Abstract
Background
Passive immunotherapy using hyperimmune intravenous immunoglobulin (hIVIG) to SARS-CoV-2, derived from recovered donors, is a potential rapidly available, specific therapy for an outbreak infection such as SARS-CoV-2. Findings from randomised clinical trials of hIVIG for the treatment of COVID-19 are limited.
Methods
In this international randomised, double-blind, placebo-controlled trial, hospitalised patients with COVID-19 who had been symptomatic for up to 12 days and did not have acute end-organ failure were randomly assigned (1:1) to receive either hIVIG or an equivalent volume of saline as placebo, in addition to remdesivir, when not contraindicated, and other standard clinical care. Randomisation was stratified by site pharmacy; schedules were prepared using a mass-weighted urn design. Infusions were prepared and masked by trial pharmacists; all other investigators, research staff, and trial participants were masked to group allocation. Follow-up was for 28 days. The primary outcome was measured at day 7 by a seven-category ordinal endpoint that considered pulmonary status and extrapulmonary complications and ranged from no limiting symptoms to death. Deaths and adverse events, including organ failure and serious infections, were used to define composite safety outcomes at days 7 and 28. Prespecified subgroup analyses were carried out for efficacy and safety outcomes by duration of symptoms, the presence of anti-spike neutralising antibodies, and other baseline factors. Analyses were done on a modified intention-to-treat (mITT) population, which included all randomly assigned participants who met eligibility criteria and received all or part of the assigned study product infusion. This study is registered with ClinicalTrials.gov, NCT04546581.
Findings
From Oct 8, 2020, to Feb 10, 2021, 593 participants (n=301 hIVIG, n=292 placebo) were enrolled at 63 sites in 11 countries; 579 patients were included in the mITT analysis. Compared with placebo, the hIVIG group did not have significantly greater odds of a more favourable outcome at day 7; the adjusted OR was 1·06 (95% CI 0·77–1·45; p=0·72). Infusions were well tolerated, although infusion reactions were more common in the hIVIG group (18·6% vs 9·5% for placebo; p=0·002). The percentage with the composite safety outcome at day 7 was similar for the hIVIG (24%) and placebo groups (25%; OR 0·98, 95% CI 0·66–1·46; p=0·91). The ORs for the day 7 ordinal outcome did not vary for subgroups considered, but there was evidence of heterogeneity of the treatment effect for the day 7 composite safety outcome: risk was greater for hIVIG compared with placebo for patients who were antibody positive (OR 2·21, 95% CI 1·14–4·29); for patients who were antibody negative, the OR was 0·51 (0·29–0·90; pinteraction=0·001).
Interpretation
When administered with standard of care including remdesivir, SARS-CoV-2 hIVIG did not demonstrate efficacy among patients hospitalised with COVID-19 without end-organ failure. The safety of hIVIG might vary by the presence of endogenous neutralising antibodies at entry.
Funding
US National Institutes of Health.
Introduction
Current effective therapies for individuals hospitalised with COVID-19 target viral replication or pathological elements of the host inflammatory response;1, 2, 3, 4 however, morbidity and mortality persist, and additional treatments are urgently needed.
Augmenting the host humoral immune response to SARS-CoV-2 via passive immunotherapy is one possible therapeutic approach. Development of endogenous neutralising antibody responses to SARS-CoV-2 appears variable and might not be present at the time of hospitalisation.5, 6, 7
Approaches using engineered monoclonal antibodies targeting viral elements have shown benefit among outpatients early in the course of COVID-19.8, 9 Results from two trials of monoclonal antibodies indicate that the clinical benefit and possibly safety of monoclonal antibodies for patients admitted to hospital with COVID-19 might depend on the presence of endogenous neutralising antibodies at the time of randomisation.10, 11, 12
Convalescent plasma from recovered donors has been studied in both non-randomised and randomised trials for a variety of infectious diseases. With few exceptions,13, 14 randomised trials have not shown consistent evidence of benefit with convalescent plasma. One small study in older outpatients early in the course of COVID-19 infection showed benefit,14 but this result has not been consistently replicated.15 A non-randomised study found that risk of death was reduced for hospitalised patients given convalescent plasma that had higher anti-SARS-CoV-2 IgG antibody levels compared with patients given convalescent plasma with lower antibody levels;16 however, overall, randomised trials have not consistently shown that convalescent plasma reduces the risk of death or improves outcomes.16, 17, 18, 19, 20, 21 Reasons for this might include variability in the titre of specific antibodies in convalescent plasma.
Research in context.
Evidence before this study
Passive immunotherapies targeting SARS-CoV-2 have been considered promising potential therapies for COVID-19 since the beginning of the pandemic. Convalescent plasma has been in wide use since early in the epidemic, whereas monoclonal antibodies directed at SARS-CoV-2 and polyclonal hyperimmune immunoglobulin (hIVIG) to SARS-CoV-2 derived from recovered donors have emerged as potential therapies.
We searched PubMed for research articles published between database inception and Dec 15, 2021, for clinical trials of anti-SARS-CoV-2 passive immunotherapies among hospitalised patients with COVID-19 using various combinations of the terms “COVID-19,” “SARS-CoV-2,” “monoclonal antibody” “convalescent plasma” “intravenous immunoglobulin” “passive immunotherapy” and “clinical trial.” No language or date restrictions were applied. One small parallel-group trial reported encouraging results for treatment with hIVIG among hospitalised patients with COVID-19. Two trials (bamlanivimab in a trial conducted by the ACTIV-3 investigators, and casirivimab in combination with imdevimab in a trial conducted by the RECOVERY investigators) reported no clinical benefit for anti-SARS monoclonal antibody therapy for the broad population of hospitalised patients with COVID-19, but suggested potential benefit for patients without endogenous anti-SARS-CoV-2 antibodies at the time of treatment. Trials for convalescent plasma varied greatly in their size, population, and rigour; taken together, these trials showed no clinical benefit of convalescent plasma in the hospitalised population.
Added value of this study
To our knowledge, this study is the first well powered, placebo-controlled clinical trial to report results of hIVIG for the treatment of hospitalised patients with COVID-19. When administered with standard of care, including remdesivir, SARS-CoV-2 hIVIG did not demonstrate efficacy among patients hospitalised with COVID-19 without end-organ failure. There was no heterogeneity of treatment effect in efficacy among patients without endogenous antibodies compared with those with endogenous antibodies, but there was heterogeneity of treatment effect for the primary safety outcome: risk was greater for hIVIG compared with placebo for patients with endogenous neutralising anti-SARS-CoV-2 antibodies at the time of treatment.
Implications of all the available evidence
Clinical trials completed to date do not support use of antibody-based passive immunotherapies including convalescent plasma, monoclonal antibodies, and hIVIG for the broad population of hospitalised patients with severe COVID-19. Unlike some trials of monoclonal antibodies, this trial did not show evidence of benefit in those patients endogenous neutralising anti-SARS-CoV-2 antibodies at the time of treatment, but did suggest that safety of hIVIG and potentially other passive immunotherapies might vary by baseline antibody status. Further evaluation could better define the appropriate target population for this and other passive immunotherapies against SARS-CoV-2.
