Supplemental Digital Content is available in the text.
Keywords: dose-response relationship, fluid balance, ventilator-associated event
OBJECTIVES:
Fluid therapy is an important component of intensive care management, however, optimal fluid management is unknown. The relationship between fluid balance and ventilator-associated events has not been well established. This study investigated the dose-response relationship between fluid balance and ventilator-associated events.
DESIGN:
Nested case-control study.
SETTING:
The study was based on a well-established, research-oriented registry of healthcare-associated infections at ICUs of West China Hospital system (Chengdu, China).
PATIENTS:
A total of 1,528 ventilator-associated event cases with 3,038 matched controls, who consistently underwent mechanical ventilation for at least 4 days from April 1, 2015, to December 31, 2018, were included.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
We calculated cumulative fluid balance within 4 days prior to ventilator-associated event occurrence. A weighted Cox proportional hazards model with restricted cubic splines was used to evaluate the dose-response relationship. A nonlinear relationship between fluid balance and all three tiers of ventilator-associated events, patients with fluid balance between –1 and 0 L had the lowest risk (p < 0.05 for nonlinear test). The risk of ventilator-associated event was significantly higher in patients with positive fluid balance (4 d cumulative fluid balance: 1 L: 1.19; 3 L: 1.92; 5 L: 2.58; 7 L: 3.24), but not in those with negative fluid balance (–5 L: 1.34; –3 L: 1.14; –1 L: 0.98).
CONCLUSIONS:
There was nonlinear relationship between fluid balance and all three tiers of ventilator-associated event, with an fluid balance between –1 and 0 L corresponding to the lowest risk. Positive but not negative fluid balance increased the risk of ventilator-associated events, with higher positive fluid balance more likely to lead to ventilator-associated events.
Ventilator-associated pneumonia (VAP) is a common complication among patients receiving invasive mechanical ventilation (MV) (1, 2). VAP may lead to longer stays in ICU and hospital and longer duration of MV, and is associated with increased healthcare costs and mortality (1, 3, 4). However, the diagnostic criteria for VAP are subjective and inconsistent. In 2013, the U.S. Centers for Disease Control and Prevention (CDC) proposed a new approach (5), called ventilator-associated events (VAEs), which is more objective and can detect a broad range of ICU complications (2, 6).
Although VAE rates are routinely monitored in over 2,000 hospitals in United States, there is limited surveillance in other countries (7), possibly because of a lack of sound evidence on risk factors and strategies for prevention (2). Although several potential strategies have been proposed (8), some of potential strategies have not been validated. For instance, the relationship between fluid balance (FB) and VAEs has only been investigated in a few studies with small sample sizes and is not fully understood (9–11).
As an important approach to optimize tissue perfusion, fluid therapy is widely used to improve organ perfusion and survival in patients with critical illness (12, 13). However, inadequate or overload fluid resuscitation is associated with poor prognosis; the former may lead to tissue hypoperfusion and exacerbate organ dysfunction, whereas the latter can increase the risk of heart failure, pulmonary edema, and pleural effusions (9, 10, 12, 14–17). Thus, the relationship between FB and adverse outcomes may not be linear. However, this has not been addressed by previous investigations, which have categorized FB using a linear regression model with different cutoff values (9–11).
Therefore, we conducted a nested case-control study using data from a registry of healthcare-associated infections of ICUs (ICU-HAIs) at West China Hospital (WCH) system, aiming to assess the dose-response relationship between FB and risk of VAEs.
MATERIALS AND METHODS
We followed the reporting standards set by Reporting of studies Conducted using Observational Routinely collected health Data in this study (18). This study was approved by the Ethical Committee of WCH in 2018 (WCH2018-409).
Data Source
The ICU-HAI registry of WCH system is a multisource database that include all patients who were admitted to any one of the six ICUs in WCH since April 1, 2015 (19). A detailed description regarding the data profile of the ICU-HAI registry has been published elsewhere (20). Briefly, the registry contains three databases (electronic medical record, ICU system, and ICU-HAI system). The ICU-HAI system is the unique system routinely used for VAE surveillance in China, with over 1,800 cases recorded between April 2015 and December 2018. Through integrating the multisource databases using unique patient identification codes, the registry includes a large number of patients and is a valuable, high quality, comprehensiveness data resource for clinical studies (21, 22) (Additional File 1, http://links.lww.com/CCM/G619).
Study Design and Cohort Identification
The disease status and treatment pattern including fluid therapy from initiation of MV to extubation varied across patients. Therefore, we used a nested case-control study design to ensure identical duration of MV treatment between cases and controls and to improve statistical efficiency (23).
We identified patients who consistently underwent MV for at least 4 days from April 1, 2015, to December 31, 2018, from the ICU-HAI registry. We excluded patients who met any of the following criteria: 1) age less than 18 years; 2) incomplete information on date of birth, sex, and discharged diagnosis; 3) extremely long hospital stays (> 365 d) and abnormal hospital bill because of medical disputes; 4) non-Chinese nationality; and 5) consecutive unstable or increasing daily minimum positive end-expiratory pressure (PEEP) or Fio2 during MV treatment. According to U.S. CDC criteria, patients with consecutive unstable or deteriorating respiratory status were judged as non-VAE; these patients were excluded as clinical characteristics could differ between non-VAE cases with and without consecutive stable or improved respiratory status. We also excluded patients who were exclusively admitted to the thoracic surgery ICU because information on FB was not electronically recorded at this unit.
