharge from the index hospitalization – these patients are excluded from analyses of readmission by 2 years post-randomization
The median length of stay (LOS) during the index hospitalization was 2 days shorter in MVS compared to MVS+TA patients in the US and Canada (US: 6 days [IQR 5,8] vs 8 [IQR 6,9];Canada: 7 days [IQR 6,11] vs 9 [IQR 7,14]). In Germany, the LOS was similar between treatment groups and overall longer than in North America (11.5 (IQR 9,15) vs 12 (IQR 9,16); E-Figure 2). The overall hospital readmission rate was 0.65 in MVS versus 0.56 in MVS+TA per 24-patient months (RRt 0.86;CI 0.58,1.27), with no between-group differences in the rate of cardiovascular or HF readmissions (RRt 0.87;CI 0.52,1.43 and 0.71;CI 0.18,2.71, respectively).
QUALITY OF LIFE AND FUNCTIONAL STATUS
There was no difference between treatment arms with respect to any QoL or functional status measure for surviving patients at 2 years (E-Figures 3 and 4). Among survivors, there was a 27.2% median (IQR 4.3, 70.0) improvement in heart failure symptoms over baseline, as measured by KCCQ, in the MVS alone and 21.4% (IQR 6.1, 57.1) in the MVS+TA group. Figure 2C depicts NYHA classification, accounting for death, over time. Diuretic use at 24 months was similar between groups (29.7%, [55/185] in MVS vs. 22.5% [41/182] in MVS+TA).
DISCUSSION
The optimal management of moderate or less TR at time of surgery for primary MR is uncertain. Current international guideline recommendations are largely based on observational data from studies conducted at single surgical centers.28–30 In this multi-center, international randomized clinical trial, patients with moderate or less TR receiving TA at the time of MVS for primary MR had a significantly lower 2-year rate of a composite endpoint of death, reoperation for TR, and progression of TR compared with patients undergoing MVS alone (3.9% vs.10.2%; p=0.02). This difference was driven by a significantly lower rate of progression of TR among patients assigned to TA. Although this trial was not powered for stratified analysis of the primary endpoint by TR severity at baseline, it is interesting to observe that progression of TR was almost exclusively among patients with moderate TR at baseline. Progression of TR among patients with lesser degrees of TR and annular dilation at baseline was seen in only one patient from the MVS alone group. This observation calls into question reliance on measurement of tricuspid annular diameter to inform surgical decision-making in patients with less than moderate TR – a question that can only be answered with additional research over a longer time-horizon. The incidence of MACCE, functional status, quality of life, heart failure events, diuretic use and hospital readmission rates at 2 years did not differ between groups, although the rate of permanent pacemaker implantation was significantly higher in recipients of TA, an outcome that should be factored into shared decision-making with patients.
Moreover, when considering moderate TR as well, patients receiving MVS alone were more likely to experience moderate or severe TR at 2 years (25.1%), compared to patients who also received TA (3.4%). The variability in symptoms and signs of right heart failure among patients with moderate or severe TR can challenge long-term management, as can the difficulties inherent in assessment of right ventricular function. At 2 years, we observed no significant between-group differences in NYHA Class III or IV HF (2.8% in MVS and 1.1% in MVS+TA patients, as compared to 33.5% and 26.9% at baseline, respectively). This finding was further reflected in the observation that the KCCQ overall summary scores, the SF-12 physical and mental health scores, the Euro-QOL and 6-minute walk test results did not differ between treatment arms. Notably, the 2-year KCCQ scores showed an average increase of 20 points in both groups from baseline, which reflects “a large-to very large” clinical improvement.31 Readmission rates, including overall cardiovascular and heart failure readmissions, were similar as well. The low heart failure readmission rates seen in this trial may have been influenced by the COVID pandemic.
Whereas the 7-fold higher prevalence of moderate to severe TR in MVS alone recipients did not affect clinical and functional outcomes at 2 years, longer-term evaluation may reveal differences in survival, functional status and health outcomes. Observational studies have suggested that moderate or severe functional TR in primary MR patients is an independent risk factor for long-term mortality.2 The incidence of severe TR may increase over time after isolated MVS and adversely affect right ventricular function.18 The long life expectancy of our relatively young trial population underscores the importance of longer-term follow-up.
