Abstract
Background
Scarce information is available on the duration of the protective effect of COVID-19 vaccination against the risk of SARS-CoV-2 infection and its severe clinical consequences. We investigated the effect of time since vaccine completion on the SARS-CoV-2 infection and its severe forms.
Methods
In this retrospective observational analysis using the vaccination campaign integrated platform of the Italian region of Lombardy, 5 351 085 individuals aged 12 years or older who received complete vaccination from Jan 17 to July 31, 2021, were followed up from 14 days after vaccine completion until Oct 20, 2021. Changes over time in outcome rates (ie, SARS-CoV-2 infection and severe illness among vaccinated individuals) were analysed with age-period-cohort models. Trends in vaccine effectiveness (ie, outcomes comparison in vaccinated and unvaccinated individuals) were also measured.
Findings
Overall, 14 140 infections and 2450 severe illnesses were documented, corresponding to incidence rates of 6·7 (95% CI 6·6–6·8) and 1·2 (1·1–1·2) cases per 10 000 person-months, respectively. From the first to the ninth month since vaccine completion, rates increased from 4·6 to 10·2 infections, and from 1·0 to 1·7 severe illnesses every 10 000 person-months. These figures correspond to relative reduction of vaccine effectiveness of 54·9% (95% CI 48·3–60·6) for infection and of 40·0% (16·2–57·0) for severe illness. The increasing infection rate was greater for individuals aged 60 years or older who received adenovirus-vectored vaccines (from 4·0 to 23·5 cases every 10 000 person-months). The increasing severe illness rates were similar for individuals receiving mRNA-based vaccines (from 1·1 to 1·5 every 10 000 person-months) and adenovirus-vectored vaccines (from 0·5 to 0·9 every 10 000 person-months).
Interpretation
Although the risk of infection after vaccination, and even more of severe illness, remains low, the gradual increase in clinical outcomes related to SARS-CoV-2 infection suggests that the booster campaign should be accelerated and that social and individual protection measures against COVID-19 spread should not be abandoned.
Funding
None.
Introduction
Evidence is available that vaccination against the SARS-CoV-2 virus protects from the infection,1 and that this is the case also for the most common variants of the virus.2 It has also been shown that both the mRNA-based and the adenovirus-vectored vaccines are protective and that the protection is greatest against the severe and lethal manifestations of the disease.3, 4, 5, 6, 7, 8 However, although several studies investigated the trend exhibited by virus neutralising antibodies after vaccine inoculation,9, 10, 11 the relationship of these antibodies with patient protection is largely unknown. Furthermore, few studies of limited duration have investigated the persistence of vaccine-dependent protection by a direct approach—ie, by assessing the rate of post-vaccination infection and severe illness over time.4, 12, 13, 14, 15
We used the integrated platform of the vaccination campaign of Lombardy, an Italian region which includes almost 9 million candidates to vaccination, for evaluating the effect of the time since receiving complete vaccination on incidence rates of SARS-CoV-2 infection and severe illness.
Methods
Study design and participants
Our retrospective observational analysis included 5 353 005 beneficiaries of the Lombardy Regional Health Service (RHS) aged 12 years or older who completed vaccination against SARS-CoV-2 from Jan 17 to July 31, 2021. All individuals were inoculated with two doses of a vaccine manufactured by Pfizer-BioNTech (approved by the Italian Drug Agency on Dec 22, 2020), Moderna (Jan 7, 2021), or Oxford-AstraZeneca (Jan 30, 2021), or one dose of a vaccine manufactured by Janssen (March 12, 2021). The two doses of vaccine were separated by an interval of 21 days (extended to 35 days after the first 2 months of the vaccination campaign) for the Pfizer-BioNTech or Moderna vaccines, and of 78 days during the entire vaccination campaign for the Oxford-AstraZeneca vaccine. In March, the Oxford-AstraZeneca vaccine was suspended in Italy for individuals younger than 65 years and in June the Italian Drug Agency recommended the Pfizer-BioNTech vaccine for individuals who had undergone the first vaccination with Oxford-AstraZeneca. Individuals receiving heterologous vaccination were classified according to the vaccine type that was last administered.
Research in context.
Evidence before this study
From Sept 1 to Oct 25, 2021, we continuously searched PubMed, using the search Medical Subject Headings term “COVID-19 vaccines”, jointly with the search terms “duration” or “protection”. We found that, although knowledge on how long the immunity triggered by vaccination protects against infection and symptomatic disease is of key public health relevance, few studies have investigated the persistence of vaccine-dependent protection by direct (clinical outcome assessment) rather than surrogate-based (neutralising antibody assay) approaches. We evaluated the effect of the time from complete vaccination on incidence rates of SARS-CoV-2 infection and severe illness up to 9 months since completion of mRNA-based and adenovirus-vectored COVID-19 vaccines, while controlling for time of vaccine completion and time of outcome occurrence. Data were collected in 5 351 085 individuals aged 12 years or older from the Italian region of Lombardy who received a complete vaccination from Jan 17 to July 31, 2021.
