Abstract
Capture vulnerability of commercial and recreational fishes has been associated with behavioral, morphological, and life‐history traits; however, relationships with non‐target species, such as sea turtles, have not been adequately studied. We examined species composition, timing of captures, morphological variables including body size and head width, and body condition of sea turtles captured from a recreational fishing pier in the northern Gulf of Mexico and of sea turtles captured in the waters adjacent to the pier. From 2014 to 2019, 148 net captures and 112 pier captures of three sea turtle species were documented. Green turtles were captured most frequently in the net and on the pier. Turtles captured from the pier were larger than those captured in the net. There was no difference in head width between net‐caught and pier‐caught turtles; however, small sample sizes limited those comparisons. The body condition index was lower for pier‐caught than net‐caught Kemp’;s ridleys but did not differ with green turtles or loggerheads. Differences were also observed in the timing of capture on the pier as compared to in the net. Finally, the relationship between size, body condition, and pier‐capture vulnerability suggests these are complex interactions. Mortality of sea turtles captured from fishing piers could be selecting against bolder individuals, which may result in changes in sea turtle population demographics over a long time period.
Keywords: boldness, capture vulnerability, Gulf of Mexico, pier, recreational fishing, sea turtle
We examined species composition, timing of captures, morphological variables including body size and head width, and body condition of sea turtles captured from a recreational fishing pier in the northern Gulf of Mexico and of sea turtles captured in the waters adjacent to the pier. Green turtles were captured most frequently in the net and on the pier. Turtles captured from the pier were larger than those captured in the net.

1. INTRODUCTION
Human population growth is increasing in coastal areas (Bounoua et al., 2018). This rapid rate of urbanization globally has led to dramatic changes to natural habitats (Gaston, 2010; Uchida et al., 2019). Consequently, some individual animals have adjusted their behaviors by foraging in new habitats or on novel prey items (Breck et al., 2019; Garamszegi et al., 2009; Tuomainen & Candolin, 2011). Drastic environmental changes often encourage individual behavioral traits such as boldness (Breck et al., 2019; Kelleher et al., 2017; Klefoth et al., 2017), which results in indirect selection against morphological and life‐history traits such as larger body size and faster growth rates (Alós et al., 2014; Enberg et al., 2012; Klefoth et al., 2017). These changes in individual traits can lead to increases in predation risk or vulnerability of capture in fishing activities of larger, bolder individuals (i.e., capture vulnerability; Klefoth et al., 2017; Phillip et al., 2009), thus ultimately leading to negative population‐level effects. As anthropogenic activities increase in coastal habitats, the response of wildlife, particularly imperiled species, could have far‐reaching consequences to ecology and conservation.
In the marine environment, one anthropogenic activity that has been shown to alter traits of target species is commercial and recreational fishing (Hamley, 1975; Klefoth et al., 2017; Lewin et al., 2006). In fact, commercial fishing is likely influencing the course of evolution for many target species (Enberg et al., 2012). Capture vulnerability has been shown to be a heritable trait in some fish species (Klefoth et al., 2017; Phillip et al., 2009), and behavioral traits such as boldness and morphological traits such as body size can impact catchability (Enberg et al., 2012; Uusi‐Heikkilä et al., 2008). Hook‐and‐line fishing, in particular, is more likely to capture individuals that are exploratory or have higher activity, boldness, or aggression levels. These behavioral traits increase encounters with fishing gear or increase the probability of ingesting certain baits or lures (Alós et al., 2012; Arlinghaus et al., 2016, 2017; Biro & Stamps, 2008; Diaz Pauli & Sih, 2017; Enberg et al., 2012; Lennox et al., 2017; Uusi‐Heikkilä et al., 2017).
Although increased capture vulnerability has been documented for many commercially and recreationally valuable fish species, many non‐target animals, including imperiled species such as sea turtles, are also often incidentally captured in fishing gear (Cook et al., 2020; Pate & Marshall, 2020). It would be expected that a suite of traits similar to those documented in fishes would increase sea turtle capture vulnerability. Sea turtles often forage in neritic waters (Bolten, 2003). Increases in incidental sea turtle captures by hook‐and‐line anglers, particularly from fishing piers, have recently been reported (Cook et al., 2020). Studies of sea turtles captured in recreational fishing activities have focused almost exclusively on individuals once they are captured (Cook et al., 2020; Rudloe & Rudloe, 2005; Seney, 2017) or after they have been released from subsequent rehabilitation (Coleman et al., 2016). Other studies have shown that juvenile green turtles (Chelonia mydas) exhibit individual differences in boldness (Griffin et al., 2017; Kudo et al., 2021). Capture vulnerability may also be affected by variations in the morphology, life‐history traits, and behavior of a species. Identifying such relationships may help managers design actions to reduce hook‐and‐line captures of protected species. For example, if a protected species co‐occurs with fishing activities during a certain time of the year, fishing activities could potentially be restricted at those times to reduce unintentional bycatch.
Globally, all sea turtle species, except the olive ridley (Lepidochelys olivacea), are listed as threatened or endangered by the U.S. Endangered Species Act and CITES. Five species of sea turtles are found in the Gulf of Mexico (GOM), including the Kemp’s ridley (Lepidochelys kempii), loggerhead (Caretta caretta), green turtle, leatherback (Dermochelys coriacea), and hawksbill (Eretmochelys imbricata) (Ward & Tunnell, 2017). The recovery plans for all of these species, except the leatherback (which is threatened by the pelagic longline fishery; Lewison et al., 2004), identify nearshore, recreational hook‐and‐line captures as a threat, and the Kemp’s ridley recovery plan specifically targets the reduction of hook‐and‐line interactions as a high‐priority action (National Marine Fisheries Service, 2011; National Marine Fisheries Service & U.S. Fish & Wildlife Service, 1991; National Marine Fisheries Service & U.S. Fish & Wildlife Service, 2008; Seminoff et al., 2015). Information on sea turtle bycatch and mortality in recreational fishing activities is minimal. We provide quantitative information on the vulnerability of sea turtles to recreational fishing, which can be used for developing management actions to reduce this source of mortality for sea turtle populations. The objectives of this project were to identify and compare characteristics that may affect capture vulnerability of sea turtles on a recreational fishing pier.
2. METHODS
We captured sea turtles along 21 km of Santa Rosa Island (SRI) owned by Eglin Air Force Base (AFB, Figure 1). The nearshore sediments in this area are predominately fine silica sand (Williams et al., 2012). The Navarre Beach (NAV) Marine Sanctuary, an artificial reef that consists of 78 structures constructed of piling‐mounted concrete disks located 340 feet offshore of the mean high tide line, lies approximately 0.5 km west of the study site. A fishing pier (NAV pier) is located on the GOM coast of SRI ~ 1 km west of the NAV Marine Sanctuary. This is the longest fishing pier along Florida’s GOM coast (Clark, 2010).
FIGURE 1.
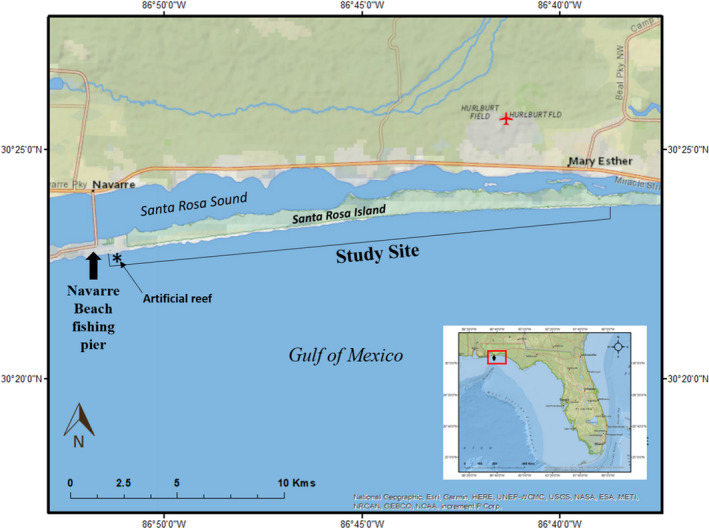
Locations where sea turtles were captured from 2014 to 2019 including the Navarre Beach fishing pier and surrounding waters off of Navarre Beach and Santa Rosa Island, Florida
At the SRI study site, we captured turtles (hereafter net‐caught turtles) between March and November 2014–2019. Capture and sampling occurred following methods described by Lamont and Johnson (2021). Briefly, we surveyed for turtles from all‐terrain vehicles ridden on the beach. Once observed, we captured turtles using a modified set‐net technique in nearshore waters typically <2 m deep and within 100 m of shore. We marked all turtles with an Inconel tag in each front flipper and a passive integrated transponder (PIT) tag in one front flipper. We measured straight carapace length measured notch to tip (SCL) using metal calipers and curved carapace length measured notch to tip (CCL) using a cloth tape measure. Additionally, we used metal calipers to determine the straight head width following Price et al. (2017) and then calculated a relative head width (hereafter head width) by dividing head width by SCL. We determined weight (kg) by placing the turtle in a harness and hanging the harness from a hand‐held Pesola spring scale.
