Supplemental Digital Content is available in the text.
Keywords: coronavirus disease 2019, extracorporeal membrane oxygenation, prone position, severe acute respiratory syndrome-related coronavirus
OBJECTIVES:
The study investigated the impact of prone positioning during venovenous extracorporeal membrane oxygenation support for coronavirus disease 2019 acute respiratory failure on the patient outcome.
DESIGN:
An observational study of venovenous extracorporeal membrane oxygenation patients. We used a multistate survival model to compare the outcomes of patients treated with or without prone positioning during extracorporeal membrane oxygenation, which incorporates the dynamic nature of prone positioning and adjusts for potential confounders.
SETTING:
Seventy-two international institutions participating in the Coronavirus Disease 2019 Critical Care Consortium international registry.
PATIENTS:
Coronavirus disease 2019 patients who were supported by venovenous extracorporeal membrane oxygenation during the study period.
INTERVENTION:
None.
MEASUREMENTS AND MAIN RESULTS:
There were 232 coronavirus disease 2019 patients at 72 participating institutions who were supported with venovenous extracorporeal membrane oxygenation during the study period from February 16, 2020, to October 31, 2020. Proning was used in 176 patients (76%) before initiation of extracorporeal membrane oxygenation and in 67 patients (29%) during extracorporeal membrane oxygenation. Survival to hospital discharge was 33% in the extracorporeal membrane oxygenation prone group versus 22% in the extracorporeal membrane oxygenation supine group. Prone positioning during extracorporeal membrane oxygenation support was associated with reduced mortality (hazard ratio, 0.31; 95% CI, 0.14–0.68).
CONCLUSIONS:
Our study highlights that prone positioning during venovenous extracorporeal membrane oxygenation support for refractory coronavirus disease 2019-related acute respiratory distress syndrome is associated with reduced mortality. Given the observational nature of the study, a randomized controlled trial of prone positioning on venovenous extracorporeal membrane oxygenation is needed to confirm these findings.
Extracorporeal membrane oxygenation (ECMO) has been recommended as rescue therapy for coronavirus disease 2019 (COVID-19)-related acute respiratory distress syndrome (ARDS) (1–4). In a recent Extracorporeal Life Support Organization (ELSO) registry analysis of 1,035 ECMO-supported patients with COVID-19, the estimated inhospital 90-day mortality after ECMO initiation was 37% (5). Clinical trials have demonstrated a survival benefit of early prone positioning for ARDS (6). For COVID-19, multiple observational reports have suggested that prone positioning of both intubated and nonintubated patients improves oxygenation and decreases the work of breathing. In some patients, the use of prone positioning has been suggested to reduce the need for escalation of care with intubation and mechanical ventilation (7–9). Prone positioning during venovenous ECMO has been shown to improve hypoxemia in selected patients and can be conducted safely (10, 11). However, it is a labor-intensive procedure and, in times of pandemic stress and limited hospital resources, may be challenging to provide.
The COVID-19 Critical Care Consortium (COVID Critical) is an international registry enrolling COVID-19 patients admitted to ICUs from more than 370 hospitals in 53 countries (12, 13). The purpose of this study is to report a global assessment of the use and the outcome of prone positioning during venovenous ECMO in patients with COVID 19-related acute respiratory failure.
METHODS
Study Design, Settings, and Patients
All participating hospitals obtained approval from their local Institutional Review Boards and waivers of informed consent were granted because the study was observational, data recorded in the central repository were de-identified, and there was minimal risk to participants. The full study protocol for the wider COVID Critical study is available (13, 14).
Site investigators and study coordinators received detailed instructions and used a data dictionary to gather and enter data. The International Severe Acute Respiratory and Emerging Infection Consortium (14) and the Short PeRiod IncideNce sTudy of Severe Acute Respiratory Infection network (15) case report forms were completed on hospital admission. Additional case report forms were completed for patients receiving mechanical ventilation and ECMO. De-identified data were entered in the Research Electronic Data Capture hosted by the University of Oxford, United Kingdom.
This study used data from February 16, 2020, to October 31, 2020. The enrollment eligibility criteria were age of 18 years old or greater, confirmed COVID-19, and need for invasive mechanical ventilation and venovenous ECMO support. The primary outcomes were survival to hospital discharge and mortality through 90 days from ECMO initiation.
Data Collection
The collected data included demographics, comorbid conditions, date of admission and discharge from the ICU, the Acute Physiology Score II, and the Sequential Organ Failure Assessment (SOFA), relevant laboratory tests, mechanical ventilation settings and the time of initiation and discontinuation of invasive mechanical ventilation, and adjunctive therapies including prone positioning before and during ECMO support. For patients supported with ECMO, the time of cannulation and decannulation, mode of ECMO support, and ECMO complications were recorded. Center-specific data included the country and location, and the number of patients on ECMO support.
Statistical Analysis
The number of ECMO patients in the sample was plotted over calendar time, together with the proportion in prone position. The average proportion of patients in prone position on ECMO over time was estimated using a locally estimated scatterplot smoothing smoother (online supplement, http://links.lww.com/CCM/G711).
The study population characteristics were described as counts (%) for categorical variables and medians (interquartile ranges [IQRs]) for continuous variables.
To examine the key changes in patients’ position during ECMO support, a multistate survival analysis was used to examine patient outcomes over time. A four states model was used where patients started in the state of “ECMO prone” or “ECMO supine” based on their position on the day of ECMO initiation (Supplement Fig. 1, http://links.lww.com/CCM/G699; legend, http://links.lww.com/CCM/G710). Patients could move between the prone and supine states during their ECMO run and then be discharged alive from the hospital or die in hospital. The multistate survival analysis allowed us to examine the transitions between states that many patients experienced during their hospital stay. We plotted the modeled number of patients over time in each state (16).