Added value of this study
Infection rates increased continuously as time from complete vaccination increased (from five to ten infections per 10 000 person-months from the first month until the ninth month after vaccination completion), especially for individuals receiving adenovirus-vectored vaccines (from four to 17 infections per 10 000 person-months). Conversely, weakly rising rates of severe illness were observed for both the mRNA-based and adenovirus-vectored vaccines, the average rates never exceeding three cases per 10 000 person-months even after 9 months from the complete vaccination.
Implications of all the available evidence
Although the risk of infection after vaccination, and even more of severe illness, remains low, the fast waning of vaccine-induced immunity, particularly for individuals who received adenovirus-vectored vaccine, suggests that the campaign of the third booster dose should be accelerated. This is supported by (1) the long time that has elapsed since the vaccine completion of elderly individuals and frail patients, (2) the expected autumn-winter recovery of the virus spread, and (3) the predominant spread of the delta variant. Social and individual protection measures should not be abandoned.
Individuals who, within 14 days after completion of the vaccine procedure experienced a SARS-CoV-2 infection, were hospitalised for COVID-19, or who died were excluded from the analysis. The remaining individuals were included in the study cohort and were followed up from 14 days after vaccine completion (under the assumption that clinically significant immunity is achieved 2 weeks after receiving the vaccine)8 until Oct 20, 2021. In a supplementary analysis, this 14-day time-window was lengthened to 28 days.
Four population-based data sources collecting individual health information were used: (1) the COVID-19 vaccination registry established from Dec 27, 2020 (ie, the date of the first vaccine administration in Lombardy), which collected individual data on the date, type, and dose of vaccine dispensed; (2) the registry of patients with a confirmed diagnosis of SARS-CoV-2 infection established on Feb 21, 2020 (ie, the date of the first confirmed diagnosis in Lombardy), for monitoring individual data on infections, and hospital admissions, emergency-room access, and deaths due to COVID-19; (3) the health-care use database, which since the year 2000 collects various types of information, including inpatient diagnoses supplied by public or private hospitals, and outpatient drug and services supplied by the RHS; and (4) the health registry, which since the year 2000 reports and updates data on Lombardy residents, including date and reasons for entry (eg, birth, immigration) and exit (eg, death, emigration) from the condition of RHS beneficiary. These electronic databases might be linked through a single individual identification code. To preserve privacy, each identification code was deidentified automatically, with this inverse process being allowed only for the RHS on request from judicial authorities.
Statistical analyses
The occurrence of post-vaccination SARS-CoV-2 infection was established by positive PCR test to the SARS-CoV-2 virus in any clinical setting regardless of the presence of symptoms.12 The rates of infection were calculated as the number of cases per 10 000 person-months. The rates of severe illness (the first occurrence of a composite outcome that included hospital admission, including patients in intensive care units, and deaths attributed to COVID-19, whichever occurred first) were calculated as the number of cases per 10 000 person-months.
At first sight, a simple measurement of the trend in outcome rates as the time since vaccination increases might appear an adequate approach for establishing the causal role of time since receiving vaccine on the probability of SARS-CoV-2 infection and disease. Two main pitfalls should be taken care of, however. First, because vaccination was implemented early in older adults and frail patients, individuals accumulating more time since the vaccination might be those with faster immunity loss. Second, the probability of infection and severe illness is affected by the epidemic spread and variant diffusion, two factors that changed considerably during the vaccination campaign. This means that the time elapsed since vaccination, the period when vaccination was completed, and the period when the outcome occurred must be all considered, although their collinearity hinders a direct estimation of their independent effects. In this Article we have made use of the age-period-cohort (APC) analysis, which is a classical approach for the measurement of the temporal trends of clinical outcomes.16 The primary temporal factor of interest was the number of months between complete vaccination and outcome occurrence (the effect of time since vaccination), because this provided direct information on how many months the immunisation coverage conferred by the vaccine lasted. However, two other temporal factors were considered: the month in which vaccination was completed (called cohort effect), which reflected the effect of the Italian Government's decision to prioritise the vaccination of high-risk groups; and the month of outcome occurrence (the period effect), which reflected the temporal heterogeneity of the epidemic spread as well as the possible influence of the predominant variants. Methodological details are reported in the appendix (p 3). Briefly, to overcome collinearity—ie, to separate the effect of time since vaccine completion (ie, the so-called age effect in a classical APC model) from the period and cohort effects, we adapted the classic APC model according to the approach of Ananth and colleagues.17 Outcome data were stratified according to 1-month age groups (considered as time from completion of vaccination, from 1 month to 9 months since completion for mRNA-based vaccines and from 1 month to 7 months since completion for adenovirus-vectored vaccines), nine period groups (from February to October), and seven cohort groups (from January to July),18 and time-related outcome rates were modelled accordingly. The effects of time since vaccination derived from APC modelling were estimated for the entire cohort and when indicated, for the age of the individuals at cohort entry (<60 and ≥60 years), and type of vaccine received (mRNA-based or adenovirus vectored). Modelling was done using the apc.fit function in the Epi package in R (version 4.0.2).