Data for turtles captured from the NAV pier (hereafter pier‐caught turtles) were collected by participants in the Florida Sea Turtle Stranding and Salvage Network using standardized protocols described by Foley et al. (2005). Pier‐caught individuals were taken to a local rehabilitation facility where they remained for varying lengths of time, depending on health status. The SCL, CCL, and weight were determined for pier‐caught turtles, but head width was not measured. Most individuals were released in nearby waters; however, some were relocated at distances >200 km.
Pairwise correlations (r) were .99 between weight, CCL, and SCL. We used SCL in all analyses. If SCL was not gathered for an individual, we converted CCL to SCL using the following regression equations from Teas (1993), where r 2 was >.95 for all modeled relationships:
| (1) |
| (2) |
| (3) |
We calculated body condition index (BCI) as Fulton’s K (BCI = body mass/SCL3 × 104; Bjorndal et al., 2000; Lamont & Johnson, 2021). The correlation between SCL and BCI was low (r = −.14).
2.1. Data analyses
2.1.1. Body size and condition
We used linear modeling to test for differences in SCL and BCI between pier‐caught and net‐caught sea turtles. We fit separate models with the same structure with SCL and BCI as the respective response variable Y. Each SCL and BCI observation was a measurement at the time of capture. Thus, turtles with more than one capture had multiple observations, each with unique measurements associated with either a net or pier capture. We could not directly account for individual fidelity in net or pier captures with SCL and BCL as Y because any lack of independence was at the observation, not turtle, level. Data plots supported a normal distribution assumption for BCI, but SCL was right‐skewed. Thus, we used a log‐normal distribution for SCL (see linear equations below). We treated species j as a factor with three levels (green turtle, Kemp’s ridley, and loggerhead) using a means parameterization (Gelman & Hill, 2007; Kéry & Royle, 2016). Pier caught or net caught was treated as an indicator (dummy) variable (pier caught = 0, net caught = 1). We accounted for variation in SCL and BCI due to time of year (hereafter day) using a covariate (i.e., continuous predictor variable). Day was quantified as an integer that coincided with the calendar day of observation i ranging from March 23 (day 82) to November 29 (day 334). We standardized day to a mean of zero and standard deviation (SD) of one. We also included a year k grouping factor (i.e., “random intercept”, Gelman & Hill, 2007) to account for unexplained annual variation in SCL and BCI. Standard deviations for both SCL and BCI were similar between pier‐caught and net‐caught turtles for each species (Figure 2). Thus, we assumed equal variances for both models. The linear equation can be written as:
| (4) |
where α is pier‐caught, α 1 is net‐caught, β 1 is the day slope, µ is mean, and σ 2 is variance. We report SCL and BCI relationships for each species as the mode (effect size) of the difference between pier‐caught and net‐caught turtles with a 90% highest density interval (HDI). Differences were considered significant if the HDI did not overlap zero (Kruschke & Liddell, 2019). The complete linear model estimates are included in Table 1. We assessed model fit using a posterior predictive check of observed versus simulated Y values (Kéry & Royle, 2016). In addition to a visual examination, we formally assessed fit using a Bayesian p‐value. A Bayesian p‐value .10–.90 supports adequate fit (Conn et al., 2018; Kéry & Royle, 2016).
FIGURE 2.
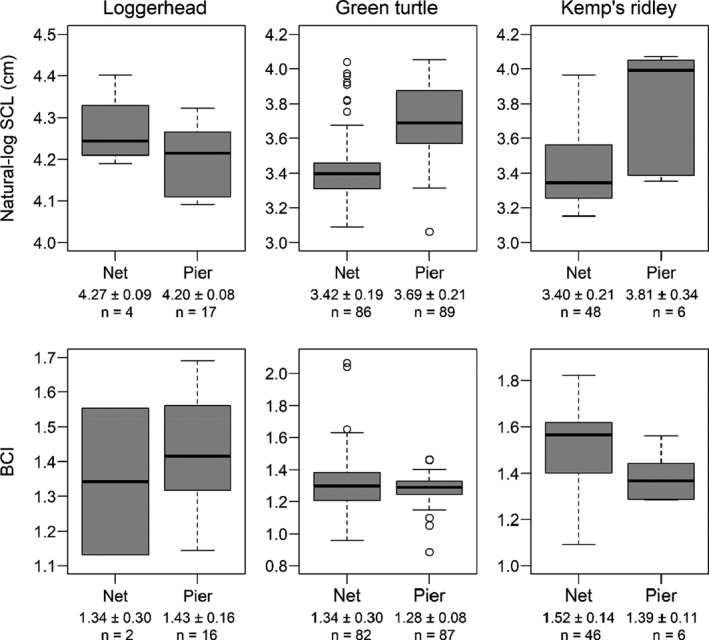
Summary statistics of pier‐caught and net‐caught sea turtles for straight carapace length (SCL) and body condition index (BCI). Values below the x‐axis labels are mean ± standard deviation (SD). Thicker vertical lines on the boxplots are medians. The number of observations (n) corresponds to the number of SCL and BCI measurements, where some turtles were observed more than once across time (see Methods). SCL is reported on a natural‐log scale to coincide with the log‐normal distribution used for the linear model. Mean ± SD in cm for net‐caught and pier‐caught turtles, respectively, was 71.73 ± 6.85 and 67.06 ± 5.52 for loggerhead, 31.06 ± 6.83 and 41.03 ± 8.30 for green turtle, and 30.79 ± 7.01 and 47.08 ± 14.07 for Kemp’s ridley
TABLE 1.
Linear model estimates for straight carapace length (SCL) and body condition index (BCI) used for the pier‐caught and net‐caught multiple comparisons tests (Table 1)
| Parameter | SCL | BCI |
|---|---|---|
| Green turtle—pier | 3.43 (3.34, 3.52) | 1.31 (1.28, 1.35) |
| Green turtle—net | −0.20 (−0.27, −0.14) | −0.03 (−0.08, 0.01) |
| Green turtle—day | 0.01 (−0.02, 0.04) | −0.01 (−0.04, 0.01) |
| Kemp’s ridley—pier | 3.36 (3.26, 3.46) | 1.52 (1.48, 1.56) |
| Kemp’s ridley—net | −0.53 (−0.68, −0.38) | −0.11 (−0.22, −0.01) |
| Kemp’s ridley—day | 0.11 (0.05, 0.17) | −0.01 (−0.06, 0.03) |
| Loggerhead—pier | 4.22 (4.03, 4.41) | 1.40 (1.24, 1.53) |
| Loggerhead—net | 0.00 (−0.18, 0.19) | 0.02 (−0.12, 0.18) |
| Loggerhead—day | 0.04 (−0.03, 0.11) | −0.01 (−0.10, 0.03) |
Coefficients are reported on the natural‐log and raw scale for SCL and BCI, respectively, as the mode (effect size) with a 90% highest density interval (HDI). Net is interpreted as the difference between net‐caught turtles and pier‐caught turtles at mean time of year (day). Year standard deviation (SD) represents unexplained annual variation in SCL and BCI across all species.
We fit the models using the program JAGS (Plummer, 2003) called from the package jagsUI (Kellner, 2018) within the statistical software R (version 3.5.3; R Development Team, 2019). Posterior distributions for coefficients were estimated with Markov chain Monte Carlo (MCMC) methods using two chains of 15,000 iterations each after a 5000‐iteration burn‐in phase (no thinning). We assessed model convergence using the Brooks–Gelman–Rubin statistic (, Gelman & Rubin, 1992). < 1.1 for all parameters indicates adequate chain mixing (Kruschke, 2015). We also examined parameter trace plots to confirm good convergence.
2.1.2. Head width and body condition
We used the same general model structure as the pier‐caught and net‐caught SCL and BCI comparisons to test for differences in head width and body condition between groups of green turtles with different numbers of pier captures. Because head width was only determined for turtles that were net‐captured, we could only use pier‐captured turtles that were initially captured by net. For turtles that were captured multiple times by net, we used the mean for head width. Loggerheads and Kemp’s ridleys were not included in this analysis because head width was not measured on the majority of individuals. We fit identical models treating head width and BCI as the respective Y variable. A plot of head width supported a normal distribution assumption. A recapture factor comprised three levels associated with each net‐caught green turtle: no pier captures (i.e., only net caught), one pier recapture of a net‐caught individual, and multiple pier recaptures of net‐caught individuals. Standard deviations for both head width and BCI were similar among the recapture levels (Figure 3). We used multiple comparisons (Kruschke, 2015; Kruschke & Liddell, 2019) to test for differences in green turtle head width and BCI between the following groups of net‐caught turtles: no pier captures versus one or multiple pier captures, no pier captures versus one pier capture, no pier captures versus multiple pier captures, and one pier capture versus multiple pier captures. The complete linear model estimates are included in Table 2. We fit models using JAGS with the same MCMC settings and assessed significance, fit, and model convergence as described for the pier‐caught and net‐caught SCL and BCI comparisons.