Case Identification and Control Selection
VAEs were identified from the ICU-HAI system at WCH. A team of experienced infection control practitioners collected related information and judged VAEs using the U.S. CDC criteria every day (5). VAEs were classified as ventilator-associated complication (VAC), infection-related ventilator-associated complication (IVAC), or the possible ventilator-associated pneumonia (PVAP) (Additional File 2, Supplementary Fig. S1, http://links.lww.com/CCM/G619). The accuracy of PVAP has been validated to be 96.2% previously (20).
We matched each case with up to two controls with the same number of MV days from the initiation of MV to VAE occurrence using an incidence density sampling approach. In addition, cases and controls were matched in terms of age (< 45, 45–59, 60–75, 75–89, and ≥ 90) and days from ICU admission to initiation of MV. Case and control selection was limited to the first episode of MV.
Exposure Assessment
We calculated cumulative FB as cumulative daily FB within 4 days prior to the event of interest. Daily FB was calculated as daily total fluid intake (sum of all IV and oral fluids) minus daily total fluid output (sum of urine, ultrafiltration fluid, drain fluid, and gastrointestinal losses). Data regarding fluid intake and output were routinely recorded every hour by well-trained specialty nurses. Due to the difficulty of a reliable assessment, we did not consider insensible fluid loss.
Measurement of Covariates
Potential confounders were identified from the registry including demographic characteristics, ICU type, chronic comorbidities, acute conditions at ICU admission, Acute Physiology and Chronic Health Evaluation (APACHE) II score, prescription, operation/procedures, and other treatments (Table 2). We identified drug treatment from prescription data, diagnosis information using the International Classification of Diseases, 10th Revision (ICD-10) code, and laboratory examinations information using specific codes developed by WCH. The completeness and accuracy of the ICD-10 code at WCH have been reported as 99% and 88%, respectively (22).
Table 2.
Associations Between Cumulative Fluid Balance Within 4 Days Prior to the Events of Interest and Ventilator-Associated Events
| Cumulative Fluid Balance | Adjusted Hazard Ratio (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| –5 L | –3 L | –1 L | 0 L | 1 L | 3 L | 5 L | 7 L | |
| Ventilator-associated event | 1.34 (0.97–1.85) | 1.14 (0.95–1.36) | 0.98 (0.94–1.02) | References | 1.19 (1.14–1.25) | 1.92 (1.67–2.21) | 2.58 (2.22–2.99) | 3.23 (2.76–3.79) |
| Infection-related ventilator-associated complication | 1.56 (0.99–2.48) | 1.24 (0.96–1.61) | 1.01 (0.95–1.07) | References | 1.16 (1.08–1.25) | 1.82 (1.46–2.28) | 2.48 (1.96–3.14) | 3.19 (2.49–4.10) |
| Possible ventilator-associated pneumonia | 1.81 (0.83–3.96) | 1.35 (0.87–2.08) | 1.03 (0.92–1.15) | References | 1.16 (0.99–1.35) | 1.83 (1.15–2.90) | 2.40 (1.46–3.92) | 2.94 (1.74–4.98) |
All models were adjusted for: age; sex; ICU type; chronicity comorbidities (malignant tumor, diabetes, cardiovascular disease, congestive heart failure, chronic lung disease, liver failure, and renal failure), acute comorbidities at ICU admission (gastrointestinal bleeding, shock, pneumonia, and acute respiratory distress syndrome); Acute Physiology and Chronic Health Evaluation II score; rehabilitation exercises; operation/procedures (cranial or cardiac surgery, fiberoptic bronchoscopy examination, tracheotomy); daily exposures to sedative, neuroleptic agents, opioids, antithrombotic agents, neuromuscular blockers, acid inhibitors, expectorant, antibiotics, intestinal probiotics, immunosuppressive agent, expectorant, and vasopressors; daily exposure to enteral nutrition, gastrointestinal decompression, mandatory ventilation, and head-of-bed elevation.
Associations between cumulative fluid balance within 4 d prior to the events of interest and the risk of all three tiers of ventilator-associated events, compared with the reference category of 0 L.
Statistical Analysis
We assessed the dose-response relationship between cumulative FB for all three tiers of VAE using a weighted Cox proportional hazards model with restricted cubic splines (RCS), which can provide greater flexibility for data fitting and modeling without the assumption of linearity and has been widely used to explore nonlinear relationships between continuous exposure and response (24–26). The hazard ratio (HR) for FB and risk of all tiers of VAE were calculated using a weighted Cox model, which has been applied to matched case-control studies with time-dependent variates and is considered superior to traditional Cox models and conditional logistic regression in terms of bias and mean squared error (27).
We adjusted all analyses using a combination of fixed and time-varying covariates. Time-varying variables included exposures to drugs, enteral nutrition, gastrointestinal decompression, mandatory ventilation, and head-of-bed elevation, which were measured as daily exposure on each of the 4 days prior to the event of interest. Missing data were imputed by stochastic regression.
Sensitivity Analyses
To examine the robustness of effect estimates, we conducted the following sensitivity analyses for VAEs: alternative definition of FB (cumulative FB within 7 d prior to the event of interest); alternative approach for missing data (without imputation); alternative approach for incidence density sampling (sampling controls from the entire risk set excluding cases); alternative inclusion and exclusion criteria (excluding patients with heart, kidney failure, and cases of VAE occurring within 4 d after initiation of MV; including patients with consecutive unstable or increasing PEEP and Fio2); and alternative statistical model (additionally adjusted stroke, trauma, sepsis, and complicated intra-abdominal infections).