Because APC estimates can vary depending on the adopted constraint,16, 17, 18 their robustness was assessed by adopting an alternative design. In accordance with the studies from Tartof and colleagues12 and Cohn and colleagues,13 we included all 9 140 390 potential candidates to receive the vaccine. Each member of this expanded cohort accumulated person-months of follow-up from the entry date (ie, the start of the vaccination campaign, Dec 27, 2020) until the earliest between outcome occurrence (measured as above described) or censuring (emigration, death unrelated to COVID-19, administration of more than two vaccine doses, or the endpoint of the study period, that is Oct 20, 2021). The person-months accumulated by each cohort member were portioned into three subperiods (ie, unvaccinated [from entry date until 14 days after the first dose], partially vaccinated [from 14 days after the first dose until 14 days after the second dose], and fully vaccinated [from 14 days after receiving the second dose until censuring]). A Cox proportional hazard model was used for separately estimating the hazard ratio of COVID-19 infection and severity, together with its 95% CI, associated with the time-dependent exposure to vaccine. Vaccine effectiveness was measured as complementary to the hazard ratio. The model was adjusted for available demographic and clinical characteristics. The durability of the vaccine effectiveness was assessed by including in the model the calendar time—ie, the monthly intervals after participants were fully vaccinated.12
With the aim of making results from APC and Cox modelling comparable, results were expressed as relative reduction of vaccine effectiveness—that is, the relative difference between rates (APC) or vaccine effectiveness (Cox) from the first to a generic month since vaccine completion. The corresponding 95% CI was calculated by means of normal approximation.
Role of the funding source
There was no funding source for this study.
Results
The 5 351 085 individuals included in the study cohort (46·9% men; mean age 57·7 years [SD 18·0]) yielded 21 205 273 person-months of observation, on average almost 4 months in each person. More than three-quarters of the entire study cohort (4 116 803 [76·9%] of 5 351 085) received an mRNA-based vaccine (Pfizer-BioNTech or Moderna), whereas the remaining 1 234 282 (23·1%) received an adenovirus-vectored vaccine (Oxford-AstraZeneca or Janssen). Among the 4 116 803 individuals classified as receiving an mRNA-based vaccine, very few (91 063 [2·2 %]) received the Oxford-AstraZeneca vaccine as the first dose. With respect to cohort members receiving an mRNA-based vaccine, those receiving an adenovirus-vectored vaccine mainly belonged to intermediate age classes (from 60 to 79 years) and suffered less frequently from diabetes, whereas there were no substantial differences for the other considered covariates (table ).
Table.
