Abstract
Antimicrobial resistance (AMR) is assigned as a menace by the WHO (World Health Organization) where diseases resulting from multidrug-resistant bacteria are refractory to treatment as a result of a scarcity of new antibiotics in the queue. Antibiotic stewardship program is one of the worldwide strategies to advertise responsible use of antibiotics to halt AMR. The world has started facing a postantibiotic era without immediate and integrated action. Common infections which were treatable for decades can once again kill. The dentistry contributions towards antibiotic resistance are substantial. Approximately 10% of all common antibiotics are prescribed by dentists. Antimicrobial stewardship is a policy that can assist us in addressing the problems of antibiotic resistance. It is a framework that promotes the truly sustainable use of antimicrobials in dentistry. It refers to the approach which is multifaceted and incorporates policies, and guidelines along with surveillance, reports of prevalence and education, and audit of practice for reducing prescribing, adopted by health care organizations. The prime strategy is to improve clinical results while mitigating unintended consequences such as toxicity, pathogenic organism selection, and resistance emergence. Such issues should be centralized and the ongoing need to identify and convert “responsible usage” into context-specific and time-specific behavior. The importance of the antibiotic stewardship program, its team, and their action has become a challenge for the dental hospital but along with it, there are numerous opportunities to achieve the goal.
Keywords: Antibiotics, antimicrobial stewardship, dental profession, dentists, leadership
Introduction
The absence of advanced antibiotics in the advancement pipeline and diseases brought about by multidrug-resistant microbes getting irreversible, antimicrobial resistance (AMR) was thus documented as a credible threat by the World Health Organization (WHO).[1,2] Antibiotic discovery also their far-reaching use in hospital and community settings have been seen in the last 50 years. Respected as viable, safe, and generally economical, antibiotics have saved a huge number of lives. In any case, this has prompted their abuse through use without a remedy and abuse for self-restricting infection.[3,4,5]
Excessive consumption or exploitation of antibiotics triggers antibiotic resistance.[6] The rate of antimicrobial misuse in hospitals is 50%, due to over prescription of broad-spectrum antibiotics and failure to reduce it. Antimicrobial stewardship programs (ASPs) are perhaps a way of bringing excessive antimicrobial use to light. ASPs have set their goal to enhance patient safety and outcomes along with health care costs through promoting judicious antibiotics use and scale back AMR. Effective ASPs have a number of key components, including leadership engagement, prescriber responsibility, medication experience, and clinician and patient education.[7,8] ASPs can include hospital staff and facilities as additional services in order to be competitive and long-lasting. As a consequence, for those who have not yet introduced an ASP, the initial cost can be a deterrent. Because of its increasing significance, there has been an increase in the number of studies evaluating the medical and financial impacts of ASPs in recent years.[9]
A microorganism’s resistance to an antimicrobial medication is known as antimicrobial resistance. Antibiotic resistance is no longer a potential prediction, as claimed by the WHO’s global surveillance of antimicrobial resistance, which announced in 2014 that it is occurring now, all over the world. In the community and hospitals, this has placed at great risk for the treatment of common infections.[10] The world has started facing a postantibiotic era without immediate and integrated action. Common infections which were treatable for decades can once again kill. The dentistry contributions towards antibiotic resistance are substantial. Approximately 10% of all common antibiotics are prescribed by dentists.[11]
Antimicrobial Stewardship Program
As suggested by O.J. Dyar et al., the Antimicrobial Stewardship Program could be defined as “a coherent set of actions which promote using antimicrobials responsibly.”[11]
Optimal antibiotics selection, dose along with the duration of treatment are included in Antimicrobial Stewardship.[12] It attributes to the responsible handling of antimicrobials at the hand of health care professionals, together with the antibiotic selection more precisely and also for a given patient the dose, duration, and administration route with a demonstration for suspected infection.[13] It refers to the approach which is multifaceted and incorporates policies, and guidelines along with surveillance, reports of prevalence and education, and audit of practice for reducing prescribing, adopted by health care organizations.[14] The prime strategy is to improve clinical results while mitigating unintended consequences such as toxicity, pathogenic organism selection, and resistance emergence.[15] Antimicrobial stewardship is a technique for reducing the number of antimicrobial agents used in clinical practice, as well as suboptimal antibiotic usage.[16] “The best possible availability of an antimicrobial drug, dosage, and time that eventuating in the elite therapeutic response for the clinical management of infection, with the minutest possible damage to the patient and the least amount of effect on consequent resistance,” is the definition of great antimicrobial stewardship.[17]
Team of Antimicrobial Stewardship Program
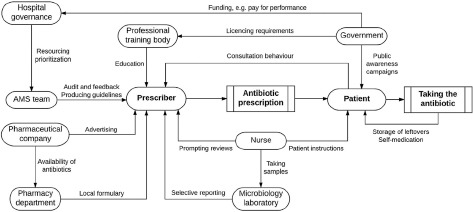
Examples of antimicrobial stewardship actors and their practices[11]
Prescriber: Meets antimicrobial recommendations by checking the need for treatment on a regular basis, allows careful diagnoses, and safely uses antimicrobials.
