Supplemental Digital Content is available in the text.
Keywords: adult, artificial, coronavirus, critical care, mortality, respiration, respiratory distress syndrome
OBJECTIVES:
To determine the association between time period of hospitalization and hospital mortality among critically ill adults with coronavirus disease 2019.
DESIGN:
Observational cohort study from March 6, 2020, to January 31, 2021.
SETTING:
ICUs at four hospitals within an academic health center network in Atlanta, GA.
PATIENTS:
Adults greater than or equal to 18 years with coronavirus disease 2019 admitted to an ICU during the study period (i.e., Surge 1: March to April, Lull 1: May to June, Surge 2: July to August, Lull 2: September to November, Surge 3: December to January).
MEASUREMENTS AND MAIN RESULTS:
Among 1,686 patients with coronavirus disease 2019 admitted to an ICU during the study period, all-cause hospital mortality was 29.7%. Mortality differed significantly over time: 28.7% in Surge 1, 21.3% in Lull 1, 25.2% in Surge 2, 30.2% in Lull 2, 34.7% in Surge 3 (p = 0.007). Mortality was significantly associated with 1) preexisting risk factors (older age, race, ethnicity, lower body mass index, higher Elixhauser Comorbidity Index, admission from a nursing home); 2) clinical status at ICU admission (higher Sequential Organ Failure Assessment score, higher d-dimer, higher C-reactive protein); and 3) ICU interventions (receipt of mechanical ventilation, vasopressors, renal replacement therapy, inhaled vasodilators). After adjusting for baseline and clinical variables, there was a significantly increased risk of mortality associated with admission during Lull 2 (relative risk, 1.37 [95% CI = 1.03–1.81]) and Surge 3 (relative risk, 1.35 [95% CI = 1.04–1.77]) as compared to Surge 1.
CONCLUSIONS:
Despite increased experience and evidence-based treatments, the risk of death for patients admitted to the ICU with coronavirus disease 2019 was highest during the fall and winter of 2020. Reasons for this increased mortality are not clear.
During the early days of the coronavirus disease 2019 (COVID-19) pandemic, mortality rates among patients with COVID-19 requiring critical care and mechanical ventilation were exceedingly high, ranging from 50% to 97% in reports from Wuhan, China, and Washington State (1–4). After the initial spring surge of COVID-19 cases in the United States, our group and others reported mortality rates of 17–39% among critically ill patients (5–10), in line with historical data from acute respiratory distress syndrome (ARDS) and previous influenza pandemics (11–13).
Multiple groups have since examined trends in critical care outcomes following that initial surge. Our own group found a drop in mortality from 34% in March 2020 to 22% in June 2020 (14). Similarly, a study from the United Kingdom found an 11% reduction in mortality after the initial surge (15), whereas a study from Philadelphia found a 24% absolute reduction in mortality from March to May, 2020 (16). A systematic review and meta-analysis also found a statistically significant decline of ~10% in the mortality rate between January and May 2020 (7). These improvements in mortality were attributed to a number of factors, including growing provider comfort, the availability of established treatments including antivirals and steroids (17, 18), and more consistent approaches to anticoagulation (19–21).
However, there are little data as to how mortality rates have since changed, including with the recent winter surge in the United States, during which time nationwide case counts routinely exceeded 200,000 cases/d (vs 30,000 and 60,000 cases/d during Spring and Summer 2020) (22). No studies have examined changes in patient characteristics over time and whether these may account for changes in mortality. We report here on mortality rates across the first 11 months of the COVID-19 pandemic, a period comprised of three surges and two lulls. We sought to characterize trends in patient characteristics over time and determine whether the time period of ICU admission was independently associated with mortality.
METHODS
Study Setting and Design
This is an observational cohort study of patients with COVID-19 admitted to an ICU at four Emory Healthcare hospitals in Atlanta, GA, from March 6, 2020, to January 31, 2021. These four hospitals have 1,800 inpatient beds, of which 276 were designated as ICU beds in January 2020. Although up to 313 beds were designated as ICU beds during the study period, all ICU beds had standard continuous monitoring of vital signs and were staffed by critical care providers and staff. During the study period, staffing was periodically supplemented with both internal and external temporary staff. Standard ICU care was provided throughout the study period, with the exception of March 2020 when, as reported previously, heated high-flow nasal cannula was not used given concerns about aerosol transmission (5, 14).
Study Population
All adults greater than or equal to 18 years admitted to an ICU with COVID-19 were included in the study. COVID-19 status was based upon a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) assay or an International Classification of Diseases, 10th Edition (ICD-10) billing code for COVID-19 (U07.1) during the hospitalization. For patients with more than one ICU admission, we only included the first admission, unless the second admission occurred within 7 days of the first discharge. In this instance, the admissions were combined and considered a single ICU stay, albeit designated a readmission for analytic purposes.
Data Collection and Statistical Analysis
Patient data, including sociodemographic information, clinical, and laboratory data, and clinical outcomes were obtained from the electronic medical record (EMR) and abstracted through March 31, 2021, at which time no patients in the cohort remained hospitalized. The Elixhauser Comorbidity Index was used as a measure of the burden of medical comorbidities (23). d-dimer and C-reactive protein (CRP) were collected at hospital admission. The Sequential Organ Failure Assessment (SOFA) score and the Pao2/Fio2 ratio were determined at ICU admission (24). Bacteremia was defined by a positive blood culture, excluding likely contaminants from a single positive culture, and bacterial pneumonia was defined by a positive respiratory culture with exclusion of common commensals and contaminants (25). Steroid administration was defined as receipt of dexamethasone greater than or equal to 6 mg daily (or the corticosteroid equivalent). Therapeutic anticoagulation was defined as administration of unfractionated heparin, low-molecular weight heparin, or direct oral anticoagulants at intermediate or full therapeutic dosing, argatroban, or bivalirudin at any point. Type of ICU was defined as either a preexisting medical/surgical ICU, a preexisting specialty ICU (e.g., cardiac, neurologic/neurosurgical), or an expansion ICU (i.e., for additional patients during the pandemic).