Hyperimmune intravenous immunoglobulin (hIVIG) is derived from healthy individuals who have recovered from COVID-19 and mounted a neutralising immune response to the infection.22 It differs from convalescent plasma in being a drug product manufactured from plasma pooled from multiple donors. It is comprised of purified immunoglobulin G in a limited volume, and is standardised to high neutralising titres to SARS-CoV-2, thereby over-coming the interunit variability of convalescent plasma. Unlike monoclonal antibodies, hIVIG is a concentrated mixture of polyclonal antibodies reflecting the diversity of the endogenous antibody response, which might provide advantages over monoclonal antibodies by mitigating immune escape by viral variants. A small single-centre trial reported encouraging results with this approach;23 parallel approaches using polyclonal product derived from non-human inoculation strategies also suggested possible benefit.24 Findings from well powered, controlled clinical trials of hIVIG to SARS-CoV-2 have not been reported.
We conducted a randomised, double-blind, placebo-controlled, phase 3 trial to evaluate the safety and clinical efficacy of anti-SARS-CoV-2 hIVIG in addition to standard of care including the antiviral remdesivir in individuals hospitalised with COVID-19 without end-organ failure between October, 2020, and March, 2021. Following the completion of the trial, stored specimens collected at study entry were analysed to address an a priori hypothesis that patients without neutralising antibodies at study entry would benefit more from hIVIG compared with placebo that those with neutralising antibodies.
Methods
Study design
INSIGHT 013 was an international, double-blind, placebo-controlled, phase 3 trial conducted by the International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) at 63 hospital sites in Argentina, Denmark, Germany, Greece, Indonesia, Israel, Japan, Nigeria, Spain, the UK, and the USA.
The protocol was approved by a central institutional review board or an ethics committee at each participating site.
The protocol and statistical analysis plan are available in the appendix (pp 80, 118).
Participants
Participants were adults (aged ≥18 years) hospitalised with documented SARS-CoV-2 infection and symptoms attributable to COVID-19 for 12 days or less. Exclusion criteria included previous passive immunotherapies, end-organ failure (including vasopressor therapy, new renal replacement therapy, and mechanical ventilation), known IgA deficiency with anti-IgA antibodies, and certain thrombotic conditions and prothrombotic disorders. Additional eligibility criteria are provided in the appendix (p 13).
Written informed consent was obtained from all participants or their legally authorised representative.
Randomisation and masking
Participants were randomly assigned (1:1) to receive either hIVIG or an equivalent volume of saline as placebo. Randomisation was stratified by site pharmacy; schedules were prepared using a mass-weighted urn design.25 Infusions were prepared by trial pharmacists and masked using opaque sleeves. All other investigators and research staff, and trial participants were masked to the treatment administered.
Procedures
A dose of hIVIG of 400 mg/kg bodyweight, capped at 40 g, was chosen based on predicted efficacy from in vitro studies of SARS-CoV-2 neutralisation activity, previous safety data for non-COVID hIVIG products, and consideration of likely tolerability of the required infusion volume in this patient population. Given the early scarcity of SARS-CoV-2 hIVIG, four products were used: CSL Behring (King of Prussia, PA, USA), Emergent BioSolutions (Gaithersburg, MD, USA), Grifols (Barcelona, Spain), and Takeda (Osaka, Japan) each manufactured hIVIG for the study using plasma collected either from fractionated whole blood or by plasmapheresis from healthy convalescent volunteers at sites in North America and Europe. Donors and plasma units were selected based on neutralisation antibody titres against SARS-CoV-2. All hIVIG lots underwent central testing and were required to meet a prespecified range of neutralising activity (ie, potency; appendix p 14).
Infusion of hIVIG or placebo was to commence at a rate of 0·5 mg/kg per min for approximately 30 min. If tolerated, the rate of infusion could be doubled after intervals of not less than 30 min up to a maximum of 4 mg/kg per min.
Each site pharmacy was allocated the same hIVIG product throughout the trial; a single-site pharmacy could serve multiple sites. Data from participants receiving each of the products and corresponding placebo were pooled for the primary analysis. Each participant receiving hIVIG received product from a single lot.
All participants received supportive care reflecting local practice and national guidelines. Standard of care background therapy included up to 10 days of study-provided remdesivir unless contraindicated. Other aspects of standard care including corticosteroids, prophylactic anti-coagulation, supplemental oxygen, and other end-organ support, as clinically indicated.
SARS-CoV-2 viral RNA levels were measured from a mid-turbinate nasal swab. Plasma samples collected at study entry were used to measure anti-spike receptor binding domain neutralising antibodies, and anti-nucleocapsid binding antibody levels. Plasma SARS-CoV-2 N antigen was measured using a microbead-based immunoassay (appendix p 17). These centrally determined measurements were used to address prespecified subgroup hypotheses.
Outcomes
The primary endpoint was an ordinal outcome based on the patient's clinical status on day 7 (appendix p 14). The seven categories of this outcome ranged from return to usual activities with no more than minimal symptoms due to COVID-19, to death. They reflect oxygen requirements and a range of organ dysfunction, and were modified from similar outcomes in earlier influenza and COVID-19 studies.1, 12, 26, 27 The primary safety outcome was a composite of death, serious adverse events, and grade 3 or 4 adverse events up to day 7. Serious adverse events included organ failure events and serious infections, which were reported as secondary endpoints separately from other serious adverse events (appendix p 16). Adverse events were graded for severity using the toxicity table of the Division of AIDS, National Institute of Allergy and Infectious Diseases, version 2.1. Adverse events were categorised according to codes in the Medical Dictionary for Regulatory Activities (MedDRA), version 23·1. The composite safety outcome at day 28 included all of the outcomes used in the day 7 safety outcome except grade 3 and 4 adverse events. Several other outcomes were specified in the protocol or statistical analysis plan; all protocol-defined outcomes are summarised in the appendix (pp 22–79).
Participants were followed up for 28 days from randomisation.
Statistical analysis
The planned sample size was 500 participants (250 per group). This sample size provided 80% power to detect an odds ratio (OR; hIVIG vs placebo) of 1·61 for a more favourable outcome at day 7 on the ordinal scale at the 0·05 (two-sided) level of significance (appendix p 18).
Analyses were done on a modified intention-to-treat (mITT) population, which included all randomly assigned participants who met eligibility criteria and received all or part of the assigned study product infusion. A proportional odds model was used to compare the primary ordinal outcome at day 7. The proportional odds model estimated a summary OR; ie, the ratio of the cumulative odds of being in a better category of the ordinal outcome for hIVIG versus placebo. ORs greater than 1·0 corresponded to more favourable outcomes for those receiving hIVIG. Models were adjusted for pulmonary status at entry and which of the four hIVIG products was provided to the site. The day 7 primary outcome was imputed for participants for whom this information was missing (appendix p 20).
A logistic regression model was used to estimate an OR for hIVIG versus placebo for the composite safety endpoint up to day 7. Percentages of participants who had infusion reactions or prematurely terminated infusions were also compared between treatment groups using logistic regression. Each of these logistic regression models adjusted for baseline ordinal outcome category and hIVIG study product provided to the site. The composite safety outcome at day 28 was summarised with hazard ratios (HRs) estimated from a proportional hazards model adjusted for baseline ordinal outcome category and hIVIG product provided to the site. ORs and HRs less than 1·0 for these safety outcomes favour hIVIG compared with placebo. We tested the proportional hazards assumption by including an interaction term between the treatment indicator and log-transformed follow-up time.
Kaplan-Meier estimates were used to summarise time to the day 28 composite safety outcome, time to the three least favourable categories of the ordinal outcome, and time to discharge or the most favourable category on the ordinal scale. For the latter outcome, Gray's test with ρ=0, the Fine-Gray model for stratified models, and the Aalen-Johansen estimator for the cumulative incidence curve which is the competing risk equivalent to the Kaplan-Meier estimator and Cox proportional hazards models are used.28, 29, 30 A recovery rate ratio (RRR) is cited for this outcome, also adjusted for baseline ordinal outcome category and hIVIG product provided to the site; estimates more than 1·0 denote superiority of hIVIG to placebo.