In this trial, 90% of enrolled patients with severe primary MR underwent MV repair. The addition of TA increased cardiopulmonary bypass time by 34 minutes on average. Importantly, this difference was not associated with a higher risk of perioperative mortality, as reported by some studies.23 Tricuspid valve annuloplasty, however, was associated with longer LOS during the index hospitalization. In fact, the median LOS was 2 days longer in the MVS+TA group than in the MVS alone group in both the US and Canada. In Germany, LOS was generally longer but similar between groups, which reflects different incentives embedded in that health care system.
An important finding in this trial was the high incidence of permanent pacemaker implantations in the TA group (14.1% versus 2.5%), with nearly 80% of these implants occurring during the index hospitalization. Among patients receiving a pacemaker, the frequency of AF surgery was similar in both groups. Permanent pacemakers are associated with the need for generator changes over time, infections and lead complications. In addition, long-term RV pacing has been associated with recurrent or progressive TR, RV remodeling and reduced survival.32,33 Although the clinical impact of permanent pacemaker implantation was not evident over 2 years in the current study, longer term follow-up will be needed to gain further insight. The potential contribution of recurrent MR to late outcomes in both treatment groups will also need consideration.
This trial has several limitations. First, the components of the composite primary endpoint are not uniformly clinical. We combined clinical and echocardiographic endpoints to achieve a manageable sample size that would allow efficient trial completion. On the other hand, our choice of TR progression was driven by observational evidence correlating it with the long-term risk of adverse clinical outcomes. Second, the trial was designed to address surgical decision-making for patients with either moderate TR or those with less TR and annular dilation, and it was not powered to draw inferences about these groups individually. Third, the COVID-19 pandemic affected the ability of patients to return to the clinical site for primary endpoint assessment. However, we achieved a primary endpoint completion rate of 93%. Finally, measuring the primary endpoint at 24 months may not fully capture the clinical impact of TR progression or pacemaker implantation over time. The trial, however, will follow patients for 5 years and offer insights into longer-term clinical outcomes.
In conclusion, the addition of concomitant TA at time of MVS reduced a composite primary endpoint event rate at 2 years, driven by less frequent progression to severe TR. This reduction in TR progression came at the cost of higher risk of permanent pacemaker implantation. Importantly, at 2 years, there were no differences in survival, MACCE, quality of life, functional status or readmissions. Whether the lower rate of TR progression and the higher rate of pacemaker implantations associated with concomitant TA will result in long-term net clinical benefits requires longer follow-up.
Supplementary Material
Funding
The “Tricuspid Valve Repair during Surgery for Primary Mitral Valve Regurgitation” trial was supported by a cooperative agreement (U01 HL088942) funded by the National Heart Lung and Blood Institute and a grant from DZHK (German Centre of Cardiovascular Research). The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; National Institutes of Health; the United States Department of Health and Human Services or the German Centre of Cardiovascular Research.
Footnotes
(ClinicalTrials.gov number: NCT02675244)
REFERENCES
- 1.Badhwar V, Rankin JS, He M, et al. Performing Concomitant Tricuspid Valve Repair at the Time of Mitral Valve Operations Is Not Associated With Increased Operative Mortality. Ann Thorac Surg 2017;103(2):587–593. DOI: 10.1016/j.athoracsur.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Essayagh B, Antoine C, Benfari G, et al. Functional tricuspid regurgitation of degenerative mitral valve disease: a crucial determinant of survival. Eur Heart J 2020;41(20):1918–1929. DOI: 10.1093/eurheartj/ehaa192. [DOI] [PubMed] [Google Scholar]