Nurse: Guarantees antimicrobial use by collecting cultures at the appropriate time and enhancing patients’ awareness of antimicrobial use upon discharge.
Patient: Strictly observing the antimicrobial courses prescribed by the prescriber when using antimicrobials.
Antimicrobial stewardship team: Guidelines for training prescribers on antibiotics and their use, as well as auditing and providing input to prescribers.
Hospital governance: Antimicrobials are used safely by ensuring that antimicrobial stewardship committees have enough long-term resources, monitoring antimicrobial usage tolerance, and using a clinical decision–making framework with health plan limitations.
Producers and farmers who diagnose selectivity and do not use antimicrobials as growth promoters use antimicrobials safely.
Pharmaceutical company: It ensures the safe use of antimicrobials by prohibiting antimicrobial ads, especially broad-spectrum antimicrobials, and ensuring an abundant array of antimicrobials.
National policymaker: National policymakers use antimicrobials by prioritizing and funding antimicrobial stewardship programs, encouraging the use of consistency criteria, and paying for performance.
Actions within antimicrobial stewardship
Within the context of inpatient hospitalization, prescribers, patients, veterinarians, and farmers’ behavior is specifically affected by either allowing judicious utilization of antimicrobial agents (e.g., tools that support decision making, audit, and feedback) or restricting excessive antimicrobial use (e.g., limited reporting of susceptibility testing, formulary restrictions)[18]. These activities may be referred to as “stewardship measures” in the context of inpatient hospitalization. A comprehensive team in charge of an antimicrobial stewardship program often collaborates on these activities and selects possible interventions from a menu of customizable and modifiable principles.[19] It is unique to the governance structures, such as in what way an antimicrobial stewardship team is integrated into the infrastructure of the hospital, who would be on the team and who assists it, what diagnostic and therapeutic evidence is accessible to the team, and the degree to which the stewardship team can influence the prescribers directly because stewardship teams are typically in process of executing antimicrobial stewardship policies, hospital quality officers or consultants may assist them by knowing how to execute them effectively.[11]
The CDC (Centers for Disease Control and Prevention)
Center for disease control and prevention (CDC) guideline “Management of multi-drug resistant organism in health care setting” in 2006 expressed that by paying incredible consideration to prudent antimicrobial use, the rise of multidrug resistance can be controlled. Essentially “Get Smart for Healthcare Campaign” was launched by CDC in 2009 to promote advanced antibiotic use in in-patient settings.
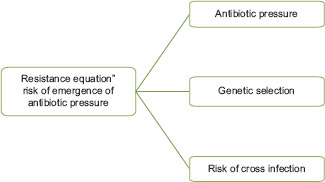
The emergence of resistance and hospital cross-infections
The following are the consequences of using antimicrobials wrongly:
Casual association between emergence of antimicrobial resistance and antimicrobial use.
The presence of multidrug-resistant organisms relates with the mortality rate.
The beneficial bacteria within the body would be reduced in number.
Collateral damage.
The 30% rule of Antimicrobial Stewardship Program:
Antibiotics are given to around 30% of all hospitalized in-patients at any point in time.
Antibiotics are prescribed incorrectly in the population in excess of 30% of the time.
About 30% of all surgical prophylaxis is unnecessary.
Antimicrobial use accounts for 30% of hospital drug costs.
Antimicrobial stewardship projects will save between 10% and 30% of antimicrobial costs.
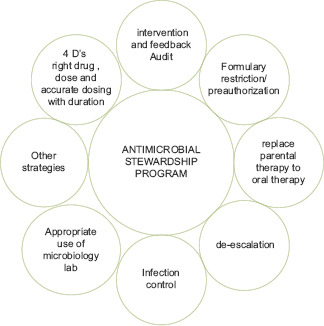
Antimicrobial Stewardship: Strategic Approaches
Rational antimicrobial therapy.
For operative procedures, improving antimicrobial prophylaxis.
Developing antibiotic policies and upholding standard treatment guidelines (STG).
Antibiotic prescriptions and reviews are streamlined by prospective auditing and prompt intervention.
Preauthorization/formulary limitation.
Antimicrobial prescribing improved by instructional and administrative methods.