The primary exposure was the time period of ICU admission. For this purpose, the study period was divided into five epochs based upon author consensus, with three “surges” and two “lulls.” The three surge periods were as follows: March to April (Surge 1), July to August (Surge 2), December to January (Surge 3). The two lull periods were May to June (Lull 1) and September to November (Lull 2). The primary outcome of the study was all-cause, in-hospital mortality. Crude daily in-hospital mortality was also described using a rolling average calculated at ±14 days. The number of deaths that occurred each day was divided by the number of individuals at risk each day (i.e., the daily ICU census). Transfers to inpatient or home hospice, of which there were 57 and 48, respectively, were not included in mortality counts.
Sociodemographic, clinical, and ICU admission and intervention data were compared across the five time periods using a chi-square or Kruskal-Wallis test for categorical and continuous variables, respectively, with a two-sided p value of less than 0.05 considered statistically significant. We next examined changes in mortality over the study time periods using a modified Poisson regression method suited for common binary outcomes with robust ses obtained using the R package “sandwich.” A 95% CI was calculated for each estimated relative risk (RR). An unadjusted model was used with the study time period as a multilevel categorical exposure, with Surge 1 (i.e., March to April 2020) as the reference group and mortality as the outcome. We then ran a “baseline” model adjusted for key sociodemographic and clinical characteristics of age, race, ethnicity, Elixhauser Comorbidity Index, and admission directly from a nursing home. A final “complete” model was adjusted for covariates significantly associated with both the exposure and outcome: SOFA score, d-dimer, CRP, receipt of remdesivir, steroids, vasopressors, renal replacement therapy, mechanical ventilation, evidence of bacteremia and/or bacterial pneumonia, and ICU length of stay, in addition to variables in the baseline model. To account for non-Gaussian distributions, d-dimer and CRP were log-transformed prior to inclusion. Complete multivariable models were compared using the Akaike information criterion to select the most parsimonious model. All analyses were performed using R Version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
Ethics
This study was approved by the Emory University Institutional Review Board (STUDY00000425).
RESULTS
Patient Characteristics at Baseline
There were 1,686 patients admitted to an ICU from March 6, 2020, to January 31, 2021. The median age was 63 years (interquartile range [IQR], 52–74 yr), and 950 (56.3%) were men (Table 1). Just over half (885; 52.5%) were Black, 525 (31.1%) were White, and 138 (8.2%) were Hispanic. The median body mass index (BMI) was 28.0 kg/m2 (IQR, 23.8–34.0 kg/m2), and the median Elixhauser Comorbidity Index was 26 (IQR, 17–35). The most common comorbidities were hypertension (n = 1,010; 59.9%), diabetes mellitus (n = 859; 50.9%), congestive heart failure (n = 574; 34.0%), and chronic/end stage kidney disease (n = 564; 33.5%); many patients had multiple comorbidities (Supplemental Fig. 1, http://links.lww.com/CCM/G596). There were 122 patients (7.2%) admitted directly from a nursing home.
Table 1.
Sociodemographic and Baseline Clinical Characteristics of Patients Admitted to the ICU With Coronavirus Disease 2019
| Characteristics | Total (N = 1,686) | Surge 1 (N = 258) | Lull 1 (N = 61) | Surge 2 (N = 503) | Lull 2 (N = 288) | Surge 3 (N = 576) | p |
|---|---|---|---|---|---|---|---|
| Age, median (IQR) | 63 (52–74) | 64 (53–73) | 59 (52–70) | 60 (49–72) | 62 (53–75) | 66 (56–77) | < 0.001 |
| Age category, n (%) | < 0.001 | ||||||