Selected characteristics of the study cohort according to vaccine type
| mRNA-based vaccines*(n=4 116 803) | Adenovirus-vectored vaccines*(n=1 234 282) | ||
|---|---|---|---|
| Men | 1 935 105 (47·0%) | 575 869 (46·7%) | |
| Age category, years | |||
| 12–49 | 1 606 712 (39·0%) | 132 399 (10·7%) | |
| 50–59 | 886 799 (21·5%) | 236 497 (19·2%) | |
| 60–69 | 528 681 (12·8%) | 422 166 (34·2%) | |
| 70–79 | 404 455 (9·8%) | 436 013 (35·3%) | |
| ≥80 | 690 156 (16·8%) | 7207 (0·6%) | |
| Previous contacts with the Regional Health Service† | |||
| <5 | 1 500 430 (36·4%) | 431 250 (34·9%) | |
| 5–99 | 2 319 122 (56·3%) | 726 786 (58·9%) | |
| ≥100 | 297 251 (7·2%) | 76 246 (6·2%) | |
| Previous SARS-CoV-2 infection | 180 619 (4·4%) | 33 304 (2·7%) | |
| Comorbidities† | |||
| Diabetes | 343 557 (8·4%) | 56 229 (4·6%) | |
| Heart failure | 31 889 (0·8%) | 2057 (0·2%) | |
| Chronic respiratory disease | 377 205 (9·2%) | 103 027 (8·4%) | |
| Malignancies | 132 713 (3·2%) | 22 005 (1·8 %) | |
| Organ transplant | 17 184 (0·4%) | 754 (0·1%) | |
| Hypertension | 627 997 (15·3%) | 217 824 (17·7%) | |
| Vascular disease | 337 537 (8·2%) | 84 978 (6·9%) | |
| Valvular disease | 12 635 (0·3%) | 2059 (0·2%) | |
| Cerebrovascular disease | 38 675 (0·9%) | 3833 (0·3%) | |
| Chronic kidney disease | 19 709 (0·5%) | 874 (0·1%) | |
| Month of vaccine completion | |||
| January | 66 739 (1·6%) | 0 (0·0%) | |
| February | 169 198 (4·1%) | 0 (0·0%) | |
| March | 271 429 (6·6%) | 54 (<0·1%) | |
| April | 430 874 (10·5%) | 11 064 (0·9%) | |
| May | 941 385 (22·9%) | 252 439 (20·5%) | |
| June | 598 948 (14·6%) | 279 985 (22·7%) | |
| July | 1 638 230 (39·8%) | 690 740 (56·0%) | |
Four vaccines are currently authorised in Italy, two of them are mRNA-based (ie, those manufactured by Pfizer-BioNTech and Moderna) and the other two are adenovirus-vectored (ie, those manufactured by Oxford-AstraZeneca and Janssen).
Measured in the 2-year period before the index date.
Overall, 14 140 infections and 2450 cases of severe illness were documented (of which 2385 hospital admissions, including 60 in intensive care units, and 229 deaths were attributed to COVID-19), corresponding to incidence rates of 6·7 (95% CI 6·6–6·8) infections and 1·2 (1·1–1·2) cases of severe illness per 10 000 person-months.
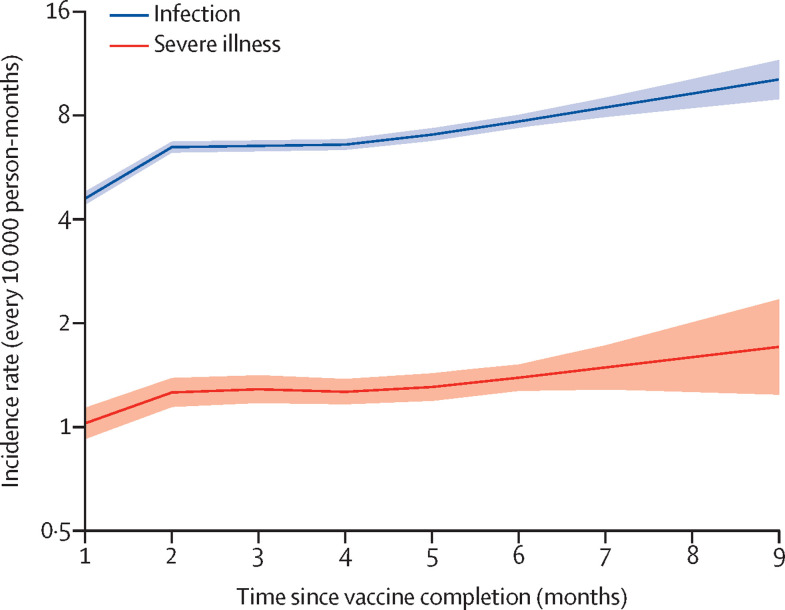
Figure 1 shows the APC modelled effects of time since vaccination on the rates of infection and severe illness. From the first to the ninth month since complete vaccination the rates increased progressively from 4·6 (95% CI 4·4–4·8) to 10·2 (8·9–11·6) infections every 10 000 person-months, with a corresponding increase in severe illness from 1·0 (0·9–1·1) to 1·7 (1·2–2·4). These figures correspond to relative reduction of vaccine effectiveness of 54·9% (48·3–60·6) for infection and of 40·0% (16·2–57·0) for severe illness.
Figure 1.
Influence of time since complete vaccination on rates of SARS-CoV-2 infection and severe COVID-19 illness
Estimates based on the cohort of 5 351 085 individuals who received complete vaccination from January to July, 2021. The figure reports the trends in age-period-cohort modelled incidence rates (and 95% CI bands) according to time since complete vaccination. Estimates are adjusted for the month of vaccine completion (cohort effect), and the month of outcome occurrence (period effect).
