Abstract
Severe and life-threatening cases of metformin-associated lactic acidosis (MALA) are treated with renal replacement therapy. Intermittent hemodialysis is recommended, as it achieves rapid more elimination of metformin compared to continuous renal replacement therapy (CRRT). This case series describes 4 patients, 2 with acute metformin intoxications and 2 with insidious metformin toxicity. All were treated using a novel approach with dual CRRT to achieve rapid elimination of metformin. Three of the 4 patients survived to hospital discharge. Dual CRRT may be an effective alternative when dialysis is not readily available.
Toxicity due to metformin can lead to metformin-associated lactic acidosis (MALA) but is rarely seen without a precipitating event such as an intentional overdose or renal failure. MALA is thought to be due to a dual effect of inhibition of mitochondrial metabolism, which increases lactate production and reduced lactate removal due to impaired gluconeogenesis.1 There is a correlation between lactate levels and MALA-associated mortality, which has been reported to be >30% in severe cases.2,3
Case reports describe the use of different renal replacement therapy modalities to enhance elimination of metformin. Intermittent hemodialysis4,5 or sustained low-efficiency dialysis6,7 is recommended, but in some intensive care units (ICUs), continuous renal replacement therapy (CRRT) is the only option. At present, the use of 2 concurrent CRRT machines has only been published in letter form.8,9
Ethics approval was obtained from the Royal Brisbane & Women’s Hospital Human Research Ethics Committee (Ref-No: LNR/2020/QRBW/60781[AM01]). The patients or their next of kin provided written consent for the publication of this report. This article adheres to the applicable Enhancing the QUALity and Transparency Of health Research guidelines.
CASE SERIES
Patients admitted to the ICU between 2016 and 2019 who received CRRT were retrospectively identified via the electronic medical record clinical information system. Patients were excluded if they did not receive dual CRRT or were under the age of 16 years.
CASE 1
A 17-year-old man presented 1.5 hours following ingestion of 48-g metformin and 82-g paracetamol. Other ingested medications included rosuvastatin, desvenlafaxine, risperidone, esomeprazole, metronidazole, ibuprofen, diclofenac, celecoxib, linagliptin, metoclopramide, methyphenidate, and fluoxetine.
His blood pressure (BP) was 116/75 mm Hg, pulse rate 132 beats/min, respiratory rate 16 breaths/min, oxygen saturation 98% on room air, temperature 36.9 °C, and Glasgow Coma Scale (GCS) 14 (eyes 3, verbal 5, and motor 6). Initial paracetamol concentration was 344 mg/L (normal range, 10–30 mg/L) and initial lactate concentration was 4.3 mmol/L (normal range, 0.5–2.2 mmol/L).
He was treated with n-acetylcysteine for paracetamol toxicity and intubated and ventilated for airway protection when his GCS decreased to 9. He was transferred to a different ICU where continuous venovenous hemodiafiltration (CVVHDF) was commenced 16 hours after ingestion. Four hours later, due to poor clearance of lactate (Figure 1), a second CVVHDF machine was started via a second dialysis catheter (Tables 1 and 2). Dual CVVHDF ran for a total of 33 hours with single CRRT continuing until day 15. He was extubated on day 9 and discharged to the ward on day 20 and home on day 27.
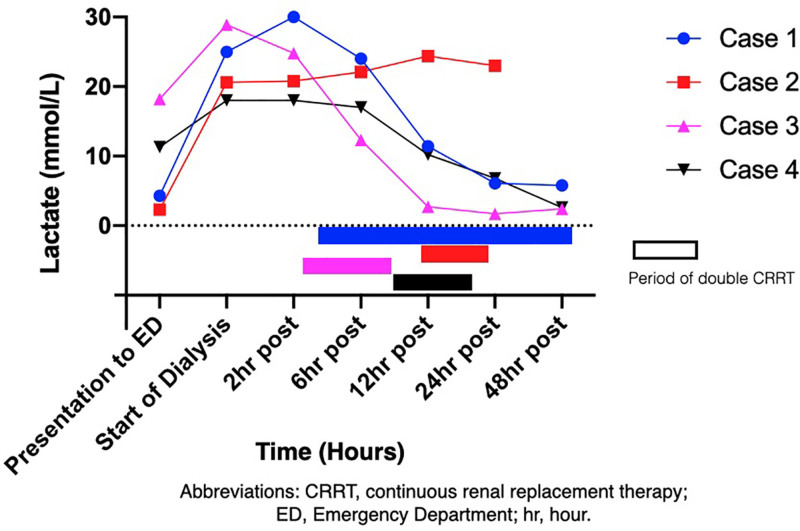
Figure 1.