Components of antimicrobial stewardship
ICMR initiatives/Government of India for AMSP
“Anti-Microbial Resistance Surveillance and Research Network” (AMRSN) started by The Indian Council of Medical Research (ICMR), New Delhi, India, across the nation in 2013 to react to national AMR emergency in India. The aim was to rationalize the antibiotic stewardship program in India. It was in line with the proposal of the “Chennai Declaration,” which addressed global efforts to combat antimicrobial resistance. Six nodal centers and twenty regional centers were compromised to begin reconnaissance of six pathogenic groups. The key objectives of the organization were to utilize the proof to guide treatment strategies thereby justifying antimicrobial use. The first phase made noteworthy progress. Now that the organization has advanced to the next phase, its focus will be on strengthening prognostic stewardship and disease prevention for the next 5 years.
Antibiotic prescribing problems in dentistry and antimicrobial resistance
Dentists, among approved health care providers, play a key role in reducing the issue of inappropriate broad-spectrum antibiotic prescriptions, as they are thought to prescribe roughly 10% of all popular antibiotics, sometimes inappropriately.[20] In an ideal situation and common practice, dental procedures including certain simple tooth extractions, and cavity restorations could be done without the use of broad-spectrum antibiotics in immune-competent people, particularly when performed under strict sterile conditions. This is not always the case, as most dentists tend to prescribe broad-spectrum antibiotics, particularly in the developing areas, after brief dental procedures for the factors outlined previously.[21]
Despite its prevalence in developing countries, the issue of dentists prescribing antibiotics maliciously is not peculiar to those countries. Basic dental services are expected to be conducted under specific ideal circumstances in today’s dynamic world, which should eradicate the superfluous prescription of antibiotics. In countries like the United Kingdom and Germany, dentists are still responsible for inappropriate antibiotic prescriptions in unreasonably high proportion.[22,23]
Role of Pharmacist in the battle against AMR
A profession that commits entire life to drugs, from discovering and dispensing is of the pharmacist. Nearly 40% of antibiotics are prescribed inappropriately. A pharmacist is the last person to be in contact with a patient before taking antibiotics. Thus, irrational use of medicines can be control by them. Collaborating with prescribing physicians and assisting antibiotic stewardship within the hospital setting in primary health care settings is the major role of the clinical pharmacist in the present situation. The pharmacist and prescriber can make the situation better by making rational use of antibiotics preceded by professional association and patient communities.[24]
Conclusion
For all who require admittance to successful antimicrobials, today and tomorrow, antibiotic stewardship is crucial to guarantee. The term “antibiotic stewardship” arose moderately as of late, and is being applied in an undeniably assorted scope of settings, especially in a dental scenario. The particular activities shift contingent upon the entertainer, however, share numerous shared characteristics at various levels inside a medical care framework, just as among human and creature well-being.
Antimicrobial stewardship is an instrument: Every entertainer can inquire as to whether they or their associations are attempted activities to utilize antimicrobials dependably, and if these activities are reasonable. Dentists are targeted for mishaps in relation to antibiotic prescription hence it is the need of the hour to practice a setting of explicit and time explicit activities.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Goossens H. Expert-proposed European strategies to monitor and control infection, antibiotic use, and resistance in health-care facilities. Lancet Infect Dis. 2011;11:338–40. doi: 10.1016/S1473-3099(11)70070-6. [DOI] [PubMed] [Google Scholar]
- 2.Carlet J, Jarlier V, Harbarth S, Voss A, Goossens H, Pittet D. Ready for a world without antibiotics?The Pensières Antibiotic Resistance Call to Action. Antimicrob Resist Infect Control. 2012;1:11. doi: 10.1186/2047-2994-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman JM, Shah ND, Vermeulen LC, Schumock GT, Grim P, Hunkler RJ, et al. Projecting future drug expenditures--2007. Am J Health Syst Pharm. 2007;64:298–314. doi: 10.2146/ajhp060545. [DOI] [PubMed] [Google Scholar]
- 4.Wise R, Hart T, Cars O, Streulens M, Helmuth R, Huovinen P, et al. Antimicrobial resistance. Is a major threat to public health. BMJ. 1998;317:609–10. doi: 10.1136/bmj.317.7159.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.John JF, Fishman NO. Programmatic role of the infectious diseases physician in controlling antimicrobial costs in the hospital. Clin Infect Dis. 1997;24:471–85. doi: 10.1093/clinids/24.3.471. [DOI] [PubMed] [Google Scholar]
- 6.Hand K. Antibiotic stewardship. Clin Med (Lond) 2013;13:499–503. doi: 10.7861/clinmedicine.13-5-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Overview |Antimicrobial stewardship:Systems and processes for effective antimicrobial medicine use |Guidance |NICE [Internet], NICE. [Last accessed on 2021 Jan 23]. Available from: https://www.nice.org.uk/guidance/ng15 .