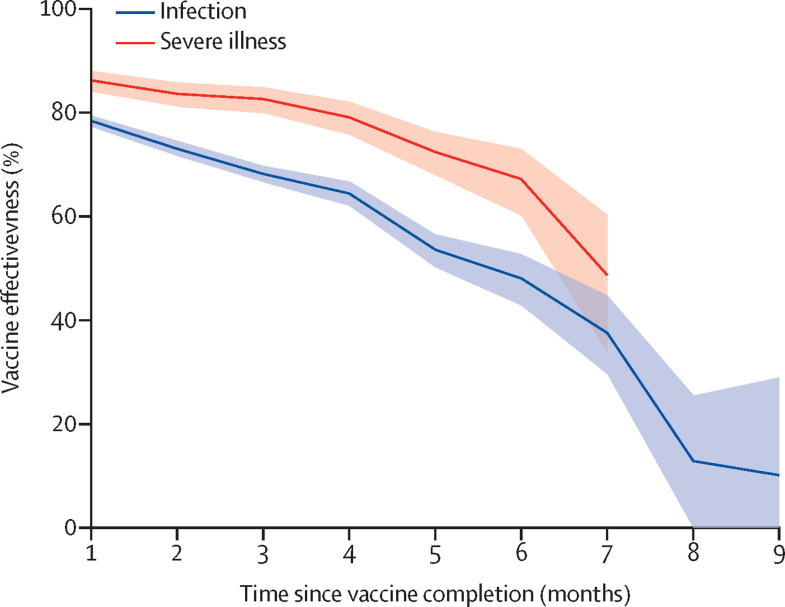
Figure 2 shows the Cox modelled effects of time since vaccination on the vaccine effectiveness. From the first to the ninth month the vaccine effectiveness against the infection decreased progressively from 78% to 10%, with a relative reduction of 87·0% (95% CI 75·8–93·0). From the first to the seventh month the vaccine effectiveness against severe illness decreased from 86% to 49%, with a relative reduction of 43·5% (95% CI 24·5–57·7). Because the models did not reach convergence, estimates beyond the seventh month were not obtained. Further details on the effect of selected covariates on the infection and severe illness hazard ratios are reported in the appendix (pp 12–13).
Figure 2.
Influence of time since complete vaccination on vaccine effectiveness against SARS-CoV-2 infection and severe COVID-19 illness
Estimates based on the cohort of 9 140 390 potential candidates who were to receive the vaccine as of Dec 27, 2020. Cox proportional hazard models were fitted for estimating hazard ratio and 95% CI. Vaccine effectiveness was directly calculated as 1 -hazard ratio.
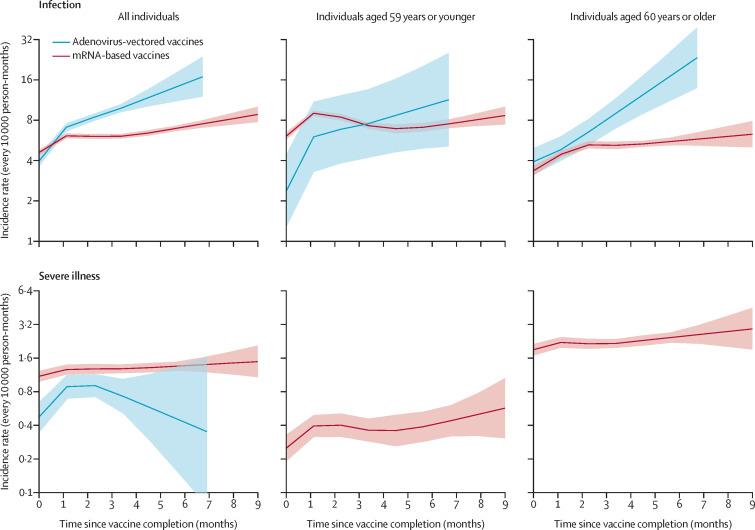
Figure 3 shows the ACP modelled effects of time since vaccination according to the vaccine-type and age at cohort entry. The rate of infection showed an increase among individuals who received mRNA-based vaccines from 4·6 (95% CI 4·4–4·8) to 8·8 (7·8–10·1) infections every 10 000 person-months (relative reduction of vaccine effectiveness 48·1% [95% CI 40·3–54·8]). This was the case, to a greater degree, among those who received adenovirus-vectored vaccines in whom the rate of infection increased from 4·0 (3·6–4·4) to 16·8 (12·0–23·7) infections every 10 000 person-months (relative reduction of vaccine effectiveness 76·5% [66·5–83·5]). The difference between vaccines was smaller in younger than in older individuals, the relative reduction of vaccine effectiveness being 46·2% (31·7–57·7) and 83·2% (70·3–90·5) according to whether they received mRNA-based or adenovirus-vectored vaccines, respectively. Compared with the rate of infection, severe illness showed a smaller increase in individuals who received mRNA-based vaccines (from 1·1 [1·0–1·2] to 1·5 [1·1–2·1] cases every 10 000 person-months, with a relative reduction of vaccine effectiveness of 26·1% [0·0–47·5]). In contrast, a nonlinear trend was observed for individuals receiving adenovirus-vectored vaccines—ie, an increase in severe infection rates during the first 3 months (from 0·5 [0·3–0·6] to 0·9 [0·7–1·1] cases every 10 000 person-months) and a decrease in the severe infection rates afterwards (up to 0·3 [0·1–1·6] cases every 10 000 person-months after 7 months). Because few severe outcomes were observed in individuals who received adenovirus-vectored vaccines, age-stratification was considered only for individuals who received mRNA-based vaccines. In these individuals, rising rates of severe infections were observed in older and younger individuals, with relative reduction of vaccine effectiveness of 35·0% (0·0–58·5) and 55·9 % (13·3–77·6) respectively.
