Supplemental Digital Content is available in the text.
Keywords: extracorporeal membrane oxygenation, pediatric, peripheral, venoarterial, venovenous, sepsis, septic shock
Abstract
Extracorporeal membrane oxygenation (ECMO) is a rescue treatment used in children and adults with reversible cardiorespiratory failure. The role of ECMO is not fully established in pediatric sepsis. In this retrospective single-center study, we aimed to investigate risk factors and survival in pediatric septic shock supported with peripheral cannulation ECMO. All patients aged 30 days to 18 years treated between 2007 and 2016 with ECMO for septic shock were included. Of 158 screened patients, 31 were enrolled in the study. The P/F ratio was 48 ± 22 mm Hg, b-lactate 8.5 ± 6.6 mmol/L, p-procalcitonin 214 (IQR 19–294) μg/L, and 2 (1–2) vasoactive drugs were infused. The number of organ failures were 3 (3–4). Ten patients were commenced on venovenous and 21 on venoarterial ECMO. Survival from ECMO was 71%, and 68% survived to hospital discharge. Hospital survival was 80% for venovenous ECMO and 62% in venoarterial support (p = 0.43). Factors associated with in-hospital mortality were high b-lactate (p = 0.015) and high creatinine (p = 0.019) at admission. Conversion between modalities was not a risk factor. Sixty percent were alive at long-term follow-up (median 6.5 years). Peripheral cannulation ECMO is feasible in pediatric septic shock. Treatment should be performed at high-volume ECMO centers experienced in sepsis, and central or peripheral type and ECMO modality according to center preference and patient’s need.
Extracorporeal membrane oxygenation (ECMO) may be used as rescue in the critically ill when conventional respiratory and/or cardiac support prove insufficient.1 For respiratory support and cardiopulmonary support, venovenous (VV) ECMO and venoarterial (VA) ECMO may be applied, respectively. Besides improved survival, ECMO has also shown not to increase severe disability compared with conventional respiratory care.2 Patient outcome may improve from treatment of these patients at high-volume ECMO centers,3–5 and consolidation of ECMO improves resource utilization and reduces costs for society.2,6 Mobile ECMO services have been developed for patient assessment, cannulation, and retrieval on ECMO for continued treatment at a dedicated ECMO center.7–10
The incidence of severe sepsis in the pediatric population is approximately 0.6–0.9/1,000 and mortality between ranges between 10% and 25%, and survivors from pediatric sepsis face the risk of disability.11–13 In the latest revision of the Surviving Sepsis Campaign, ECMO was briefly mentioned as a rescue therapy in experienced centers.14 Since 2002, the American College of Critical Care Medicine and the Society of Critical Care Medicine has published guidelines concerning pediatric sepsis recommending ECMO as a last resort in refractory septic shock.15–17 Kawasaki et al.12 underlined that this recommendation relied on only three publications.18–20 International guidelines are published and revised on a regular basis by the Extracorporeal Life Support Organization (ELSO, Ann Arbor, MI).1
Concerning the utilization and management of ECMO in sepsis and septic shock in the neonate, survival has been reasonably good. However, in pediatric and adult patients, mortality has been >50% with the exception of a limited number of single-center publications.18–22 Studies on sepsis in children are few and the populations are limited. One of the earliest studies (1994) included nine patients of whom five survived.23 Another study, based on ELSO Registry data including 76 patients reported a survival rate of 36.5%, although this study included some neonates.24 It was concluded that sepsis per se not was a risk factor and ECMO should thus not be withheld in these populations. In the first study by MacLaren et al.,18 45 children with septic shock were included with an overall survival of 47%. In their later treated subgroup of 11 centrally cannulated patients, 73% survived.18 A second study by the same researchers on 23 centrally cannulated children with septic shock showed a survival to discharge of 74%.19 Ever since, the recommendation for septic shock in children has been central cannulation VA ECMO. However, reference data on peripheral cannulation were based on the historical data from their first study.18
The aims of this study were to assess survival and identify risk factors for mortality in pediatric patients supported with peripheral cannulation VV and VA ECMO in septic shock.
Materials and Methods
In this single-center retrospective observational study, all patients between 30 days and 18 years of age treated with ECMO for septic shock according International Consensus Conference on Pediatric Sepsis25 were eligible for inclusion. This definition of septic shock in children requires two or more of the systemic inflammatory syndrome criteria, suspected or proven infection, and need of vasoactive support to reach a mean arterial blood pressure (age targeted) despite adequate fluid resuscitation. Data were collected from January 2007 to December 2016 from the department’s databases and crosschecked against ECMO transport records and medical charts. Patients with partial treatment at another national or foreign ECMO center, subjects to extracorporeal cardiopulmonary resuscitation, or central cannulation were excluded (Figure 1).