- 8.Core Elements of Hospital Antibiotic Stewardship Programs |Antibiotic Use |CDC [Internet] [Last acessed on 2021 Jan 23]. Available from: https://www.cdc.gov/antibiotic-use/core-elements/hospital.html .
- 9.Value of hospital antimicrobial stewardship programs [ASPs]:A systematic review |Antimicrobial Resistance and Infection Control |Full Text [Internet] [Last accessed on 2021 Jan 23]. Available from: https://aricjournal.biomedcentral.com/articles/10.1186/s13756-019-0471-0 . [DOI] [PMC free article] [PubMed]
- 10.Antimicrobial resistance [Internet] [Last accessed on 2021 Jan 23]. Available from: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance .
- 11.Dyar OJ, Huttner B, Schouten J, Pulcini C. What is antimicrobial stewardship? Clin Microbiol Infect. 2017;23:793–8. doi: 10.1016/j.cmi.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 12.Dm S, Dn G, Jf J, Wa C, Dl B, Ra D, et al. Society for Healthcare Epidemiology of America and Infectious Diseases Society of America Joint Committee on the Prevention of Antimicrobial Resistance:Guidelines for the prevention of antimicrobial resistance in hospitals. Clin Infect Dis. 1997;25:584–99. doi: 10.1086/513766. [DOI] [PubMed] [Google Scholar]
- 13.Goff DA. Antimicrobial stewardship:Bridging the gap between quality care and cost. Curr Opin Infect Dis. 2011;24(Suppl 1):S11–20. doi: 10.1097/01.qco.0000393484.17894.05. [DOI] [PubMed] [Google Scholar]
- 14.Charani E, Cooke J, Holmes A. Antibiotic stewardship programmes--What's missing? J Antimicrob Chemother. 2010;65:2275–7. doi: 10.1093/jac/dkq357. [DOI] [PubMed] [Google Scholar]
- 15.Dellit TH, Owens RC, McGowan JE, Gerding DN, Weinstein RA, Burke JP, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–77. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 16.Van Schooneveld T. Antimicrobial stewardship:Attempting to preserve a strategic resource. J Community Hosp Intern Med Perspect. 2011;1 doi: 10.3402/jchimp.v1i2.7209. doi:10.3402/jchimp.v1i2.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerding DN. The search for good antimicrobial stewardship. Jt Comm J Qual Improv. 2001;27:403–4. doi: 10.1016/s1070-3241(01)27034-5. [DOI] [PubMed] [Google Scholar]
- 18.Davey P, Marwick CA, Scott CL, Charani E, McNeil K, Brown E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2017;2:CD003543. doi: 10.1002/14651858.CD003543.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Septimus EJ, Owens RC. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53(Suppl 1):S8–14. doi: 10.1093/cid/cir363. [DOI] [PubMed] [Google Scholar]
- 20.Pallasch TJ. Global antibiotic resistance and its impact on the dental community. J Calif Dent Assoc. 2000;28:215–33. [PubMed] [Google Scholar]
- 21.Oyiborhoro O. Challenges of inappropriate antibiotic prescription in dentistry and threat of antimicrobial resistance in developing countries-Medicinal plants to the rescue. Altern Integr Med. 2020;9:8. [Google Scholar]
- 22.Löffler C, Böhmer F, Hornung A, Lang H, Burmeister U, Podbielski A, et al. Dental care resistance prevention and antibiotic prescribing modification-the cluster-randomised controlled DREAM trial. Implement Sci. 2014;9:27. doi: 10.1186/1748-5908-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zahabiyoun S, Sahabi M, Kharazi MJ. Improving knowledge of general dental practitioners on antibiotic prescribing by raising awareness of the faculty of general dental practice (UK) guidelines. J Dent (Tehran) 2015;12:171–6. [PMC free article] [PubMed] [Google Scholar]
- 24.The role of pharmacist in encouraging prudent use of antibiotic medicines and averting antimicrobial resistance –A review of current policies and experiences in Europe (2014) [Internet] [Last accessed on 2021 Jan 27]. Available from: https://www.euro.who.int/en/health-topics/Health-systems/health-technologies-and-medicines/publications/2014/the-role-of-pharmacist-in -encouraging-prudent-use-of-antibiotic-medicines -and-averting-antimicrobial-resistance-a-review -of-current-policies-and-experiences-in-europe-2014 .