- 3.Topilsky Y, Maltais S, Medina Inojosa J, et al. Burden of Tricuspid Regurgitation in Patients Diagnosed in the Community Setting. JACC Cardiovasc Imaging 2019;12(3):433–442. DOI: 10.1016/j.jcmg.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg 2021. DOI: 10.1093/ejcts/ezab389. [DOI] [Google Scholar]
- 5.Writing Committee M, Otto CM, Nishimura RA, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Thorac Cardiovasc Surg 2021;162(2):e183–e353. DOI: 10.1016/j.jtcvs.2021.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Jeganathan R, Armstrong S, Al-Alao B, David T. The risk and outcomes of reoperative tricuspid valve surgery. Ann Thorac Surg 2013;95(1):119–24. DOI: 10.1016/j.athoracsur.2012.08.058. [DOI] [PubMed] [Google Scholar]
- 7.Pfannmuller B, Moz M, Misfeld M, et al. Isolated tricuspid valve surgery in patients with previous cardiac surgery. J Thorac Cardiovasc Surg 2013;146(4):841–7. DOI: 10.1016/j.jtcvs.2012.07.096. [DOI] [PubMed] [Google Scholar]
- 8.Desai RR, Vargas Abello LM, Klein AL, et al. Tricuspid regurgitation and right ventricular function after mitral valve surgery with or without concomitant tricuspid valve procedure. J Thorac Cardiovasc Surg 2013;146(5):1126–1132 e10. DOI: 10.1016/j.jtcvs.2012.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sordelli C, Lancellotti P, Carlomagno G, et al. Tricuspid Annular Size and Regurgitation Progression After Surgical Repair for Degenerative Mitral Regurgitation. Am J Cardiol 2016;118(3):424–31. DOI: 10.1016/j.amjcard.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Benedetto U, Melina G, Angeloni E, et al. Prophylactic tricuspid annuloplasty in patients with dilated tricuspid annulus undergoing mitral valve surgery. J Thorac Cardiovasc Surg 2012;143(3):632–8. DOI: 10.1016/j.jtcvs.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Bertrand PB, Koppers G, Verbrugge FH, et al. Tricuspid annuloplasty concomitant with mitral valve surgery: effects on right ventricular remodeling. J Thorac Cardiovasc Surg 2014;147(4):1256–64. DOI: 10.1016/j.jtcvs.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Chikwe J, Itagaki S, Anyanwu A, Adams DH. Impact of Concomitant Tricuspid Annuloplasty on Tricuspid Regurgitation, Right Ventricular Function, and Pulmonary Artery Hypertension After Repair of Mitral Valve Prolapse. J Am Coll Cardiol 2015;65(18):1931–8. DOI: 10.1016/j.jacc.2015.01.059. [DOI] [PubMed] [Google Scholar]
- 13.Dreyfus GD, Corbi PJ, Chan KM, Bahrami T. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg 2005;79(1):127–32. DOI: 10.1016/j.athoracsur.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy PM, Szlapka M, Kruse J, et al. The relationship of atrial fibrillation and tricuspid annular dilation to late tricuspid regurgitation in patients with degenerative mitral repair. J Thorac Cardiovasc Surg 2021;161(6):2030–2040 e3. DOI: 10.1016/j.jtcvs.2019.11.098. [DOI] [PubMed] [Google Scholar]
- 15.Brescia AA, Ward ST, Watt TMF, et al. Outcomes of Guideline-Directed Concomitant Annuloplasty for Functional Tricuspid Regurgitation. Ann Thorac Surg 2020;109(4):1227–1232. DOI: 10.1016/j.athoracsur.2019.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calafiore AM, Gallina S, Iaco AL, et al. Mitral valve surgery for functional mitral regurgitation: should moderate-or-more tricuspid regurgitation be treated? a propensity score analysis. Ann Thorac Surg 2009;87(3):698–703. DOI: 10.1016/j.athoracsur.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 17.Calafiore AM, Iaco AL, Romeo A, et al. Echocardiographic-based treatment of functional tricuspid regurgitation. J Thorac Cardiovasc Surg 2011;142(2):308–13. DOI: 10.1016/j.jtcvs.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 18.Goldstone AB, Howard JL, Cohen JE, et al. Natural history of coexistent tricuspid regurgitation in patients with degenerative mitral valve disease: implications for future guidelines. J Thorac Cardiovasc Surg 2014;148(6):2802–9. DOI: 10.1016/j.jtcvs.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Van de Veire NR, Braun J, Delgado V, et al. Tricuspid annuloplasty prevents right ventricular dilatation and progression of tricuspid regurgitation in patients with tricuspid annular dilatation undergoing mitral valve repair. J Thorac Cardiovasc Surg 2011;141(6):1431–9. DOI: 10.1016/j.jtcvs.2010.05.050. [DOI] [PubMed] [Google Scholar]