Graph showing change in lactate during double CRRT. CRRT indicates continuous renal replacement therapy; ED, emergency department.
Table 1.
Settings for Each CRRT Machine
| Dialysis parameters | Case 1 | Case 2 | Case 3 | Case 4 | ||||
|---|---|---|---|---|---|---|---|---|
| CRRT 1 | CRRT 2 | CRRT 1 | CRRT 2 | CRRT 1 | CRRT 2 | CRRT 1 | CRRT 2 | |
| Weight, kg | 61 | 110 | 72 | 118 | ||||
| CRRT catheter location | Right femoral vein | Right IJ vein | Left IJ Vein | Right femoral vein | Right femoral vein | Right IJ vein | Right femoral vein | Right IJ vein |
| Mode | CVVH-DF | CVVH-DF | CVVH-DF | CVVH-DF | CVVH-DF | CVVH-DF | CVVH-DF | CVVH-DF |
| Anticoagulation | Heparin | Heparin | Heparin | Heparin | Heparin | Heparin | Heparin | Heparin |
| Filter size | ST 150 | ST 150 | ST 150 | ST 150 | ST 150 | ST 150 | ST 150 | ST 150 |
| Blood flow, mL/min | 250 | 250 | 350 | 350 | 150 | 200 | 200 | 200 |
| Prepump replacement, mL/h | 2500 | 2500 | 1500 | 1500 | 2000 | 1000 | 1500 | 1500 |
| Dialysate, mL/h | 2000 | 2000 | 2750 | 2750 | 2000 | 2000 | 1500 | 1500 |
| Postpump replacement, mL/h | 2000 | 2000 | 2000 | 2000 | 1500 | 2000 | 1000 | 1000 |
| Total CRRT dose, mL·kg–1·h–1 | 165 | 76 | 156 | 57 | ||||
| Filtration fraction, % | 40 | 43 | 23 | 27 | ||||
Abbreviations: CRRT, continuous renal replacement therapy; CVVHDF, continuous venovenous hemodiafiltration; IJ, internal jugular vein; ST, ST 150, disposable extracorporeal circuit for use with the Baxter Prismaflex or PrisMax CRRT machines.
Table 2.
Renal Function and Acid Base Balance at Commencement of CRRT
| Blood results | Case 1 | Case 2 | Case 3 | Case 4 | ||||
|---|---|---|---|---|---|---|---|---|
| CRRT 1 | CRRT 2 | CRRT 1 | CRRT 2 | CRRT 1 | CRRT 2 | CRRT 1 | CRRT 2 | |
| Time, h | 0 | +4 | 0 | +12 | 0 | +3 | 0 | +9 |
| Urea, mmol/L | 4.5 | 8.9 | 4.6 | 22.8 | 31.9 | 25.3 | ||
| Creatinine, μmol/L | 163 | 224 | 160 | 489 | 1230 | 931 | ||
| pH | 7.00 | 6.99 | 7.14 | 6.99 | 6.74 | 7.02 | 6.78 | 6.91 |
| Fio2 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.3 | 0.7 | 0.3 |
| Pao2, mm Hg | 249 | 240 | 142 | 89 | 246 | 124 | 258 | 96 |
| Paco2, mm Hg | 33 | 34 | 45 | 45 | 21 | 18 | 24 | 24 |
| Bicarbonate, mmol/L | 8 | 8 | 15 | 11 | 3 | 4 | 4 | 5 |
| Base excess, mmol/L | –22.3 | –22.8 | –13.7 | –20.3 | –31.0 | –24.7 | –30.0 | –26.6 |
| Anion gap, mmol/L | 43 | 41 | 29 | 34 | 44 | 38 | 38 | 35 |
| Lactate, mmol/L | 25.4 | 29.2 | 22.2 | 25.0 | 28.1 | 24.8 | 17.0 | 17.0 |
Abbreviations: Fio2, fraction of inspired oxygen; Paco2, partial pressure carbon dioxide in arterial blood; Pao2, partial pressure oxygen in arterial blood.
Normal range: anion gap, 4 to 13 mmol/L; base excess, −2 to +2 mmol/L; bicarbonate, 22 to 28 mmol/L; creatinine, 36 to 73 μmol/L; lactate, 0.5 to 2.2 mmol/L; Paco2, 35 to 45 mm Hg; Pao2, 83 to 108 mm Hg; pH, 7.35 to 7.45; and urea, 2.9 to 8.2 mmol/L.