A Weibull survival model was used in the multistate model to examine the instantaneous cause-specific hazards of death or hospital discharge and whether this depended on prone positioning, while adjusting for potential confounders. In an alternative survival model, the effect of prone position was modeled as a time-dependent variable that accumulated during a patient’s time on ECMO (0, 1, 2 d, etc.). We assumed prone position had a nonlinear cumulative effect. Hence, we tried four nonlinear transformations of the cumulative prone variable (squared inverse, inverse, log, and squared) and a linear version. We compared the five alternative models using the Akaike Information Criterion, which is a measure of model fit. To visualize the cumulative effect of prone position, we plotted the estimated hazard ratios by cumulative days on prone. We also examined the timing of initiation of prone position during ECMO run (either early or late) by highlighting the interaction between cumulative prone and the day of ECMO run.
The potential confounders were identified based on previously published studies, the clinically relevant factors for decision making, and differences in baseline characteristics (17, 18). We adjusted for each ICU using a random intercept, which allowed ICUs to vary in their baseline risks of death and discharge. We scaled continuous variables so that the hazard ratios were more clinically relevant, for example, age per 10-year increase. The Weibull survival models examined a patients’ instantaneous risk of death and discharge, and to give a complete picture of risk, we also used multiple variable models of the subdistribution hazard to examine cumulative risks while adjusting for the same potential confounders (19).
The Weibull survival model estimates were made using a Bayesian framework, and so results are presented with a 95% credible interval (20). We used standard statistical modeling for the cumulative risk models, and hence these are presented using CIs. We used intervals instead of p values because this allows us to focus on the size of the estimated effects and their uncertainty, which is more clinically relevant than tests of statistical significance (21). We handled missing data in the confounding variables by conducting multiple imputation. Patients still alive at 90 days were censored and so still contributed to the estimates of risk for death and discharge.
We used R Version 4.0.2 (2020-06-22) (R Core Team 2020) and WinBUGS Version 1.4.2 for all analyses (20). The cumulative frequency models were fitted using the “cmprsk” package (https://www.r-project.org/). and the Cox models using the “survival” package (22, 23). All the R and WinBUGS code used in the analysis is available online (24).
RESULTS
A total of 232 COVID-19 patients received venovenous ECMO for respiratory support at 72 collaborating sites from February 16, 2020, to October 31, 2020. Most cases were in the United States, Italy, Germany, or Japan (Supplement Table 1, http://links.lww.com/CCM/G700). Patients were commonly males (69%) and White (41%). The median (IQR) age was 53 years (43–60 yr) (Table 1). The overall frequency of proning during ECMO was 29% (67/232 patients). Comorbidities, the severity of illness as reflected by the Acute Physiology Score II and SOFA, and the time of onset of symptoms were comparable between those who received prone positioning during venovenous ECMO and those who did not. Pre-ECMO positive end-expiratory pressure was higher in the prone position group with median (IQR) 14 cm H2O (10–16 cm H2O) versus 12 cm H2O (10–16 cm H2O) cm H2O in the supine group (Table 2). The respiratory compliance was lower in the prone position group with median (IQR) 21 mL/cm H2O (20–29 mL/cm H2O) in comparison to those in the supine group 29 mL/cm H2O (22–37 mL/cm H2O). The median (IQR) Pao2/Fio2 was 85 (70–136) in the prone position group versus 83 (61–124) in the supine group.
Table 1.
Patients Characteristics Comparing Patients Who Experienced Prone Positioning Post-Extracorporeal Membrane Oxygenation Initiation Versus Those Who Were Always Supine
| Variable | All Patients (n = 232) | Prone Position (n = 67) | Supine Position (n = 165) |
|---|---|---|---|
| Age, yr | 53 (43–60) | 52 (43–60) | 53 (44–60) |
| Sex | |||
| Male | 160 (69%) | 47 (70%) | 113 (68%) |
| Female | 72 (31%) | 20 (30%) | 52 (32%) |
| Race/ethnicity | |||
| White | 95 (41%) | 34 (51%) | 61 (37%) |
| Hispanic | 50 (22%) | 15 (22%) | 35 (21%) |
| Asian | 30 (13%) | 8 (12%) | 22 (13%) |
| Black | 29 (12%) | 4 (6%) | 25 (15%) |
| Others | 28 (12%) | 6 (9%) | 22 (13%) |
| Body mass index, kg/cm2 | 30 (27–36) | 29 (26–33) | 31 (27–36) |
| Acute Physiology Score II | 19 (12–25) | 19 (12–24) | 19 (12–26) |
| Sequential Organ Function Assessment | 8 (5–10) | 7 (4–9) | 8 (5–10) |
| Pregnancy | 3 (1%) | 0 | 3 (2%) |
| Comorbidities | |||
| Obesity | 114 (49%) | 33 (49%) | 81 (49%) |
| Immunocompromised | 113 (49%) | 34 (51%) | 79 (48%) |
| Hypertension | 96 (41%) | 31 (46%) | 65 (39%) |
| Diabetes | 58 (25%) | 19 (28%) | 39 (24%) |
| Active smoker | 42 (18% | 13 (19%) | 29 (18%) |
| Chronic cardiac disease | 18 (8%) | 4 (6%) | 14 (8%) |
| Chronic kidney disease | 6 (4%) | 2 (3%) | 8 (3%) |
| Alcohol abuse | 7 (3%) | 4 (6%) | 3 (2%) |
| Malignancy | 5 (2%) | 3 (4%) | 2 (1%) |
| Time from first symptoms to hospital admission, d | 10 (6–16) | 10 (6–16) | 10 (6–16) |
| Time from intubation to extracorporeal membrane oxygenation, d | 4 (2–6) | 4 (2–8) | 4 (2–6) |
| Duration of mechanical ventilation, d | 24 (14–36) | 28 (16–42) | 24 (14–34) |
| Hospital length of stay, d | 32 (20–47) | 36 (24–58) | 30 (19–44) |
Data are median (interquartile range) or n (%).