FIGURE 3.
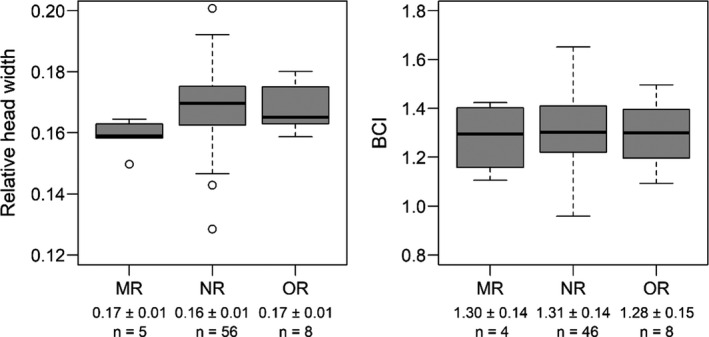
Summary statistics of relative head width and body condition index (BCI) for net‐caught green turtles with different numbers of pier captures. MR is multiple pier captures, NR is no pier captures, and OR is one pier capture. Values below the x‐axis labels are mean ± standard deviation (SD). Thicker vertical lines on the boxplots are medians. The number of observations (n) corresponds to the number of turtles, where only one relative head width and BCI value was assigned to each (see Methods)
TABLE 2.
Linear model estimates for green turtle relative head width (head width) and body condition index (BCI) used for the multiple comparisons tests (Table 2)
| Parameter | Head width | BCI |
|---|---|---|
| No pier captures | 0.17 (0.17, 0.17) | 1.31 (1.28, 1.35) |
| One pier capture | 0.17 (0.16, 0.17) | 1.30 (1.21, 1.38) |
| Multiple pier captures | 0.16 (0.15, 0.17) | 1.28 (1.16, 1.40) |
| SD | 0.01 (0.01, 0.01) | 0.15 (0.12, 0.17) |
Coefficients are reported on the raw scale as the mode (effect size) with a 90% highest density interval (HDI). SD is standard deviation.
2.1.3. Pier‐capture vulnerability
We examined green turtle pier‐capture vulnerability in relation to SCL, BCI, and day. Day provided a surrogate for both general tendencies of being pier caught at certain times of year (e.g., increased boldness) and seasonal variation in fishing effort. We did not include loggerhead or Kemp’s ridley in this analysis due to the small number of captures from the pier. Pier‐capture vulnerability Ψ was treated as a Bernoulli process (Y = 0 if net caught, Y = 1 if pier caught) and modeled as a linear function of covariates. Straight carapace length was natural‐log transformed due to a right‐skewed distribution. All covariates were standardized to a mean of zero and SD of one. We also included an individual s grouping factor to account for pseudoreplication and unexplained individual variation associated with repeat measurements of individuals (Wagner et al., 2006). The linear model can be written as:
| (5) |
We considered a covariate significant if the 90% HDI for the slope did not overlap zero. We fit the model using JAGS with MCMC settings as four chains of 50,000 iterations each after a 25,000‐iteration burn‐in phase (thinning = 10). We assessed model convergence using and parameter trace plots.
3. RESULTS
3.1. Net‐caught turtles
From October 2014 to October 2019, 148 captures of 102 unique individuals occurred in the nearshore waters at our study site (Figure 2). Captures were attempted in every month from May to October. Sampling effort was greatest in September (27% of sampling days), but most turtles were captured in October (44%; Figure 4). Green turtles were caught most frequently followed by Kemp’s ridleys and loggerheads.
FIGURE 4.
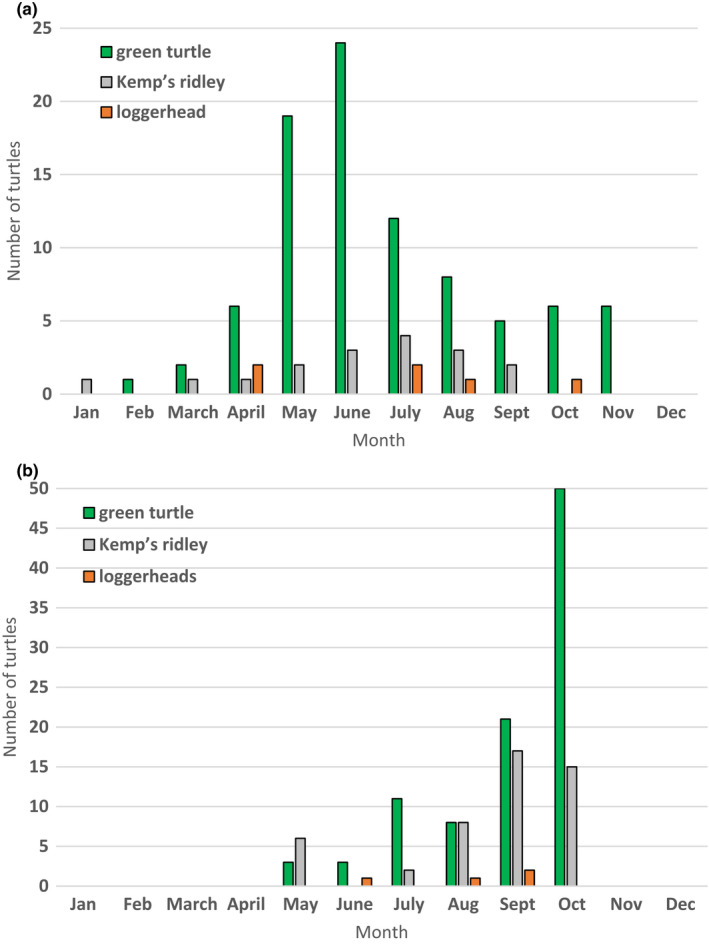
Number of turtles by species captured per month on the Navarre Beach fishing pier (a) and by net off of Santa Rosa Island, Florida (b)
Twelve net‐caught turtles were also caught (either before or after) on the pier. Our overall recapture rate (i.e., either net or pier recaptures) of net‐caught turtles was 29%. Most recaptured turtles (67%) were only recaptured once, 30% were recaptured twice, and 4% were recaptured three times. We recaptured green turtles more frequently (72%) than Kemp’s ridleys (28%); we did not recapture any loggerheads. Mean recapture interval was 111 d and was longer for Kemp’s ridleys (185 d; range 12–366 d) than green turtles (72 days; range 1–391 d). Of turtles recaptured in the net, 12 were originally tagged after being caught by hook and line on the NAV pier (27%). Mean weight ± SD for net‐caught turtles was 5.34 ± 6.39 kg (range: 1.40–50.40).
3.2. Pier‐caught turtles
From 2014 to 2019, 112 captures of 78 unique individuals were captured by hook‐and‐line on the NAV pier (Figure 2). Captures occurred in every month except December; most (24%) occurred in June. On the pier, green turtles were captured most frequently followed by loggerheads and Kemp’s ridleys. Green turtles were captured most frequently in June (27%) and May (21%), whereas Kemp’s ridleys were captured most frequently in April (33%) and July (33%) and loggerheads in July (23%; Figure 4). Most (58%) pier‐caught turtles were foul‐hooked, whereas 30% were hooked in the mouth, and the remaining 12% were hooked in unreported locations. Hooking locations were similar among species with the fewest mouth‐hooked individuals reported in loggerheads (24%) as compared to green turtles (30%) and Kemp’s ridleys (33%). Mean weight ± SD for pier‐caught turtles was 15.04 ± 12.89 kg (range: 1.30–56.90).
Of all pier‐caught turtles, 30% were already tagged (i.e., they were recaptures). Of those individuals, 14% (n = 16) were originally net caught and then recaptured from the pier; mean recapture interval for those individuals (i.e., net caught to pier caught) was 832 days (SD = 487.8, range 57–1422 days). Of all turtles tagged after being pier caught, most were captured only once (55%), whereas 30% were captured twice, 10% were captured three times, and one individual (5%) was captured 5 times during the study period. Of all pier recaptures (individuals captured at least twice from the pier), most (85%) were green turtles followed by loggerheads (16%); no Kemp’s ridleys were recaptured from the pier. Mean pier‐recapture interval for all turtles was 333 d and was longer for green turtles (347 d; range 26–1422 d) than for loggerheads (266 d; range 42–473 d).