Figure 3.
Influence of time since complete vaccination on rates of SARS-CoV-2 infection (top boxes) and severe COVID-19 illness (bottom boxes) in the entire cohort and according to age and vaccine type
Estimates based on the cohort of 5 351 085 individuals who received complete vaccination from January to July, 2021. The figure reports the trends in age-period-cohort modelled incidence rates (and 95% CI bands) according to time since complete vaccination. Estimates are adjusted for the month of vaccine completion (cohort effect), and the month of outcome occurrence (period effect).
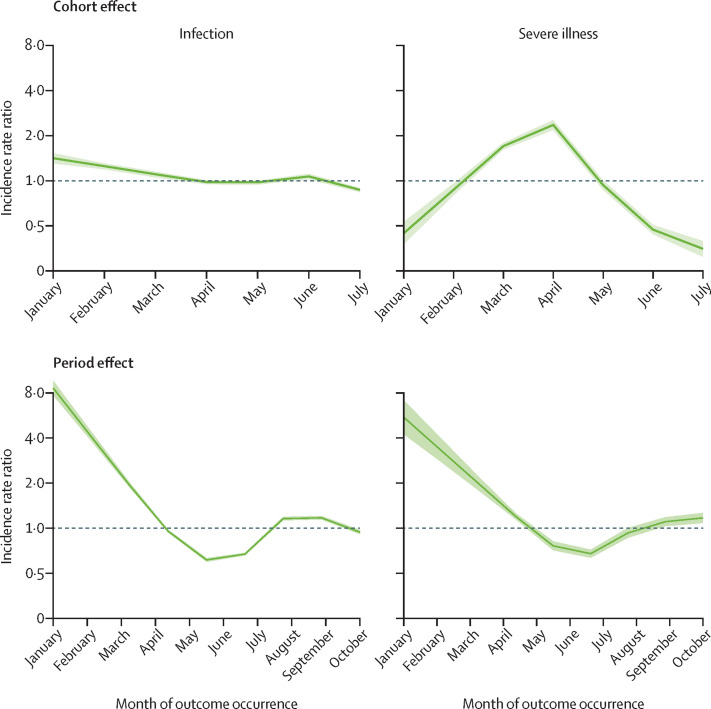
The APC modelled effects of the month of vaccine completion (cohort effect) and of outcome occurrence (period effect) on outcomes are shown in figure 4 . Regarding cohort effects, different patterns were observed for infection rate and severe illness rate. Individuals who completed vaccination in January or February were at significantly higher rate of infection, whereas those who completed vaccination in March or April were at higher rate of severe illness. Period effects exhibited similar patterns for both infection and the severe illness rates, with the highest value in February, a sharp drop until June–July, and an increased risk thereafter. Infection rate was reasonably stable from August to October, whereas a slight increasing rate of severe illness was noticed in October.
Figure 4.
Influence of month of vaccination completion (cohort effect) and month of outcome occurrence (period effect) on rates of SARS-CoV-2 infection and severe COVID-19
Estimates based on the cohort of 5 351 085 individuals who received complete vaccination from January to July, 2021. The figure reports the trends in age-period-cohort modelled incidence rate ratios (and 95% CI bands) according to the cohort (month of vaccine completion) and the period (month of outcome occurrence) while controlling for the months since complete vaccination.
The patterns described in Figure 1, Figure 4 did not change substantially when the post-final vaccination window was lengthened to 28 days (appendix pp 15–16).
