Abstract
Shoulder injury related to vaccine administration (SIRVA) is a rare but potentially debilitating injury characterized by persistent shoulder pain, typically occurring within 48 hours of intramuscular deltoid vaccine administration. With over 150 million flu vaccines being administered in the United States each year, and the US Centers for Disease Control’s goal of immunizing greater than 70% of the population for the coronavirus disease 2019 virus, cases of SIRVA can be expected to rise. A search of current literature was done to identify published material corresponding to incidence, diagnosis, and treatment of SIRVA. Most events have been associated with poor needle placement and/or a local reaction to the delivered serum during vaccine administration. Shoulder injury related to vaccine administration events can lead to persistent and possibly permanent injury. Clinical evaluation involves a thorough history, physical examination, and often diagnostic studies including radiographs, magnetic resonance imaging, and nerve studies. Treatment is individually directed and should initially consist of observation and local symptom management. Recalcitrant cases or infections may warrant surgical intervention. Published outcomes vary widely, and our understanding of SIRVA remains limited. Large-scale studies are necessary to better understand the pathophysiology of SIRVA, its treatment, and its outcomes. Overall, the initial priority in managing SIRVA should be awareness and prevention.
Key words: COVID-19, Shoulder pain, SIRVA, Vaccination, VICP
Mild shoulder discomfort is a common and well-recognized side effect of intramuscular deltoid injection associated with vaccination administration. Most cases are typically self-limited.1 However, individuals occasionally experience severe and persistent shoulder pain that requires further workup and management.2 These injuries, referred to as “shoulder injury related to vaccine administration” (SIRVA), typically occur moments to days after vaccine injection and can result in prolonged and even permanent shoulder dysfunction.3 Although uncommon, with influenza vaccine–based studies showing an incidence of 1 to 2 per million, SIRVA is expected to become more prevalent as vaccination numbers grow worldwide.4 Effective treatment for SIRVA begins with prevention, followed by accurate diagnosis and timely treatment.
Background
Nearly 50% of the United States population receives the flu shot annually, representing over 150 million vaccinations per year.5 Now, with the coronavirus disease 2019 (COVID-19) pandemic, the US Centers for Disease Control and Prevention is aiming for a vaccination goal of 70% of the population.6 Moreover, depending on which vaccination is given, the initial COVID-19 vaccination requires up to 2 injections.6 Additionally, a booster injection, which will likely become annual, is now being recommended for adults.7 These 2 vaccinations, for influenza and COVID-19, will represent the most common annual vaccinations. It is currently unclear whether the Centers for Disease Control and Prevention will recommend children under the age of 16 years receive the COVID-19 booster.7 However, as it stands, it is estimated that Americans will receive nearly 500 million vaccinations annually.5,8 As such, it can be anticipated that cases of SIRVA will be expected to climb.
Epidemiology
The incidence of SIRVA is not well-known, but is assumed to be uncommon. Shoulder injury related to vaccine administration was not officially added to the National Vaccine Injury Compensation Program (VICP) Vaccine Injury Table until 2017. However, shoulder injury claims had been substantially increasing for over a decade.4 Petitions to the VICP increased from 2.5% of total claims of SIRVA in 2011 to 41.9% of total claims in 2016.9 Of these, the majority of reported SIRVA cases were female, making up 82.8% of SIRVA petitioner claims to the VICP from 2010 to 2016.9 This majority was reflected in a large cohort study done by Hesse et al,10 in which 69% of cases were female. The age associated with SIRVA varied widely, ranging from 19 to 89 years.11,12 The type of vaccine also varied; however, the influenza vaccine predominated.9
Some studies suggest that the growing incidence of SIRVA reports may not be due to increasing injury, but instead to previously underreported events.4 A search of the US Vaccine Adverse Event Reporting System showed that of the approximately 996 million doses of influenza vaccine distributed in the United States from July 2010 to June 2017, there were 1,220 reports of atypical shoulder pain that began within 48 hours of vaccine administration and lasted for greater than 7 days. This made up 2% of all reported Vaccine Adverse Event Reporting System cases.4 Since then, the percent of cases reported has remained relatively consistent at 2%.4 Contrarily, Hesse et al10 suggests that the incidence is much lower. A population-based study of nearly 3 million persons receiving an influenza vaccine during the 2016 to 2017 influenza season only identified 16 cases of bursitis.10 This study, though, limited its evaluation to subacromial bursitis, a single type of SIRVA injury. The incidence of SIRVA due to COVID-19 vaccination is still unknown, although a number of COVID-19 vaccine SIRVA case studies have recently been published.13,14 A larger study is still necessary to understand the association of SIRVA and COVID-19 vaccination to determine whether it differs in presentation and incidence from other vaccines, such as influenza.