Case 2
A 59-year-old man presented following a polypharmacy overdose of 50 g of metformin, perindopril, venlafaxine, isosorbide mononitrate, atorvastatin, and esomeprazole. He had hypertension, dyslipidemia, type 2 diabetes mellitus (DM), and ischemic heart disease.
His BP was 160/120 mm Hg, pulse rate 102 beats/min, respiratory rate 22 breaths/min, oxygen saturation 96% on room air, temperature 36.5 °C, and GCS 13 (eyes 3, verbal 4, and motor 6). He was intubated when his GCS fell and for worsening acidosis. The initial lactate was 2.3 mmol/L and peaked at 22.2 mmol/L. He was transferred to an alternate ICU where CRRT was started 12 hours after initial presentation. The lactate remained elevated, so dual CRRT, via a second dialysis catheter, was started 24 hours after ingestion.
CRRT ran for 22 hours that included 10 hours of 2 machines concurrently running. The patient continued to deteriorate with escalation of vasopressors, including norepinephrine, epinephrine, vasopressin, and methylene blue. After a family meeting, life-sustaining therapies were withdrawn, and he passed away 33 hours after hospital presentation.
Case 3
A 65-year-old woman presented with altered consciousness following 4 days of persistent vomiting and diarrhea while continuing to take her regular metformin. She had type 2 DM and hypothyroidism.
Her BP was 87/54 mm Hg, pulse rate 97 beats/min, oxygen saturations 97% on room air, respiratory rate 40 breaths/min, and GCS 10 (eyes 3, verbal 2, and motor 5). Initial lactate was 18.2 mmol/L and peaked at 28.9 mmol/L.
Bicarbonate 300 mEq was administered, and invasive monitoring and central access were established. CVVHDF was commenced within 4 hours of presentation to hospital followed by a second dialysis catheter and CVVHDF 3 hours later. This was associated with hemodynamic instability requiring a norepinephrine infusion up to 0.3 μg·kg–1·min–1.
After 8 hours of 2 dialysis machines concurrently running, 1 filter clotted. Her lactate and pH had normalized, so the second CVVHDF machine was not restarted. The single CRRT machine clotted 9 hours later, but there was no indication for ongoing dialysis. She was discharged from the ICU on day 5 and home on day 10.
Case 4
A 61-year-old man presented to the emergency department with a 3-day history of generalized abdominal pain, diarrhea, and vomiting. He had ischemic heart disease, hypertension, stage 3b chronic kidney disease and type 2 DM, for which he took metformin.
On arrival, his BP was 75/50 mm Hg, pulse rate 65 beats/min, respiratory rate 36 breaths/min, oxygen saturations 99% on room air, temperature 35.7 °C, and GCS 14 (eyes 4, verbal 4, and motor 6). Initial lactate was 11.3 mmol/L, peaking at 18 mmol/L.
He had a pulseless electrical cardiac arrest possibly due to hyperkalemia (K+ 5.6 mmol/L [normal range, 3.5–5.2 mmol/L]) and achieved return of spontaneous circulation after 5 minutes of advanced life support. Immediately postarrest, 4 hours after hospital presentation, a dialysis catheter was inserted and CVVHDF commenced. After 9 hours, a second CRRT machine was started for a rising lactate. Both machines ran for approximately 10 hours with 1 machine discontinued once his pH was consistently above 7.3. Single CRRT continued for 2 more days. The patient was extubated on day 5 and discharged to the ward on day 7 and home on day 50.
DISCUSSION
We report a case series of 4 patients with MALA and severe high anion gap acidosis treated with dual CRRT. Metformin can lead to increased lactate production but not always acidosis. The physiology is incompletely understood, but MALA represents the most severe form and likely requires a precipitating event.10 Further cardiovascular compromise can lead to increased lactate production due to hypoxia (type A lactic acidosis), worsening the acidosis and subsequently the patient’s cardiovascular state.11
The EXtracorporeal TReatments in Poisoning (EXTRIP) working group recommends dialysis, compared to CRRT, as the preferred elimination technique for metformin toxicity due to its superior correction of acid-base and removal of metformin.12 EXTRIP also states that dialysis is the most available form of extracorporeal treatments. However, the survey by Ricci et al (2006)13 is still likely representative of current practice in Australian ICUs with CRRT being mostly used. It is a more familiar technique to the ICU team and not dependent on external staff or equipment. CRRT can be rapidly initiated at any time, which is a major advantage over dialysis.