Center Characteristics
Our unit is a dedicated respiratory ECMO intensive care unit (ICU) treating all ages and diagnoses except postcardiotomy. Sepsis is the most common diagnosis in both the pediatric and adult patient groups. No selection of mode of extracorporeal life support was made due to the departments clinical profile offering support based on patient’s need, not diagnosis.
Data Collection.
Data on demography were collected regarding age, body weight, sex, and comorbidities. Data retrieved before implantation of ECMO were diagnoses, microbial cultures, PaO2/FiO2 (P/F ratio), number of vasoactive drugs, Pediatric Index of Mortality (PIM 2/3) score, cardiopulmonary resuscitation (CPR) before ECMO initiation, number of organ failures,25 echocardiography findings, blood lactate, biochemistry, blood gases and use of inhaled nitric oxide. Extracorporeal membrane oxygenation-related data were mode, ECMO blood flow and oxygen delivery, conversion to other modes, adjuvant treatments (i.e., continuous renal replacement therapy [CRRT] and plasmapheresis), length of stay (LOS) defined as days to discharge from the ECMO ICU or pediatric ICU (PICU), or death, complications and cause of death. Patient data was entered onto a spreadsheet and anonymized. Data was aggregated, and analysis thus performed at the group level.
Statistics.
Descriptive statistics included numbers and frequencies. Data were assessed for normal distribution using Shapiro–Wilk test. Normally distributed data are presented as mean ± SD and nonparametric data as median (IQR). Comparison of continuous data was performed using Student’s T-test or Mann–Whitney U test, accordingly. Categorical data were analyzed using or Fisher’s exact test. A p value <0.05 was considered a significant difference.
Ethical Considerations.
Ethical approval was received from the Regional Ethical Review Board in Stockholm: DNR 2015/2342-31/2. Informed consent was waived due to the retrospective character of the study.
Results
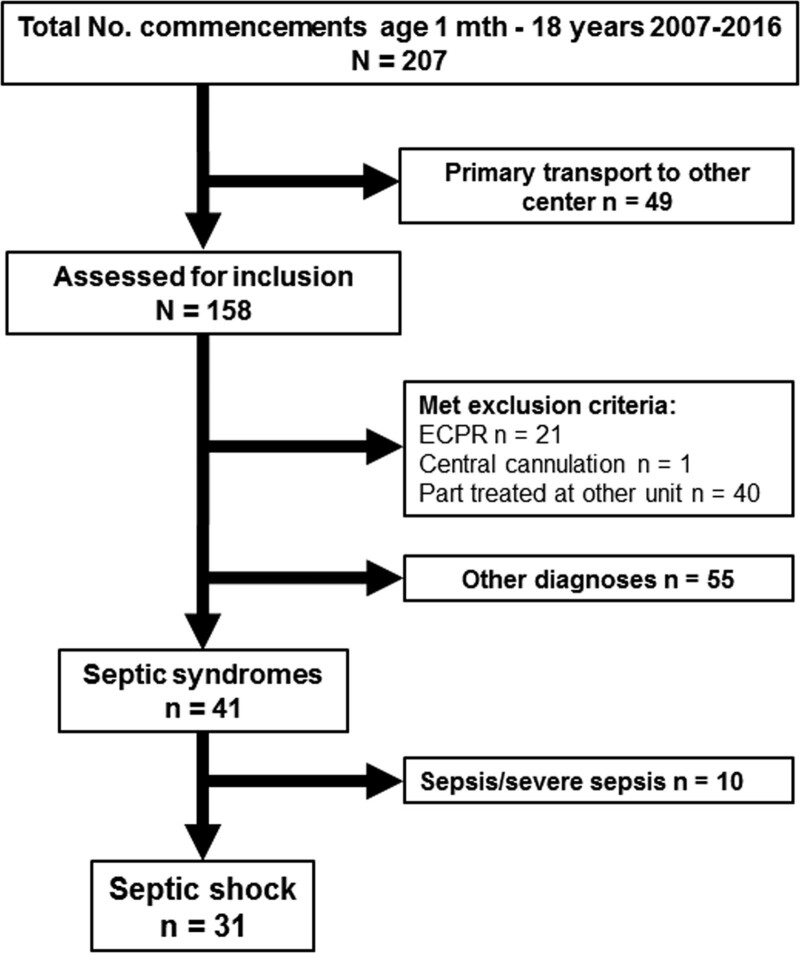