Discussion
The current study based on real-world data from more than 5 million individuals who had completed the vaccination against COVID-19 shows that the rate of SARS-CoV-2 infection increased continuously as time from the vaccination increased. Nearly 5 infections per 10 000 person-months occurred already in the first month after vaccination, the continuous increase leading to approximately doubling of the infection rate at 9 months after complete vaccination. It further shows that the rate of severe COVID-19 was very low immediately after vaccination (1 case per 10 000 person-months in the first month) but that thereafter it also exhibited a continuous increase reaching 1·7 cases per 10 000 person-months at the ninth month. Finally, although our study was not primarily designed for measuring vaccination effectiveness, our results also show a decline of vaccine protection during the months after complete vaccination that was greater for the risk of infection (from 78% to 10%) than for its more severe and lethal forms (from 86% to 49%). These findings provide robust documentation that the protection against COVID-19 offered by currently available vaccines undergoes a clear decline during the following months and that this occurs both for protection against the infection and protection against the more severe and lethal consequences of the infection. They further clearly documented, however, that the vaccine-dependent protection does not disappear but that a residual protection is still present 9 months after the vaccination, particularly against the severe forms of COVID-19. This is consistent with the conclusion of two recent US real-world investigations12, 13 that reported, over a shorter follow-up (5–7 months), a decline of the SARS-CoV-2 infection vaccination effectiveness from 88% to 48%, a lower decline of the risk for the clinically severe or fatal consequences of the infection, and a somewhat heterogeneous decline among different vaccine types.13 The data are also consistent with a recent modelling study, which predicted that the decay of the titre of neutralising antibodies over the first 250 days after COVID-19 immunisation is interpretable as a significant loss of protection from SARS-CoV-2 infection, albeit with a substantial preservation of protection against severe COVID-19 disease.19 Our study extends this evidence to vaccination with two types of vaccine platforms and offers a longer temporal extension to currently available real-world data.
An additional important finding of the present study is that protection against the risk and the severity of SARS-CoV-2 infection was not superimposable between mRNA-based and adenovirus-vectored vaccines. Both vaccine types exhibited increasing infection rates over time. However, although the increase was relatively small for mRNA-based vaccines, a greater increase was observed for adenovirus-vectored vaccines. The absolute between-vaccine difference, however, was much less evident for the risk of developing severe illness. These findings strongly suggest that both the mRNA-based and the adenovirus-vectored vaccines exert a sustained protective effect against the severe forms of the SARS-CoV-2 infection while the mRNA type seems to be better equipped to prolong the initial protective effect against the risk of developing milder forms of the infection.
A major determinant of SARS-CoV-2 infection and of its severe or lethal forms in individuals who were fully vaccinated was the level of epidemic spread in the area in which they lived. In fact, among vaccinated individuals, rates were highest from January to March when the infection incidence in Lombardy reached 132 positive swabs every 10 000 person-months (and very restrictive containment measures were therefore imposed) and lowest in June and July when only 7 positive swabs per 10 000 person-months were found (so justifying the adoption of mild containment measures in that period). Among vaccinated individuals the infection rate increased again in August and September, 2021, when the delta variant (B.1.617.2), which is reportedly associated with a higher viral load than previous variants,20 became dominant.
A cohort effect was also clearly documented by our findings because the infection rates were higher in individuals who completed vaccination in January or February, and a higher incidence of severe illness was seen in those who completed vaccination in March or April. Because in Italy the vaccination campaign addressed health workers first (approximately up to February), and involved elderly individuals and frail individuals soon afterwards (starting from March), these cohort effects probably reflect the categories of people at higher risk of infection (younger and healthy individuals) and those at higher risk of developing severe clinical forms of the infection (older and frail individuals).
Our study does not provide information on the factors and mechanisms involved in the faster or slower decline of the protective effect of the vaccines on the risk and severity of SARS-CoV-2 infection. In this context, however, differential patterns of immunogenicity have been reported from available vaccine platforms, the antibody responses being 2·9-times higher following the Pfizer-BioNTech vaccine and the cellular responses 1·4-times higher following the Oxford-AstraZeneca vaccine.21 It has also been reported that a rapid protection against symptomatic infection is provided by humoral immunity through control of viral replication, whereas long-term protection against the severe forms of the infection is more closely related to cellular immunity.22 We can tentatively speculate that this reported dichotomy between humoral and cellular responses to the two vaccine types accounts for their similarly persistent protection against severe illness and perhaps also for the more rapid reduction of the protection against the risk of the infection with the adenovirus-vectored vaccines.
The present study has several strengths. First, the study provides the largest and most robust available evidence of the durability of vaccine protection against the risk of infection with SARS-CoV-2 and its clinical consequences. It also extends this information on the longest time course so far explored. Second, the study was based on a very large population and included all ages that were regarded as suitable for the COVID-19 vaccination. This allowed a large accumulation of person-months, which meant that although postvaccination infections and cases of severe illness were very rare events, the study was sufficiently powered to address its primary goal. Third, our use of the APC analysis (a widely applied analysis in descriptive epidemiology) allowed us to accurately separate the effects of the different temporal components on the outcomes of interest. Finally, the robustness of our main findings was confirmed by an alternative, and more conventional design.