| Age < 55 | 492 (29.2) | 72 (27.9) | 20 (32.8) | 185 (36.8) | 87 (30.2) | 128 (22.2) | |
| Age 55–64 | 402 (23.8) | 62 (24.0) | 20 (32.8) | 111 (22.1) | 68 (23.6) | 141 (24.5) | |
| Age 65–74 | 374 (22.2) | 65 (25.2) | 9 (14.8) | 117 (23.3) | 58 (20.1) | 125 (21.7) | |
| Age > 74 | 418 (24.8) | 59 (22.9) | 12 (19.7) | 90 (17.9) | 75 (26.0) | 182 (31.6) | |
| Gender, n (%) | 0.843 | ||||||
| Female | 736 (43.7) | 113 (43.8) | 25 (41.0) | 212 (42.1) | 133 (46.2) | 253 (43.9) | |
| Male | 950 (56.3) | 145 (56.2) | 36 (59.0) | 291 (57.9) | 155 (53.8) | 323 (56.1) | |
| Race, n (%) | < 0.001 | ||||||
| African American or Black | 885 (52.5) | 175 (67.8) | 33 (54.1) | 279 (55.5) | 126 (43.8) | 272 (47.2) | |
| Caucasian or White | 525 (31.1) | 48 (18.6) | 10 (16.4) | 134 (26.6) | 107 (37.2) | 226 (39.2) | |
| Asian | 65 (3.9) | 12 (4.7) | 2 (3.3) | 12 (2.4) | 11 (3.8) | 28 (4.9) | |
| Other | 15 (0.9) | 1 (0.4) | 1 (1.6) | 7 (1.4) | 1 (0.3) | 5 (0.9) | |
| Unknown | 196 (11.6) | 22 (8.5) | 15 (24.6) | 71 (14.1) | 43 (14.9) | 45 (7.8) | |
| Ethnic group, n (%) | < 0.001 | ||||||
| Hispanic or Latino | 138 (8.2) | 13 (5.0) | 10 (16.4) | 67 (13.3) | 16 (5.6) | 32 (5.6) | |
| Non-Hispanic or Latino | 1362 (80.8) | 220 (85.3) | 45 (73.8) | 384 (76.3) | 231 (80.2) | 482 (83.7) | |
| Unknown | 186 (11.0) | 25 (9.7) | 6 (9.8) | 52 (10.3) | 41 (14.2) | 62 (10.8) | |
| BMI (kg/m2), median (IQR) | 28.0 (23.8–34.0) | 27.9 (23.8–33.6) | 26.3 (22.5–31.7) | 28.7 (24.4–34.4) | 27.6 (23.9–32.8) | 27.6 (23.3–34.2) | 0.168 |
| BMI ≥ 40 kg/m2, n (%) | 189 (11.6) | 24 (9.6) | 5 (8.6) | 53 (10.9) | 26 (9.6) | 81 (14.5) | 0.121 |
| Hypertension, n (%) | 1010 (59.9) | 163 (63.2) | 34 (55.7) | 289 (57.5) | 176 (61.1) | 348 (60.4) | 0.544 |
| Coronary artery disease, n (%) | 364 (21.6) | 50 (19.4) | 14 (23.0) | 95 (18.9) | 79 (27.4) | 126 (21.9) | 0.066 |
| Congestive heart failure, n (%) | 574 (34.0) | 89 (34.5) | 25 (41.0) | 155 (30.8) | 103 (35.8) | 202 (35.1) | 0.365 |
| Diabetes mellitus, n (%) | 859 (50.9) | 147 (57.0) | 27 (44.3) | 240 (47.7) | 143 (49.7) | 302 (52.4) | 0.105 |
| Chronic kidney disease/end-stage renal disease, n (%) | 564 (33.5) | 98 (38.0) | 19 (31.1) | 142 (28.2) | 94 (32.6) | 211 (36.6) | 0.023 |
| Asthma, n (%) | 158 (9.4) | 25 (9.7) | 3 (4.9) | 40 (8.0) | 32 (11.1) | 58 (10.1) | 0.405 |
| Chronic obstructive pulmonary disease, n (%) | 213 (12.6) | 23 (8.9) | 10 (16.4) | 60 (11.9) | 33 (11.5) | 87 (15.1) | 0.100 |
| Elixhausera, median (IQR) | 26 (17–35) | 26 (18–35) | 30 (19–36) | 24 (17–34) | 27 (18–36) | 26 (16–34) | 0.240 |
| Admission from nursing home, n (%) | 122 (7.2) | 30 (11.6) | 9 (14.8) | 26 (5.2) | 21 (7.3) | 36 (6.2) | 0.002 |
BMI = body mass index, IQR = interquartile range.
aElixhauser comorbidities indices were calculated using the van Walraven algorithm for the weighting process.
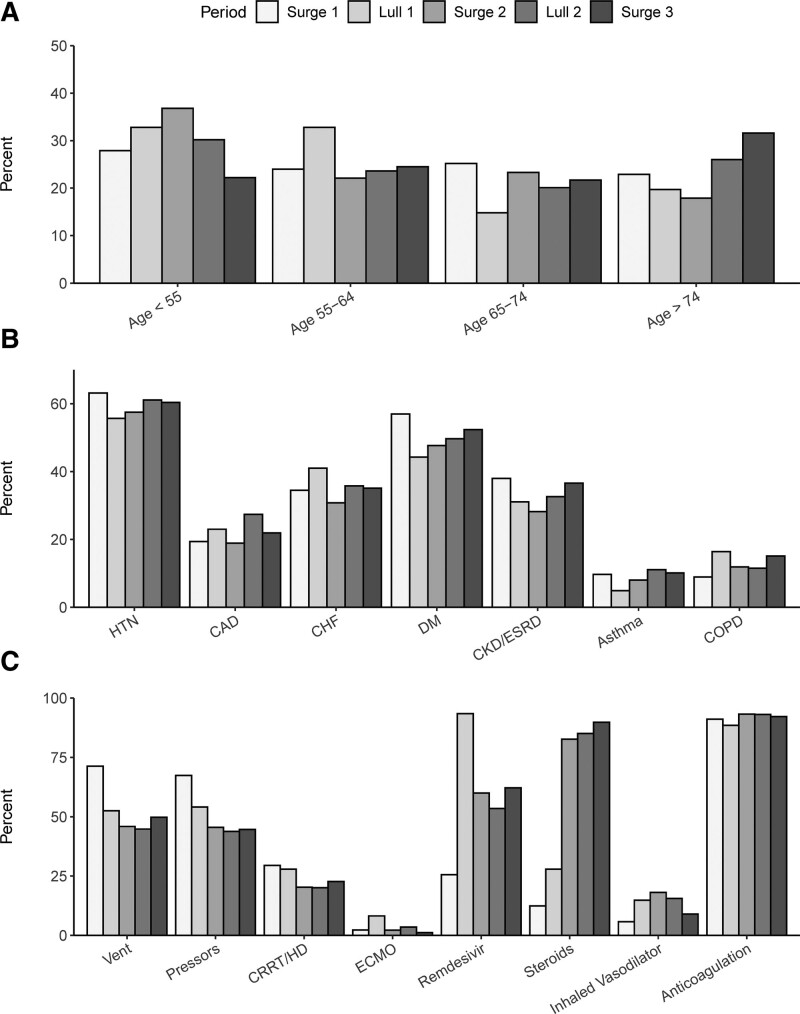
There were 258 patients (15.3%) admitted during Surge 1, 61 (3.6%) admitted during Lull 1, 503 (29.8%) admitted during Surge 2, 288 (17.1%) admitted during Lull 2, and 576 (34.2%) admitted during Surge 3. The median age in Surge 1 was 64 years (IQR, 53–73 yr) and then dropped during the two Lulls and Surge 2 before rising to 66 years (IQR, 55–77 yr) in Surge 3 (p < 0.001) (Fig. 1A). Gender did not significantly differ across the study time periods. However, race did significantly differ, with the proportion of Black patients highest in Surge 1 at 67.8% and lowest in Lull 2 and Surge 3 at 43.8% and 47.2%, respectively (p < 0.001). The proportion of Hispanic patients also differed across study, from 5.0% in Surge 1 to 16.4% in Lull 1 (p < 0.001). Although neither BMI nor the Elixhauser Index significantly differed over time, the proportion of patients with chronic or end-stage renal disease ranged from 38.0% in Surge 1 to 28.2% in Surge 2 (p = 0.023) (Fig. 1B).