Compared to single CRRT, dual CRRT doubles the blood volume and flow rate of the extracorporeal circuits. These factors partially negate the advantage of single CRRT being more hemodynamically stable than dialysis. This can be managed by starting the 2 machines in a sequential manner, which allows for less rapid fluid shifts and titration of vasopressors. The additional cost of requiring 2 dialysis catheters and running a second CRRT machine needs to be considered. The total “CRRT dose” for cases 1 and 3 was over 150 mL/kg/h, whereas for case 2 was 76 mL/kg/h and for case 4 was 57 mL/kg/h (Table 1), an average of 2724 mL/kg/d. This is 2.7 times the mean dialysis dose (977.7 mL/kg/d) described by Mariano et al14 in their retrospective analysis of MALA treated with dialysis. These dialysis doses correlate with EXTRIP’s recommendation of using a technique that achieves high flux and high efficiency to rapidly clear metformin and lactate.12
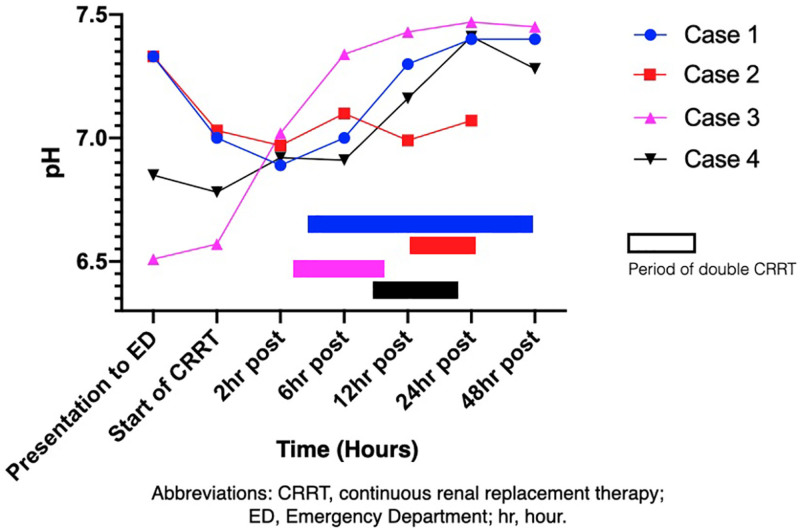
For cases 1 and 2 with acute intoxication, there was a time delay of >12 hours from the overdose to initiating CRRT. For cases 3 and 4 with insidious intoxication, dual CRRT therapy was initiated rapidly after hospital presentation. Cases 1, 3, and 4 all survived and had a fairly rapid restoration of their acid base balance. Case 2 had the greatest delay in starting the second CRRT machine and never achieved normalization of his pH (Figure 2). He died of multiorgan failure. Given the limited number of patients treated, we are unable to make a recommendation on when to initiate the second CRRT machine. However, for case 2, it is unknown whether there would have been an alternate outcome had the second CRRT machine been initiated earlier.
Figure 2.
Graph showing change in pH during double CRRT. CRRT indicates continuous renal replacement therapy; ED, emergency department.
LIMITATIONS
The utility of measuring metformin concentration is not well established12 and as such is not routine practice in our institution. The initial high dialysis dose achieved by using dual CRRT will rapidly clear metformin from the central compartment. Ongoing single-machine CRRT will continually clear metformin, as it equilibrates from the tissues back into the central compartment. Despite the absence of metformin concentrations to test this theory, the clinical improvements in our patients suggest that there was rapid clearance of metformin and correction of pH. This case series only reviews dual CRRT for patients with MALA. There may be scope to investigate its use in other settings of severe metabolic acidosis.
CONCLUSIONS
In the setting of metformin toxicity, dual CRRT is an acceptable alternative to dialysis. The accessibility and ICU staff familiarity with CRRT offer an advantage of this technique.
ACKNOWLEDGMENTS
We thank Patrick Young, RN, Clinical Information Systems Manager, Intensive Care Unit, Redcliffe Hospital, ANZAC Avenue, Redcliffe QLD 4020, Australia, for his assistance with data curation.
DISCLOSURES
Name: Hannah V. Reynolds, FCICM, FANZCA, MBChB.
Contribution: This author helped with methodology, data curation, writing—original draft—review, editing, and submission.
Name: Hamish H. G. Pollock, FCICM, FANZCA, MBBS.
Contribution: This author helped with concept, writing—review, and editing.
Name: Yogesh V. Apte, FCICM, MClinEd, MBBS.
Contribution: This author helped with concept, writing—review, and editing.
Name: Alexis Tabah, FCICM, MD.
Contribution: This author helped with methodology, writing—review, editing, and supervision.