RESULTS
Characteristics of the Study Population
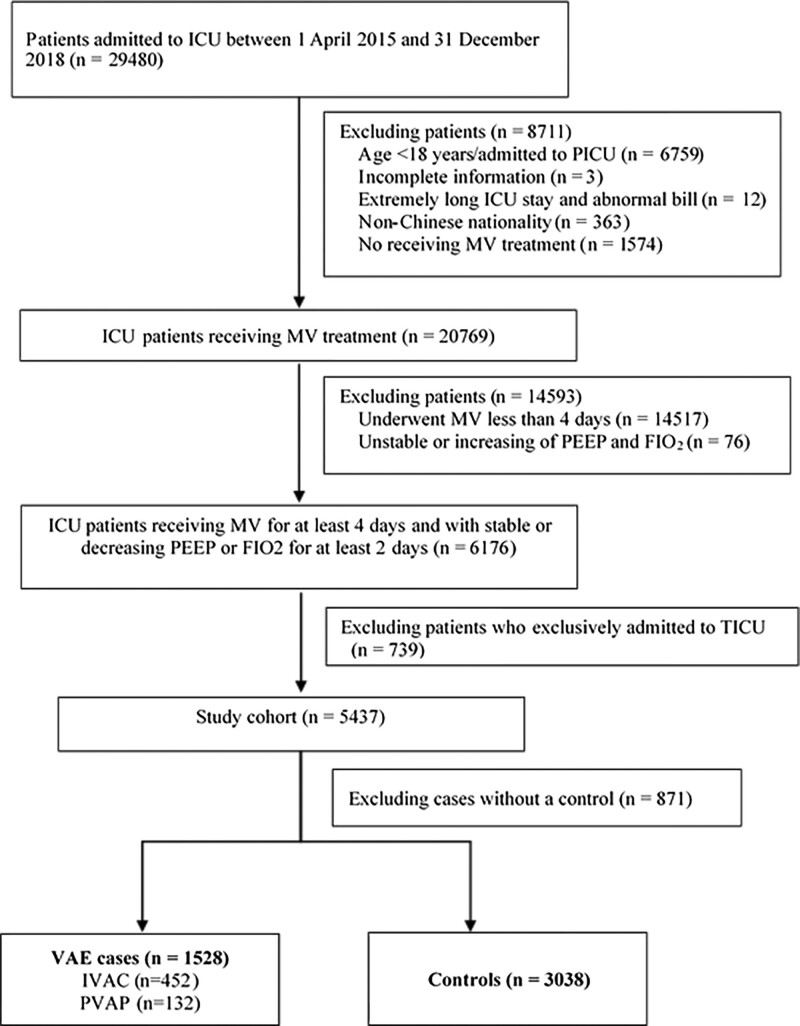
The initial cohort included 6,176 consecutive patients who consistently underwent MV for at least 4 days and with stable or decreasing PEEP or Fio2 for at least 2 days between April 1, 2015, and December 31, 2018. We excluded 739 patients who were exclusively admitted to the thoracic surgery ICU. After performing incidence density sampling, 1,528 VAE cases with 3,038 matched controls were finally included. Of 1,528 VAEs, 452 were IVACs and 132 were PVAP (Fig. 1).
Figure 1.
Study flow chart. IVAC = infection-related ventilator-associated complication, MV = mechanical ventilation, PEEP = positive end-expiratory pressure, PVAP = possible ventilator-associated pneumonia, TICU = thoracic surgery ICU, VAE = ventilator-associated event.
Among VAE cases, the median age was 58 years and median APACHE II score was 20; 64.5% of patients were male and 30% were admitted to the surgical ICU. The most common comorbidities were hypertension (21.5%), kidney failure (9.4%), and malignant tumor (8.4%) among VAEs. Most VAE cases occurred early in the course of the MV episode and the median day from the initiation of MV to the VAE occurrence was 4 days (2–7 d). There were 519 (34.0%) VAE cases occurred on calendar day 3 of MV and 260 (17.0%) cases occurred on calendar day 4 of MV. There were no significant differences between VAE cases and controls in terms of age, sex, APACHE II score, ICU type, and acute conditions at admission (p > 0.05). Comorbidities were similar among cases and controls with the exception of malignant tumors, which were more common in control patients compared with patients with VAEs (10.3% vs 8.4%; p = 0.043). Compared with controls, patients with VAEs had longer median duration of hospitalization (27 vs 24 d; p < 0.001), ICU stay (19 vs 15 d; p < 0.001), and time on MV (13 vs 9 d; p < 0.001); crude mortality rate was also higher (21.1% vs 15.2%; p < 0.001) (Table 1).
Table 1.