Subgroup analyses were carried out for the primary ordinal outcome at day 7 and the composite safety outcome at day 7. Heterogeneity was assessed by including interaction terms in the proportional odds and logistic regression models. Key a priori defined subgroups are described in the appendix (p 21).
Statistical analyses were performed with the SAS software, version 9·4. All p values reported are two-sided.
This study is registered with ClinicalTrials.gov, NCT04546581.
Role of the funding source
The study was funded by the US National Institutes of Health. Except for named members of the writing and study group, the funder had no role in data collection, analysis, interpretation, writing of the manuscript, or the decision to submit.
Results
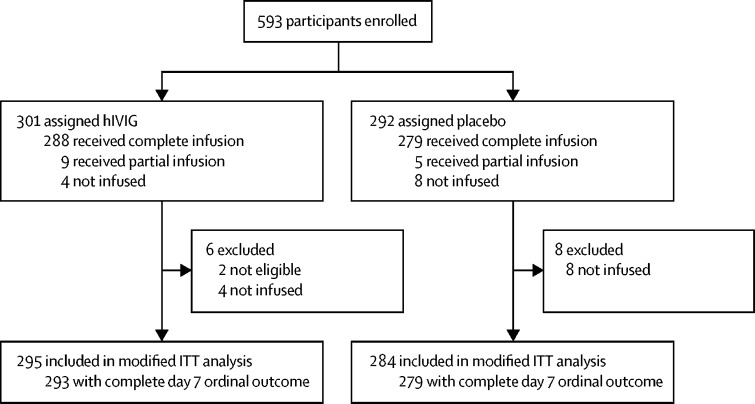
From Oct 8, 2020 to Feb 10, 2021, 593 participants (n=301 hIVIG, n=292 placebo) were enrolled (figure 1 ); 295 participants in the hIVIG group and 284 in the placebo group (n=579) were in the mITT analysis cohort. The number of participants given each of the four products or its matching placebo and potency levels for lots of the products were similar (appendix pp 29–30).
Figure 1.
Trial profile
ITT=intention to treat.
Demographic and clinical characteristics were similar between groups, with the exception of sex—women comprised 49% of the hIVIG group and 37% of the placebo group (table 1 ).
Table 1.
Participant characteristics at time of randomisation
| hIVIG (n=295) | Placebo (n=284) | Total (N=579) | ||
|---|---|---|---|---|
| Age, years | 58 (48–70) | 60 (50–70) | 59 (49–70) | |
| Sex | ||||
| Female | 146 (49%) | 104 (37%) | 250 (43%) | |
| Male | 149 (51%) | 180 (63%) | 329 (57%) | |
| Race | ||||
| White | 170 (58%1) | 155 (55%) | 325 (56%) | |
| Hispanic | 41 (14%) | 47 (16%) | 88 (15%) | |
| Black | 41 (14%) | 46 (16%) | 87 (15%) | |
| Asian | 38 (13%) | 31 (11%) | 69 (12%) | |
| Other | 5 (2%) | 5 (2%) | 10 (2%) | |
| Oxygen requirement | ||||
| Not receiving supplementary oxygen | 79 (27%) | 81 (29%) | 160 (28%) | |
| Supplementary oxygen <4 L/min | 107 (36%) | 92 (32%) | 199 (34%) | |
| Supplementary oxygen ≥4 L/min | 84 (28%) | 78 (27%) | 162 (28%) | |
| High-flow oxygen | 25 (8%) | 33 (12%) | 58 (10%) | |
| Days since symptom onset | 8 (5–10) | 8 (6–10) | 8 (6–10) | |
| C-reactive protein, mg/L | 61 (21–111) | 63 (28–120) | 62 (23–112) | |
| Lymphocytes, 109 cells per L | 0·95 (0·70–1·39) | 0·89 (0·61–1·30) | 0·92 (0·65–1·35) | |
| Body-mass index, kg/m2 | ||||
| ≥30 | 145 (50%) | 136 (48%) | 281 (49%) | |
| ≥40 | 34 (12%) | 29 (10%) | 63 (11%) | |
| History (yes) | ||||
| Hypertension requiring medication | 125 (42%) | 122 (43%) | 247 (43%) | |
| Diabetes requiring medication | 84 (28%) | 80 (28%) | 164 (28%) | |
| Renal impairment | 17 (6%) | 24 (8%) | 41 (7%) | |
| Asthma | 32 (11%) | 26 (9%) | 58 (10%) | |
| COPD | 23 (8%) | 16 (6%) | 39 (7%) | |
| Heart failure | 17 (6%) | 10 (4%) | 27 (5%) | |
| Compromised immune function* | 15 (5%) | 14 (5%) | 29 (5%) | |
| At least one comorbidity | 186 (63%) | 168 (59%) | 354 (61%) | |
| SARS-CoV-2 vaccination before enrolment | 9 (3%) | 3 (1%) | 12 (2%) | |
| Use of Remdesivir before enrolment | 144 (49%) | 140 (49%) | 284 (49%) | |
| Concomitant medications | ||||
| Corticosteroids | 172 (58%) | 155 (55%) | 327 (56%) | |
| Antibacterial | 124 (42%) | 118 (42%) | 242 (42%) | |
| Heparin | 179 (61%) | 173 (61%) | 352 (61%) | |
| Other antiplatelets or anticoagulants | 38 (13%) | 39 (14%) | 77 (13%) | |
| ACE inhibitor or ARB | 56 (19%) | 67 (24%) | 123 (21%) | |
| NSAID | 24 (8%) | 20 (7%) | 44 (8%) | |
| Laboratory assessments | ||||
| Plasma nucleocapsid antigen, ng/L | 1407 (241–4371) | 1270 (201–4254) | 1368 (206–4335) | |
| ≥3 ng/L, positive | 259 (94%) | 248 (94%) | 507 (94%) | |
| Nasal swab fluid viral RNA, positive | 217 (82%) | 221 (88%) | 438 (85%) | |
| Viral load if positive, copies/mL | 267 671 (7400–2 934 985) | 105 743 (7037– 1 564 469) | 169 979 (7261– 2 147 457) | |
| Neutralising antibodies, positive | 133 (49%) | 128 (48%) | 261 (48%) | |
| Anti-nucleocapsid antibodies, positive | 185 (68%) | 189 (71%) | 374 (69%) | |
Data are median (IQR) or n (%). ACE=angiotensin converting enzyme. ARB=angiotensis receptor blocker. COPD=chronic obstructive pulmonary disease. hIVIG=hyperimmune intravenous immunoglobulin. NSAID=non-steroidal anti-inflammatory drugs.
HIV, an immunosuppressive condition other than HIV, taking anti-rejection medication, immune modulators, or biological treatment for autoimmune disease or cancer.
The median time from onset of first COVID-19 symptoms to participant randomisation was 8 days (IQR 6–10); 38% were receiving either supplemental oxygen at 4 L/min or more or high-flow oxygen. 96% of participants received remdesivir; 49% had started remdesivir before randomisation. 56% were receiving corticosteroids and 61% received at least a prophylactic dose of heparin before randomisation (table 1; appendix pp 28, 31).