Out of the 158 patients screened, 31 were included in the study (Figure 1). The mean age was 5.5 years (range: 2 months–15.1 years) and mean body weight was 23.4 ± 17.9 kg. Thirteen (42%) patients were initiated on ECMO by a mobile ECMO team at the referring hospital. Median transport distance was 401 km (range: 40–1,370 km) and aircraft used in 62% of the transports. Comorbidities were present in 16 (52%) patients: genetic disorder (n = 9, 56%) and leukemia (n = 5, 31%) being most common. Cardiopulmonary resuscitation was performed in nine (29%) patients within the last 24 hours before commencement of ECMO. The P/F ratio was 48 ± 22 mm Hg, b-lactate 8.5 ± 6.6 mmol/L, p-procalcitonin (PCT) 214 (19–294) μg/L, and 2 (1–2) vasoactive drugs were infused. The number of organ failures was 3 (3–4). For further demographic data, see Table 1. Concerning hemodynamic echocardiographic evaluation, pre-ECMO data were available in 87% of the cases. Reduced single- or bi-ventricular cardiac function was reported in 55% and a hyperdynamic circulation in 14% of the whole population. Cardiac impairment tended to be more frequent among VA patients; however, no patient needed left ventricular unloading. The mostly used vasoactive agent was norepinephrine and the VA group showed an insignificantly higher need for inotropic support (Table 2).
Figure 1.
Selection of pediatric patients with septic shock. ECPR, extracorporeal cardiopulmonary resuscitation.
Table 1.
Patient Characteristics
| Demographic Data Before ECMO | Patients (N = 31) | Missing Data |
|---|---|---|
| n (%) | n | |
| Age, years | 5.5 ± 5.0 | |
| Body weight, kg | 23.4 ± 17.9 | |
| Sex, male | 16 (51.6) | |
| Cardiopulmonary resuscitation before ECMO | 9 (29) | |
| Immunosuppression | 8 (26) | |
| pH | 7.2 (7.09–7.27) | |
| MAP, mm Hg | 51 ± 14.6 | |
| PaO2/FiO2 ratio, mm Hg | 48 ± 22 | 5 |
| PIM2/3, EMR% | 31.6 (14.8–52.4) | 2 |
| b-Lactate, mmol/L | 8.5 ± 6.6 | |
| b-WBC, ×109/L | 7.1 ± 9.6 | 9 |
| p-Creatinine, μmo/L | 65 (43–131) | 1 |
| PT, INR | 1.9 ± 0.74 | |
| p-CRP, mg/L | 94 (47–166) | 9 |
| p-procalcitonin, μg/L | 214 (19–294) | 14 |
| Number of vasoactive substances | 2 (1–2) | |
| Inhaled nitric oxide | 7 (23) | |
| Cardiac function by echocardiography | 5 | |
| Right ventricular dysfunction | 3 (12) | |
| Left ventricular dysfunction | 5 (19) | |
| Bi-ventricular dysfunction | 7 (27) |
Patient demography and characteristics at decision for extracorporeal membrane oxygenation.
CPR, cardiopulmonary resuscitation; CRP, c-reactive protein; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; PIM, Pediatric Index of Mortality revision 2 or 3.
Table 2.