Clinical Characteristics of Cases and Matched Controls
| Characteristics | Overall (n = 4,566) | Cases (n = 1,528) | Controls (n = 3,038) | p |
|---|---|---|---|---|
| Age, median (IQR) | 59 (46–70) | 58 (46–70) | 59 (46–70) | 0.456 |
| 18–44 | 1,026 (22.5) | 344 (22.5) | 682 (22.4) | 0.85 |
| 45-64 | 1,794 (39.3) | 608 (39.8) | 1,186 (39.0) | |
| ≥ 65 | 1,746 (38.2) | 576 (37.7) | 1,170 (38.5) | |
| Sex, male (%) | 2,895 (63.4) | 985 (64.5) | 1,910 (62.9) | |
| ICU type (%) | < 0.001 | |||
| General ICU | 1,704 (37.3) | 555 (36.3) | 1,149 (37.8) | |
| Neurologic ICU | 1,007 (22.1) | 344 (22.5) | 663 (21.8) | |
| Respiratory ICU | 831 (18.2) | 171 (11.2) | 660 (21.7) | |
| Surgical ICU | 1,024 (22.4) | 458 (30.0) | 566 (18.6) | |
| Acute Physiology and Chronic Health Evaluation II, median (IQR) | 20 (16–25) | 20 (16–25) | 20 (15–25) | 0.56 |
| Cardiac surgery | 22 (0.5) | 14 (0.5) | 8 (0.5) | 0.95 |
| Cranial surgery | 418 (9.2) | 294 (9.7) | 124 (8.1) | 0.094 |
| Acute conditions (%) | ||||
| Acute respiratory distress syndrome at ICU admission | 56 (1.2) | 16 (1.0) | 40 (1.3) | 0.523 |
| Shock at ICU admission | 287 (6.3) | 96 (6.3) | 191 (6.3) | 1 |
| Gastrointestinal bleeding at ICU admission | 75 (1.6) | 22 (1.4) | 53 (1.7) | 0.521 |
| Pneumonia at ICU admission | 486 (10.6) | 159 (10.4) | 327 (10.8) | 0.75 |
| Chronic comorbidities (%) | ||||
| Diabetes | 250 (5.5) | 72 (4.7) | 178 (5.9) | 0.124 |
| Cardiovascular disease | 19 (0.4) | 9 (0.6) | 10 (0.3) | 0.297 |
| Heart failure | 244 (5.3) | 86 (5.6) | 158 (5.2) | 0.592 |
| Chronic lung disease | 274 (6.0) | 78 (5.1) | 196 (6.5) | 0.081 |
| Malignant tumor | 441 (9.7) | 128 (8.4) | 313 (10.3) | 0.043 |
| Liver failure | 105 (2.3) | 42 (2.7) | 63 (2.1) | 0.183 |
| Hypertension | 974 (21.3) | 329 (21.5) | 645 (21.2) | 0.845 |
| Kidney failure | 383 (8.4) | 143 (9.4) | 240 (7.9) | 0.105 |
| Outcomes | ||||
| Days of hospitalization, median (IQR) | 25 (16–38) | 27 (16–42) | 24 (16–37) | < 0.001 |
| Days of ICU stays, median (IQR) | 16 (10–26) | 19 (12–30.25) | 15 (10–24) | < 0.001 |
| Days of mechanical ventilation, median (IQR) | 11 (7–18) | 13 (8–21) | 9 (6–16) | < 0.001 |
| Hospital mortality (%) | 784 (17.2) | 323 (21.1) | 461 (15.2) | < 0.001 |
IQR = interquartile range.
The clinical characteristics of included patients and compares these between patients with ventilator-associated events and matched controls.
The clinical characteristics of included patients varied among different ICU units. The proportion of cases with acute respiratory distress syndrome (2.8%), shock (10.2%), gastrointestinal bleeding (2.6%), complicated intra-abdominal infections (28.4%), sepsis (10.2%), and pneumonia (14.7%) were highest in the general ICU. The proportion of patient with stoke (61.5%) was highest in neurologic ICU and trauma (23.8%) were highest in surgical ICU. With respect to chronic comorbidities, patients admitted to the neurologic ICU had the highest proportion of hypertension (35.6%) and diabetes (7.1%), while proportion of heart failure (9.7%) and chronic lung disease (19.6%) were highest among patients admitted to the respiratory ICU (Additional File 3, Supplementary Table S1, http://links.lww.com/CCM/G619).
FB Over Time
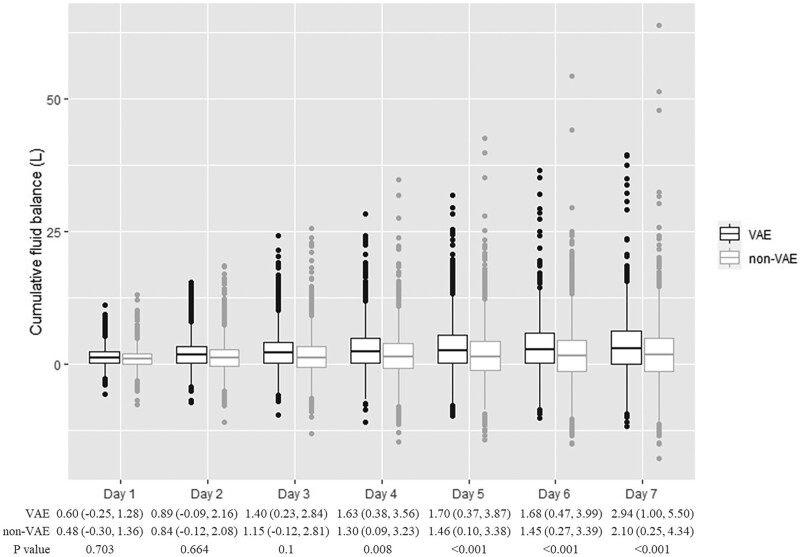
Cumulative FBs within 7 days after ICU admission are shown in Figure 2. Compared with non-VAE cases, the cumulative FB was significantly higher among VAE cases 1–7 days after ICU admission (1 d: 1.08 vs 0.83 L, p < 0.001; 2 d: 1.65 vs 1.14 L, p < 0.001; 3 d: 2.06 vs 1.20 L, p < 0.001; 4 d: 2.33 vs 1.31 L, p < 0.001; 5 d: 2.57 vs 1.38 L, p < 0.001; 6 d: 2.69 vs 1.47 L, p < 0.001; 7 d: 2.93 vs 1.71 L, p < 0.001). The cumulative FB during ICU stays was also higher among VAE cases than non-VAE cases (7.66 vs 4.37 L; p < 0.001). We also summarized cumulative FBs within 7 days prior to events of interest and daily fluid input and output with 14 days after ICU admission in Additional File 4, Supplementary Figs. S1 and S2 (http://links.lww.com/CCM/G619). The fluid output increased within 7 days after ICU admission both in VAE cases and non-VAE cases. However, after 7 days, the fluid output gradually stabilized.