3.3. Data analyses
3.3.1. Pier‐caught versus net‐caught size and body condition
Both SCL and BCI differed significantly between pier‐caught and net‐caught turtles for at least one species (Table 3). Straight carapace length was significantly greater for both pier‐caught green turtles and Kemp’s ridleys, with a larger effect size for Kemp’s ridley (Table 3). In contrast, BCI was significantly lower for pier‐caught Kemp’s ridley. Neither SCL nor BCI differed significantly for loggerheads, with a small effect size for both relationships. Diagnostic plots indicated good fit, with a Bayesian p‐value of .51 and .49 for the SCL and BCI model, respectively. was <1.05 for all model parameters, and trace plots confirmed adequate convergence.
TABLE 3.
Comparisons of straight carapace length (SCL) and body condition index (BCI) between pier‐caught (pier) and net‐caught individuals (net) for green turtle, Kemp’s ridley, and loggerhead (also see Table 1)
| Comparison | SCL (cm) | BCI |
|---|---|---|
| Green turtle—pier versus net | *6.94 (4.77, 9.22) | −0.03 (−0.08, 0.01) |
| Kemp’s ridley—pier versus net | *20.10 (13.00, 27.68) | *−0.11 (−0.22, −0.01) |
| Loggerhead—pier versus net | 0.21 (−12.43, 12.79) | 0.02 (−0.12, 0.18) |
Coefficients for SCL and BCI reported as the mode (effect size) of the difference with a 90% highest density interval (HDI). The direction of the tests is left minus right. *Highlights HDIs that did not overlap zero. For example, the finding for “green turtle—pier versus net” is that mean SCL was greater for pier‐caught green turtles than net caught, and the difference was significant. Mean BCI was greater for net‐caught green turtles; there it was not significant difference from pier‐caught turtles.
3.3.2. Pier recaptures head width and body condition
There were no significant differences in either head width or BCI among green turtles with different numbers of pier recaptures (Table 4). Diagnostic plots indicated good fit, with a Bayesian p‐value of .53 and .50 for the head width and BCI model, respectively. was <1.05 for all model parameters, and trace plots confirmed adequate convergence.
TABLE 4.
Multiple comparisons of relative head width (head width) and body condition index (BCI) between groups of net‐caught green turtles with different numbers of pier captures (also see Table 2)
| Comparison | Head width | BCI |
|---|---|---|
| No pier captures versus one or multiple pier captures | 0.01 (−0.01, 0.01) | 0.02 (−0.06, 0.11) |
| No pier captures versus one pier capture | 0.00 (−0.01, 0.01) | 0.02 (−0.07, 0.11) |
| No pier captures versus multiple pier captures | 0.01 (−0.01, 0.02) | 0.03 (−0.09, 0.16) |
| One pier captures versus multiple pier captures | 0.01 (−0.01, 0.02) | 0.02 (−0.13, 0.16) |
Coefficients are reported on the raw scale as the mode (effect size) with a 90% highest density interval (HDI). The direction of the tests is left minus right.
3.3.3. Pier‐capture vulnerability
Green turtle pier‐capture vulnerability was significantly related to both size and time of year, with strong linear relationships (Table 5, Figure 5). Ψ increased significantly with increasing SCL and decreased significantly with increasing day. The HDI for the BCI slope overlapped zero; however, the posterior distribution supported a negative relationship with Ψ (Figure 6). was <1.05 for all model parameters, and trace plots confirmed adequate convergence.
TABLE 5.
Green turtle pier‐capture vulnerability model estimates
| Parameter | Mode (90% HDI) |
|---|---|
| Intercept | 0.24 (−0.27, 0.83) |
| SCL | *1.76 (1.10, 2.91) |
| BCI | −0.23 (−0.76, 0.28) |
| Day | *−1.60 (−2.73, −0.94) |
| Individual SD | 0.98 (0.01, 2.92) |
Coefficients are reported on the logit scale as the mode (effect size) with a 90% highest density interval (HDI). The intercept is interpreted as estimated capture vulnerability at mean straight carapace length (SCL, 36 cm), body condition index (BCI, 1.29), and day of year (day, August 9). *Highlights covariate slope HDIs that did not overlap zero. Individual standard deviation (SD) represents unexplained individual variation in capture vulnerability.
FIGURE 5.
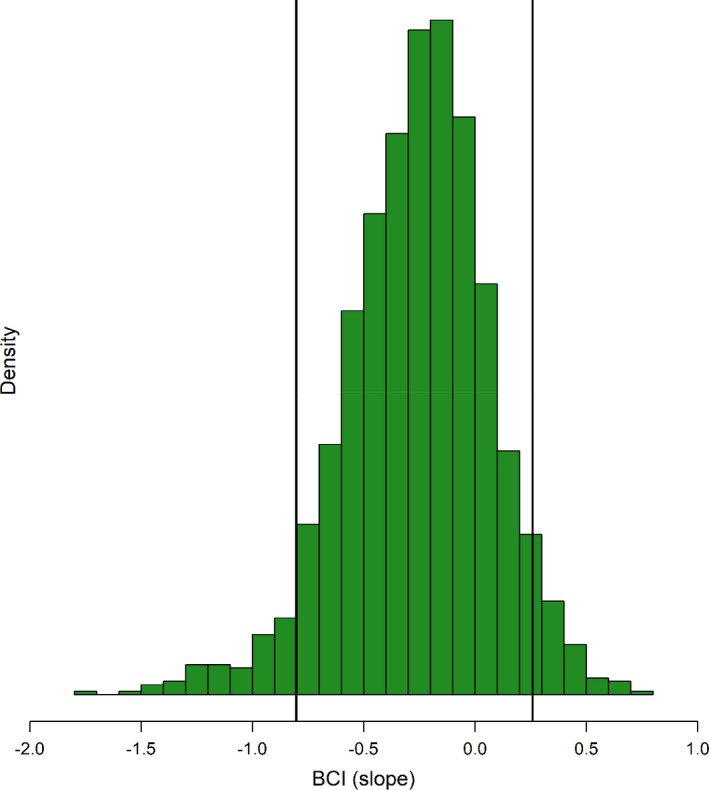
Posterior distribution of the body condition index (BCI) slope with a 90% highest density interval vertical lines from the green turtle pier‐use model
FIGURE 6.
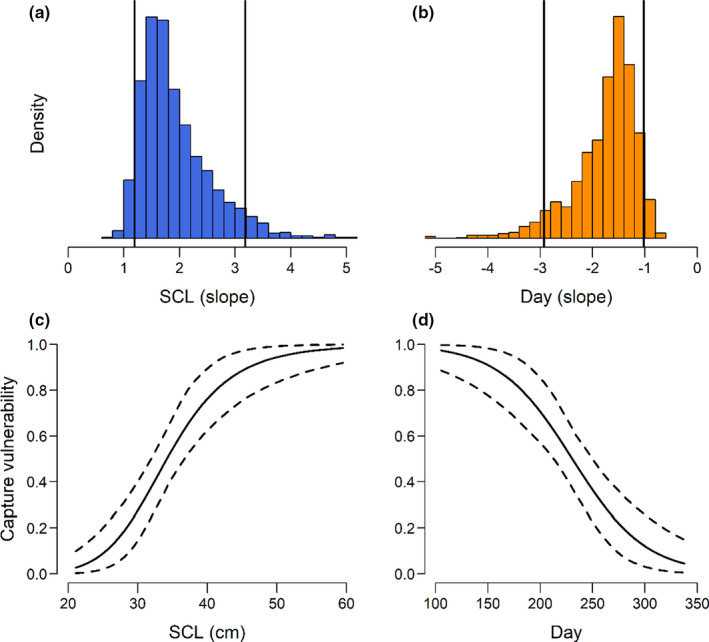
Posterior distribution of the slope with a 90% highest density interval (HDI, vertical lines) for straight carapace length (SCL, panel a) and day of year (day, panel b) and associated pier‐capture vulnerability estimates with 90% confidence limits (dashed lines) across a range of covariate values (panels c and d). The SCL slope and estimates are interpreted as the relationship with pier use with day of year held at mean levels (and vice versa). The curvature in the slopes is an artifact of the scaling to a probability between 0 and 1 from the logit scale and back transformation of SCL from the natural‐log scale. These are straight‐line relationships on the model scale
4. DISCUSSION
Morphological and behavioral traits are commonly used to assess capture vulnerability of fish in commercial fishing activities (Alós et al., 2014; Klefoth et al., 2017), and results of this study suggest similar forces also affect hook‐and‐line capture of non‐target species such as sea turtles. We identified factors, including size and time of year when individuals are captured, that affect vulnerability of sea turtles to hook‐and‐line capture from a recreational fishing pier in Northwest Florida. Additional characteristics of sea turtles, such as body condition and head width, may also play a role. Individual variability in pier use has also been observed with other marine species including manta rays (Mobula birostrus; Pate & Marshall, 2020) and blacktip sharks (Carcharhinus limbatus; Spencer, 2017). While direct mortality of these species may not occur in large numbers on fishing piers (Adimey et al., 2014), sublethal wounds and stress from capture and injury may have long‐term, and still unknown, repercussions (Pate & Marshall, 2020).