Table 2.
Patients Pre-Extracorporeal Membrane Oxygenation Respiratory Support Characteristics and Outcomes, Comparing Patients Who Experienced Prone Positioning Post-Extracorporeal Membrane Oxygenation Initiation Versus Those Who Were Always Supine
| Variable | All Patients (n = 232) | Prone Position (n = 67) | Supine Position (n = 165) |
|---|---|---|---|
| Ventilatory parameters | |||
| Fio2 | 100 (60–100) | 100 (92–100) | 99 (50–100) |
| Positive end-expiratory pressure, cm H2O | 12 (10–16) | 14 (10–16) | 12 (10–16) |
| Static compliance, mL/cm H2O | 27 (21–36) | 21 (20–29) | 29 (22–37) |
| Pao2/Fio2 | 84 (61–126) | 85 (70–136) | 83 (61–124) |
| Pco2 | 49 (37–59) | 49 (39–62) | 48 (35–58) |
| Pre-ECMO prone positioning | 176 (76%) | 49 (73%) | 127 (77%) |
| Duration of prone positioning on ECMO, d | 0 (0–2) | 6 (2–14) | 0 |
| Outcome | |||
| Discharged from hospital alive | 59 (25%) | 22 (33%) | 37 (22%) |
| Discharged to other facilities | 40 (17%) | 12 (18%) | 28 (17%) |
| Remain in the hospital | 9 (4%) | 4 (6%) | 5 (3%) |
| Inhospital death | 90 (39%) | 23 (34%) | 67 (41%) |
| Unknown | 34 (15%) | 6 (9%) | 28 (17%) |
ECMO = extracorporeal membrane oxygenation.
Data are median (interquartile range) or n (%).
The number of patients receiving ECMO peaked in late April, followed by a decrease in the number of cases in mid-summer of the Northern hemisphere. The percentage of ECMO patients receiving prone positioning decreased over time from a high of just under 50% in February to a nadir of under 25% in June (Supplement Fig. 2, http://links.lww.com/CCM/G701; legend, http://links.lww.com/CCM/G710).
Models of Death and Discharge
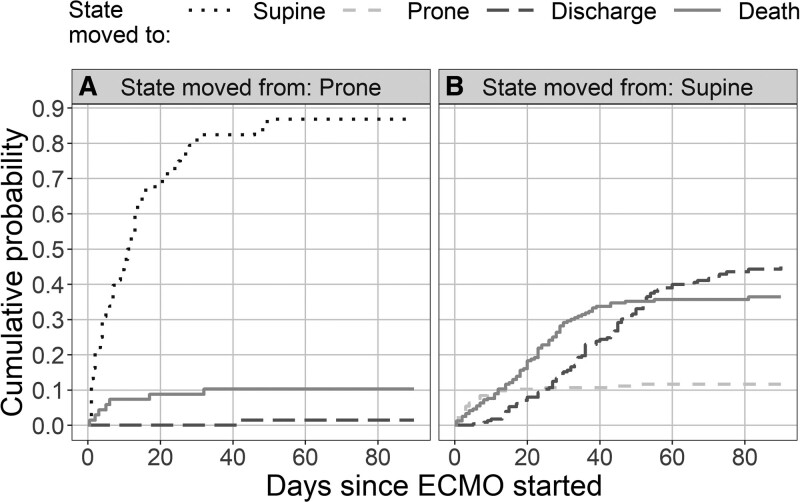
The number of patients on ECMO decreased during the first 40 days after ECMO initiation as patients died or were discharged from hospital (Supplement Fig. 3, http://links.lww.com/CCM/G702; legend, http://links.lww.com/CCM/G710). During the ECMO run, patients in prone position were often moved to supine (Fig. 1), and this occurred mainly in the first 40 days (Fig. 1, dotted line), while transitions from supine position to prone were mainly in the first 2 weeks (Fig. 1, dashed gray line). There was a steady accumulation of deaths among patients in the supine position that slowed after 40 days (Fig. 1B, solid line).
Figure 1.
Cumulative probabilities over time for the four states. The plots show the cumulative probability over time of moving between states. A, The probabilities for patients in the “ECMO and prone” state. B, The probabilities for patients in the “ECMO and supine” state. There is a steady accumulation of deaths in patients in the supine group (solid line [B]), although this slows after around 40 d. The movement of patients from the supine to the prone group greatly reduces after around 12 d (dashed gray line [B]). ECMO = extracorporeal membrane oxygenation.