Baseline endogenous anti-SARS-CoV-2 antibody and antigen levels were completed for 539 (93%) patients; 261 (48%) were positive for anti-spike neutralising antibodies and 374 (69%) were positive for anti-nucleocapsid antibodies; 507 (94%) had detectable plasma nucleocapsid antigen levels. The median for antigen was 1368 ng/L (IQR 206–4335). Viral RNA was detected in the central reference laboratory among 438 of 513 with mid-turbinate swab material; the median RNA viral load among those who were RNA positive was 169 979 copies/mL (IQR 7261–2 147 457).
The presence of endogenous neutralising antibodies varied by duration of symptoms at entry, ranging from 27% for those with symptoms for less than 6 days to 67% for those with symptoms for 10–12 days (appendix p 33).
All but two patients were infused with hIVIG or placebo on the day of randomisation. Randomisation occurred within 2 days of admission for 466 (80%) of 579 patients.
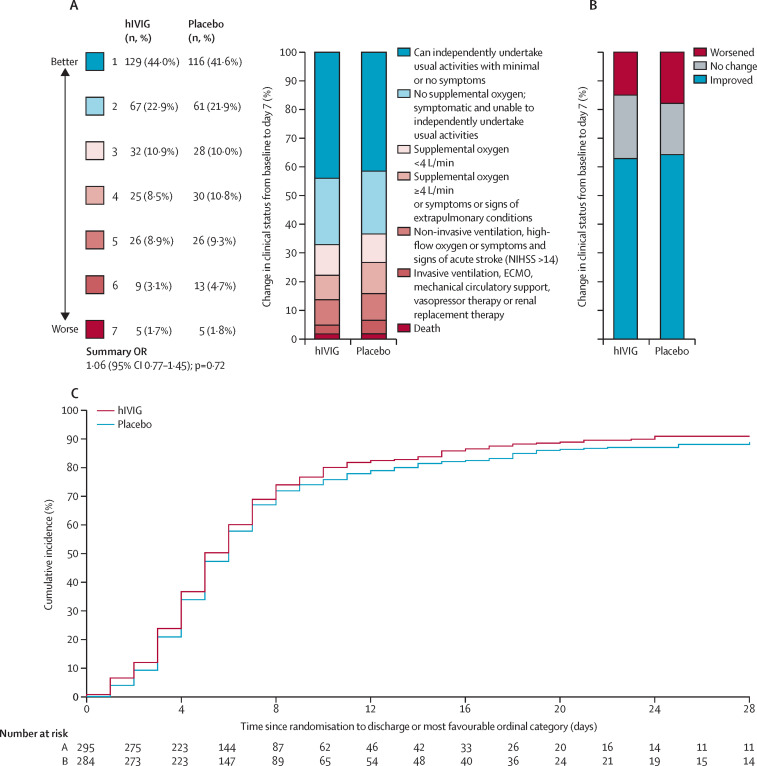
The primary ordinal outcome at day 7 was available for all but seven participants. Outcomes were imputed for these seven participants (appendix p 20). The OR for being in a more favourable outcome in the hIVIG group compared with placebo on day 7 was 1·06 (95% CI 0·77–1·45; p=0·72; figure 2 ; table 2 ). The proportional odds assumption was met (p=0·97). Planned sensitivity analyses for the primary endpoint analysis given in the appendix (p 34) yielded consistent results.
Figure 2.
Clinical efficacy of hIVIG
(A) Clinical status at day 7. (B) Change in clinical status from baseline to day 7. (C) Time to discharge or most favourable ordinal category. hIVIG=hyperimmune intravenous immunoglobulin. NIHSS=National Institute of Health Stroke Scale. ECMO=Extra-corporeal membrane oxygenation. OR=odds ratio.
Table 2.
Summary of major outcomes by treatment group
| hIVIG (n=295) | Placebo (n=284) | OR, RRR, or HR (95% CI) | p value | ||
|---|---|---|---|---|---|
| Efficacy outcomes | |||||
| Primary | |||||
| Ordinal outcome at day 7* | NA | NA | 1·06 (0·77–1·45) | 0·72 | |
| Secondary | |||||
| Number reaching one of the two most favourable categories (categories 1 and 2)† | 178 | 160 | 1·11 (0·91–1·35) | 0·30 | |
| Number discharged from hospital or reached most favourable category (category 1)‡ | 268 | 252 | 1·07 (0·92–1·26) | 0·37 | |
| Safety outcomes | |||||
| Number with infusion reaction grade 2 or above§ | 38 (13%) | 17 (6%) | 2·27 (1·24–4·16) | 0·008 | |
| Number with infusion reaction grade 3 or above§ | 17 (6%) | 4 (1%) | 4·20 (1·38–12·78) | 0·012 | |
| Number with composite safety outcome up to day 7§ | 71 (24%) | 70 (25%) | 0·98 (0·66–1·46) | 0·91 | |
| Number of deaths up to day 28¶ | 18 (6%) | 22 (8%) | 0·80 (0·42–1·51) | 0·49 | |
| Number with composite safety outcome up to day 28¶ | 63 (21%) | 76 (27%) | 0·79 (0·57–1·11) | 0·18 | |
Data are n (%) unless otherwise specified. hIVIG=hyperimmune intravenous immunoglobulin. OR=odds ratio. RRR=recovery rate ratio. HR=hazard ratio. NA=not applicable.
Ordinal outcome is based on a seven-category ordinal scale (1=can independently undertake usual activities with minimal or no symptoms; 2=no supplemental oxygen, symptomatic and unable to undertake usual activities; 3=supplemental oxygen <4 L/min; 4=supplemental oxygen ≥4 L/min or symptoms/signs of extra-pulmonary conditions; 5=non-invasive ventilation, high-flow oxygen, or symptoms and signs of acute stroke (National Institute of Health Stroke Scale >14); 6=invasive ventilation, extra-corporeal membrane oxygenation, mechanical circulatory support, vasopressor therapy or renal replacement therapy; 7=death). The summary statistic cited is a proportional OR based on use of multiple imputation.
Among people not in the category at baseline. The summary statistic cited is RRR.
The summary statistic cited is RRR.
The summary statistic is OR.
The summary statistic cited is HR.
The summary ORs for the ordinal outcome on days 3, 5, 14, and 28 ranged from 0·96 to 1·09 (appendix p 35). When comparing the day 7 ordinal category with baseline ordinal category, 184 (63%) of 293 participants in the hIVIG group and 179 (64%) of 279 in the placebo group were in a better category; 44 (15%) and 50 (18%), respectively were in a worse category (figure 2; appendix p 36).
Treatment differences were not significant for any of the other efficacy outcomes (table 2; appendix pp 37–40). Although rates of hospitalisation or death were not significantly different between treatment groups overall at days 7, 14, or 28, a post-hoc analysis restricted to participants without endogenous neutralising antibodies showed lower rates of hospitalisation or death only at day 7 in participants who received hIVIG compared with participants who received placebo (OR 0·64, 95% CI 0·38–1·07). In contrast, in participants who had endogenous neutralising antibodies, the OR at this day 7 timepoint was 1·50 (0·87–2·58; pinteraction=0·02; appendix p 39). The RRR for time to discharge or the most favourable category of the primary ordinal outcome was 1·07 (0·92–1·26; p=0·37; figure 2; table 2).
Infusion reactions were significantly more common in the hIVIG group. 19% of participants in the hIVIG group had reactions of any grade, compared with 10% of participants in the placebo group (p=0·002; appendix p 41). 6% of participants in the hIVIG group had reactions of grade 3 or higher, compared with 1% of participants in the placebo group (p=0·012; table 2). Infusions were paused for an adverse event in 7% of participants in the hIVIG group and 3% in the placebo group (p=0·01; appendix p 42). These differences between treatment groups remained significant after the exclusion of one site which infused at a faster rate for all their participants (appendix pp 43–46).