Kemp’s ridleys are commonly documented as incidental captures on fishing piers (Coleman et al., 2016; Cook et al., 2020; Seney, 2017); however, during our study, green turtles dominated pier captures. Species composition of pier captures most likely reflects the composition and abundance of species using surrounding waters; green turtles are frequently captured in nearshore waters off of northwest Florida (Lamont & Johnson, 2021). Additionally, although the genetic origin of juvenile green turtles captured on the pier is unknown, green turtle nesting has increased exponentially in Florida in recent years (Ceriani et al., 2019) while loggerhead nesting has remained stable (Ceriani et al., 2019) and Kemp’s ridley nesting appears to have declined (Caillouet et al., 2018; Gallaway et al., 2016). Capture of Kemp’s ridleys and loggerheads on recreational fishing piers may not be unexpected considering that the bait typically used by recreational anglers (e.g., fish, shrimp) are known diet items of these species (Molter et al., 2021; Ramirez et al., 2020; Shaver, 1991). However, capture of green turtles on the NAV pier was unexpected as juvenile green turtles in neritic habitats are generally considered herbivores (Williams et al., 2014). Most of the pier‐caught green turtles were foul‐hooked (67%), which may suggest turtles were foraging on algae growing on the pier pilings rather than targeting bait. However, turtles may have also been foul‐hooked while attempting to take bait. Probability of pier capture for green turtles increased with size, which may simply reflect a larger surface area for foul hooking to occur. The six pier‐caught Kemp’s ridleys were also larger than our net‐caught individuals and were also larger than Kemp’s ridleys captured from piers in Mississippi Sound (mean SCL ± SD: 36.0 ± 7.5 cm, Coleman et al., 2016).
Exploratory behavior has been linked to body size, and perhaps larger turtles are bolder and more willing to risk foraging around the pier (Darby & McGhee, 2019; Kelleher et al., 2017; Maillet et al., 2015). In our study, green turtles captured both from the pier and in the net were on average 14.5 cm larger when they were pier‐captured (n = 11). It is difficult to directly examine boldness in juvenile sea turtles either in a laboratory (Klefoth et al., 2017; but see Kudo et al., 2021) or in the wild (Breck et al., 2019; Hertel et al., 2019). Griffin et al. (2017) were able to observe green turtle behavior in relation to snorkelers; however, in general, sea turtles move large distances, inhabit deep waters, and may remain submerged at depth for several hours. Fine‐scale tracking of individuals has been used to assess boldness, primarily with terrestrial species (Breck et al., 2019; Hertel et al., 2019); however, satellite tags that transmit GPS‐quality locations are not readily available in sizes small enough for juvenile sea turtles. In addition, tracking durations for small turtles are not long enough to assess behavior due to rapid carapace growth (Lamont & Iverson, 2018). Acoustic telemetry using a passive receiver array installed on and in the vicinity of an active fishing pier (such as NAV pier) would provide useful data, particularly when installed as part of a cooperative acoustic network such as iTAG (Friess et al., 2021; Hertel et al., 2019).
Body condition of pier‐caught green turtles and loggerheads did not differ from net‐caught turtles. After being caught on the pier, turtles go to a rehabilitation center where they are examined and treated by a veterinarian (sometimes for weeks or months) before being released, and this care may improve their BCI (Hughes et al., 2019). However, measurements of pier‐caught turtles were collected immediately after capture and, as such, should not have been impacted by rehabilitation efforts, except perhaps for turtles caught multiple times on the pier. We found no difference in BCI between green turtles with multiple pier captures and those captured in the net or captured only once on the pier. Interestingly, BCI of net‐caught green turtles from SRI was lower than BCI of green turtles captured in nearby seagrass habitats of St. Joseph Bay, Florida, approximately 150 km east of SRI. This suggests green turtles in the SRI area are foraging in a less optimal habitat, regardless of whether they are foraging naturally or at the pier (Lamont & Johnson, 2021). Examining stable isotope values for net‐caught and pier‐caught turtles would help clarify the diet of these individuals, including whether pier‐caught turtles are only foraging on bait or also foraging on algae and epibionts growing on the pier structure (Vander Zanden et al., 2013). Our small numbers of recaptured turtles (particularly those with recapture intervals >180 days; Lamont & Johnson, 2021) prohibited us from comparing growth rates of pier‐caught and net‐caught turtles.
As opposed to green turtles, body condition of pier‐caught Kemp’s ridleys was lower than their net‐caught counterparts. However, this may simply reflect variation in body shape as Kemp’s ridley grow. Lamont and Johnson (2021) reported lower BCI values for Kemp’s ridleys >40 cm SCL at SRI as compared to those <40 cm SCL. Mean BCI of our pier‐caught turtles (1.39) was similar to mean BCI reported for 40–49 cm SCL Kemp’s ridleys by Lamont and Johnson (2021; 1.41).
Although there were no statistical differences between head width of net‐caught and pier‐caught turtles, conclusions from those analyses should be taken with caution because of small sample size. Head width is not a standard measurement collected at the rehabilitation facilities; therefore, we were only able to evaluate head width of turtles first captured in the net and then recaptured from the pier (n = 5). That being said, head width was lower for individuals captured only in the net (1.69), those captured once on the pier (1.64), and those captured >2 times on the pier (1.586). Head width is a function of bite force (Marshall et al., 2014), and as such, it is reasonable to suspect differences among individuals that forage in different habitats, as has been shown for nesting loggerheads (Price et al., 2017). For example, turtles foraging on algae along rock jetties may have narrower head widths that those foraging in seagrass habitat because seagrass blades are relatively tough and must be torn by the turtle (Marshall et al., 2014). Head width is easy to measure, and we suggest it might be beneficial to add to the suite of data collected from turtles captured from fishing piers.
In addition to body size, pier‐capture vulnerability was also influenced by time of year. Individuals were more likely to be caught on the pier earlier in the year. This corresponds well with angler surveys collected by the National Marine Fisheries Service Marine Recreational Fishery Statistics Survey (MRFSS) that showed shore‐based fishing effort along Florida’s GOM coast was greatest in March–April and lowest in July–August (Braun‐McNeill & Epperly, 2002). We do not have fishing effort data for the NAV pier and were unable to determine whether the seasonal pattern of turtle captures documented on the pier was a function of fishing effort or turtle behavior (or both). A similar pattern was observed in both Apalachicola Bay, Florida, and on piers in Galveston County, Texas, where approximately 50% of hook‐and‐line captures occurred in May and June (Rudloe & Rudloe, 2005; Seney, 2017). However, anglers on piers reported capturing most turtles between June and August in Mississippi (Cook et al., 2020). Seasonal variation in pier captures could also reflect movements of juvenile turtles into neritic, summer foraging areas from deep‐water, wintering home ranges (Lamont & Iverson, 2018; Metz et al., 2020). This type of movement was not reflected in our net captures, however.
Although we identified characteristics that differed between pier‐caught and net‐caught turtles, our results suggest that the capture vulnerability of sea turtles at fishing piers may be driven by a relatively complex suite of factors. Although it was not significant in our dataset, the results support a potential negative relationship between BCI and capture vulnerability with size held constant. Thus, larger turtles with lower body condition may be more likely to be pier‐captured than similar‐sized individuals with higher body condition. The complexity in relationships between turtle behavior, morphology, and life‐history traits appears to exist in the factors that contribute to capture vulnerability of sea turtles at fishing piers.
4.1. Conservation implications
It has been suggested that mortality in recreational fishing activities is resulting in selection against specific morphological traits, such as body depth and mouth size in target fishes (Alós et al., 2014). Mortality of sea turtles captured from fishing piers could be having a similar impact on sea turtle populations, albeit over a much longer time scale. Additionally, sublethal injuries and stress from capture and handling may have population‐level impacts. In a 6‐year period (2010–2015), more than 1000 sea turtles were captured by recreational anglers in Mississippi alone (Cook et al., 2020). Capture in recreational fishing activities may result in selection against bolder turtles and that personality trait could impact the overall population, not just pier‐captured turtles, by increasing exposure to threats such as predators (Griffin et al., 2017) or altering a turtle’s ability to adjust to changing temperatures (Clark et al., 2017; Pich et al., 2019). On the other hand, bolder turtles may be more exploratory and that could benefit range expansion in populations as they adapt to a changing climate (Osland et al., 2021). Populations that contain a mix of behavior types may be most resilient to anthropogenic and natural pressures that affect these species over time (Griffin et al., 2017; Schindler et al., 2010).