Figure 1.
Distribution of age (A), baseline comorbidities (B), and ICU interventions (C) over the study time periods (i.e., surges and lulls) for all patients admitted to the ICU with coronavirus disease 2019 (n = 1,686). CAD = coronary artery disease, CHF = congestive heart failure, CKD = chronic kidney disease, COPD = chronic obstructive pulmonary disease, CRRT = continuous renal replacement therapy, DM = diabetes mellitus, ECMO = extracorporeal membrane oxygenation, ESRD = end-stage renal disease, HD = hemodialysis, HTN = hypertension, Vent = ventilator.
Clinical Characteristics at ICU Admission
The median SOFA score for all patients was 6.0 (IQR, 3.0–9.8), the median Pao2/Fio2 ratio was 128.1 (IQR, 89.7–211.8), the median d-dimer was 1,186 ng/mL (IQR 672–2,494 ng/mL), and the median CRP was 118 mg/L (IQR, 66–183 mg/L) (Table 2). Although a majority of patients (n = 1,310; 77.7%) were admitted to an existing medical/surgical ICU, 143 (8.5%) were admitted to an existing specialty ICU, and 231 (13.7%) were admitted to an expansion ICU. There were 143 patients (8.5%) readmitted to an ICU within seven days of a prior ICU discharge.
Table 2.
Clinical Characteristics at ICU Admission, ICU Interventions, and Mortality of Patients Admitted to the ICU With Coronavirus Disease 2019
| Characteristics | Total (N = 1,686) | Surge 1 (N = 258) | Lull 1 (N = 61) | Surge 2 (N = 503) | Lull 2 (N = 288) | Surge 3 (N = 576) | p |
|---|---|---|---|---|---|---|---|
| Sequential Organ Failure Assessmenta, median (IQR) | 6.0 (3.0–9.8) | 8.0 (5.0–11.0) | 6.0 (4.0–9.0) | 5.0 (2.0–9.0) | 5.0 (2.0–8.2) | 6.0 (3.0–9.0) | < 0.001 |
| Pao2/Fio2 ratioa (mm Hg), median (IQR) | 128.1 (89.7–211.8) | 131.1 (90.6–194.0) | 150.0 (106.4–272.1) | 132.0 (89.4–215.6) | 125.0 (85.3–196.0) | 123.1 (88.7–216.4) | 0.421 |
| d-dimerb (ng/mL), median (IQR) | 1,186 (672–2,494) | 1,343 (765–3,535) | 1,088 (639–3,269) | 1,009 (601–1,919) | 1,141 (701–2,387) | 1,303 (716–3,634) | < 0.001 |
| C-reactive proteinb (mg/L), median (IQR) | 118 (66–183) | 145 (82–216) | 104 (43–169) | 117 (68–193) | 113 (65–161) | 111 (59–173) | < 0.001 |
| Mechanical ventilation, n (%) | 863 (51.2) | 184 (71.3) | 32 (52.5) | 231 (45.9) | 129 (44.8) | 287 (49.8) | < 0.001 |
| Days on ventilator, median (IQR) | 11 (5–19) | 11 (6–16) | 11 (7–20) | 14 (7–25) | 10 (5–21) | 8 (3–18) | < 0.001 |
| Vasopressors, n (%) | 819 (48.6) | 174 (67.4) | 33 (54.1) | 229 (45.5) | 126 (43.8) | 257 (44.6) | < 0.001 |
| Continuous renal replacement therapy/hemodialysis, n (%) | 384 (22.8) | 76 (29.5) | 17 (27.9) | 102 (20.3) | 58 (20.1) | 131 (22.7) | 0.035 |
| Extracorporeal membrane oxygenation, n (%) | 39 (2.3) | 6 (2.3) | 5 (8.2) | 11 (2.2) | 10 (3.5) | 7 (1.2) | 0.007 |
| Remdesivir, n (%) | 937 (55.6) | 66 (25.6) | 57 (93.4) | 302 (60.0) | 154 (53.5) | 358 (62.2) | < 0.001 |
| Steroidsc, n (%) | 1,227 (72.8) | 32 (12.4) | 17 (27.9) | 416 (82.7) | 245 (85.1) | 517 (89.8) | < 0.001 |
| Inhaled vasodilator, n (%) | 212 (12.6) | 15 (5.8) | 9 (14.8) | 91 (18.1) | 45 (15.6) | 52 (9.0) | < 0.001 |
| Anticoagulationd, n (%) | 1,557 (92.3) | 235 (91.1) | 54 (88.5) | 469 (93.2) | 268 (93.1) | 531 (92.2) | 0.613 |
| Emergency department LOS (hr), median (IQR) | 6.2 (4.4–9.0) | 4.7 (3.7–6.3) | 6.2 (3.9–7.9) | 6.2 (4.6–8.8) | 5.6 (4.4–7.9) | 7.3 (5.0–12.0) | < 0.001 |
| ICU type, n (%) | < 0.001 | ||||||
| Existing medical/surgical ICU | 1,310 (77.7) | 186 (72.1) | 51 (83.6) | 400 (79.5) | 235 (81.6) | 438 (76.0) | |
| Existing specialty ICUe | 143 (8.5) | 50 (19.5) | 6 (9.8) | 27 (5.4) | 20 (7.0) | 40 (6.9) | |
| Expansion ICUf | 231 (13.7) | 21 (8.2) | 4 (6.6) | 76 (15.1) | 32 (11.1) | 98 (17.0) | |
| Readmission, n (%) | 143 (8.5) | 29 (11.2) | 6 (9.8) | 37 (7.4) | 32 (11.1) | 39 (6.8) | 0.083 |
| Bacteremiag, n (%) | 220 (13.0) | 22 (8.5) | 8 (13.1) | 74 (14.7) | 36 (12.5) | 80 (13.9) | 0.177 |
| Bacterial pneumoniah, n (%) | 310 (18.4) | 53 (20.5) | 9 (14.8) | 94 (18.7) | 52 (18.1) | 102 (17.7) | 0.815 |
| ICU LOS (d), median (IQR) | 6 (2–15) | 10 (4–16) | 7 (2–15) | 6 (2–15) | 6 (2–13) | 5 (2–12) | < 0.001 |
| Crude mortality, n (%) | 501 (29.7) | 74 (28.7) | 13 (21.3) | 127 (25.2) | 87 (30.2) | 200 (34.7) | 0.007 |
IQR = interquartile range, LOS = length of stay.