Figure 2.
Cumulative fluid balances over the 7 d after ICU admission for ventilator-associated events (VAEs) and non-VAEs.
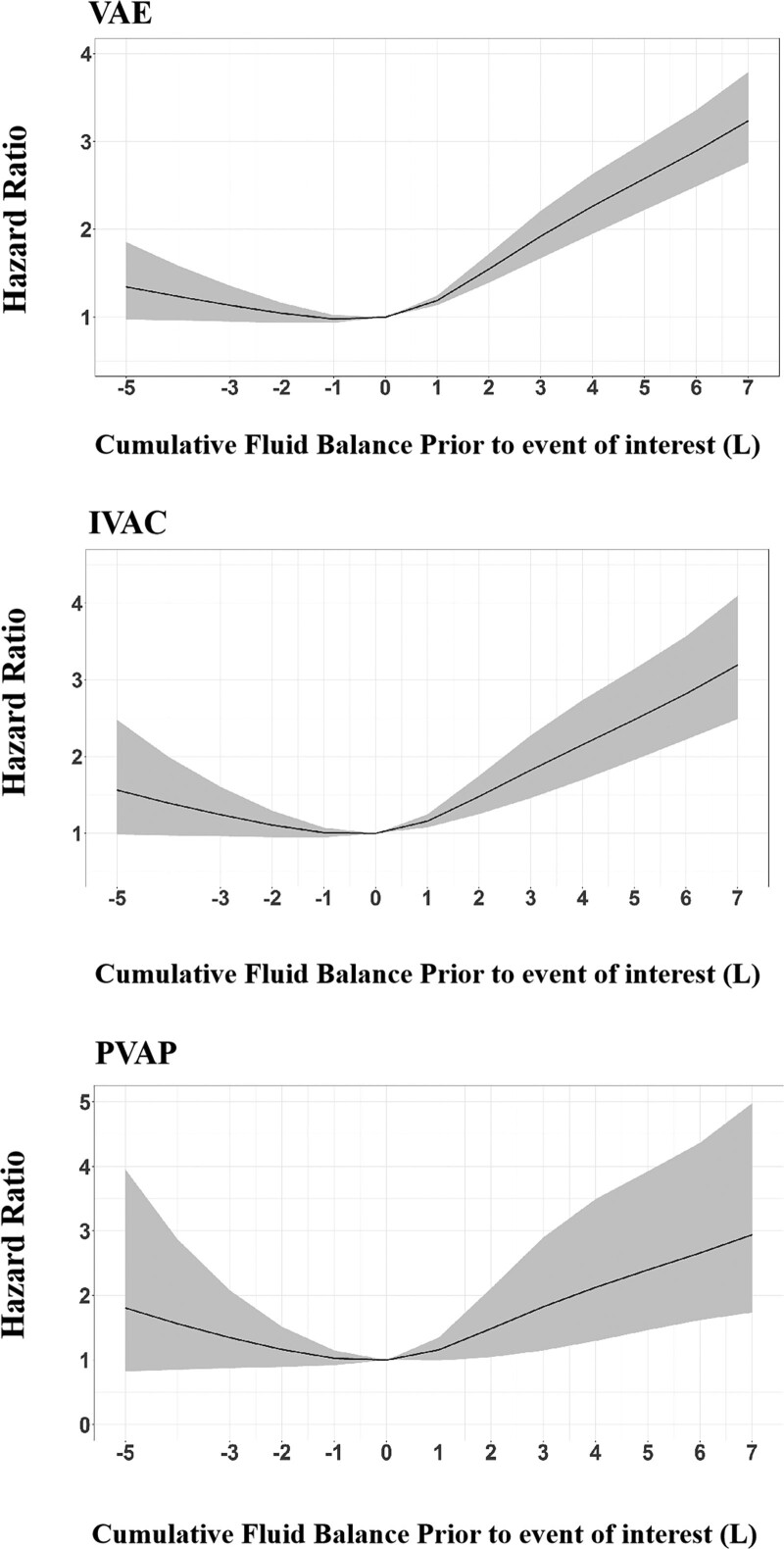
Dose-Response Relationship Between FB and VAEs
The Cox model with RCS showed a J shape relationship between cumulative FB within 4 days prior to events of interest and VAEs, an FB between –1 and 0 L had the lowest risk of VAEs (p < 0.0001 for nonlinear test) (Fig. 3). Compared with the reference value of 0 L, a positive FB significantly increased the risk of VAEs, and higher FB was associated with an elevated risk for VAEs in patients with positive FB (4 d cumulative FB at 1 L: HR 1.19, 95% CI: 1.14–1.25, p < 0.0001; 3 L: HR 1.92, 95% CI: 1.67–2.21, p < 0.0001; 5 L: HR 2.58, 95% CI: 2.22–2.99, p < 0.0001; 7 L: HR 3.24, 95% CI: 2.76–3.79, p < 0.0001). Although there was a trend toward more VAE cases in patients with negative FB, but the result was not statistically significant (4 d cumulative FB at –1 L: HR 0.98, 95% CI: 0.94–1.02, p = 0.358; –3 L: HR 1.14, 95% CI: 0.95–1.36, p = 0.167; –5 L: HR 1.34, 95% CI: 0.97–1.85, p = 0.076) (Fig. 3 and Table 2). Among patients with cumulative positive FB, those with VAEs had longer duration of MV (13 vs 9 d; p < 0.001), ICU stays (19 vs 15 d; p < 0.001), and hospital stay (25 vs 23 d; p < 0.001) compared with those without VAEs. The hospital mortality was higher in patients with VAEs than those without (21.3% vs 15.0%) (Additional File 3, Supplementary Table S2, http://links.lww.com/CCM/G619).