CONFLICT OF INTEREST
The authors declare no conflicts of interest. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. government.
AUTHOR CONTRIBUTIONS
Margaret M. Lamont: Conceptualization (lead); data curation (lead); formal analysis (supporting); funding acquisition (lead); investigation (lead); methodology (equal); project administration (lead); writing – original draft (lead); writing – review & editing (equal). Robert Mollenhauer: Conceptualization (supporting); data curation (supporting); formal analysis (lead); writing – original draft (supporting); writing – review & editing (equal). Allen M. Foley: Data curation (supporting); project administration (supporting); writing – original draft (supporting); writing – review & editing (equal).
DISCLOSURE
Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. government. This draft manuscript is distributed solely for purposes of scientific peer review. Its content is deliberative and pre‐decisional, so it must not be disclosed or released by reviewers. Because the manuscript has not yet been approved for publication by the U.S. Geological Survey (USGS), it does not represent any official USGS finding or policy.
ACKNOWLEDGMENTS
We are grateful to Kathy Gault, Justin Johnson, and Bruce Hagedorn and the Natural Resources staff at Eglin Air Force Base for their field efforts and continued support of this project. We thank Cathy and Jim Holmes, Ralph Agnew, Lisa Bradley, Alexandra Douglas, and other volunteers at the Navarre Beach Sea Turtle Conservation Center for field assistance. This work was conducted under National Marine Fisheries Service Permit 21366 and State of Florida permit 118. All turtle handling and sampling were performed according to the Institutional Animal Care Protocols USGS‐SESC‐IACUC‐2011‐05 and USGS‐WARC‐IACUC‐2019‐15.
Lamont, M. M. , Mollenhauer, R. , & Foley, A. M. (2022). Capture vulnerability of sea turtles on recreational fishing piers. Ecology and Evolution, 12, e8473. 10.1002/ece3.8473
DATA AVAILABILITY STATEMENT
The datasets generated for this study will not be made publicly available. Restrictions apply to the datasets. Raw data are exempt from publication due to the sensitivity of endangered species information. Requests to access the datasets should be directed to the corresponding author. All other data used for analyses are presented in the manuscript.
REFERENCES
- Adimey, N. M. , Hudak, C. A. , Powell, J. R. , Bassos‐Hull, K. , Foley, A. , Farmer, N. A. , White, L. , & Minch, K. (2014). Fishery gear interactions from stranded bottlenose dolphins, Florida manatees and sea turtles in Florida, U.S.A. Marine Pollution Bulletin, 81, 103–115. 10.1016/j.marpolbul.2014.02.008 [DOI] [PubMed] [Google Scholar]
- Alós, J. , Palmer, M. , & Arlinghaus, R. (2012). Consistent selection towards low activity phenotypes when catchability depends on encounters among human predators and fish. PLoS One, 7, e48030. 10.1371/journal.pone.0048030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alós, J. , Palmer, M. , Catalan, I. A. , Alonso‐Fernández, A. , Basterretxea, G. , Jordi, A. , Buttay, L. , Morales‐Nin, B. , & Arlinghaus, R. (2014). Selective exploitation of spatially structured coastal fish populations by recreational anglers may lead to evolutionary downsizing of adults. Marine Ecology Progress Series, 503, 219–233. 10.3354/meps10745 [DOI] [Google Scholar]
- Arlinghaus, R. , Alós, J. , Klefoth, T. , Laskowski, K. L. , Monk, C. T. , Nakayama, S. , & Schröder, A. (2016). Consumptive tourism causes timidity rather than boldness syndromes: A response to Geoffrey et al Trends in Ecology and Evolution, 31, 92–94. 10.1016/j.tree.2015.11.008 [DOI] [PubMed] [Google Scholar]
- Arlinghaus, R. , Laskowski, K. L. , Alós, J. , Klefoth, T. , Monk, C. T. , Nakayama, S. , & Schröder, A. (2017). Passive gear‐induced timidity syndrome in wild fish populations and its potential ecological and managerial implications. Fish and Fisheries, 18, 360–373. 10.1111/faf.12176 [DOI] [Google Scholar]
- Biro, P. A. , & Stamps, J. A. (2008). Are animal personality traits linked to life‐history productivity? Trends in Ecology and Evolution, 23, 361–368. 10.1016/j.tree.2008.04.003 [DOI] [PubMed] [Google Scholar]
- Bjorndal, K. A. , Bolten, A. B. , & Chaloupka, M. Y. (2000). Green turtle somatic growth model: Evidence for density dependence. Ecological Applications, 10, 269–282. [Google Scholar]
- Bolten, A. B. (2003). Variation in sea turtle life history patterns: Neritic vs. oceanic developmental stages. In Lutz P. L., Musick J., & Wyneken J. (Eds.), The biology of sea turtles (Vol. II, pp. 243–257). CRC Press. [Google Scholar]
- Bounoua, L. , Nigro, J. , Thome, K. , Zhang, P. , Fathi, N. , & Lachir, A. (2018). A method for mapping future urbanization in the United States. Urban Science, 2, 40. 10.3390/urbansci2020040 [DOI] [Google Scholar]
- Braun‐McNeill, J. , & Epperly, S. P. (2002). Spatial and temporal distribution of sea turtles in the western North Atlantic and the US Gulf of Mexico from Marine Recreational Fishery Statistics Survey (MRFSS). Marine Fisheries Review, 64, 50–56. http://spo.nwr.noaa.gov/mfr644/mfr6444.pdf [Google Scholar]
- Breck, S. W. , Poessel, S. A. , Mahoney, P. , & Young, J. K. (2019). The intrepid urban coyote: A comparison of bold and exploratory behavior in coyotes from urban and rural environments. Scientific Reports, 9, 2104. 10.1038/s41598-019-38543-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillouet, C. W. , Raborn, S. W. , Shaver, D. J. , Putman, N. F. , Galloway, B. J. , & Mansfield, K. L. (2018). Did declining carrying capacity for the Kemp’s ridley sea turtle population within the Gulf of Mexico contribute to the nesting setback in 2010–2017? Chelonian Conservation and Biology, 17, 123–133. 10.2744/CCB-1283.1 [DOI] [Google Scholar]
- Ceriani, S. A. , Casale, P. , Brost, M. , Leone, E. H. , & Witherington, B. E. (2019). Conservation implications of sea turtle nesting trends: Elusive recovery of a globally important loggerhead population. Ecosphere, 10, e02936. 10.1002/ecs2.2936 [DOI] [Google Scholar]
- Clark, R. R. (2010). Fishing pier design guidance Part 1: Historical pier damage in Florida. Florida Department of Environmental Protection, Bureau of Beaches and Coastal Systems. https://floridadep.gov/sites/default/files/FishingPierDesignGuidance‐Part1.pdf [Google Scholar]
- Clark, T. D. , Messmer, V. , Tobin, A. J. , Hoey, A. S. , & Pratchett, M. S. (2017). Rising temperatures may drive fishing‐induced selection of low‐performance phenotypes. Scientific Reports, 7, 40571. 10.1038/srep40571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, A. T. , Pulis, E. E. , Pitchford, J. L. , Crocker, K. , Heaton, A. J. , Carron, A. M. , Hatchett, W. , Shannon, D. , Austin, F. , Dalton, M. , Clemons‐Chevis, C. L. , & Solangi, M. (2016). Population ecology and rehabilitation of incidentally captured Kemp’s ridley sea turtles (Lepidochelys kempii) in the Mississippi Sounds, USA. Herpetological Conservation and Biology, 11, 253–264. [Google Scholar]
- Conn, P. B. , Johnson, D. S. , Williams, P. J. , Melin, S. R. , & Hooten, M. B. (2018). A guide to Bayesian model checking for ecologists. Ecological Monographs, 88, 526–542. 10.1002/ecm.1314 [DOI] [Google Scholar]
- Cook, M. , Dunch, V. S. , & Coleman, A. T. (2020). An interview‐based approach to assess angler practices and sea turtle captures on the Mississippi fishing piers. Frontiers in Marine Science, 7, 655. 10.3389/fmars.2020.00655 [DOI] [Google Scholar]
- Darby, N. A. , & McGhee, K. E. (2019). Boldness is affected by recent experience with predation cues and body size in mosquitofish. Behavioural Processes, 164, 143–149. 10.1016/j.beproc.2019.05.007 [DOI] [PubMed] [Google Scholar]