Several weaknesses should also be taken into consideration, however. First, our study is a retrospective investigation, since secondary data were used. Second, power limitations prevented the investigation of severe illness rates according to age and vaccine type. Third, outcome misclassification might have affected the study, because not all individuals in whom the infection occurred were tracked and some patients with severe symptoms might have been treated at home. Last, as with any observational study, the investigation might have been affected by differences between groups—for example, by sociodemographic, behavioural, and clinical factors. It is unlikely, however, that this limitation substantially affected the time-related post-vaccination trends because these factors remained relatively constant over time.
The present study has public health implications for the policies to adopt to achieve pandemic control. Although the risk of SARS-CoV-2 infection after vaccination, and even more its severe illness, remains low, the fast waning of vaccine-induced immunity, particularly for individuals who received adenovirus-vectored vaccines, suggests that the campaign of the third booster dose should be accelerated.23 This recommendation is supported also by (1) the long time that has elapsed since the vaccine completion of elderly individuals and frail patients, (2) the expected autumn-winter recovery of the virus spread, and (3) the predominant spread of the delta variant. It should be nevertheless emphasised that these considerations should be taken with caution because individual persistence of vaccine-dependent protection might vary markedly from average data. Markers that can assess the protective effect of the COVID-19 vaccines in individual represent an urgent goal of research.
Data sharing
The data that support the findings of this study are available from Lombardy Regional Health Service, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the Lombardy Regional Health Service upon reasonable request.
Declaration of interests
GC received research support from the European Community, the Italian Agency of Drugs and the Italian Ministry for University and Research. He took part in a variety of projects that were funded by pharmaceutical companies (ie, Novartis, GSK, Roche, AMGEN, and BMS). He also received honoraria as a member of the advisory board to Roche. All other authors declare no competing interests.
Acknowledgments
Contributors
GC led the study conceptualisation and the development of the research question, supported by DC, AZ, OL, MG, GB, and GM. FB, GP, AB, and ME were responsible for data collection. ME and JJ accessed and verified the data. MF developed the statistical analysis plan and performed the analyses. GC supervised the analyses and wrote the first draft of the paper. All authors contributed to the interpretation and discussion of the results, critically revised the manuscript for intellectual content, and approved the final version and the submission of the manuscript. The corresponding author had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.McDonald I, Murray SM, Reynolds CJ, Altmann DM, Boyton RJ. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines. 2021;6:74. doi: 10.1038/s41541-021-00336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373 doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall VJ, Foulkes S, Saei A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397:1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doria-Rose N, Suthar MS, Makowski M, et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for COVID-19. N Engl J Med. 2021;384:2259–2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voysey M, Costa Clemens SA, Madhi SA, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baraniuk C. How long does COVID-19 immunity last? BMJ. 2021;373 doi: 10.1136/bmj.n1605. [DOI] [PubMed] [Google Scholar]
- 12.Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2021 doi: 10.1126/science.abm0620. published online Nov 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas SJ, Moreira ED, Jr, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Sahly HM, Baden LR, Essink B, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021;385:1774–1785. doi: 10.1056/NEJMoa2113017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell A. Age period cohort analysis: a review of what we should and shouldn't do. Ann Hum Biol. 2020;47:208–217. doi: 10.1080/03014460.2019.1707872. [DOI] [PubMed] [Google Scholar]
- 17.Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. BMJ. 2013;347 doi: 10.1136/bmj.f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carstensen B. Age-period-cohort models for the Lexis diagram. Stat Med. 2007;26:3018–3045. doi: 10.1002/sim.2764. [DOI] [PubMed] [Google Scholar]
- 19.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 20.Teyssou E, Delagrèverie H, Visseaux B, et al. The delta SARS-CoV-2 variant has a higher viral load than the beta and the historical variants in nasopharyngeal samples from newly diagnosed COVID-19 patients. J Infect. 2021;83:e1–e3. doi: 10.1016/j.jinf.2021.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parry H, Bruton R, Stephens C, et al. Differential immunogenicity of BNT162b2 or ChAdOx1 vaccines after extended-interval homologous dual vaccination in older people. Immun Ageing. 2021;18:34. doi: 10.1186/s12979-021-00246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ukey R, Bruiners N, Mishra H, et al. Dichotomy between the humoral and cellular responses elicited by mRNA and adenoviral vector vaccines against SARS-CoV-2. medRxiv. 2021 doi: 10.1101/2021.09.17.21263528. published online Sep 21. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against COVID-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from Lombardy Regional Health Service, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the Lombardy Regional Health Service upon reasonable request.