Anatomy and Mechanism of Injury
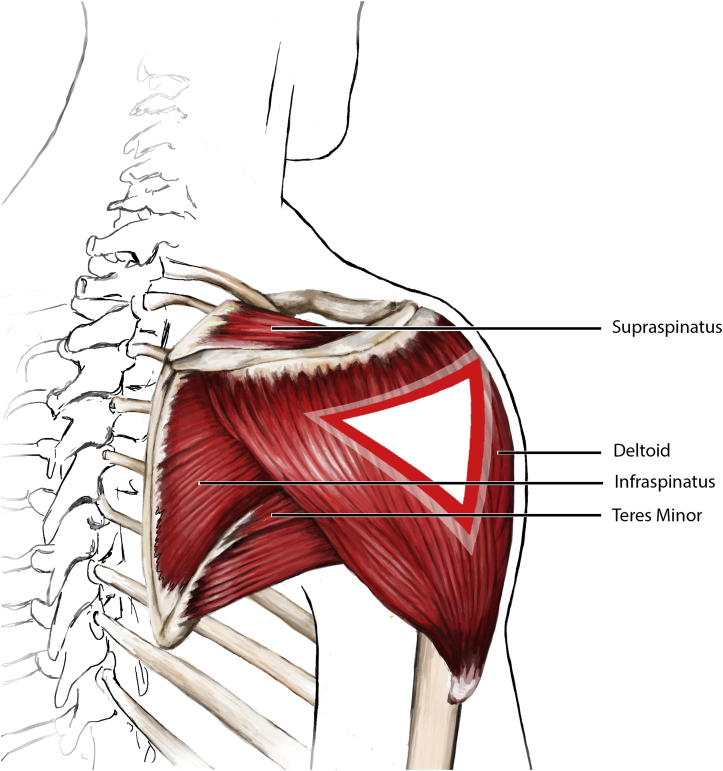
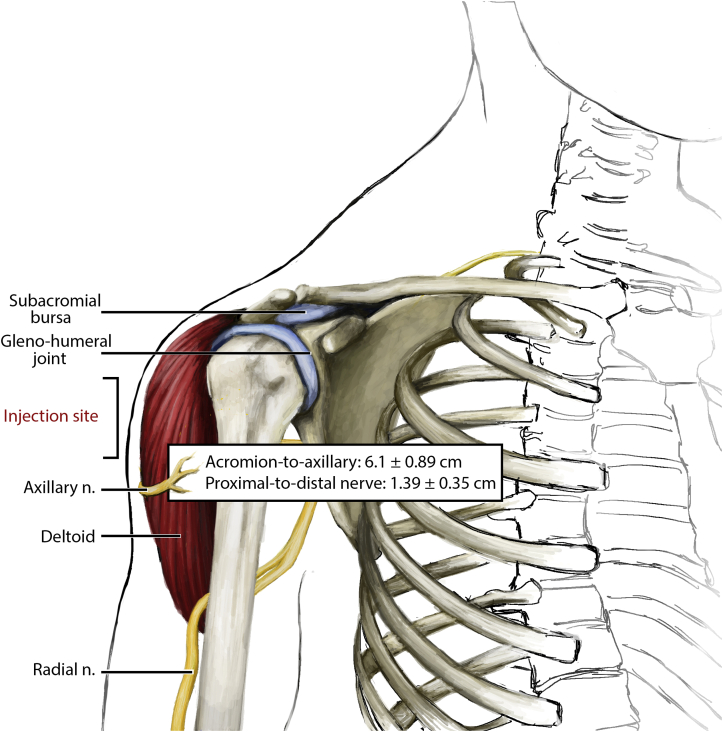
Most instances of SIRVA are associated with errant placement of the needle and/or local reaction to the delivered serum during administration of the vaccine.3 Most vaccinations, including those for influenza and COVID-19, require intramuscular placement of the vaccine into the deltoid muscle of the shoulder. The deltoid muscle is the main driver of shoulder joint motion. The joint itself consists of the glenohumeral “ball and socket” joint, the surrounding shoulder capsule, and the overlying rotator cuff and surrounding subacromial and subdeltoid bursa (Figure 1, Figure 2). Traveling inferior to the shoulder joint and then wrapping around posteriorly and laterally is the axillary nerve supplying the deltoid. The distance of the acromion-to-axillary nerve distance is approximately 6.1 ± 0.89 cm (men, 6.57 ± 0.83 cm; women, 5.72 ± 0.75 cm).15 An errant injection can potentially injure any of these structures (Table 1). An injection placed too deep into the shoulder capsule can cause shoulder joint or bursal inflammation (synovitis) or an infection (septic arthritis or bursitis). An injection placed into the rotator cuff can cause a rotator cuff injury (tendonitis or tear of the rotator cuff). An injection placed into the subacromial or subdeltoid space can cause painful inflammation (bursitis) and/or frozen shoulder (adhesive capsulitis). An injection placed into or near the axillary nerve can cause nerve irritation, numbness and tingling (paresthesia), and even weakness of the arm from temporary anterior or middle deltoid paralysis. An injection at any location can potentially cause a local infection, and can result in cellulitis, abscess, septic bursitis, septic arthritis, or osteomyelitis, depending on the depth and location of the infection and the host immune status. Lastly, a local inflammatory reaction to the delivered serum can cause local irritation and pain, including myositis of the deltoid and bursitis of the subacromial space. This adverse reaction may be due to tissue injury or an allergic response to the vaccine or various vaccine components. The most common sources of the adverse allergic reactions are proteins, such as egg products and gelatin. Other sources include yeast, commonly found in the human papillomavirus vaccine, and latex.16 The most common reactive source found in the influenza vaccine is egg protein.16 Both COVID-19 messenger RNA vaccines, from Pfizer-BioNTech and Moderna, contain polyethylene glycol as a possible source of allergy. The J&J/Janssen COVID-19 vaccine contains polysorbate, another potential source.7
Figure 1.
Proper vaccine injection site and rotator cuff anatomy.
Figure 2.
Anatomic structures at risk for SIRVA. The overall mean acromion-to-nerve distance is 6.1 ± 0.89 cm. The interval between the most proximal and distal borders of the axillary nerve is 1.39 ± 0.35 cm.15
Table 1.
Anatomic Location and Potential Injuries Caused by SIRVA