aValues captured at admission into an ICU.
bValues captured at initial hospital admission.
cSteroids include receipt of dexamethasone ≥ 6 mg daily or the corticosteroid equivalent for other steroid agents.
dAnticoagulants include unfractionated heparin, low-molecular weight heparin, or direct oral anticoagulants (i.e., dabigatran, rivaroxaban, apixaban) at intermediate or full therapeutic dosing, argatroban, or bivalirudin at any point during hospitalization.
eCardiac care units and neurologic/neurosurgical ICUs.
fICUs created to accommodate additional patients during the coronavirus disease 2019 pandemic.
gAny positive blood culture, excluding those with likely contaminants that were only positive in a single culture.
hPositive respiratory culture.
In looking across the study periods, the SOFA score differed significantly from a peak of 8 in Surge 1 to a nadir of 5 in Surge 2 and Lull 2 (p < 0.001). d-dimer and CRP were also highest in Surge 1 and differed significantly across study periods (p < 0.001). Although the proportion of patients admitted to an expansion ICU differed significantly across the study, peaking at 18.9% in Surge 3 (p < 0.001), the proportion of readmissions did not differ over time.
ICU Interventions and Care
During their ICU stay, 863 patients (51.2%) received mechanical ventilation, 819 (48.6%) required vasopressors, 384 (22.8%) received renal replacement therapy, and 39 (2.3%) received extracorporeal membrane oxygenation (ECMO) (Table 2). Over half of patients received remdesivir (n = 937; 55.6%), whereas nearly three quarters (n = 1,227; 72.8%) received steroids. The vast majority of patients (n = 1,557; 92.3%) received intermediate or full-dose anticoagulation, whereas 212 patients (12.6%) received inhaled vasodilator therapy. There were 220 patients (13.0%) with bacteremia and 310 (18.4%) with bacterial pneumonia.
The proportion of patients who received mechanical ventilation was highest in Surge 1 at 71.3% (during which time heated high-flow nasal cannula was initially not used) and then remained between 44% and 53% for the remainder of the study period (p < 0.001) (Fig. 1C). The use of vasopressors and renal replacement therapy were also highest during Surge 1 (p < 0.001, p = 0.035, respectively). Although the administration of remdesivir and steroids increased after Surge 1 (p values<0.001), there was no difference in the use of anticoagulation across the study (p = 0.613). There was also no difference in the rates of bacteremia or bacterial pneumonia over time.
All-Cause, In-Hospital Mortality
Overall, 501 patients (29.7%) died during their hospitalization. Mortality was significantly associated with older age, with a median age of 72 years (IQR, 61–80 yr) for patients who died versus 60 years (IQR, 49–71 yr) for those who survived (p < 0.001) (Supplemental Table 1, http://links.lww.com/CCM/G596). Mortality did not significantly differ by gender, although it trended toward being significantly higher in White versus Black patients (p = 0.062), and it was significantly lower for patients of Hispanic ethnicity (p = 0.040). Median BMI was significantly lower in patients who died than those who survived (26.7 kg/m2 [IQR, 23.1–32.1 kg/m2] vs 28.5 kg/m2 [IQR, 24.1–34.7 kg/m2]; p < 0.001); severe obesity (BMI > 40 kg/m2) was also associated with improved survival (p = 0.006). Patients who died also had a greater burden of comorbidities at baseline, with a median Elixhauser Comorbidity Index of 32 (IQR, 24–39) as compared to 23 (IQR, 14–32) among those who survived (p < 0.001).
SOFA score, d-dimer, and CRP levels were all significantly higher in patients who died (p < 0.01), whereas the Pao2/Fio2 was significantly lower in patients who died (p < 0.001) (Supplemental Table 2, http://links.lww.com/CCM/G596). Organ failure requiring ICU interventions including mechanical ventilation, vasopressors, renal replacement therapy, and inhaled vasodilators were all associated with greater mortality (p < 0.001). Among 39 patients who received ECMO, 22 (56%) survived. The type of ICU, whether an existing or expansion unit, was not significantly associated with mortality.