In the hIVIG group, 24% of participants achieved the composite safety outcome up to day 7 compared with 25% in the placebo group (OR 0·98, 95% CI 0·66–1·46; p=0·91; table 2).
Components of the composite safety outcome up to day 7 are summarised in the appendix (pp 47, 49–50). Grade 3 or 4 adverse events and organ failure or serious infection outcomes were the most commonly occurring components of the composite safety outcome up to day 7. The most commonly reported safety outcomes were respiratory (appendix pp 49–50). Respiratory events, including respiratory failure, defined as an increase in oxygen requirements to high-flow nasal cannula, non-invasive ventilation, or mechanical ventilation, and grade 3 or 4 adverse events corresponding to medDRA Preferred Terms of dyspnoea, hypoxia, and respiratory failure, occurred in 14% of hIVIG patients and 18% of placebo participants (OR 0·72, 0·45–1·16; p=0·18). The HR for the day 28 composite safety outcome was 0·79 (95% CI 0·57–1·11; tables 2; appendix p 47). There was no evidence that the proportional hazards assumption was violated (p=0·33). Similar to the events occurring up to day 7, most events up to day 28 were due to respiratory failure (appendix pp 51–52).
Up to day 28, 18 deaths (6%) occurred in the hIVIG group and 22 deaths (8%) occurred in the placebo group (HR 0·80, 95% CI 0·42–1·51; p=0·49; table 2).
Adverse events of any grade severity at days 1, 3, 7 and 28 are summarised in the appendix (pp 53–56).
Changes in laboratory safety parameters and in concomitant medications are summarised in the appendix (pp 57–59).
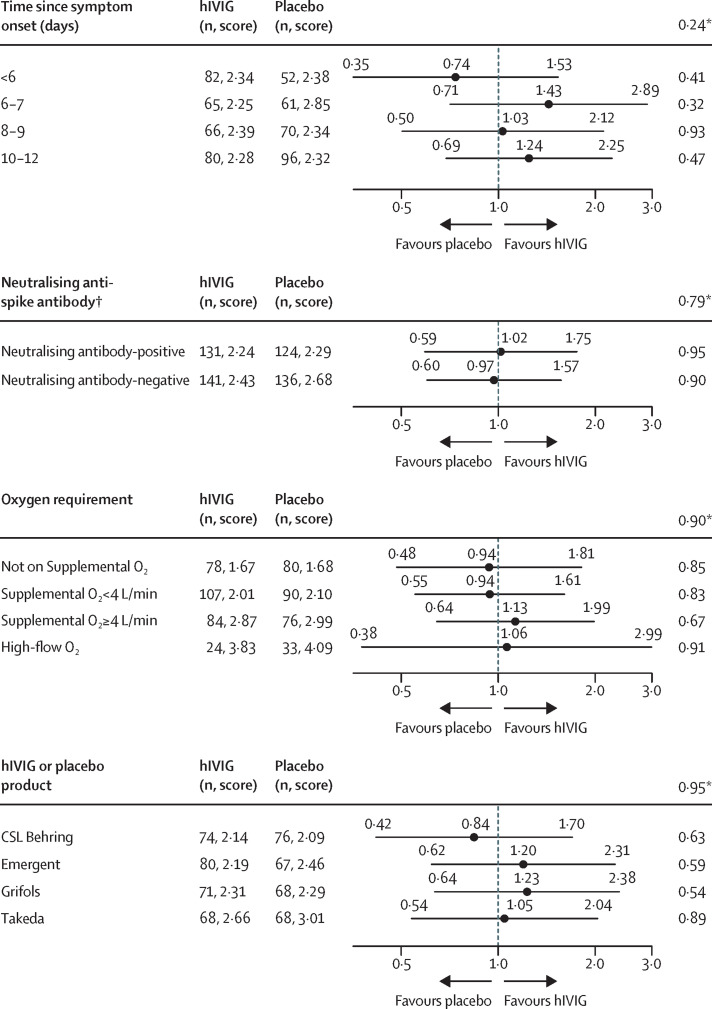
Subgroup analyses for the primary ordinal outcome at day 7 is summarised in figure 3 and the appendix (p 60) the composite safety outcome at day 7 is summarised in the appendix (pp 62–63). For the primary ordinal outcome at day 7 there was no evidence of treatment effect modification for any of the subgroups considered. As expected, the ORs for each of the hIVIG products did not vary (pinteraction=0·95). Contrary to our hypothesis, when considering days from symptom onset to randomisation, the OR was less than 1·0 (OR 0·74), favouring placebo, for those with symptom onset less than 6 days (the category for which the most favourable treatment effect was expected). ORs exceeded 1·0, favouring hIVIG, for those with later symptom onset (categorised as 6–7 days, 8–9 days, and 10–12 days). There was also no evidence for a different treatment effect for those neutralising antibody-negative (OR 0·97) and positive (OR 1·02; pinteraction=0·79) at baseline. Significant treatment effect heterogeneity was also not found for subgroups defined by the presence of anti-nucleocapsid antibodies, antigen level, viral RNA level, and the combination of neutralising antibody levels and antigen level and viral RNA levels (appendix p 61).
Figure 3.
Subgroup analyses for primary endpoint
Data are odds ratio (95% CI), unless stated otherwise. Score=mean score, 7=death, and 1=return to normal activities with minimal or no symptoms. hIVIV=hyperimmune intravenous immunoglobulin.*p value for interaction between subgroup and treatment group. In addition to heterogeneity across the 4 days since symptom onset categories, a test for linear trend was conducted (p=0·99). †Antibody data available for 272 hIVIG and 260 placebo participants.
By contrast, for the composite safety outcome up to day 7, a significant interaction was evident by neutralising antibody status (appendix pp 62–63). Among those neutralising antibody-positive at baseline, 26·3% of patients in hIVIG group and 16·4% in the placebo group had at least one event included in the composite safety outcome (OR 2·21; 95% CI 1·14–4·29). For those neutralising antibody-negative at baseline 22·7% of patients in hIVIG group and 34·3% in the placebo group experienced at least one event included in the composite safety outcome (OR 0·51, 0·29–0·90; pinteraction=0·001). The increased risk of the composite safety outcome among those neutralising antibody-positive was evident for those with high and low antigen or viral RNA levels (appendix p 64).
At day 28, the HRs for composite safety outcome did not differ for the subgroups defined by neutralising antibody status (pinteraction=0·18; appendix p 66). The HR for the day 28 composite safety outcome (which no longer included grade 3 and 4 events as a component) for neutralising antibody-positive participants was 1·01 (95% CI 0·57–1·79); the percentages with a composite safety outcome at day 28 were 18·8% and 20·3% for the hIVIG and placebo groups, respectively (appendix pp 66, 69). By contrast, for those neutralising antibody-negative at baseline, the day 7 reduced risk of the composite safety outcome persisted up to day 28 (HR 0·62, 95% CI 0·39–0·98); by day 7 the percentages with a composite safety outcome were 22·7% and 34·3% for the hIVIG and placebo groups, respectively, and 24·8% and 35·8% at day 28 (appendix pp 66, 70). As is evident from these percentages and comparing the curves on figures S3 and S4 (appendix pp 67–68), risk of the composite safety outcome was greater for those neutralising antibody-negative than those neutralising antibody-positive in both treatment groups.