Using the Weibull survival model, which predicts the instantaneous effect of proning during ECMO, prone position was not associated with reduced mortality (hazard ratio, 0.85; 95% credible interval, 0.34–1.95) (Supplement Table 2, http://links.lww.com/CCM/G703). In the cumulative outcome model, however, prone positioning was associated with significant reduced mortality (hazard ratio, 0.31; 95% CI, 0.14–0.68) and reduced discharge (hazard ratio for discharge, 0.03; 95% CI, 0.00–0.21) (Table 3). The higher probability of discharge from the supine position on ECMO state is expected because the model allows the natural progression from the prone position to the supine position on ECMO as patients recover and are removed from ECMO (Fig. 1B). It is less likely for patients in the prone position on venovenous ECMO to be discharged from proning without crossing to the supine state. The cumulative predicted probability of death and hospital discharge for a patient of average age and body mass index (BMI) diverged quickly over time and had substantial separation between prone and supine (Supplement Fig. 4, http://links.lww.com/CCM/G704; legend, http://links.lww.com/CCM/G710).
Table 3.
Hazard Ratios and 95% CIs Using a Cumulative Probability Regression Model
| Variable | Death | Hospital Discharge |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Male | 1.19 (0.77–1.86) | 0.73 (0.48–1.12) |
| Age (+10 yr) | 1.46 (1.19–1.78) | 0.78 (0.66–0.93) |
| Date of ECMO initiation (+30 d) | 1.10 (0.99–1.23) | 0.74 (0.63–0.87) |
| Body mass index (+5 kg/m2) | 1.13 (0.98–1.29) | 0.99 (0.86–1.13) |
| Prone position during ECMO | 0.31 (0.14–0.68) | 0.03 (0.00–0.21) |
| Prone position before ECMO | 1.17 (0.7–1.95) | 1.26 (0.77–2.07) |
ECMO = extracorporeal membrane oxygenation, HR = hazard ratio.
Timing and Duration of Prone Position
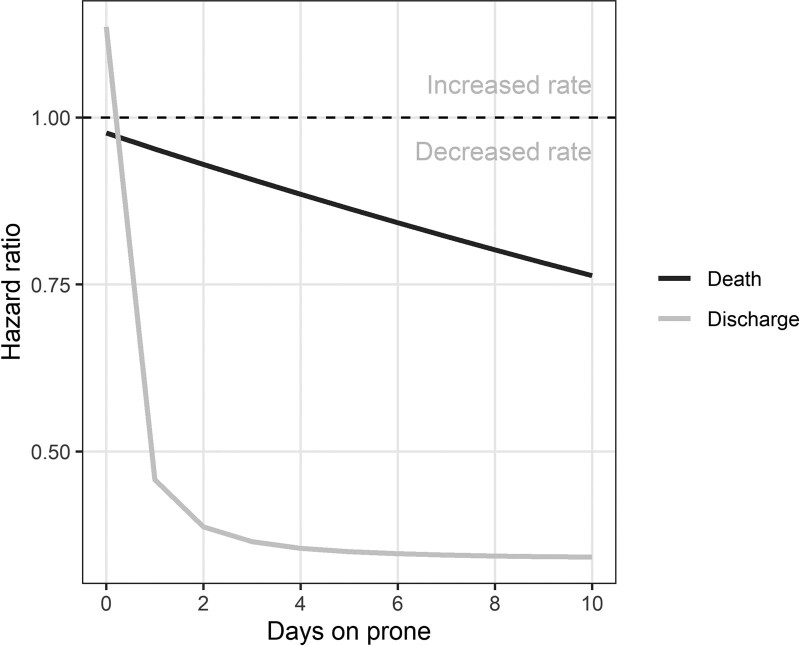
To visualize the nonlinear effect of timing of initiation of proning on death and discharge, we included the interaction between cumulative prone and the day of ECMO run. There is gradual decrease in the probability of death associated with the duration of prone position (Fig. 2). Also, prone positioning is associated with the probability of discharge. Prone positioning continued to be associated with lower mortality (hazard ratio, 0.95; 95% CI, 0.92–0.98) (Supplement Table 3, http://links.lww.com/CCM/G705).
Figure 2.
Estimated risk of death and discharge by time in prone position. There is gradual decrease in the probability of death associated with the duration of prone position.
Patient Characteristics Associated With Prone Positioning
A cumulative probability plot was used to examine the timing of the initial prone position during ECMO (Supplement Fig. 5, http://links.lww.com/CCM/G706; legend, http://links.lww.com/CCM/G710). Many patients were treated with prone position from the day of ECMO initiation. Older age and higher BMI were associated with a reduced probability of experiencing proning (Supplement Table 4, http://links.lww.com/CCM/G707). By contrast, gender, date of ECMO initiation during the pandemic, and prone positioning before ECMO did not clearly affect the probability of receiving prone positioning during ECMO.
Potential Confounders
Prone positioning prior to ECMO was associated with an increased likelihood of hospital discharge (Supplement Table 2, http://links.lww.com/CCM/G703). Older age was associated with an increased hazard of death and a decreased hazard of hospital discharge (increasing length of stay). Date of ECMO initiation (by increments of advancing forward an additional 30 d in the pandemic) had a strong effect on hospital discharge, leading to longer ECMO times (and reduced discharge hazard) over calendar time (Table 3). ICU effects were explored to determine whether the decision to prone during ECMO was dependent on the ICU. Across 72 ICUs, five ICUs were identified as being prolific users (increased hazard) of prone positioning (Supplement Fig. 6, http://links.lww.com/CCM/G708; legend, http://links.lww.com/CCM/G710). Hazard ratios for death associated with proning were similar across countries in the leave-one-country-out sensitivity analysis, indicating that the reduced hazard of death for prone positioning was not dependent on one country (Supplement Fig. 7, http://links.lww.com/CCM/G709; legend, http://links.lww.com/CCM/G710). The one patient discharged in prone position was influential in the estimated hazard of discharge for prone patients, and without that patient, the hazard of discharge became even smaller (Supplement Fig. 7, http://links.lww.com/CCM/G709; legend, http://links.lww.com/CCM/G710). Because most patients move into a supine state on ECMO as they recover before they get discharged. This patient did not influence the estimated reduced hazard of death associated with prone positioning.