| Anatomic Location | Potential Injuries |
|---|---|
| Deltoid muscle | Myositis Abscess |
| Subacromial space | Bursitis Septic bursitis |
| Rotator cuff | Tendonitis Tear or rupture |
| Glenohumeral joint | Adhesive capsulitis Septic arthritis |
| Axillary nerve | Neuritis Neuropathy |
Wiesel and Keeling17 suggest that SIRVA should be treated as a chronic, idiopathic inflammatory response of the shoulder. This response has the potential to lead to further tissue injury. Inflammation is known to play a role in the development and progression of tendon injury, making it possible for an errant injection causing tendonitis to lead to tissue breakdown and rotator cuff injury.18 Additionally, as there is an in increase in asymptomatic chronic rotator cuff injury with progressing age, it is possible for an initial injury to already be present prior to immunization and to become symptomatic secondary to synovial inflammation and irritation.19 Greater than 50% of the population over the age of 65 has a rotator cuff tear; hence, the chances of an acute-on-chronic event increases with age.18
Presentation
Most vaccine injection–related shoulder complaints are self-limited and resolve within 24 to 48 hours.20 A small subset of patients go on to experience prolonged and debilitating shoulder pain, often diagnosed as SIRVA. This term was first coined by Atanasoff et al19 in a 2010 case series describing 13 petitioners to the VICP who all experienced shoulder pain and decreased range of motion for greater than 6 months after vaccine injection. Additional diagnostic criteria for SIRVA varies between resources. These often include a lack of prior symptomatic shoulder pain, rapid onset of pain that usually occurs either immediately or less than 4 days following vaccination, and symptoms localized to the vaccinated shoulder.19 The typical presenting complaints could include shoulder pain, decreased range of motion, or general shoulder weakness, as well as paresthesia or tingling (Table 2). Hesse et al9 identified the following shoulder complaints: shoulder pain (93.9%), range of motion limitation (31.1%), tingling or paresthesia (7.8%), erythema (5.5%), and shoulder weakness (4.8%). The type of vaccine for this study varied, with the influenza vaccine being the most prevalent. Most cases (68.7%) reported that the pain began the day of vaccination, while 13.1% of cases experienced pain starting the second day. The time to resolution was not specified.9 Similarly, in a case series of 13 patients published by Atanasoff et al,19 shoulder pain was reported in 100% of patients, limited range of motion was reported in 85%, altered sensation was reported in 31%, and weakness was reported in 31%. The type of vaccine varied, with the influenza vaccine being the most prevalent. The onset of pain was immediate for 54% of patients, occurred within 24 hours for 39% of patients, and occurred within 4 days for 8% of patients. Symptoms persisted between 6 months and many years.19
Table 2.