- Diaz Pauli, B. , & Sih, A. (2017). Behavioural responses to human‐induced change: Why fishing should not be ignored. Evolutionary Applications, 10, 231–240. 10.1111/eva.12456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enberg, K. , Jørgensen, C. , Dunlop, E. S. , Varpe, Ø. , Boukal, D. S. , Baulier, L. , Eliassen, S. , & Heino, M. (2012). Fishing‐induced evolution of growth: Concepts, mechanisms and the empirical evidence. Marine Ecology, 33, 1–25. 10.1111/j.1439-0485.2011.00460 [DOI] [Google Scholar]
- Foley, A. M. , Schroeder, B. A. , Redlow, A. E. , Fick‐Child, K. J. , & Teas, W. G. (2005). Fibropapillomatosis in stranded green turtles (Chelonia mydas) from the eastern United States (1980–98): Trends and associations with environmental factors. Journal of Wildlife Diseases, 41, 29–41. 10.7589/0090-3558-41.1.29 [DOI] [PubMed] [Google Scholar]
- Friess, C. , Lowerre‐Barbieri, S. K. , Poulakis, G. R. , Hammerschlag, N. , Gardiner, J. M. , Kroetz, A. M. , Bassos‐Hull, K. , Bickford, J. , Bohaboy, E. C. , Ellis, R. D. , Menendez, H. , Patterson, W. F. , Price, M. E. , Rehage, J. S. , Shea, C. , Smukall, M. J. , Walters Burnsed, S. , Wilkinson, K. A. , Young, J. , … Griffin, L. P. (2021). Regional‐scale variability in the movement ecology of marine fishes revealed by an integrative acoustic tracking network. Marine Ecology Progress Series, 663, 157–177. 10.3354/meps13637 [DOI] [Google Scholar]
- Gallaway, B. J. , Gazey, W. J. , Caillouet, C. W. Jr , Plotkin, P. T. , Abreu Grobois, F. , Amos, A. F. , Burchfield, P. M. , Carthy, R. R. , Castro Martínez, M. A. , Cole, J. G. , Coleman, A. T. , Cook, M. , DiMarco, S. , Epperly, S. P. , Fujiwara, M. , Gomez Gamez, D. , Graham, D. L. , Griffin, W. L. , Illescas Martínez, F. , … Zapata Najera, B. M. (2016). Development of a Kemp’s ridley sea turtle stock assessment model. Gulf of Mexico Science, 33, 138–157. 10.18785/goms.3302.03 [DOI] [Google Scholar]
- Garamszegi, L. Z. , Eens, M. , & Török, J. (2009). Behavioural syndromes and trappability in free‐living collared flycatchers, Ficedula albicollis . Animal Behaviour, 77, 803–812. 10.1016/j.anbehav.2008.12.012 [DOI] [Google Scholar]
- Gaston, K. J. (2010). Urban ecology. Cambridge University Press. [Google Scholar]
- Gelman, A. , & Hill, J. (2007). Data analysis using regression and multilevel/ hierarchical models. Cambridge University Press. [Google Scholar]
- Gelman, A. , & Rubin, R. B. (1992). Inference from iterative simulation using multiple sequences. Statistical Science, 7(4), 457–472. 10.1214/ss/1177011136 [DOI] [Google Scholar]
- Griffin, L. P. , Brownscombe, J. W. , Gangé, T. O. , Wilson, A. D. M. , Cooke, S. J. , & Danylchuk, A. J. (2017). Individual‐level behavioral responses of immature green turtles to snorkeler disturbance. Oecologica, 183, 909–917. 10.1007/s00442-016-3804-1 [DOI] [PubMed] [Google Scholar]
- Hamley, J. M. (1975). Review of gillnet selectivity. Journal of the Fisheries Research Board of Canada, 3, 1943–1969. 10.1139/f75-233 [DOI] [Google Scholar]
- Hertel, A. G. , Leclerc, M. , Warren, D. , Pelletier, F. , Zedrosser, A. , & Mueller, T. (2019). Don’t poke the bear: Using tracking data to quantify behavioural syndromes in elusive wildlife. Animal Behaviour, 147, 91–104. 10.1016/j.anbehav.2018.11.008 [DOI] [Google Scholar]
- Hughes, G. N. , Kennedy, M. Q. , & Litzgus, J. D. (2019). Preliminary assessment of the success of rehabilitation in snapping turtles (Chelydra serpentina) through post‐release measures of spatial behavior and body condition. Herpetological Review, 50, 58–62. [Google Scholar]
- Kelleher, S. R. , Silla, A. J. , Dingemanse, N. J. , & Byrne, P. G. (2017). Body size predicts between‐individual differences in exploration behaviour in the southern corroboree frog. Animal Behaviour, 129, 161–170. 10.1016/j.anbehav.2017.05.013 [DOI] [Google Scholar]
- Kellner, K. (2018). jagsUI: A wrapper around ‘rjags’ to streamline JAGS analyses. https://cran.r‐project.org/web/packages/jagsUI/index.html [Google Scholar]
- Kéry, M. , & Royle, J. A. (2016). Applied hierarchical modeling in ecology: Analysis of distribution, abundance and species richness in R and BUGS: Volume 1: Prelude and static models. Elsevier Science. [Google Scholar]
- Klefoth, T. , Skov, C. , Kuparinen, A. , & Arlinghaus, R. (2017). Toward a mechanistic understanding of vulnerability to hook‐and‐line fishing: Boldness as the basic target of angling‐induced selection. Evolutionary Applications, 10, 994–1006. 10.1111/eva.12504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruschke, J. K. (2015). Doing Bayesian analysis: A tutorial with R, JAGS, and Stan. Elsevier Science. [Google Scholar]
- Kruschke, J. K. , & Liddell, T. M. (2019). The Bayesian New Statistics: Hypothesis testing, estimation, meta‐analysis, and power analysis from a Bayesian perspective. Psychonomic Bulletin and Review, 25, 178–206. 10.3758/s13423-016-1221-4 [DOI] [PubMed] [Google Scholar]
- Kudo, H. , Nishizawa, H. , Uchida, K. , & Sato, K. (2021). Boldness‐exploration behavioral syndrome in wild sub‐adult green sea turtles caught at Oita, Japan. Applied Animal Behaviour Science, 236, 105216. 10.1016/j.applanim.2021.105216 [DOI] [Google Scholar]
- Lamont, M. M. , & Iverson, A. R. (2018). Shared habitat use by juveniles of three sea turtle species. Marine Ecology Progress Series, 606, 187–200. 10.3354/meps12748 [DOI] [Google Scholar]
- Lamont, M. M. , & Johnson, D. (2021). Variation in species composition, size and fitness of two multi‐species sea turtle assemblages using different neritic habitats. Frontiers in Marine Science, 7, 608740. 10.3389/fmars.2020.608740 [DOI] [Google Scholar]
- Lennox, R. J. , Alós, J. , Arlinghaus, R. , Horodysky, A. , Klefoth, T. , Monk, C. T. , & Cooke, S. J. (2017). What makes fish vulnerable to capture by hooks? A conceptual framework and a review of key determinants. Fish and Fisheries, 18, 986–1010. 10.1111/faf.12219 [DOI] [Google Scholar]
- Lewin, W. C. , Arlinghaus, R. , & Mehner, T. (2006). Documented and potential biological impacts of recreational fishing: Insights for management and conservation. Reviews in Fisheries Science, 14, 305–367. 10.1080/10641260600886455 [DOI] [Google Scholar]
- Lewison, R. L. , Freeman, S. A. , & Crowder, L. B. (2004). Quantifying the effects of fisheries on threatened species: The impact of pelagic longlines on loggerhead and leatherback sea turtles. Ecology Letters, 7, 221–231. 10.1111/j.1461-0248.2004.00573.x [DOI] [Google Scholar]
- Maillet, Z. , Halliday, W. D. , & Blouin‐Demers, G. (2015). Exploratory and defensive behaviours change with sex and body size in eastern garter snakes (Thamnophis sirtalis). Journal of Ethology, 33, 47–54. 10.1007/s10164-014-0416-2 [DOI] [Google Scholar]
- Marshall, C. D. , Wang, J. , Rocha‐Olivares, A. , Godinez‐Reyes, C. , Fisler, S. , Narazaki, T. , Sato, K. , & Sterba‐Boatwright, B. D. (2014). Scaling of bite performance with head and carapace morphometrics in green turtles (Chelonia mydas). Journal of Experimental Marine Biology and Ecology, 451, 91–97. 10.1016/j.jembe.2013.11.004 [DOI] [Google Scholar]
- Metz, T. L. , Gordon, M. , Mokrech, M. , & Guillen, G. (2020). Movements of juvenile green turtles (Chelonia mydas) in the nearshore waters of the northwestern Gulf of Mexico. Frontiers in Marine Science, 7, 647. 10.3389/fmars.2020.00647 [DOI] [Google Scholar]