Hemodynamic Data Before Commencement of Extracorporeal Membrane Oxygenation
| All (N = 31) | VV ECMO (n = 10) | VA ECMO (n = 21) | p | |
|---|---|---|---|---|
| Pre-ECMO Echocardiography | n (% of All) | n (% of VV) | n (% of VA) | |
| Examination data reported and recovered | 27 (87) | 8 (80) | 19 (90.5) | 0.58 |
| Missing data | 4 (13) | 2 (20) | 2 (9.5) | 0.58 |
| Right ventricular dysfunction | 3 (10) | 0 | 3 (14) | 0.53 |
| Left ventricular dysfunction | 4 (13) | 0 | 4 (19) | 0.29 |
| Bi-ventricular dysfunction | 10 (32) | 3 (30) | 7 (33) | 1.0 |
| Right-, left- or bi-ventricular dysfunction | 17 (55) | 3 (30) | 14 (67) | 0.10 |
| Hyperdynamic cardiac function | 4 (13) | 1 (10) | 3 (14) | 1.0 |
| Vasoactive agent administered | 2 (1–2) | 1(1–2) | 2(1–3) | 0.17 |
| Missing or unspecified i.v. doses | 17 (55) | 6 (60) | 11 (52) | 0.72 |
| Number of vasoactive substances | 2 (1–2) | 1(1–2) | 2(1–3) | 0.17 |
| Combination of inopressor and inodilator | 12 (39) | 2 (20) | 10 (48) | 0.24 |
| Norepinephrine; range μg/kg min−1 | 24 (77) | 9 (90); 0.12–0.4 | 15 (71); 0.2–0.8 | 0.38 |
| Dopamine; range μg/kg min−1 | 8 (26) | 2 (20); 10 | 6 (29); 5–20 | 1.0 |
| Epinephrine; range μg/kg min−1 | 12 (39) | 2 (20); n/a | 10 (32); 0.05–1 | 0.24 |
| Dobutamine; range μg/kg min−1 | 2 (6) | 0 | 2 (9.5); 15 | 0.55 |
| Milrinone; range μg/kg min−1 | 6 (19) | 2 (20); 0.2–0.25 | 4 (19); 0.25–0.5 | 1.0 |
| Levosimendan | 1 (3.2) | 0 | 1 (5); n/a | 1.0 |
| Inhaled nitric oxide | 7 (23) | 2 (20) | 5(24) | 1.0 |
Available data before extracorporeal membrane oxygenation from echocardiography assessments and medical records on the use of i.v. infused vasoactive drugs. Defined inopressors are norepinephrine, dopamine and epinephrine, and inodilators are epinephrine, dobutamine, milrinone and levosimendan. In combination of inopressor and inodilator, epinephrine is only counted once. Vasopressin was not reported in any of the cases.
i.v., intravenous; VA, venoarterial; VV, venovenous.
Positive microbiological cultures and/or serology sampled before or at admission were obtained in 28 (90%) patients (see Table 1, Supplemental Digital Content 1, http://links.lww.com/ASAIO/A656). In 19 (61%) subjects, two or more infectious agents were recovered. In 19 cases, the empirical antimicrobial treatment targeted the correct pathogen. However, in four patients, initial antimicrobial treatment was ineffective and the lack of infectious control evident. In eight cases, data were incomplete, and in two cases, the pathogens were identified but the on-going treatment could not be verified. One of these patents died.
ECMO Run Data and Outcome
Ten patients were commenced on VV and 21 on VA ECMO. Eight of the nine patients that had arrested before implantation were offered VA support. Three VV ECMO patients were converted to VA ECMO due to right ventricular failure. Hence, 24 (77%) patients needed cardiorespiratory support for at least part of their extracorporeal life support time. There were no age or weight differences between the VV and VA groups.
The VV ECMO blood flow day 1 was 100 (81–117) mL/kg min−1, and net extracorporeal oxygen delivery was 3.3 (2.7–4.6) mL/kg min−1. However, the recirculation fraction during VV support was not assessed; thus, the effective ECMO flow could not be determined.
Patients submitted to VA ECMO showed a lower mean arterial blood pressure and had a higher PIM score at admission compared with VV patients (Table 2, Supplemental Digital Content 1, http://links.lww.com/ASAIO/A656). The Platelet count and P/F ratio tended to be lower in VA; however, the number of vasoactive agents was similar. Extracorporeal membrane oxygenation blood flow day 1 was 90 (68–116) mL/kg min−1, that is, similar to those offered VV support (P = 0.58), (Table 2, Supplemental Digital Content 1, http://links.lww.com/ASAIO/A656). The patients that survived from VA ECMO had an extracorporeal blood flow of 100 (70–125) as compared with the deceased with 73 (68–109) mL/kg min−1 (p = 0.26). More importantly, the net ECMO oxygen delivery was similar, 4.0 (3.0–7.6) and 3.8 (2.7–5.3) mL/kg min−1 in survivors and deceased, respectively (p = 0.43) (Table 3, Supplemental Digital Content 1, http://links.lww.com/ASAIO/A656).
Continuous renal replacement therapy was used in 27 (87%) patients and the number of days on CRRT was 8 (4–12). Plasmapheresis was offered to seven (23%) patients and the number of sessions per patient ranged from one to five. Median LOS in the ECMO ICU was 7 (5–12) days and 12 (8–19) days with the continuance in the PICU. However, the distribution was skewed towards longer LOS as illustrated by means of 10.9 and 18.8 days, respectively.