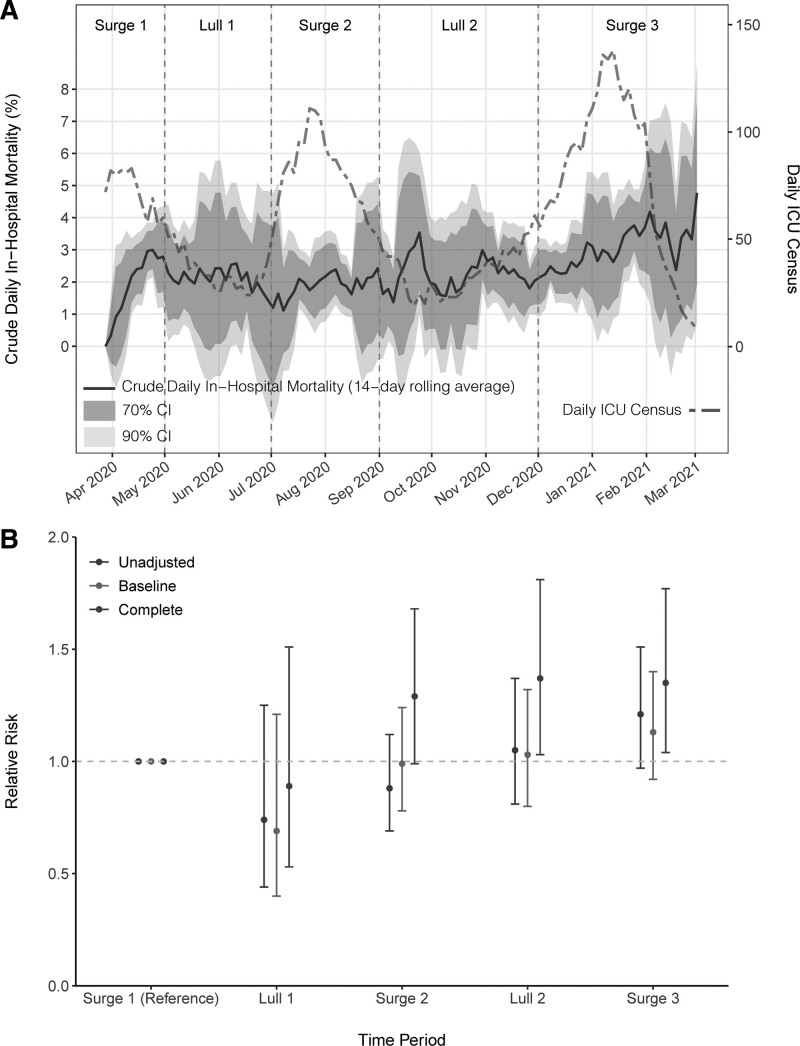
Crude in-hospital mortality differed significantly across the study time periods, initially peaking at 28.7% in Surge 1 before declining to 21.3% in Lull 1, rising to 25.2% in Surge 2, 30.2% in Lull 2, and then peaking again at 34.7% in Surge 3 (p = 0.007). Crude daily in-hospital mortality rose to 3% in Surge 1 and then remained around 1–2.5% through Lull 1 and Surge 2 (Fig. 2A). During Lull 2, there were two peaks in crude mortality in late September and early November and then mortality steadily increased throughout Surge 3, climbing from 2% to above 4% in February 2021.
Figure 2.
All-cause, in-hospital mortality over time for patients admitted to the ICU with coronavirus disease 2019 (n = 1,686). A, Crude daily in-hospital mortality (black solid line) with 70% and 90% CIs in gray and daily ICU census (red dashed line) over the study time periods. B, Relative risk of death across study time periods in unadjusted, baseline, and complete models. The baseline model is adjusted for age, race, ethnicity, Elixhauser Comorbidity Index, and admission from a nursing home. The complete model is adjusted for all variables in the baseline model in addition to d-dimer, C-reactive protein, Sequential Organ Failure Assessment score, mechanical ventilation, vasopressors, remdesivir, steroids, renal replacement therapy, bacteremia, bacterial pneumonia, and ICU length of stay.
As compared to Surge 1, the RR of all-cause, in-hospital mortality did not significantly differ across the subsequent surges and lulls in an unadjusted model (Table 3 and Fig. 2B). The RR of mortality also did not differ in our baseline adjusted models. However, after additional adjustment for clinical status at the time of ICU admission, the receipt of ICU interventions, ICU length of stay, and the presence of secondary bacterial infections in a complete adjusted model, there was a significantly greater risk of death associated with admission during Lull 2 (RR, 1.37; 95% CI, 1.03–1.81) and Surge 3 (RR, 1.35; 95% CI, 1.04–1.77). Full results of the regression models are available in Supplemental Tables 3–5 (http://links.lww.com/CCM/G596).
Table 3.
Relative Risk of All-Cause, In-Hospital Mortality Over Time
| Study Periods | Sample Size | No. of Deaths | Unadjusted Model RR (95% CI) | Baseline Model RR (95% CI) | Complete Model RR (95% CI) |
|---|---|---|---|---|---|
| Surge 1 (March to April 2020) | 258 | 74 | Reference | Reference | Reference |
| Lull 1 (May to June 2020) | 61 | 13 | 0.74 (0.44–1.25) | 0.69 (0.40–1.21) | 0.89 (0.53–1.51) |
| Surge 2 (July to August 2020) | 503 | 127 | 0.88 (0.69–1.12) | 0.99 (0.78–1.24) | 1.29 (0.99–1.68) |
| Lull 2 (September to November 2020) | 288 | 87 | 1.05 (0.81–1.37) | 1.03 (0.80–1.32) | 1.37 (1.03–1.81) |
| Surge 3 (December 2020 to January 2021) | 576 | 200 | 1.21 (0.97–1.51) | 1.13 (0.92–1.40) | 1.35 (1.04–1.77) |
RR = relative risk.