Figure 3.
Dose-response relationship between cumulative fluid balance within 4 d prior to events of interest and all three tiers of ventilator-associated events (VAEs). IVAC = infection-related ventilator-associated complication, PVAP = possible ventilator-associated pneumonia.
The dose-response analysis also showed a J shape relationship between cumulative FB and IVACs, with FB between –1 and 0 L corresponding to the lowest risk of IVACs (p < 0.0001 for the nonlinear test). Compared with the reference value of 0 L, a higher positive FB significantly increased the risk of IVACs (4 d cumulative FB at 1 L: HR 1.16, 95% CI: 1.08–1.25, p < 0.0001; 3 L: HR 1.82, 95% CI: 1.46–2.28, p < 0.0001; 5 L: HR 2.48, 95% CI: 1.96–3.14, p < 0.0001; 7 L: HR 3.19, 95% CI: 2.49–4.10, p < 0.0001). There was a nonlinear relationship between cumulative FB and PVAPs (p = 0.02 for the nonlinear test); positive but not negative FB was associated with increased risk of PVAP (Fig. 3 and Table 2).
Sensitivity Analyses
All sensitivity analyses showed nonlinear association between FB and risk of VAEs (Additional File 5, Figs. S1–S8, http://links.lww.com/CCM/G619).
DISCUSSION
There was a nonlinear relationship between FB and all three tiers of VAE, with an FB between –1 and 0 L having the lowest risk of VAEs. Compared with the reference value of 0 L, positive but not negative FB was associated with a significantly higher risks of all three tiers of VAE. A higher positive FB was more likely to increase the risk of VAEs. The effect estimates were robust using alternative definition of FB, alternative approaches for sampling and missing data, and alternative inclusion and exclusion criteria.
Similar to our findings, several observational studies and randomized controlled trials (RCTs) have reported that positive FB was associated with a higher risk of adverse outcomes (9–11, 14, 16, 17). An RCT of 304 patients at nine ICUs who received MV showed that restrictive fluid management was associated with 50% fewer VAEs and VAPs compared with the usual practice (28). In another trial of patients with acute lung injury, the fluid output increased after ICU admission among patients received liberal strategy; conservative fluid resuscitation improved lung function and increased the number of ventilator-free days compared with liberal resuscitation strategies (29). A multicenter RCT involved patients admitted to PICUs with acute lung injury/acute respiratory distress syndrome and found that increased fluid accumulation was associated with worsening oxygenation and increased mortality (30). Among critically ill patients, adequate fluid resuscitation is essential for restoring cardiac output and renal perfusion, however, excess fluid can lead to cardiopulmonary complications including pulmonary edema and congestive heart failure (8, 31, 32), resulting in impaired gas exchange and contractility, conduction disturbance, reduced compliance, and diastolic dysfunction, which may further increase the risk of adverse outcomes including VAEs (16, 33). Indeed, 20–40% of VAEs may be attributable to congestive heart failure and pulmonary edema caused by fluid overload (8, 9, 34). In addition, patients with pulmonary edema and impaired respiratory function are also more susceptible to bacterial infection, leading to IVACs and VAPs (28).
Although all of the abovementioned studies have demonstrated the adverse effects of fluid overload, the effect estimates vary markedly between studies (11, 16, 28, 35, 36). For instance, in the study by Liu et al (11), 30 of the 428 patients received MV developed VAEs and the risk was eight times higher in patients with daily FB greater than or equal to 50 mL versus less than 50 mL. Another study of 192 pairs of patients with VACs and matched controls showed that FB between 27 and 93 mL/kg was associated with two-fold increase in VAE compare to FB less than or equal to –11 mL/kg (10). However, it was also reported that a positive FB (≥ 5%) had a higher mortality rate than an even FB between 0 % and less than 5% (HR, 1.72; 95% CI, 1.55–1.92) (36). One potential reason for the inconsistent results may be small sample size overall and the paucity of VAEs in these studies (9, 10). Another possible reason may be differences in cutoff values for positive FB and reference groups. Dichotomizing a continuous variable by means rather than their original scale may reduce statistical power and increase bias (37). Furthermore, the relationship between FB and adverse events may not be linear. In this study, we used RCS to explore the nonlinear dose-response relationship in a larger sample. We documented a nonlinear relationship between FB and VAEs, with a higher FB linked to an elevated risk for VAEs in patients with a net positive FB but not in those with negative FB. This presumably reflects the greater risk of pulmonary edema and congestive heart failure among patients with cumulative positive FBs, which more often requires ventilatory support.