Complications
Complication during ECMO treatment was reported in seven cases (23%). Cannula clotting problems developed in four patients. In one patient, the development of thrombi in the femoral veins forced conversion to VA with cannulation on the neck. A later intrathoracic bleeding led to need for decompression by opening of the thorax to prevent thoracic compartment syndrome. That patient survived to hospital discharge. There was no association between complications on ECMO and death.
Outcome and Risk Factors
Twenty-two (71%) patients survived ECMO and 21 (68%) survived to hospital discharge. The VV group showed a hospital survival of 80% (8/10) compared with 62% (13/21) in the VA group (p = 0.43) (Table 2, Supplemental Digital Content 1, http://links.lww.com/ASAIO/A656). The primary cause of death was irreversible brain damage; ischemic, hypoxic, hemorrhagic (n = 5, 50%), uncurable lung disease (n = 2, 17%), multiorgan failure (n = 1, 8%), and thoracic compartment syndrome (n = 1). In all cases, ECMO support was discontinued due to futility. One patient suffered intestinal ischemia 5 days after discharge from the ECMO ICU and critical care was withdrawn. Among the deceased, two groups could be identified. Patients that suffered cerebral events died day 3 (2–5) (range: 1–8) and those who died from incurable disease/multiorgan failure after 20 (12–72) days (p = 0.014; range: 12–72). Factors associated with in-hospital death were high b-lactate (p = 0.015) and high creatinine (p = 0.019) at admission.
At long-term follow-up, median of 6.5 years, 60% were still alive; two former VV patients died of unknown causes 2.5 and 4.5 years after admission. One foreign patient was lost to follow up.
Discussion
This single-center retrospective study showed a survival to discharge from hospital of 68% in a mixed pediatric septic shock population submitted to peripheral cannulation ECMO therapy. The estimated risk for hospital mortality assessed from PCT, lactate, and P/F ratio before commencement of ECMO was very high.26,27 Peak lactate and creatinine on day 1 were associated with mortality. The most common causes of death were intracranial bleeding and ischemic events.
Extracorporeal membrane oxygenation treatment in pediatric sepsis is less well established than in neonates as the early data not were very encouraging.24 The two single-center studies by MacLaren et al. favored central access VA ECMO in the septic child. The first study showed an overall survival to discharge of 47%, including a centrally cannulated subgroup of 11 patients with a 73% survival rate.18 Their second retrospective study on 23 centrally applied VA ECMO cases that followed confirmed their earlier results, but no patients were peripherally cannulated to compare.19 However, these studies have had a great impact on clinical management for almost a decade and the general perception is that central cannulation VA ECMO is the type and mode for support in these children. However, 2 years after the first publication, the Leicester team commented on the drawback of retrospective studies and different management as they presented their data showing a high mortality rate in Meningococcal sepsis.28,29 In the current investigation, three of the four patients with Meningococcal sepsis survived.
It may not be the cannulation method per se, but rather the management of initial high ECMO blood flow for treatment of the shock state that is crucial. In the second MacLaren study, the maximum VA ECMO flow was 157 ml/kg min−1 in the survivors and 111 ml/kg min−1 in the nonsurvivors (p = 0.055).19 For many years, the Stockholm experience, brought to light by the current study, indicated comparable results concerning survival in a program where all patients are cannulated peripherally. In fact, this was the major driver to conduct this study. As emphasized by the current investigation, survival is likely to be influenced by additional factors besides initial extracorporeal flow or cannulation type. In the current study, the blood flows were similar between survivors and nonsurvivors. In fact, the flows were lower in all groups compared with the nonsurvivors reported in the Australian study.19 The core concept of ECMO is to deliver enough oxygen to the tissues determined by continuous recovery of lactate and organ function. In VV, this is monitored by an arterial oxygen saturation (>80%–85%),30 and in VA with a premembrane lung saturation >65%. If this can be achieved with a less high extracorporeal blood flow negative effects of blood trauma could be reduced (e.g., hemolysis, platelet activation).31,32 In VA, support lower ECMO flow benefits the heart in terms of reduced afterload and need of ventricular unloading. Survival was high by this approach and, although, we are open to that these patients may not have been as sick as earlier reported centrally cannulated patients, the key may not only be related to the hypothesis of ultrahigh oxygen flow to the tissues. We hypothesize that one important factor is not only to treat the underlying infection with correct antibiotic/s but to be aggressive and proactive in this strategy using continuous intravenous infusions of beta-lactam/carbapenem antibiotics with monitoring of targeted plasma concentrations and repeated frequent culturing during ECMO support.33,34 Other contributing factors are active management of fluid balance/withdrawal using CRRT, plasmapheresis in the patients that cannot be substantially weaned from vasoactive support in the first day of ECMO, and awake ECMO. Start of extracorporeal support may provide secondary changes in milieu interieur when tissue perfusion is restored, leading to tissue lactate, pH, and other metabolic factors to subsequently recover, which may be the major cause of benefit of ECMO in distributive septic shock.21 In ultrahigh-flow ECMO, if these metabolic changes occur rapidly, would that impact enzymatic processes negatively? Such an oxidative burst may be positive contributing with increased kill rate of bacteria, viruses, and activated neutrophils. The optimum flow at any given time may depend on the phase of illness and interact with other factors such as adjunct treatments.