The baseline model is adjusted for age, race, ethnicity, Elixhauser, and admission from a nursing home. The complete model is adjusted for variables from the baseline model and d-dimer, C-reactive protein, Sequential Organ Failure Assessment, mechanical ventilation, vasopressors, remdesivir, steroids, renal replacement therapy, secondary bacterial infections (i.e., bacteremia or bacterial pneumonia), and days in the ICU.
Boldface values are statistically significant.
DISCUSSION
In this large cohort of 1,686 critically ill patients, mortality rates varied significantly over the first 11 months of the COVID-19 pandemic. Our group previously reported that mortality rates during March and April of 2020 were commensurate with historical death rates from pandemic influenza and ARDS (5) and later that mortality declined in the months immediately following the initial surge (14). We attributed this decline in mortality to greater experience with the novel coronavirus alongside a growing evidence base of effective treatments. However, with additional data covering 6 more months and a second and third surge of cases, we report now that in-hospital mortality rates rose again after the summer surge and ultimately peaked at 35% during the third surge in December 2020 and January 2021. The reasons for this increase in mortality during the latter 5 months of the study period, which persisted after adjustment for baseline and clinical characteristics, are not clear.
Although several studies have examined mortality trends for COVID-19, we are not aware of any with data from the recent winter surge of cases in the United States. Rather, previous reports, including our own, described improvements in outcomes following the first surge in early 2020 (14). A study of nearly 500 patients with COVID-19 from Philadelphia attributed declines in ICU mortality from March to May of 2020 to a “learning effect” (16). Similar improvements were seen in a cohort of over 10,000 patients admitted to ICUs in the United Kingdom, with an 11.8% decline in mortality between February and July 2020 (15). In another large cohort of over 8,000 critically ill patients admitted to a U.S. Veterans Affairs hospital from March to August 2020, ICU mortality peaked in April and May before declining thereafter and was, notably, significantly associated with ICU demand (26).
This study makes an important addition to the literature by including data through the recent winter peak of cases. Although we anticipated that admission during a surge period would be associated with higher mortality given the stress on healthcare systems and clinical staff during these periods, we were surprised that mortality rates rose in the fall of 2020, prior to the third surge of cases. We were also surprised that mortality during the fall and winter of 2020 exceeded that of the first surge in early 2020, at which time there were no established therapies for COVID-19 and providers had no experience treating COVID-19. We did not find an association between the type of ICU (i.e., existing vs expansion) and mortality. However, unmeasured healthcare system factors may have played a role in the observed variability in ICU outcomes, including staff burnout with the prolonged nature of the pandemic and reliance on temporary staff members. Likewise, the spread of viral variants of concern could play a role, as several variants have been associated with higher mortality (27, 28).
As with other cohorts, we found that mortality was significantly associated with increasing age. However, contrary to some reports, there was a trend toward improved survival among Black patients. This finding echoes that of two recent studies, one in New York City where Black patients were more likely to be infected with COVID-19 but less likely to die, and a second in Detroit where people of color who were critically ill with COVID-19 had lower mortality than White patients (29, 30).
There are several limitations to this study. First, we used data from a single academic healthcare network, and so the data may not be broadly generalizable. Second, the analysis leveraged data available in the EMR. Certain variables, such as prone positioning, are not collected and thus were not included in our analysis. However, we have no reason to believe that the use of established interventions for ARDS such as prone positioning and neuromuscular blockade varied over the course of the study. Third, a diagnosis of COVID-19 was based on SARS-CoV-2 test results or an ICD-10 code, and so it is possible some patients in the analysis were in fact admitted for postacute COVID-19 syndrome or that other patients with COVID-19 were not included in the analysis (31). Fourth, viral sequencing was not performed, and so the prevalence of viral variants of concern in this cohort is not known. Fifth, this study was conducted prior to the widespread availability of vaccines against SARS-CoV-2, and so the impact of vaccinations on mortality is not known.
In conclusion, in this observational cohort of 1,686 patients critically ill with COVID-19, we found that the RR of death increased in the fall and Winter of 2020. Reasons for this increase in mortality during the third surge remain unclear but raise a cautionary note. Unlike the early days of the pandemic when confusion abounded, high mortality during this third surge of cases, and the lull preceding it, cannot be attributed to a lack of familiarity with the unique pathophysiology of COVID-19 nor a lack of established therapies. Whether this peak in mortality was attributable to viral variants, seasonal variation, an overstressed healthcare system, or other unmeasured factors, the potential impact of temporal trends and surges should be considered when preparing for future pandemics.
ACKNOWLEDGMENTS
We would like to extend our most profound thanks and gratitude to our colleagues in the Emory Critical Care Center and Emory Healthcare who have worked so hard to provide excellent clinical care during this global pandemic. Emory COVID-19 Quality and Clinical Research Collaborative Members (in alphabetical order): Max W. Adelman, Scott Arno, Sara C. Auld, Theresa Barnes, William Bender, James M. Blum, Gaurav Budharani, Stephanie Busby, Laurence Busse, Mark Caridi-Scheible, David Carpenter, Nikulkumar Chaudhari, Craig M. Coopersmith, Lisa Daniels, Johnathan A. Edwards, Jane Fazio, Babar Fiza, Eliana Gonzalez, Ria Gripaldo, Charles Grodzin, Robert Groff, Alfonso C. Hernandez-Romieu, Max Hockstein, Dan Hunt, Craig S. Jabaley, Jesse T. Jacob, Colleen Kraft, Greg S. Martin, Samer Melham, Nirja Mehta, Chelsea Modlin, David J. Murphy, Mia Park, Deepa Patel, Cindy Powell, Amit Prabhaker, Jeeyon Rim, Ramzy Rimawi, Chad Robichaux, Nicholas Scanlon, Milad Sharifpour, Bashar Staitieh, Michael Sterling, Jonathan Suarez, Colin Swenson, Nancy Thakkar, Alexander Truong, Hima Veeramachaneni, Alvaro Velasquez, Michael Waldmann, Max Weinmann, Thanushi Wynn, and Joel Zivot.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Supported, in part, by the following grants: the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases K23 AI134182 (to Dr. Auld), NIH/Clinical and Translational Science Alliance UL1TR002378.