DISCUSSION
The main finding of this analysis of 232 COVID-19 ECMO patients is that prone positioning while on venovenous ECMO was associated with a substantially decreased mortality in the cumulative regression analysis. Randomized controlled trials and meta-analyses suggest a mortality benefit of early prone positioning of 12 hours or greater duration for patients with moderate to severe ARDS (25–28). Similarly, observational studies of COVID-19 patients have demonstrated that prone positioning is associated with improved lung recruitment and oxygenation (7, 29, 30). Our large, multicenter report provides novel data on the potential benefits of prone positioning during ECMO. These results add to the evidence that COVID-19–associated respiratory failure may be particularly responsive to prone positioning.
Other investigators have reported observations based on combining prone positioning with ECMO for severe ARDS. Several case reports and retrospective studies demonstrated the safety and the feasibility of prone positioning during ECMO support (31–33). In an observational analysis of 25 patients with COVID-19 pneumonia who required venovenous ECMO support (34), prone positioning led to the improvement of oxygenation without a change in lung mechanics. Notably, the prone group had a higher mortality rate that was attributed to the severity of illness (34). In the multinational COVID Critical ECMO data, prone positioning was often done at the commencement of ECMO. By contrast, the patients selected for proning by Garcia et al (34) had more severe hypoxemia compared with the patients who did not have prone positioning during ECMO. In another observational analysis of 83 patients with COVID-19 and venovenous ECMO support, prone positioning was implemented in 80% of the study population without reported complications, and the estimated probability of death 60 days post-ECMO initiation was 31% (35). Finally, in a multicenter propensity-matched study, prone positioning during venovenous ECMO support was associated with improved oxygenation and reduction of inhospital mortality (10).
Our study is a multicenter, international study, which enhances its generalizability. The use of a dynamic survival analysis has the advantage of studying the events of interest in the context of time on ECMO and is appropriate for the situation that the decision to use prone positioning depended on clinician discretion at each ICU throughout the course of critical care. Furthermore, the multistate survival model avoids biases from simple comparisons of any versus no proning. These comparisons will be biased if prone positioning is eventually tried for patients with long ICU stays, which would mean that an “any vs none” prone position binary variable is then confounded with time and potentially also with patient severity. Survival models examine patients over time and account for the changing make-up of the cohort over time when patients either die or are discharged. Finally, by applying such a model, we could focus on the possible association between prone positioning and the clinical outcome of interest. This model has been shown to be useful in determining resource needs and mortality for critically ill COVID-19 patients (16). Nevertheless, the model is not a substitute or a surrogate for a randomized trial of proning during ECMO.
There are multiple observations from our analysis. The rate of prone positioning on venovenous ECMO is 31%, which is higher than previously reported (18). As we progress into the pandemic and with the increased number of patients on ECMO, there has been a reduction in the rate of proning on ECMO, which could be attributed to staff shortage, burnout, or limited resources (Fig. 2). Our model indicates more patients in the supine position group get discharged; however, that might reflect an improvement in patient condition. As a patient improves clinically, they move from a prone position on ECMO to the supine position and subsequently removed from ECMO and discharged. The Weibull survival model of proning did not show a reduction in the hazard of death, but this model is reflective of the instantaneous effect of prone position on the patient outcome that can be strongly influenced by competing risks, and in particular, the instantaneous hazard of discharge is very different in prone and supine. Prone position appears particularly protective (relative to supine) before 40 days of support. According to an analysis from the ELSO registry, the majority of deaths happen during the first 40 days on ECMO (5). Many prone patients in our study were later moved to supine and then discharged, but while they were in prone position, they were relatively “static” with no discharges and fewer transitions to death. Hence the final cumulative probability of death in the prone group is much lower, with no reduction in the instantaneous hazard of death.
Our study has several limitations related to its observational nature and the presence of residual and unmeasured confounders. Furthermore, our estimates for the hazard of death associated with prone position had wide 95% CIs indicating uncertainty in the effect size. There are more missing outcome data in the supine versus prone group. We attempted to overcome this limitation by applying multiple imputation and censoring patients at 90 days. Prone position on ECMO might be an indication of the institutional expertise in ECMO and ARDS management. We tried to account for these institutional variations by examining the ICU effect. In addition, we ran sensitivity analyses without site effects and obtained similar results. Also, tolerance of prone position could be a marker of lower severity of illness, which could have contributed to the mortality benefit we found. However, in our analysis, the severity of illness scores (SOFA and Acute Physiology and Chronic Health Evaluation II) was similar in the prone position group. The multistate survival model does not include the expected clinical progression from prone position on ECMO to supine and then discharge or death. We did not account for those transitional states (e.g., non-ECMO supine or non-ECMO prone) to avoid added complexity of the model by creating small groups and losing the statistical power of the study design. Also, patients in the supine position group are more likely to be discharged from the hospital, which is a reflective of a degree of lung recovery and transitioning from prone position. However, the probability of death remains high in the supine group.