The components of the day 7 and day 28 composite safety outcomes by neutralising antibody category are summarised in the appendix (pp 69–78). Up to both days 7 and 28, end-organ disease events, specifically respiratory failure, were the most common events for people who were neutralising antibody positive and neutralising antibody negative.
Discussion
In this randomised, double-blind, placebo-controlled, phase 3 trial among hospitalised patients with COVID-19 with up to 12 days of symptoms and no end-organ failure, there was no evidence that patients who received a single infusion of hIVIG in addition to remdesivir and other standard of care had better clinical outcomes at day 7 after randomisation than patients who received placebo plus remdesivir and standard of care. This finding was mirrored in secondary efficacy outcomes, with no differences observed in clinical status at other timepoints, or in time to discharge or to the most favourable category of ordinal outcome up to day 28. Overall, these findings indicate that hIVIG confers no clinical benefit for hospitalised patients with COVID-19.
Infusion reactions were more common in patients receiving hIVIG compared with placebo, but most were of low-grade severity. The percentage who had the composite safety outcome (including deaths, serious adverse events, end-organ disease and serious infections, and grade 3 and 4 events) up to day 7 did not differ between the treatment groups.
The failure to observe efficacy of hIVIG in this study could be explained in several ways. A key possibility is that antibody therapy might not benefit patients who have already mounted an immune response. Thus, the null result overall could reflect the balance of a positive response in the antibody-negative subgroup and a neutral or unfavourable response in the antibody-positive subgroup. In addition, it is possible that other characteristics of progressive COVID-19 affect the utility of hIVIG: systemically infused antibody might not effectively penetrate lung tissue in the pneumonic phase of the illness, while some patients might have progressed the inflammatory phase of COVID-19 in which augmenting the humoral immune response might not be useful.31 It is also possible that the antiviral effects of hIVIG beyond those of remdesivir are insufficient to be detected.
We hypothesised that there might be a crucial time-dependency of the impact of antibody therapy in patients with COVID-19. A priori-defined subgroup analyses were used to address this. Contrary to our prespecified hypotheses, there was no evidence of benefit based on the day 7 ordinal outcome in patients treated earliest or in patients without endogenous neutralising antibodies at entry. Among the patients treated within 6 days of symptom onset (23% of participants) the odds of a favourable outcome with hIVIG was lower than placebo, giving a relative odds of 0·74. Among the 48% of patients who were neutralising antibody-negative, the relative odds (hIVIG vs placebo) of a favourable outcome was 0·99 and not different from patients who were neutralising antibody-positive at study entry. There was no difference in treatment effect by baseline measures of systemic inflammation: subgroup analyses by C-reactive protein at entry did not reveal a differential treatment effect for either the day 7 ordinal outcome or the composite safety outcomes assessed at days 7 and 28.
Comparison of these subgroup findings with two recent studies of monoclonal antibodies in similar hospitalised populations demonstrate significant differences. In the RECOVERY study of combination casirivimab and indevimab, there was no benefit in overall mortality for the general hospitalised population; however, an analysis population of patients negative for SARS-CoV-2 binding antibodies at baseline showed a significant mortality reduction in the monoclonal antibody group.11 Similarly, the ACTIV-3/TICO study of bamlanivimab showed no overall benefit in a hospitalised population, but an improvement in time to sustained recovery in patients who were neutralising antibody-negative at entry.12
Evaluation of any impact of differences in the viral variants and the antibody responses to those variants in the hIVIG also requires consideration in understanding the overall lack of clinical benefit. Plasma for IVIG was collected in North America and Europe during the summer of 2020 and the trial enrolled across those and other regions during the winter of 2020–21. However, enrolment was largely complete before widespread emergence in enrolling countries of SARS-CoV-2 variants with potential immune escape characteristics such as B.1.1.7 (alpha) and B.1.351 (beta).3
It is possible that the infusion of hIVIG led to harm in some patients. Although there was no overall difference in the composite safety outcome at days 7 or 28, among patients who were neutralising antibody-positive at entry, an increased risk of safety events was observed, giving a relative odds for a safety event of 2·21 at day 7—although no difference was seen when considering safety events up to day 28. At both days 7 and 28 for neutralising antibody-negative participants, the risk of the composite safety outcome was lower in the hIVIG compared with the placebo group. Most safety events were of a respiratory nature in both the neutralising antibody-positive and neutralising antibody-negative groups, including increasing dyspnoea, increasing oxygen requirements, and respiratory failure. The treatment effect for a similar outcome also varied according neutralising antibody status in the placebo-controlled trial of the monoclonal antibody bamlanivimab in hospitalised patients.12 Taken together, the findings of this trial of hIVIG and of other trials of passive immunotherapies suggest that such therapies could be associated with harm in some hospitalised patients and benefit in others.
Elucidating the mechanisms of any possible harm of hIVIG in neutralising antibody-positive individuals will require further study. One study of convalescent plasma in hospitalised patients suggested certain antibody compositions, specifically the presence of IgG against the full transmembrane spike protein, could be associated with adverse events.19 Pre-existing antibodies to type I interferons have been associated with risk of COVID-19 progression and it is also possible that passive transfer of these antibodies could have adverse effects,32 although any impact would be expected to be mitigated in the pooled hIVIG product. Other theoretical possibilities include development of antibody-dependent enhancement with exaggerated viral infectivity and inflammation,33, 34, 35 formation of antibody complexes in patients with pre-existing neutralising antibodies to SARS-CoV-2, or adverse inflammatory effects via Fc-mediated antibody functions.36
These findings have limitations. Although the sample size was sufficient to exclude an OR in favour of hIVIG of 1·61 with 95% CI, the sample size might not have allowed detection of a positive treatment effect smaller than that specified. Similarly, the sample size provided limited power to explore certain clinical and immunological subgroups in whom benefit might be apparent. Finally, although the timing of enrolment makes it unlikely that participants were infected with immune-evasive SARS-CoV-2 strains, the study is limited by the lack of data on viral strains in participants.35
These results have implications beyond the hIVIG products studied here. Convalescent plasma was used widely early-on in the COVID-19 pandemic. Based on the hypothesised antiviral effects of neutralising antibodies, hIVIG would be expected to confer greater and more consistent benefit: at the dose studied here, these hIVIG products contain levels of neutralising antibodies that are generally above those seen with high titre convalescent plasma.16 The overall lack of benefit, lack of any differential treatment effect by hIVIG product potency, and potential safety signal in neutralising antibody-positive participants argue that convalescent plasma is also unlikely to be providing benefit to hospitalised patients and raise concerns about harms in certain groups. A lesson for both COVID-19 and future pandemics is that there is no evidence of efficacy for convalescent plasma or hIVIG among hospitalised patients.21
Finally, although there was no evidence of clinical benefit in this hospitalised group when used with standard of care that includes remdesivir, a potential role for hIVIG might still be found in earlier disease stages of COVID-19 or special populations. As with other passive immunotherapies it is possible that a population treated very early in the onset of disease might benefit, as might groups with persistent failure to mount humoral immune responses to infection.
Data sharing
Deidentified data from the ITAC trial will be made available 1 year after publication of final results from the trial. Supporting documents will be made available, including the protocol, statistical analysis plan, informed consent document, and data dictionary. Data will be made available to researchers after approval of a proposal for use of the data. Proposals for data use should be submitted using the research proposal Form on the INSIGHT website (www.insight-trials.org).