Dr. Auld received support for article research from the National Institutes of Health. The remaining authors have disclosed that they do not have any potential conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Arentz M, Yim E, Klaff L, et al. : Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020; 323:1612–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. : COVID-19 in critically ill patients in the Seattle Region — case series. N Engl J Medicine. 2020; 382:2012–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C, Chen X, Cai Y, et al. : Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020; 180:934–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, et al. : Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020; 395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auld SC, Caridi-Scheible M, Blum JM, et al. : ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med. 2020; 48:e799–e804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziehr DR, Alladina J, Petri CR, et al. : Respiratory pathophysiology of mechanically ventilated patients with COVID-19: A cohort study. Am J Respir Crit Care Med. 2020; 201:1560–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong RA, Kane AD, Cook TM: Outcomes from intensive care in patients with COVID-19: A systematic review and meta-analysis of observational studies. Anaesthesia. 2020; 75:1340–1349 [DOI] [PubMed] [Google Scholar]
- 8.Cummings MJ, Baldwin MR, Abrams D, et al. : Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet. 2020; 395:1763–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta S, Hayek SS, Wang W, et al. ; STOP-COVID Investigators: Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020; 180:1436–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maatman TK, Jalali F, Feizpour C, et al. : Routine venous thromboembolism prophylaxis may be inadequate in the hypercoagulable state of severe coronavirus disease 2019. Crit Care Med. 2020; 48:e783–e790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estenssoro E, Ríos FG, Apezteguía C, et al. ; Registry of the Argentinian Society of Intensive Care SATI: Pandemic 2009 influenza A in Argentina: A study of 337 patients on mechanical ventilation. Am J Respir Crit Care Med. 2010; 182:41–48 [DOI] [PubMed] [Google Scholar]
- 12.Domínguez-Cherit G, Lapinsky SE, Macias AE, et al. : Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009; 302:1880–1887 [DOI] [PubMed] [Google Scholar]
- 13.Zambon M, Vincent JL: Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008; 133:1120–1127 [DOI] [PubMed] [Google Scholar]
- 14.Auld SC, Caridi-Scheible M, Robichaux C, et al. : Declines in mortality over time for critically ill adults with coronavirus disease 2019. Crit Care Med. 2020; 48:e1382–e1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doidge JC, Gould DW, Ferrando-Vivas P, et al. : Trends in intensive care for patients with COVID-19 in England, Wales, and Northern Ireland. Am J Respir Crit Care Med. 2021; 203:565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anesi GL, Jablonski J, Harhay MO, et al. : Characteristics, outcomes, and trends of patients with COVID-19-related critical illness at a learning health system in the United States. Ann Intern Med. 2021; 174:613–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beigel JH, Tomashek KM, Dodd LE, et al. ; RECOVERY Collaborative Group: Remdesivir for the treatment of COVID-19 — preliminary report. N Engl J Med. 2020; 383:1813–1826 [DOI] [PubMed] [Google Scholar]
- 18.Horby P, Lim WS, Emberson JR, et al. : Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2020; 384:693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paranjpe I, Fuster V, Lala A, et al. : Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020; 76:122–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang N, Bai H, Chen X, et al. : Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020; 18:1094–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rentsch CT, Beckman JA, Tomlinson L, et al. : Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: Cohort study. BMJ. 2021; 372:n311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention: COVID Data Tracker. 2021. Available at: https://covid.cdc.gov/covid-data-tracker/#global-counts-rates. Accessed March 31, 2021
- 23.van Walraven C, Austin PC, Jennings A, et al. : A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009; 47:626–633 [DOI] [PubMed] [Google Scholar]
- 24.Lyu PF, Hockenberry JM, Gaydos LM, et al. : Impact of a sequential intervention on albumin utilization in critical care. Crit Care Med. 2016; 44:1307–1313 [DOI] [PubMed] [Google Scholar]
- 25.Adelman MW, Bhamidipati DR, Hernandez-Romieu AC, et al. : Secondary bacterial pneumonias and bloodstream infections in patients hospitalized with COVID-19. Ann Am Thorac Soc. 2021. Apr 6. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bravata DM, Perkins AJ, Myers LJ, et al. : Association of Intensive Care Unit Patient Load and Demand With Mortality Rates in US Department of Veterans Affairs Hospitals During the COVID-19 Pandemic. JAMA Netw Open. 2021; 4:e2034266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grint DJ, Wing K, Williamson E, et al. : Case fatality risk of the SARS-CoV-2 variant of concern B.1.1.7 in England, 16 November to 5 February. Eurosurveillance. 2021; 26:2100256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies NG, Jarvis CI, van Zandvoort K, et al. : Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. 2021; 59:270–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogedegbe G, Ravenell J, Adhikari S, et al. : Assessment of Racial/Ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Netw Open. 2020; 3:e2026881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazar MH, Fadel R, Gardner-Gray J, et al. : Racial differences in a detroit, MI, ICU population of coronavirus disease 2019 patients. Crit Care Med. 2021; 49:482–489 [DOI] [PubMed] [Google Scholar]
- 31.Nalbandian A, Sehgal K, Gupta A, et al. : Post-acute COVID-19 syndrome. Nat Med. 2021; 27:601–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.