CONCLUSIONS
Our study demonstrates that prone positioning during venovenous ECMO for COVID-19 associated severe ARDS is associated with 90-day improved survival. A strong association between prone positioning and reduced risk of death was suggested by a multistate survival model in an international registry of critically ill COVID-19 patients. A randomized controlled trial of prone positioning during venovenous ECMO support should be performed in patients with severe ARDS due to COVID-19.
ACKNOWLEDGMENTS
We recognize the crucial importance of the International Severe Acute Respiratory and Emerging Infection Consortium and Short PeRiod IncideNce sTudy of Severe Acute Respiratory Infection networks for the development and expansion of the Coronavirus Disease 2019 (COVID-19) Critical Care Consortium. We thank the generous support we received from the Extracorporeal Life Support Organization and the International Extracorporeal Membrane Oxygenation Network. We acknowledge all members of the COVID-19 Critical Care Consortium and various collaborators: Samuel Hinton, PhD; Simon Forsyth, PhD; Mauro Panigada, MD; John Laffey, MD, PhD; Daniel Brodie, MD; Eddy Fan, MD, PhD; Antoni Torres, MD, PhD, FERS; Davide Chiumello, MD; Amanda Corley, RN, MAdvPrac; India Pearse, RN; Alyaa Elhazmi; Carol Hodgson, RPT, PhD; Shingo Ichiba, MD; Carlos Luna, MD; Srinivas Murthy, MD; Alistair Nichol, MD, PhD; Pauline Yeung Ng, MD; Mark Ogino, MD; Antonio Pesenti, MD; Huynh Trung Trieu, MD; Robert Bartlett, MD; Gabriella Abbate, RN, MSc; Ryuzo Abe, MD; Shingo Adachi, MD; Takako Akimoto, MD; Abdulrahman Al-Fares, MD; Huda Alfoudri, MD; Abdullah Al-Hudaib, MD; Massimo Antonelli, MD; Christel Arnold-Day, MD; Lovkesh Arora, MD; Michaela Barnikel, MD; Peter Barrett, MD; Aleksandr Beljantsev, MD; Suzanne Bennett, MD; Amar Bhatt, MD; Pablo Blanco-Schweizer, MD; Luca Brazzi, MD; Nicolas Brozzi, MD; Sara F. Buabbas, MD; Nina Buchtele, MD; Erlina Burhan, MD; Hergen Buscher, MD; Aidan Burrell PhD, Mara Caroline, MD; Edmund G. Carton, MD; Enrique Chicote Álvarez, MD; Adrian Ceccato, MD; Silvia Coppola, MD; Hwa Jin Cho, MD; Sung-Min Cho, DO, MHS; Young-Jae Cho, MD; Anna Ciullo, MD; Sebastiano Colombo, MD; Sofía Contreras, MD; Alberto Cucino, MD; Al-Awwab M, Dabaliz, MD; Andrea Dell’Amore, MD; Mehul Desai, MD; Esther Dreier, MD; Lucian Durham, MD; Tarek B, El-Shazly, MD; Octavio Falcucci, MD; Shu Fang, MPH; Arie Zainul Fatoni, MD; Mohamed Fayed, MD; Ricard Ferrer Roca, MD, PhD; Kirsten M. Fiest, PhD; Cathleen Forney, MD; Giuseppe Foti, MD; Shigeki Fujitani, MD; Masahiro Fukuda, MD; Sérgio Gaião, MD; Johannes Gebauer, MD; Marco Giani, MD; Eric Gnall, MD; Jerónimo Graf, MD; E. Wilson Grandin, MD; Giacomo Grasselli, MD; Anna Greti, MD; Halah Hassan, BMSc; Ibrahim Hassan, MD; Jaime Hernandez-Montfort, MD; Kanako Horibe, MD; Koji Hoshino, MD; Kota Hoshino, MD; Ali Ait Hssain, MD; Jeffrey Javidfar, MD; In Seok Jeong, MD; Juan Masa Jiménez, MD; Anne Joosten, MD; Mark Joseph, MD; Jae-Seung Jung, MD, PhD; Ruth Noemí Jorge García, MD; Varun A. Karnik, BSc; Daisuke Kasugai, MD; Christy Kay, MD; Katrina Ki, PhD; Irfan Khan, MD; Jae-Bum Kim, MD; Ethan Kurtzman, MBA, RRT-NPS; Su Hwan Lee, MD; Keibun Liu, MD, PhD; Michela Leone, MD; Antonio Loforte, MD; Roberto Lorusso, MD, PhD; Gösta Lotz, MD; Olavi Maasikas, MD; Lars Maier, MD; Maximilian Malfertheiner, MD, PhD; Angela Maria Marulanda Yantén, MD; Eva Marwali, MD; Danny McAuley, MD, PhD; Colin McCloskey, MD; Angela McBride, MD; Dan Meyer, MD; Brook Mitchell, MD; Nahush A. Mokadam, MD; Giorgia Montrucchio, MD; Rita Moreno, MD; Naoki Moriyama, MD; Andrea Moscatelli, MD; Ana Motos, MSc; Paolo Navalesi, MD; Wing Yiu Ng, MD; Hollier F. O’Neill, BMSc; Nchafatso Obonyo, MD, PhD; Tawnya Ogston, MD; Getter Õigus, MD; Shinichiro Ohshimo, MD; Erik Osborn, MD; Javier Osatnik, MD; Clark Owyang, MD; Ken Kuljit S. Parhar, MD, MSc; Paolo Pelosi, MD; Leticia Pretti Pimenta, MD; Mohammed Abraar Quraishi, MD; Kollengode Ramanathan, MD; Indrek Rätsep, MD; Janice D. Reid, BSc; Jordi Riera, MD, PhD; Diego Fernando Bautista Rincón, MD; Roberto Roncon-Albuquerque, MD; Kristina Rudolph, BSN, RN; Angel Sanchez, MD; Gabriele Sales, MD; S. Veena Satyapriya, MD; Kei Sato, MD; Stephan Schroll, MD; Michael Schwameis, MD; Tamara Seitz, MD; Timothy Shapiro, MD; Kiran Shekar, MD, PhD; Hiroaki Shimazu, MD; Naoki Shimizu, MD; Hoi-Ping Shum, MD; Malcolm Sim, MBBch, MD; Dominic So, MD; Tae Song, MD; Henry T. Stelfox MD, PhD; Madhu Subramanian, MD; Stephanie-Susanne Stecher, MD; Subbarao Elapavaluru, MD; Konstanty Szuldrzynski, MD; Hiro Tanaka, MD; Hayato Taniguci, MD; Azhari Taufik, MD; Shaun Thompson, MD; David Thomson, MD; Yuka Uchinami, MD; Asad Ali Usman, MD, MPH; Jorge Velazco, MD; Aapeli Vuorinen, BMath, MSc; Karin