Declaration of interests
MNP reports grants from University of Minnesota (Minneapolis, MN, USA) during the conduct of the study, grants from National Institutes of Health (NIH) during the conduct of the study, Gilead Sciences, ViiV, Celgene, and Janssen Pharmaceuticals outside the submitted work. AGB reports grants from University of Minnesota during the conduct of the study, grants from UK Research and Innovation (UKRI) outside of the submitted work. MKJ reports donation of trial medications from Regeneron Pharmaceuticals, Janssen Pharmaceuticals, and from Merck; and grants, personal fees, and donation of trial medications from Gilead Sciences, outside the submitted work. SLP reports grants from University of Minnesota during the conduct of the study, European and Developing Countries Clinical Trials Partnership, UKRI, Academy of Medical Sciences, ViiV Healthcare, Medical Research Council, and Gilead Sciences outside the submitted work. MKD reports being an employee of CSL Behring. SP reports being an employee of CSL Behring. CH reports being an employee of Emergent; Ramanathan reports being an employee of Emergent. HC reports being an employee of Gilead Sciences. EM reports being an employee of Grifols. TW reports being an employee of Grifols. JVT reports being an employee of Takeda. LY reports being an employee of Takeda. JDN reports grants from NIH during the conduct of the study. All other members of the writing group declare no competing interests.
Acknowledgments
Acknowledgments
We thank all participants and their families for their invaluable contribution to the ITAC study. We also thank the members of the ITAC data and safety monitoring board (Graeme A Meintjes, Merlin L Robb, Wendy Armstrong, David Glidden, Abuhaerah Instiaty, Jonathan Kimmelman, Yvonne [Bonnie)] Maldonado, Barbara E Murray, Stuart Campbell Ray, Valeria Cavalcanti Rolla, Haroon Saloojee, Anastasios A Tsiatis, Paul A Volberding, and Sally Hunsberger) for their review of the protocol and their guidance based on interim reviews of the data. Support for INSIGHT was primarily provided by the US Operation Warp Speed Program, the National Institute of Allergy and Infectious Diseases (HHSN261200800001E) via Leidos Biomedical Research (Task Order 18X107C); and grants from the governments of Denmark (number 126, National Research Foundation), Australian National Health and Medical Research Council (number 1110067), and the UK Medical Research Council (MRC_UU_12023/23). Trial medications were donated by CSL Behring, Emergent BioSolutions, Grifols, Takeda, and Gilead Sciences.
Writing group members
Mark N Polizzotto, Jacqueline Nordwall, Abdel G Babiker, Andrew Phillips, David M Vock, Nnakelu Eriobu, Vivian Kwaghe, Roger Paredes, Lourdes Mateu, Srikanth Ramachandruni, Rajeev Narang, Mamta K Jain, Susana M Lazarte, Jason V Baker, Anne E P Frosch, Garyfallia Poulakou, Konstantinos N Syrigos, Gretchen S Arnoczy, Natalie A McBride, Philip A Robinson, Farjad Sarafian, Sanjay Bhagani, Hassan S Taha, Thomas Benfield, Sean T H Liu, Anastasia Antoniadou, Jens Ulrik Stæhr Jensen, Ioannis Kalomenidis, Adityo Susilo, Prasetyo Hariadi, Tomas O Jensen, Jose Luis Morales-Rull, Marie Helleberg, Sreenath Meegada, Isik S Johansen, Daniel Canario, Eduardo Fernández-Cruz, Simeon Metallidis, Amish Shah, Aki Sakurai, Nikolaos G Koulouris, Robin Trotman, Amy C Weintrob, Daria Podlekareva, Usman Hadi, Kathryn M Lloyd, Birgit Thorup Røge, Sho Saito, Kelly Sweerus, Jakob J Malin, Christoph Lübbert, Jose Muñoz, Matthew J Cummings, Marcelo H Losso, Dan Turner, Kathryn Shaw-Saliba, Robin Dewar, Helene Highbarger, Perrine Lallemand, Tauseef Rehman, Norman Gerry, Dona Arlinda, Christina C Chang, Birgit Grund, Michael R Holbrook, Horace P Holley, Fleur Hudson, Laura A McNay, Daniel D Murray, Sarah L Pett, Megan Shaughnessy, Mary C Smolskis, Giota Touloumi, Mary E Wright, Mittie K Doyle, Sharon Popik, Christine Hall, Roshan Ramanathan, Huyen Cao, Elsa Mondou, Todd Willis, Joseph V Thakuria, Leman Yel, Elizabeth Higgs, Virginia L Kan, Jens D Lundgren, James D Neaton, H Clifford Lane
Contributors
MNP, JDN, and HCL were responsible for the decision to submit the manuscript for publication. JDN, DMV, and JN directly accessed and verified the underlying data. MNP, JN, AP, AGB, and JDN composed the initial manuscript. MNP, AGB, VLK, JDL, JDN, and HCL conceptualised the study. MNP, JN, AGB, AP, DMV, NE, VK, RP, LM, SR, RN, MKJ, SML, JVB, AEPF, GP, KNS, GSA, NAM, PAR, FS, SB, HST, TB, STHL, AA, JUSJ, IK, ASu, PH, TOJ, JLM-R, MH, SM, ISJ, DC, EF-C, SM, ASh, ASa, NGK, RT, ACW, DP, UH, KML, BTR, SS, KS, JJM, CL, JM, MJC, MHL, DT, KS-S, RD, HH, PL, TR, NG, DA, CCC, BG, MRH, HPH, FH, LAM, DDM, SLP, MS, MCS, GT, MEW, MKD, SP, CH, RR, HC, EM, TW, JVT, LY, EH, VLK, JDL, JDN, and HCL did the investigations. JN, DMV, and JDN curated the data. JN, DMV, AGB, AP, and JDN did the formal analysis. MNP, AGB, VLK, JDL, JDN, and HCL acquired funding. MNP, AGB, VLK, EH, JDL, JDN, and HCL supervised the study. MNP, JN, AGB, AP, DMV, NE, VK, RP, LM, SR, RN, MKJ, SML, JVB, AEPF, GP, KNS, GSA, NAM, PAR, FS, SB, HST, TB, STHL, AA, JUSJ, IK, ASu, PH, TOJ, JLM-R, MH, SM, ISJ, DC, EF-C, SM, ASh, ASa, NGK, RT, ACW, DP, UH, KML, BTR, SS, KS, JJM, CL, JM, MJC, MHL, DT, KS-S, RD, HH, PL, TR, NG, DA, CCC, BG, MRH, HPH, FH, LAM, DDM, SLP, MS, MCS, GT, MEW, MKD, SP, CH, RR, HC, EM, TW, JVT, LY, EH, VLK, JDL, JDN, and HCL reviewed and edited the manuscript. All members of the writing group assume responsibility for the overall content and integrity of this article. The affiliations and degrees of members of the writing group are listed in the appendix.