Center volume is known to influence outcome over time,3,5 and ECMO is not performed the same way in different centers.35 Thus, the results from single-center studies cannot be generalized to other centers with limited experience and different case-mix. The current investigation with 3.1 treatments/year during the study period, and the MacLaren study’s (2.6/year),19 were performed at high-volume ECMO centers. The results were similar although the difference in cannulation approaches paramount, central vs. peripheral. Complication panoramas differ. Central cannulation in general carries a higher risk for complications especially over longer treatments.36 Central cannulation required reoperation in 30% of the cases due to bleeding from the sternotomy19 while also carrying a risk for mediastinitis. In central cannulation and peripheral atriocarotic and femoral VA ECMO with low native cardiac output, the risk is higher for gaseous and thrombotic emboli to the brain as compared with femoral return VA with high cardiac output or VV ECMO. On the other hand, in peripheral femorofemoral VA ECMO differential hypoxemia may be pronounced and could lead to watershed infarctions, brain ischemia and heart hypoxia, and peripheral VA ECMO carries a higher overall complication risk compared with VV.36,37 At this point, to favor one configuration method over the other is premature. Multicenter trials are needed, but these cases are not the most common diagnoses treated. Some centers do not treat children with septic syndromes at all. However, the current investigation shows peripheral cannulation ECMO to be feasible as support in septic children with a high risk of death. ECMO should be considered and the treatment method put into center context, available resources, patient’s illness and requirement of cardiorespiratory support. Early intervention and correct aggressive treatment against primary cause may prohibit escalation from peripheral VV to VA or central ECMO. Each center performs probably best in its own setting from the experiences gained over the years.
Limitations of this study were its retrospective design and the lack of consistent data on doses of vasoactive agents before ECMO that denied calculations of vasopressor and inotropic scores. Another limitation is the generalizability of results from one experienced high-volume ECMO center to the less experienced centers. The strength was the uniform patient management in a high number of septic patients, since septic syndrome also was the major indication for ECMO in adults as well, in which similar management strategies were used.21
Future prospective multicenter observational studies should be encouraged and supported. However, as wisely stated by Professor Bartlett, to expose this small population for randomization cannot be contemplated without ethical discussions due to the high risk for mortality in the arm not offered extracorporeal support.38
Conclusions
The current investigation shows peripheral cannulation ECMO to be feasible for support in septic children with a high risk of mortality. Treatment of these patients should be concentrated to high-volume ECMO centers experienced in sepsis, and the type (central or peripheral cannulation) of extracorporeal support according to center preference and the patient’s need. Larger multicenter prospective studies are warranted.
Declarations
The datasets generated and analyzed in the current study are not publicly available but are available from the corresponding author on reasonable request.
G.M. collected and analyzed the data and critically revised the manuscript for intellectual content. S.G. assisted in data collection and critically revised the manuscript for intellectual content. L.M.B. designed the concept, analyzed the data, and drafted the manuscript. All authors approved the manuscript before submission.
Acknowledgment
The authors are grateful to Rita Bexar and Tanja Dobrosavljevic, ECMO Centre Karolinska, Stockholm, Sweden for excellent administrative support. The authors are also grateful to Associate Professor Peter Radell, Department of Pediatric Perioperative Medicine and Intensive Care, Karolinska University Hospital, for editing of the English language.
Supplementary Material
Footnotes
Disclosure: L.M.B. is a member of the Medical Advisory Boards of Eurosets Srl., Medolla, Italy, and of Xenios AG, Heilbronn, Germany. The other authors have no conflicts of interest to report.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML and PDF versions of this article on the journal’s Web site (www.asaiojournal.com).
Ethical approval was received from the Regional Ethical Review Board in Stockholm: DNR 2015/2342-31/2. Informed consent was waived as no patients were to be contacted for additional questions or samples. All participants were anonymized.
Contributor Information
Georgy Melnikov, Email: georgym.official@gmail.com.