S. Wildi MD; Emily S. Wood; Masaki Yamasaki, MD; Minlan Yang, MD; Stephanie Yerkovich, PhD; Toshiki Yokoyama, MD; Saptadi Yularito, MD; Ana Loza Vazquez, MD; Bishoy Zakhary, MD; Alberto Zanella, MD; Navy Long, MD; Vera Irawany, MD; Kohar Hari Santoso, MD; Susanthy Djajalaksana, MD; Gezy Giwangkancana, MD; Erwin Pradian, MD; Jose M. Mandei, MD; Keiki Shimizu, MD; Nobuya Kitamura, MD; Simone Carelli, MD; Antonia Arcadipane, MD; Vincenzo Russotto, MD; Yuri Kida, MSW, Jai Madhok, MD, MSE, Terese Hammond, MD, Mark Epler, MD; Louise Kruse, RN; Ryan Kennedy, MD; Timothy Evans, MD, PhD; John Adam Reich, MD; David Dean, MD; Keith Wille, MD; Marc Csete, MD, MBA, Glenn Whitman, MD; Michael Piagnerelli, PhD; Patrick Biston, MD; Juan Fernando Masa Jiménez; Leonardo Salazar, MD, MSc; Rodrigo Diaz Gómez, MD; Josefa Valenzuela Sarrazin; Piotr Bielański, MD, Malcom Miller, MD; Lisa Seymore, MD; Mariano Esperatti, MD, PhD; Andrea Villoldo, MD; A. Dave Nagpal, MSc, MD; Gaenor Cross; Hannah Marrinan; and Courtney Dwyer.
Supplementary Material
Footnotes
*See also p. 343.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http:/journals.lww.com/ccmjournal).
Drs. Zaaqoq, Barnett, Griffee, Dalton, and Peek conceived the study. Drs. Heinsar, Suen, Bassi, Fraser, and Dalton collected the data. Drs. Barnett, Zaaqoq, Griffee, and Peek conducted data analysis. Drs. Zaaqoq, Barnett, Griffee, MacLaren, Jacobs, Dalton, and Peek drafted the article. All authors helped to revise the draft of the article. All authors read and approved the final article.
Dr. Jacobs received funding from SpecialtyCare and the American Academy of Dermatology. Dr. Heinsar received funding via PhD scholarship from the University of Queensland. Dr. Bassi’s institution received funding from Fisher & Paykel. Dr. Dalton received funding from Innovative Extracorporeal Membrane Oxygenation (ECMO) Concepts; she disclosed the off-label product use of ECMO equipment. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Code availability: https:/github.com/agbarnett/covid_cccc.
REFERENCES
- 1.Alhazzani W, Møller MH, Arabi YM, et al. : Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020; 48:e440–e469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett RH, Ogino MT, Brodie D, et al. : Initial ELSO guidance document: ECMO for COVID-19 patients with severe cardiopulmonary failure. ASAIO J. 2020; 66:472–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobs JP, Stammers AH, St Louis J, et al. : Extracorporeal membrane oxygenation in the treatment of severe pulmonary and cardiac compromise in coronavirus disease 2019: Experience with 32 patients. ASAIO J. 2020; 66:722–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alnababteh M, Hashmi MD, Vedantam K, et al. : Extracorporeal membrane oxygenation for COVID-19 induced hypoxia: Single-center study. Perfusion. 2021; 36:564–572 [DOI] [PubMed] [Google Scholar]
- 5.Barbaro RP, MacLaren G, Boonstra PS, et al. ; Extracorporeal Life Support Organization: Extracorporeal membrane oxygenation support in COVID-19: An international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020; 396:1071–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guérin C, Reignier J, Richard JC, et al. ; PROSEVA Study Group: Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013; 368:2159–2168 [DOI] [PubMed] [Google Scholar]
- 7.Coppo A, Bellani G, Winterton D, et al. : Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): A prospective cohort study. Lancet Respir Med. 2020; 8:765–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munshi L, Fralick M, Fan E: Prone positioning in non-intubated patients with COVID-19: Raising the bar. Lancet Respir Med. 2020; 8:744–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paul V, Patel S, Royse M, et al. : Proning in non-intubated (PINI) in times of COVID-19: Case series and a review. J Intensive Care Med. 2020; 35:818–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giani M, Martucci G, Madotto F, et al. : Prone positioning during venovenous extracorporeal membrane oxygenation in acute respiratory distress syndrome. A multicenter cohort study and propensity-matched analysis. Ann Am Thorac Soc. 2021; 18:495–501 [DOI] [PubMed] [Google Scholar]
- 11.Guervilly C, Prud’homme E, Pauly V, et al. : Prone positioning and extracorporeal membrane oxygenation for severe acute respiratory distress syndrome: Time for a randomized trial? Intensive Care Med. 2019; 45:1040–1042 [DOI] [PubMed] [Google Scholar]
- 12.Rubin R: Global effort to collect data on ventilated patients with COVID-19. JAMA. 2020; 323:2233–2234 [DOI] [PubMed] [Google Scholar]
- 13.COVID-19 Critical Care Consortium. . Available at: https://www.covid-critical.com/. Accessed November 26, 2020.