- Molter, C. M. , Norton, T. M. , Hoopes, L. A. , Nelson, S. E. , Kaylor, M. , Hupp, A. , Thomas, R. , Kemler, E. , Kass, P. H. , Arendt, M. D. , & Koutsos, E. A. (2021). Health and nutrition of loggerhead sea turtles (Caretta caretta) in the southeastern United States. Journal of Animal Physiology and Animal Nutrition, 1, 1–15. 10.1111/jpn.13575 [DOI] [PubMed] [Google Scholar]
- National Marine Fisheries Service (2011). Bi‐national recovery plan for the Kemp’s ridley sea turtle (Lepidochelys kempii), 2nd revision. https://www.fws.gov/kempsridley/Finals/kempsridley_revision2.pdf [Google Scholar]
- National Marine Fisheries Service and U.S. Fish and Wildlife Service (1991. ). Recovery plan for the U.S. population of Atlantic green turtle. National Marine Fisheries Service. [Google Scholar]
- National Marine Fisheries Service and U.S. Fish and Wildlife Service (2008). Recovery plan for the Northwest Atlantic population of loggerhead sea turtle (Caretta caretta), second revision. National Marine Fisheries Service. [Google Scholar]
- Osland, M. J. , Stevens, P. W. , Lamont, M. M. , Brusca, R. C. , Hart, K. M. , Waddle, J. H. , Langtimm, C. A. , Williams, C. M. , Keim, B. D. , Terando, A. J. , Reyier, E. A. , Marshall, K. E. , Loik, M. E. , Boucek, R. E. , Lewis, A. B. , & Seminoff, J. A. (2021). Tropicalization of temperate ecosystems in North America: The northward range expansion of tropical organisms in response to warming winter temperatures. Global Change Biology, 27, 3009–3034. 10.1111/gcb.15563 [DOI] [PubMed] [Google Scholar]
- Pate, J. H. , & Marshall, A. D. (2020). Urban manta rays: Potential manta ray nursery habitat along a highly developed Florida coastline. Endangered Species Research, 43, 51–64. 10.3354/esr01054 [DOI] [Google Scholar]
- Phillip, D. P. , Cooke, S. J. , Claussen, J. E. , Koppelman, J. B. , Suski, C. D. , & Burkett, D. P. (2009). Selection for vulnerability to angling in largemouth bass. Transactions of the American Fisheries Society, 138, 189–199. 10.1577/T06-243.1 [DOI] [Google Scholar]
- Pich, J. M. , Belden, A. J. , & Carlson, B. E. (2019). Individual variation in boldness in turtles is consistent across assay conditions and behavioural measures. Behaviour, 156, 1039–1056. 10.1163/1568539X-00003555 [DOI] [Google Scholar]
- Plummer, M. (2003). JAGS: A program for analysis of Bayesian graphical models using Gibbs sampling. In Proceedings of the 3rd international workshop on distributed statistical computing. https://sourceforge.net/projects/mcmc‐jags/ [Google Scholar]
- Price, J. T. , Pfaller, J. B. , Vander Zanden, H. B. , Williams, K. L. , Bolten, A. B. , & Bjorndal, K. A. (2017). Foraging area, not trophic position, is linked to head size in adult female loggerhead turtles. Journal of Zoology, 302, 279–287. 10.1371/journal.pone.0177642 [DOI] [Google Scholar]
- R Development Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing. http://www.R‐project.org/ [Google Scholar]
- Ramirez, M. D. , Avens, L. , Goshe, L. R. , Snover, M. L. , Cook, M. , & Heppell, S. S. (2020). Regional variation in Kemp’s ridley sea turtle diet composition and its potential relationship with somatic growth. Frontiers in Marine Science, 7, 253. 10.3389/fmars.2020.00253 [DOI] [Google Scholar]
- Rudloe, A. , & Rudloe, J. (2005). Site specificity and the impact of recreational fishing activity on subadult endangered Kemp’s ridley sea turtles in estuarine foraging habitats in the northeastern Gulf of Mexico. Gulf of Mexico Science, 23, 186–191. 10.18785/goms.2302.05 [DOI] [Google Scholar]
- Schindler, D. E. , Hilborn, R. , Chasco, B. , Boatright, C. P. , Quinn, T. P. , Rogers, L. A. , & Webster, M. S. (2010). Population diversity and the portfolio effect in an exploited species. Nature, 465, 609–612. 10.1038/nature09060 [DOI] [PubMed] [Google Scholar]
- Seminoff, J. A. , Allen, C. D. , Balazs, G. H. , Dutton, P. H. , Eguchi, T. , Haas, H. L. , Hargrove, S. A. , Jensen, M. , Klemm, D. L. , Lauritsen, A. M. , MacPherson, S. L. , Opay, P. , Possardt, E. E. , Pultz, S. L. , Seney, E. E. , Van Houtan, K. S. , & Waples, R. S. (2015). Status review of the green turtle (Chelonia mydas) under the U.S. Endangered Species Act. NOAA Technical Memorandum, NOAA‐NMFS‐SWFSC‐539 (571pp). [Google Scholar]
- Seney, E. E. (2017). Diet of Kemp’s ridley sea turtles incidentally caught on recreational fishing gear in the northwestern Gulf of Mexico. Chelonian Conservation and Biology, 15, 132–137. 10.2744/CCB-1191.1 [DOI] [Google Scholar]
- Shaver, D. J. (1991). Feeding ecology of wild and head‐started Kemp’s ridley sea turtles in South Texas waters. Journal of Herpetology, 25, 327–334. 10.2307/1564592 [DOI] [Google Scholar]
- Spencer, K. L. (2017). Habitat use of blacktip sharks (Carcharhinus limbatus) at fishing piers (51 pp.). Master of Science Thesis at Coastal Carolina University. https://digitalcommons.coastal.edu/etd/47 [Google Scholar]
- Teas, W. G. (1993). Species composition and size class distribution of marine turtle strandings on the Gulf of Mexico and southeast United States coasts, 1985–1991. NOAA Technical Memorandum, NMFSSEFSC‐315. https://repository.library.noaa.gov/view/noaa/3093 [Google Scholar]
- Tuomainen, U. , & Candolin, U. (2011). Behavioural responses to human‐induced environmental change. Biological Reviews, 86, 640–657. 10.1111/j.1469-185X.2010.00164.x [DOI] [PubMed] [Google Scholar]
- Uchida, K. , Suzuki, K. K. , Shimamoto, R. , Yanagawa, H. , & Koizumi, I. (2019). Decreased vigilance or habituation to humans? Mechanisms on increased boldness in urban animals. Behavioral Ecology, 30, 1583–1590. 10.1093/beheco/arz117 [DOI] [Google Scholar]
- Uusi‐Heikkilä, S. , Sävilammi, T. , Leder, E. , Arlinghaus, R. , & Primmer, C. R. (2017). Rapid, broad‐scale gene expression evolution in experimentally harvested fish populations. Molecular Ecology, 26, 3954–3967. 10.1111/mec.14179 [DOI] [PubMed] [Google Scholar]
- Uusi‐Heikkilä, S. , Wolter, C. , Klefoth, T. , & Arlinghaus, R. (2008). A behavioral perspective on fishing‐induced evolution. Trends in Ecology and Evolution, 23, 419–421. 10.1016/j.tree.2008.04.006 [DOI] [PubMed] [Google Scholar]
- Vander Zanden, H. B. , Arthur, K. E. , Bolten, A. B. , Popp, B. N. , Lagueux, C. J. , Harrison, E. , Campbell, C. L. , & Bjorndal, K. A. (2013). Trophic ecology of a green turtle breeding population. Marine Ecology Progress Series, 476, 237–249. 10.3354/meps10185 [DOI] [Google Scholar]
- Wagner, T. , Hayes, D. B. , & Bremigan, M. T. (2006). Accounting for multilevel data structures in fisheries data using mixed models. Fisheries, 31, 180–187. [Google Scholar]
- Ward, C. H. , & Tunnell, J. W. Jr (2017). Habitats and biota of the Gulf of Mexico: An overview. In Ward C. H. (Ed.), Habitats and biota of the Gulf of Mexico: Before the Deepwater horizon oil spill (Vol. 1, pp. 1–51). Springer. 10.1007/978-1-4939-3447-8 [DOI] [Google Scholar]
- Williams, N. C. , Bjorndal, K. A. , Lamont, M. M. , & Carthy, R. R. (2014). Winter diets of immature green turtles (Chelonia mydas) on a northern feeding ground: Integrating stomach contents and stable isotope analyses. Estuaries and Coasts, 37, 986–994. 10.1007/s12237-013-9741 [DOI] [Google Scholar]
- Williams, S. J. , Flocks, J. , Jenkins, C. , Khalil, S. , & Moya, J. (2012). Offshore sediment character and sand resource assessment of the northern Gulf of Mexico, Florida to Texas. Journal of Coastal Research, 60, 30–44. 10.2112/SI_60_4 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study will not be made publicly available. Restrictions apply to the datasets. Raw data are exempt from publication due to the sensitivity of endangered species information. Requests to access the datasets should be directed to the corresponding author. All other data used for analyses are presented in the manuscript.