The optimal fluid management of ICU patients has not been established. Clinical characteristics vary among ICU patients, making is difficulties to detect fluid overload for individual patients. Several RCTs have showed that biomarker-guided protocols for fluid management, such as a B-type natriuretic peptide-guided strategy for depletive fluid management, may have benefits on improving respiratory function (28, 38). However, large simple size RCTs are needed to further compare the effectiveness and feasibility of these protocols.
Our study has several strengths. Our sample size was very large, with thousands of VAE cases. We used RCS functions to explore the nonlinear relationship between FB and all three tiers of VAE and performed multiple sensitivity analyses to assess the robustness of effect estimates. However, our findings should be interpreted with caution. First, this study was an observational study. Although we used multiple statistical methods and adjusted for an extensive array of potential confounders, the results may be attributable to residual confounders. Second, we only calculated FB during ICU admission and did not take into account FB in the emergency department or ward and insensible losses, which may have resulted in the misclassification of FB. Third, due to the stringent requirements of dose-response analysis and relatively small number of patients for each type of respiratory failure, we did not conduct subgroup analyses by type of respiratory failure. Finally, our findings were exclusively based on data from a homogeneous healthcare system and may not be generalizable to other settings.
CONCLUSIONS
We documented a strong but nonlinear relationship between FB and risk of all three tiers of VAE, with an FB between –1 and 0 L corresponding to the lowest risk. Positive but not negative FB was associated with significantly higher risk of all three tiers of VAEs. Patients with higher positive FB were more likely to develop VAEs. Although this study mirrors and extends findings from prior investigations, it should be interpreted with caution given the observational nature of the study.
ACKNOWLEDGMENTS
We thank Dr. Yan Ren for assistance with statistical analyses.
Supplementary Material
Footnotes
*See also p. 349.
This work was performed at Chinese Evidence-based Medicine Center and CREAT Group, West China Hospital, Sichuan University, Chengdu, 610041, China.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Drs. Zong and Sun contributed equally and shared corresponding authorship.
Dr. Wang received support from National Key Research and Development Program (Grant No. 2020YFC2009003). Dr. Sun received support for article research from the Sichuan Youth Science and Technology Innovation Research Team (Grant No. 2020JDTD0015), and the 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Grant No. ZYYC08003). Dr. Zong received support from the 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Grant No. ZYYC08006). Drs. Zou and Zong received support for article research from the National Natural Science Foundation of China. Dr. Klompas’ institution received funding from the Centers for Disease Control and Prevention, the Agency for Healthcare Research and Quality, and the Massachusetts Department of Public Health; he received funding from UpToDate. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Zhu S, Cai L, Ma C, et al. : The clinical impact of ventilator-associated events: A prospective multi-center surveillance study. Infect Control Hosp Epidemiol. 2015; 36:1388–1395 [DOI] [PubMed] [Google Scholar]
- 2.Klein Klouwenberg PM, van Mourik MS, Ong DS, et al. ; MARS Consortium: Electronic implementation of a novel surveillance paradigm for ventilator-associated events. Feasibility and validation. Am J Respir Crit Care Med. 2014; 189:947–955 [DOI] [PubMed] [Google Scholar]
- 3.Bouadma L, Sonneville R, Garrouste-Orgeas M, et al. ; OUTCOMEREA Study Group: Ventilator-associated events: Prevalence, outcome, and relationship with ventilator-associated pneumonia. Crit Care Med. 2015; 43:1798–1806 [DOI] [PubMed] [Google Scholar]
- 4.Ramírez-Estrada S, Lagunes L, Peña-López Y, et al. ; the EU-VAE Study Investigators Group: Assessing predictive accuracy for outcomes of ventilator-associated events in an international cohort: The EUVAE study. Intensive Care Med. 2018; 44:1212–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention: Surveillance for Ventilator-Associated Events. 2014. Available at: http://www.cdc.gov/nhsn/acute-care-hospital/vae/index.html. Accessed March 25, 2014
- 6.Klompas M: Barriers to the adoption of ventilator-associated events surveillance and prevention. Clin Microbiol Infect. 2019; 25:1180–1185 [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention: 2016 National and State Healthcare-Associated Infections Progress Report. 2016. Available at: https://www.cdc.gov/hai/data/archive/2016-HAI-progress-report.html#2016_HAI_Progress_Report. Accessed January 1, 2020
- 8.Klompas M: Potential strategies to prevent ventilator-associated events. Am J Respir Crit Care Med. 2015; 192:1420–1430 [DOI] [PubMed] [Google Scholar]
- 9.Lewis SC, Li L, Murphy MV, et al. ; CDC Prevention Epicenters: Risk factors for ventilator-associated events: A case-control multivariable analysis. Crit Care Med. 2014; 42:1839–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cocoros NM, Priebe G, Gray JE, et al. : Factors associated with pediatric ventilator-associated conditions in six U.S. hospitals: A nested case-control study. Pediatr Crit Care Med. 2017; 18:e536–e545 [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Zhang S, Chen J, et al. : Risk factors for ventilator-associated events: A prospective cohort study. Am J Infect Control. 2019; 47:744–749 [DOI] [PubMed] [Google Scholar]