Funding: None.
This manuscript was handled by: BobbieJean Sweitzer, MD, FACP.
GLOSSARY
- BP
- blood pressure
- CVVHDF
- continuous venovenous hemodiafiltration
- CRRT
- continuous renal replacement therapy
- DM
- diabetes mellitus
- ED
- emergency department
- EXTRIP
- EXtracorporeal TReatments in Poisoning
- GCS
- Glasgow Coma Scale
- ICU
- intensive care unit
- IJ
- internal jugular vein
- K+
- potassium
- MALA
- metformin associated lactic acidosis
- ST
- ST 150, disposable extracorporeal circuit for use with the Baxter Prismaflex or PrisMax CRRT machines
The authors declare no conflicts of interest.
REFERENCES
- 1.DeFronzo R, Fleming GA, Chen K, Bicsak TA. Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metabolism. 2016;65:20–29. [DOI] [PubMed] [Google Scholar]
- 2.Yeh HC, Ting IW, Tsai CW, Wu JY, Kuo CC. Serum lactate level and mortality in metformin-associated lactic acidosis requiring renal replacement therapy: a systematic review of case reports and case series. BMC Nephrol. 2017;18:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boucaud-Maitre D, Ropers J, Porokhov B, et al. Lactic acidosis: relationship between metformin levels, lactate concentration and mortality. Diabet Med. 2016;33:1536–1543. [DOI] [PubMed] [Google Scholar]
- 4.Lalau JD, Westeel PF, Debussche X, et al. Bicarbonate haemodialysis: an adequate treatment for lactic acidosis in diabetics treated by metformin. Intensive Care Med. 1987;13:383–387. [DOI] [PubMed] [Google Scholar]
- 5.Rifkin SI, McFarren C, Juvvadi R, Weinstein SS. Prolonged hemodialysis for severe metformin intoxication. Ren Fail. 2011;33:459–461. [DOI] [PubMed] [Google Scholar]
- 6.Greco P, Regolisti G, Maggiore U, et al. Sustained low-efficiency dialysis for metformin-associated lactic acidosis in patients with acute kidney injury. J Nephrol. 2019;32:297–306. [DOI] [PubMed] [Google Scholar]
- 7.Angioi A, Cabiddu G, Conti M, et al. Metformin associated lactic acidosis: a case series of 28 patients treated with sustained low-efficiency dialysis (SLED) and long-term follow-up. BMC Nephrol. 2018;19:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friesecke S, Abel P, Kraft M, Gerner A, Runge S. Combined renal replacement therapy for severe metformin-induced lactic acidosis. Nephrol Dial Transplant. 2006;21:2038–2039. [DOI] [PubMed] [Google Scholar]
- 9.Panzer U, Kluge S, Kreymann G, Wolf G. Combination of intermittent haemodialysis and high-volume continuous haemofiltration for the treatment of severe metformin-induced lactic acidosis. Nephrol Dial Transplant. 2006;19:2157–2158.. [DOI] [PubMed] [Google Scholar]
- 10.Lalau JD, Kajbaf F, Protti A, Christensen MM, De Broe ME, Wiernsperger N. Metformin-associated lactic acidosis (MALA): moving towards a new paradigm. Diabetes Obes Metab. 2017;19:1502–1512. [DOI] [PubMed] [Google Scholar]
- 11.Lalau J-D, Lacroix CCB, De Cagny B, Fournier A. Metformin-associated lactic acidosis in diabetic patients with acute renal failure: a critical analysis of its pathogenesis and prognosis. Nephrol Dial Transplant. 1994;9(suppl 4):126–129.. [PubMed] [Google Scholar]
- 12.Calello DP, Liu KD, Wiegand TJ, et al. ; Extracorporeal Treatments in Poisoning Workgroup. Extracorporeal treatment for metformin poisoning: systematic review and recommendations from the Extracorporeal Treatments in Poisoning Workgroup. Crit Care Med. 2015;43:1716–1730. [DOI] [PubMed] [Google Scholar]
- 13.Ricci Z, Ronco C, D’Amico G, et al. Practice patterns in the management of acute renal failure in the critically ill patient: an international survey. Nephrol Dial Transplant. 2006;21:690–696. [DOI] [PubMed] [Google Scholar]
- 14.Mariano F, Pozzato M, Inguaggiato P, et al. Metformin-associated lactic acidosis undergoing renal replacement therapy in intensive care units: A Five-Million Population-Based Study in the North-West of Italy. Blood Purif. 2017;44:198–205. [DOI] [PubMed] [Google Scholar]