- 20.David TE, David CM, Fan CS, Manlhiot C. Tricuspid regurgitation is uncommon after mitral valve repair for degenerative diseases. J Thorac Cardiovasc Surg 2017;154(1):110–122 e1. DOI: 10.1016/j.jtcvs.2016.12.046. [DOI] [PubMed] [Google Scholar]
- 21.Helmers MR, Shin M, Iyengar A, et al. Permanent pacemaker implantation following mitral valve surgery: a retrospective cohort study of risk factors and long-term outcomes. Eur J Cardiothorac Surg 2021;60(1):140–147. DOI: 10.1093/ejcts/ezab091. [DOI] [PubMed] [Google Scholar]
- 22.Jouan J, Mele A, Florens E, et al. Conduction disorders after tricuspid annuloplasty with mitral valve surgery: Implications for earlier tricuspid intervention. J Thorac Cardiovasc Surg 2016;151(1):99–103. DOI: 10.1016/j.jtcvs.2015.09.063. [DOI] [PubMed] [Google Scholar]
- 23.LaPar DJ, Mulloy DP, Stone ML, et al. Concomitant tricuspid valve operations affect outcomes after mitral operations: a multiinstitutional, statewide analysis. Ann Thorac Surg 2012;94(1):52–7; discussion 58. DOI: 10.1016/j.athoracsur.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JW, Song JM, Park JP, Lee JW, Kang DH, Song JK. Long-term prognosis of isolated significant tricuspid regurgitation. Circ J 2010;74(2):375–80. DOI: 10.1253/circj.cj-09-0679. [DOI] [PubMed] [Google Scholar]
- 25.Ro SK, Kim JB, Jung SH, Choo SJ, Chung CH, Lee JW. Mild-to-moderate functional tricuspid regurgitation in patients undergoing mitral valve surgery. J Thorac Cardiovasc Surg 2013;146(5):1092–7. DOI: 10.1016/j.jtcvs.2012.07.100. [DOI] [PubMed] [Google Scholar]
- 26.Yilmaz O, Suri RM, Dearani JA, et al. Functional tricuspid regurgitation at the time of mitral valve repair for degenerative leaflet prolapse: the case for a selective approach. J Thorac Cardiovasc Surg 2011;142(3):608–13. DOI: 10.1016/j.jtcvs.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 27.Dion RA. Is the air in Toronto, Rochester, and Cleveland different from that in London, Monaco, Leiden, Genk, Milan, and New York? J Thorac Cardiovasc Surg 2015;150(5):1040–3. DOI: 10.1016/j.jtcvs.2015.08.048. [DOI] [PubMed] [Google Scholar]
- 28.Chan V, Burwash IG, Lam BK, et al. Clinical and echocardiographic impact of functional tricuspid regurgitation repair at the time of mitral valve replacement. Ann Thorac Surg 2009;88(4):1209–15. DOI: 10.1016/j.athoracsur.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 29.Kim JB, Yoo DG, Kim GS, et al. Mild-to-moderate functional tricuspid regurgitation in patients undergoing valve replacement for rheumatic mitral disease: the influence of tricuspid valve repair on clinical and echocardiographic outcomes. Heart 2012;98(1):24–30. DOI: 10.1136/heartjnl-2011-300403. [DOI] [PubMed] [Google Scholar]
- 30.Navia JL, Brozzi NA, Klein AL, et al. Moderate tricuspid regurgitation with left-sided degenerative heart valve disease: to repair or not to repair? Ann Thorac Surg 2012;93(1):59–67; discussion 68-9. DOI: 10.1016/j.athoracsur.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 31.Spertus JA, Jones PG, Sandhu AT, Arnold SV. Interpreting the Kansas City Cardiomyopathy Questionnaire in Clinical Trials and Clinical Care: JACC State-of-the-Art Review. J Am Coll Cardiol 2020;76(20):2379–2390. DOI: 10.1016/j.jacc.2020.09.542. [DOI] [PubMed] [Google Scholar]
- 32.Mar PL, Angus CR, Kabra R, et al. Perioperative predictors of permanent pacing and long-term dependence following tricuspid valve surgery: a multicentre analysis. Europace 2017;19(12):1988–1993. DOI: 10.1093/europace/euw391. [DOI] [PubMed] [Google Scholar]
- 33.McCarthy PM, Bhudia SK, Rajeswaran J, et al. Tricuspid valve repair: durability and risk factors for failure. J Thorac Cardiovasc Surg 2004;127(3):674–85. DOI: 10.1016/j.jtcvs.2003.11.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