Simon Grabowski, Email: simon@grabowski.pm.
References
- 1.Richardson AC, Tonna JE, Nanjayya V, et al. Extracorporeal Cardiopulmonary Resuscitation in Adults. Interim Guideline Consensus Statement From the Extracorporeal Life Support Organization., ASAIO J 2021.67: 221–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peek GJ, Mugford M, Tiruvoipati R, et al. ; CESAR trial collaboration: Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet 2009.374: 1351–1363 [DOI] [PubMed] [Google Scholar]
- 3.Karamlou T, Vafaeezadeh M, Parrish AM, et al. : Increased extracorporeal membrane oxygenation center case volume is associated with improved extracorporeal membrane oxygenation survival among pediatric patients. J Thorac Cardiovasc Surg 2013.145: 470–475 [DOI] [PubMed] [Google Scholar]
- 4.Freeman CL, Bennett TD, Casper TC, et al. : Pediatric and neonatal extracorporeal membrane oxygenation: Does center volume impact mortality? Crit Care Med 2014.42: 512–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbaro RP, Odetola FO, Kidwell KM, et al. : Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med 2015.191: 894–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Combes A, Brodie D, Bartlett R, et al. : Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med 2014.190: 488–496 [DOI] [PubMed] [Google Scholar]
- 7.Broman LM, Dirnberger DR, Malfertheiner MV, et al. : International survey on extracorporeal membrane oxygenation transport. ASAIO J 2020.66: 214–225 [DOI] [PubMed] [Google Scholar]
- 8.Fletcher-Sandersjöö A, Frenckner B, Broman M: A single-center experience of 900 interhospital transports on extracorporeal membrane oxygenation. Ann Thorac Surg 2019.107: 119–127 [DOI] [PubMed] [Google Scholar]
- 9.Bryner B, Cooley E, Copenhaver W, et al. : Two decades’ experience with interfacility transport on extracorporeal membrane oxygenation. Ann Thorac Surg 2014.98: 1363–1370 [DOI] [PubMed] [Google Scholar]
- 10.Enger TB, Philipp A, Lubnow M, et al. : Long-term survival in adult patients with severe acute lung failure receiving veno-venous extracorporeal membrane oxygenation. Crit Care Med 2017.45: 1718–1725 [DOI] [PubMed] [Google Scholar]
- 11.Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC: The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med 2003.167: 695–701 [DOI] [PubMed] [Google Scholar]
- 12.Kawasaki T: Update on pediatric sepsis: A review. J Intensive Care 2017.5: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss SL, Fitzgerald JC, Pappachan J, et al. : Global epidemiology of pediatric severe sepsis: The sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 2015. 191: 1147–1157, Erratum in: Am J Respir Crit Care Med 193:223–224, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhodes A, Evans LE, Alhazzani W, et al. : Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017.43: 304–377 [DOI] [PubMed] [Google Scholar]
- 15.Carcillo JA, Fields AI; American College of Critical Care Medicine Task Force Committee Members: Clinical practice parameters for hemodynamic support of pediatric and neonatal patients in septic shock. Crit Care Med 2002.30: 1365–1378 [DOI] [PubMed] [Google Scholar]
- 16.Davis AL, Carcillo JA, Aneja RK, et al. : American College of Critical Care Medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock. Crit Care Med 2017.45: 1061–1093 [DOI] [PubMed] [Google Scholar]
- 17.Davis AL, Carcillo JA, Aneja RK, et al. : The American College of Critical Care Medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: executive summary. Pediatr Crit Care Med 2017.18: 884–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maclaren G, Butt W, Best D, Donath S, Taylor A: Extracorporeal membrane oxygenation for refractory septic shock in children: One institution’s experience. Pediatr Crit Care Med 2007.8: 447–451 [DOI] [PubMed] [Google Scholar]
- 19.MacLaren G, Butt W, Best D, Donath S: Central extracorporeal membrane oxygenation for refractory pediatric septic shock. Pediatr Crit Care Med 2011.12: 133–136 [DOI] [PubMed] [Google Scholar]