- 12.Oddo M, Poole D, Helbok R, et al. : Fluid therapy in neurointensive care patients: ESICM consensus and clinical practice recommendations. Intensive Care Med. 2018; 44:449–463 [DOI] [PubMed] [Google Scholar]
- 13.Singer M, Deutschman CS, Seymour CW, et al. : The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acheampong A, Vincent JL: A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit Care. 2015; 19:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klompas M, Kleinman K, Murphy MV: Descriptive epidemiology and attributable morbidity of ventilator-associated events. Infect Control Hosp Epidemiol. 2014; 35:502–510 [DOI] [PubMed] [Google Scholar]
- 16.Silversides JA, Fitzgerald E, Manickavasagam US, et al. ; Role of Active Deresuscitation After Resuscitation (RADAR) Investigators: Deresuscitation of patients with iatrogenic fluid overload is associated with reduced mortality in critical illness. Crit Care Med. 2018; 46:1600–1607 [DOI] [PubMed] [Google Scholar]
- 17.Boyd JH, Forbes J, Nakada TA, et al. : Fluid resuscitation in septic shock: A positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011; 39:259–265 [DOI] [PubMed] [Google Scholar]
- 18.Benchimol EI, Smeeth L, Guttmann A, et al. ; RECORD Working Committee: The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015; 12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West China Hospital of Sichuan University: About Us. 2018. Available at: http://english.cd120.com/fastFacts/index.jhtml. Accessed December 11, 2018
- 20.Wang W, Zhu S, He Q, et al. : Developing a registry of healthcare-associated infections at intensive care units in West China: Study rationale and patient characteristics. Clin Epidemiol. 2019; 11:1035–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu S, Kang Y, Wang W, et al. : The clinical impacts and risk factors for non-central line-associated bloodstream infection in 5046 intensive care unit patients: An observational study based on electronic medical records. Crit Care. 2019; 23:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W, Liu Y, Yu C, et al. : Cefoperazone-sulbactam and risk of coagulation disorders or bleeding: A retrospective cohort study. Expert Opin Drug Saf. 2020; 19:339–347 [DOI] [PubMed] [Google Scholar]
- 23.Essebag V, Genest J, Jr, Suissa S, et al. : The nested case-control study in cardiology. Am Heart J. 2003; 146:581–590 [DOI] [PubMed] [Google Scholar]
- 24.Salazar MC, Rosen JE, Wang Z, et al. : Association of delayed adjuvant chemotherapy with survival after lung cancer surgery. JAMA Oncol. 2017; 3:610–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becher H: The concept of residual confounding in regression models and some applications. Stat Med. 1992; 11:1747–1758 [DOI] [PubMed] [Google Scholar]
- 26.Desquilbet L, Mariotti F: Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010; 29:1037–1057 [DOI] [PubMed] [Google Scholar]
- 27.Leffondre K, Wynant W, Cao Z, et al. : A weighted Cox model for modelling time-dependent exposures in the analysis of case-control studies. Stat Med. 2010; 29:839–850 [DOI] [PubMed] [Google Scholar]
- 28.Mekontso Dessap A, Katsahian S, Roche-Campo F, et al. : Ventilator-associated pneumonia during weaning from mechanical ventilation: Role of fluid management. Chest. 2014; 146:58–65 [DOI] [PubMed] [Google Scholar]
- 29.Wiedemann HP, Wheeler AP, Bernard GR, et al. : Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006; 354:2564–2575 [DOI] [PubMed] [Google Scholar]
- 30.Willson DF, Thomas NJ, Tamburro R, et al. ; Pediatric Acute Lung and Sepsis Investigators Network: The relationship of fluid administration to outcome in the pediatric calfactant in acute respiratory distress syndrome trial. Pediatr Crit Care Med. 2013; 14:666–672 [DOI] [PubMed] [Google Scholar]
- 31.Claure-Del Granado R, Mehta RL: Fluid overload in the ICU: Evaluation and management. BMC Nephrol. 2016; 17:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyle A, Maurer MS, Sobotka PA: Myocellular and interstitial edema and circulating volume expansion as a cause of morbidity and mortality in heart failure. J Card Fail. 2007; 13:133–136 [DOI] [PubMed] [Google Scholar]
- 33.Rass V, Gaasch M, Kofler M, et al. : Fluid intake but not fluid balance is associated with poor outcome in nontraumatic subarachnoid hemorrhage patients. Crit Care Med. 2019; 47:e555–e562 [DOI] [PubMed] [Google Scholar]
- 34.Boyer AF, Schoenberg N, Babcock H, et al. : A prospective evaluation of ventilator-associated conditions and infection-related ventilator-associated conditions. Chest. 2015; 147:68–81 [DOI] [PubMed] [Google Scholar]
- 35.Ertmer C, Van Aken H: Fluid therapy in patients with brain injury: What does physiology tell us? Crit Care. 2014; 18:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balakumar V, Murugan R, Sileanu FE, et al. : Both positive and negative fluid balance may be associated with reduced long-term survival in the critically ill. Crit Care Med. 2017; 45:e749–e757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hingorani AD, Windt DA, Riley RD, et al. ; PROGRESS Group: Prognosis research strategy (PROGRESS) 4: Stratified medicine research. BMJ. 2013; 346:e5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mekontso Dessap A, Roche-Campo F, Kouatchet A, et al. : Natriuretic peptide-driven fluid management during ventilator weaning: A randomized controlled trial. Am J Respir Crit Care Med. 2012; 186:1256–1263 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.