- 14.Li Bassi G, Suen J, Barnett AG, et al. : The COVID-19 Critical Care Consortium observational study: Design and rationale of a prospective, international, multicenter, observational study. medRxiv. Preprint posted online June 2, 2020. doi: 10.1136/bmjopen-2020-041417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunning JW, Merson L, Rohde GGU, et al. ; ISARIC Working Group 3, ISARIC Council: Open source clinical science for emerging infections. Lancet Infect Dis. 2014; 14:8–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murthy S: Using research to prepare for outbreaks of severe acute respiratory infection. BMJ Global Health. 2019; 4:e001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazard D, Kaier K, von Cube M, et al. : Joint analysis of duration of ventilation, length of intensive care, and mortality of COVID-19 patients: A multistate approach. BMC Med Res Methodol. 2020; 20:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noah MA, Peek GJ, Finney SJ, et al. : Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA. 2011; 306:1659–1668 [DOI] [PubMed] [Google Scholar]
- 19.Rilinger J, Zotzmann V, Bemtgen X, et al. : Prone positioning in severe ARDS requiring extracorporeal membrane oxygenation. Crit Care. 2020; 24:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolkewitz M, Cooper BS, Bonten MJ, et al. : Interpreting and comparing risks in the presence of competing events. BMJ. 2014; 349:g5060. [DOI] [PubMed] [Google Scholar]
- 21.Lunn D, Jackson C, Best N, et al. : The Bugs Book: A Practical Introduction to Bayesian Analysis. Chapman & Hall/Crc Texts in Statistical Science. New York, Taylor & Francis, 2012 [Google Scholar]
- 22.Wasserstein RL, Lazar NA: The ASA statement on p-values: Context, process, and purpose. Am Stat. 2016; 70:129–133 [Google Scholar]
- 23.Therneau TM: A Package for Survival Analysis in R. 2020. Available at: https://CRAN.R-project.org/package=survival. Accessed November 11, 2020
- 24.Gray B: Cmprsk: Subdistribution analysis of Competing risks. 2020. Available at: https://CRAN.R-project.org/package=cmprsk. Accessed November 11, 2020
- 25.Barnett A: Analysis for CCCC. 2020. Available at: https://github.com/agbarnett/covid_cccc. Accessed November 11, 2020
- 26.Guerin C, Gaillard S, Lemasson S, et al. : Effects of systematic prone positioning in hypoxemic acute respiratory failure: A randomized controlled trial. JAMA. 2004; 292:2379–2387 [DOI] [PubMed] [Google Scholar]
- 27.Bloomfield R, Noble DW, Sudlow A: Prone position for acute respiratory failure in adults. Cochrane Database Syst Rev. 2015; 2015:CD008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mora-Arteaga JA, Bernal-Ramírez OJ, Rodríguez SJ: The effects of prone position ventilation in patients with acute respiratory distress syndrome. A systematic review and metaanalysis. Med Intensiva. 2015; 39:359–372 [DOI] [PubMed] [Google Scholar]
- 29.Munshi L, Del Sorbo L, Adhikari NKJ, et al. : Prone position for acute respiratory distress syndrome. A systematic review and meta-analysis. Ann Am Thorac Soc. 2017; 14:S280–S288 [DOI] [PubMed] [Google Scholar]
- 30.Pan C, Chen L, Lu C, et al. : Lung recruitability in COVID-19-associated acute respiratory distress syndrome: A single-center observational study. Am J Respir Crit Care Med. 2020; 201:1294–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elharrar X, Trigui Y, Dols AM, et al. : Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMA. 2020; 323:2336–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voelker MT, Jahn N, Bercker S, et al. : [Prone positioning of patients during venovenous extracorporeal membrane oxygenation is safe and feasible]. Anaesthesist. 2016; 65:250–257 [DOI] [PubMed] [Google Scholar]
- 33.Kredel M, Bischof L, Wurmb TE, et al. : Combination of positioning therapy and venovenous extracorporeal membrane oxygenation in ARDS patients. Perfusion. 2014; 29:171–177 [DOI] [PubMed] [Google Scholar]
- 34.Culbreth RE, Goodfellow LT: Complications of prone positioning during extracorporeal membrane oxygenation for respiratory failure: A systematic review. Respir Care. 2016; 61:249–254 [DOI] [PubMed] [Google Scholar]
- 35.Garcia B, Cousin N, Bourel C, et al. ; Lille Intensive Care COVID-19 group: Prone positioning under VV-ECMO in SARS-CoV-2-induced acute respiratory distress syndrome. Crit Care. 2020; 24:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.