- 20.Skinner SC, Iocono JA, Ballard HO, et al. : Improved survival in venovenous vs venoarterial extracorporeal membrane oxygenation for pediatric noncardiac sepsis patients: A study of the Extracorporeal Life Support Organization registry. J Pediatr Surg 2012.47: 63–67 [DOI] [PubMed] [Google Scholar]
- 21.Falk L, Hultman J, Broman LM: Extracorporeal membrane oxygenation for septic shock. Crit Care Med 2019.47: 1097–1105 [DOI] [PubMed] [Google Scholar]
- 22.Bréchot N, Luyt CE, Schmidt M, et al. : Venoarterial extracorporeal membrane oxygenation support for refractory cardiovascular dysfunction during severe bacterial septic shock. Crit Care Med 2013.41: 1616–1626 [DOI] [PubMed] [Google Scholar]
- 23.Beca J, Butt W: Extracorporeal membrane oxygenation for refractory septic shock in children. Pediatrics 1994.93: 726–729 [PubMed] [Google Scholar]
- 24.Meyer DM, Jessen ME: Results of extracorporeal membrane oxygenation in children with sepsis. The Extracorporeal Life Support Organization. Ann Thorac Surg 1997.63: 756–761 [DOI] [PubMed] [Google Scholar]
- 25.Goldstein B, Giroir B, Randolph A; International Consensus Conference on Pediatric Sepsis: International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005.6: 2–8 [DOI] [PubMed] [Google Scholar]
- 26.Hatherill M, Tibby SM, Turner C, Ratnavel N, Murdoch IA: Procalcitonin and cytokine levels: Relationship to organ failure and mortality in pediatric septic shock. Crit Care Med 2000.28: 2591–2594 [DOI] [PubMed] [Google Scholar]
- 27.ELSO: Extracorporeal Life Support Organization (ELSO): Guidelines for Neonatal Respiratory Failure. 2020. Available at: https://www.elso.org/Portals/0/Files/Guideline/Neonatal%20resp%20guideline_Published.pdf. Accessed April 29, 2021. [DOI] [PubMed]
- 28.Tembo M, Harvey C, Duthie M, et al. : Extracorporeal membrane oxygenation for refractory septic shock in children: One institution’s experience. Pediatr Crit Care Med 2009.10: 534–5; author reply 535 [DOI] [PubMed] [Google Scholar]
- 29.Luyt DK, Pridgeon J, Brown J, Peek G, Firmin R, Pandya HC: Extracorporeal life support for children with meningococcal septicaemia. Acta Paediatr 2004.93: 1608–1611 [PubMed] [Google Scholar]
- 30.Maratta C, Potera RM, van Leeuwen G, Castillo Moya A, Raman L, Annich GM: Extracorporeal life support organization (ELSO): 2020 pediatric respiratory ELSO guideline. ASAIO J 2020.66: 975–979 [DOI] [PubMed] [Google Scholar]
- 31.Pedersen TH, Videm V, Svennevig JL, et al. : Extracorporeal membrane oxygenation using a centrifugal pump and a servo regulator to prevent negative inlet pressure. Ann Thorac Surg 1997.63: 1333–1339 [DOI] [PubMed] [Google Scholar]
- 32.Fuchs G, Berg N, Broman LM, Prahl Wittberg L: Flow-induced platelet activation in components of the extracorporeal membrane oxygenation circuit. Sci Rep 2018.8: 13985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts JA, Abdul-Aziz MH, Davis JS, et al. : Continuous versus intermittent β-lactam infusion in severe sepsis. A meta-analysis of individual patient data from randomized trials. Am J Respir Crit Care Med 2016.194: 681–691 [DOI] [PubMed] [Google Scholar]
- 34.Abdul-Aziz MH, Sulaiman H, Mat-Nor MB, et al. : Beta-lactam infusion in severe sepsis (BLISS): A prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med 2016.42: 1535–1545 [DOI] [PubMed] [Google Scholar]
- 35.Malfertheiner MV, Broman LM, Belliato M, et al. : Management strategies in venovenous extracorporeal membrane oxygenation: A retrospective comparison from five European centres. Crit Care Resusc 2017.19(Suppl 1): 76–81 [PubMed] [Google Scholar]
- 36.Saeed D, Stosik H, Islamovic M, et al. : Femoro-femoral versus atrio-aortic extracorporeal membrane oxygenation: Selecting the ideal cannulation technique. Artif Organs 2014.38: 549–555 [DOI] [PubMed] [Google Scholar]
- 37.Prodhan P: Brogan TV, Lequier L, Lorusso R, MacLaren G, Peek G. (eds). ECLS outcomes, complications, and followup of children with respiratory failure. In: The ELSO Red Book. 2017, pp. 5th ed. Ann Arbor, Extracorporeal Life Support Organization, 297–305. [Google Scholar]
- 38.Bartlett RH: Extracorporeal support for septic shock. Pediatr Crit Care Med 2007.8: 498–499 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.