Contributor Information
The ITAC (INSIGHT 013) Study Group:
Mark N. Polizzotto, Jacqueline Nordwall, Abdel G. Babiker, Andrew Phillips, David M. Vock, Nnakelu Eriobu, Vivian Kwaghe, Roger Paredes, Lourdes Mateu, Srikanth Ramachandruni, Rajeev Narang, Mamta K. Jain, Susana M. Lazarte, Jason V. Baker, Anne E.P. Frosch, Garyfallia Poulakou, Konstantinos N. Syrigos, Gretchen S. Arnoczy, Natalie A. McBride, Philip A. Robinson, Farjad Sarafian, Sanjay Bhagani, Hassan S. Taha, Thomas Benfield, Sean T.H. Liu, Anastasia Antoniadou, Jens Ulrik Stæhr Jensen, Ioannis Kalomenidis, Adityo Susilo, Prasetyo Hariadi, Tomas O. Jensen MD, Jose Luis Morales-Rull, Marie Helleberg, Sreenath Meegada, Isik S. Johansen, Daniel Canario, Eduardo Fernández-Cruz, Simeon Metallidis, Amish Shah, Aki Sakurai, Nikolaos G. Koulouris, Robin Trotman, Amy C. Weintrob, Daria Podlekareva, Usman Hadi, Kathryn M. Lloyd, Birgit Thorup Røge, Sho Saito, Kelly Sweerus, Jakob J. Malin, Christoph Lübbert, Jose Muñoz, Matthew J. Cummings, Marcelo H. Losso, Dan Turner, Kathryn Shaw-Saliba, Robin Dewar, Helene Highbarger, Perrine Lallemand, Tauseef Rehman, Norman Gerry, Dona Arlinda, Christina C. Chang, Birgit Grund, Michael R. Holbrook, Horace P. Holley, Fleur Hudson, Laura A. McNay, Daniel D. Murray, Sarah L. Pett, Megan Shaughnessy, Mary C. Smolskis, Giota Touloumi, Mary E. Wright, Mittie K. Doyle, Sharon Popik, Christine Hall, Roshan Ramanathan, Huyen Cao, Elsa Mondou, Todd Willis, Joseph V. Thakuria, Leman Yel, Elizabeth Higgs, Virginia L. Kan, Jens D. Lundgren, James D. Neaton, and H. Clifford Lane
Supplementary Material
References
- 1.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19 - final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horby PW, Pessoa-Amorim G, Peto L, et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. Lancet. 2021;97:637–645. [Google Scholar]
- 4.Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 6.Gharbharan A, Jordans CCE, GeurtsvanKessel C, et al. Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection. Nat Commun. 2021;12 doi: 10.1038/s41467-021-23469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Beltran WF, Lam EC, Astudillo MG, et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021;184:476–488. doi: 10.1016/j.cell.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325:632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lundgren JD, Grund B, Barkauskas CE, et al. A neutralizing monoclonal antibody for hospitalized patients with COVID-19. N Engl J Med. 2021;384:905–914. doi: 10.1056/NEJMoa2033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.RECOVERY Collaborative Group. Horby PW, Mafham M, et al. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv. 2021 doi: 10.1016/S0140-6736(22)00163-5. http://medrxiv.org/lookup/doi/10.1101/2021.06.15.21258542 published online June 16. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ACTIV-3/TICO Bamlanivimab Study Group Responses to a neutralizing monoclonal antibody for hospitalized patients with COVID-19 according to baseline antibody and antigen levels: a randomized controlled trial. Ann Intern Med. 2021 doi: 10.7326/M21-3507. published online Dec 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enria DA, Briggiler AM, Sánchez Z. Treatment of Argentine hemorrhagic fever. Antiviral Res. 2008;78:132–139. doi: 10.1016/j.antiviral.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Libster R, Pérez Marc G, Wappner D, et al. Early high-titer plasma therapy to prevent severe COVID-19 in older adults. N Engl J Med. 2021;384:610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korley FK, Durkalski-Mauldin V, Yeatts SD, et al. Early convalescent plasma for high-risk outpatients with COVID-19. N Engl J Med. 2021;385:1951–1960. doi: 10.1056/NEJMoa2103784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joyner MJ, Carter RE, Senefeld JW, et al. Convalescent plasma antibody levels and the risk of death from COVID-19. N Engl J Med. 2021;384:1015–1027. doi: 10.1056/NEJMoa2031893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simonovich VA, Burgos Pratx LD, Scibona P, et al. A randomized trial of convalescent plasma in COVID-19 severe pneumonia. N Engl J Med. 2021;384:619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abani O, Abbas A, Abbas F, et al. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet. 2021;397:2049–2059. doi: 10.1016/S0140-6736(21)00897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bégin P, Callum J, Jamula E, et al. Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial. Nat Med. 2021;27:2012–2024. doi: 10.1038/s41591-021-01488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avendaño-Solá C, Ramos-Martínez A, Muñez-Rubio E, et al. A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia. J Clin Invest. 2021;131 doi: 10.1172/JCI152740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janiaud P, Axfors C, Schmitt AM, et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA. 2021;325:1185–1195. doi: 10.1001/jama.2021.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vandeberg P, Cruz M, Diez JM, et al. Production of anti-SARS-CoV-2 hyperimmune globulin from convalescent plasma. Transfusion. 2021;61:1705–1709. doi: 10.1111/trf.16378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali S, Uddin SM, Shalim E, et al. Hyperimmune anti-COVID-19 IVIG (C-IVIG) treatment in severe and critical COVID-19 patients: a phase I/II randomized control trial. EClinicalMedicine. 2021;36 doi: 10.1016/j.eclinm.2021.100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopardo G, Belloso WH, Nannini E, et al. RBD-specific polyclonal F(ab')2 fragments of equine antibodies in patients with moderate to severe COVID-19 disease: a randomized, multicenter, double-blind, placebo-controlled, adaptive phase 2/3 clinical trial. EClinicalMedicine. 2021;34 doi: 10.1016/j.eclinm.2021.100843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao W. Mass weighted urn design—a new randomization algorithm for unequal allocations. Contemp Clin Trials. 2015;43:209–216. doi: 10.1016/j.cct.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davey RT, Jr, Fernández-Cruz E, Markowitz N, et al. Anti-influenza hyperimmune intravenous immunoglobulin for adults with influenza A or B infection (FLU-IVIG): a double-blind, randomised, placebo-controlled trial. Lancet Respir Med. 2019;7:951–963. doi: 10.1016/S2213-2600(19)30253-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall JC, Murthy S, Diaz J, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 29.Zhou B, Latouche A, Rocha V, Fine J. Competing risks regression for stratified data. Biometrics. 2011;67:661–670. doi: 10.1111/j.1541-0420.2010.01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aalen OO, Johansen S. An empirical transition matrix for non-homogeneous Markov chains based on censored observations. Scand J Stat. 1978;5:141–150. [Google Scholar]
- 31.Griffin DO, Brennan-Rieder D, Ngo B, et al. The importance of understanding the stages of COVID-19 in treatment and trials. AIDS Rev. 2021;23:40–47. doi: 10.24875/AIDSRev.200001261. [DOI] [PubMed] [Google Scholar]
- 32.Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arvin AM, Fink K, Schmid MA, et al. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature. 2020;584:353–363. doi: 10.1038/s41586-020-2538-8. [DOI] [PubMed] [Google Scholar]
- 34.Lee WS, Wheatley AK, Kent SJ, DeKosky BJ. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol. 2020;5:1185–1191. doi: 10.1038/s41564-020-00789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 36.Vanderven HA, Wragg K, Ana-Sosa-Batiz F, et al. Anti-influenza hyperimmune immunoglobulin enhances Fc-functional antibody immunity during human influenza infection. J Infect Dis. 2018;218:1383–1393. doi: 10.1093/infdis/jiy328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data from the ITAC trial will be made available 1 year after publication of final results from the trial. Supporting documents will be made available, including the protocol, statistical analysis plan, informed consent document, and data dictionary. Data will be made available to researchers after approval of a proposal for use of the data. Proposals for data use should be submitted using the research proposal Form on the INSIGHT website (www.insight-trials.org).