Reported Symptoms or Diagnosis, Treatment, and Resolution of SIRVA
| Author | Patients, n | Reported Symptoms or Diagnosis | % | Treatments | n (%) | Resolution, n (%) |
|---|---|---|---|---|---|---|
| Atanasoff et al19 | 13 | Shoulder pain Limited ROM Altered sensation Weakness |
100 84.6 30.8 30.8 |
NSAIDs Steroid injection Physical therapy Surgery |
8 (61.5) 8 (61.5) 6 (46.2) 4 (30.8) |
4 (30.8) |
| Chuaychoosakoon et al13 | 1 | Shoulder pain Limited ROM Fever |
100 | Intravenous antibiotics | 1(100) | 1(100) |
| Cross et al29 | 2 | Shoulder pain Limited ROM Supraspinatus tear |
100 100 50 |
Surgical joint washout Steroid injection |
1 (50) 1 (50) |
2 (100) |
| Hesse et al10 | 16 | Subdeltoid bursitis | 100 | Surgery Steroid injection |
4 (25) 1 (6.3) |
2 (12.5) |
| Hesse et al9 | 476 | Shoulder pain Rotator cuff problems Bursitis Local reaction Adhesive capsulitis Adverse effects of vaccination Neuritis Impingement Other Not specified |
31.9 13.9 11.8 8.2 5.5 2.5 2.3 1.9 9.7 18.5 |
Physical or occupational therapy Steroid injections NSAIDs or other analgesics Surgery Oral steroids Exercise routine Opiates Chiropractic treatment Muscle relaxant Acupuncture |
381 (80) 286 (61.1) 240 (50.4) 155 (32.5) 130 (27.3) 111 (23.3) 65 (13.7) 30 (6.3) 29 (6.1) 18 (3.8) |
116 (24.3) |
| Hibbs et al4 | 1,220 (symptoms) 546 (treatments) |
Shoulder pain Limb mobility decreased Joint ROM decreased Rotator cuff syndrome Bursitis Arthralgia Frozen shoulder Pain in joint involving shoulder region Injection site joint pain Stiffness shoulder Shoulder bursitis Injection site joint movement impairment |
44.1 40.8 22.1 9.2 9.0 8.8 5.2 3.2 2.9 2.9 2.8 2.4 |
Nonnarcotic analgesics Physical therapy Corticosteroid injection Hot and cold therapy Oral steroids Narcotic analgesics Home shoulder exercise Pain medication, not specified Surgery Muscle relaxants Topical analgesics Massage Chiropractic Sling Acupuncture Shoulder manipulation with sedation Antibiotics Muscle stimulators Other |
254 (46.5) 215 (39.4) 109 (20.0) 89 (16.3) 79 (14.5) 28 (5.1) 19 (3.5) 17 (3.1) 16 (2.9) 15 (2.7) 10 (1.8) 10 (1.8) 7 (1.3) 6 (1.1) 4 (0.7) 2 (0.4) 2 (0.4) 2 (0.4) 41 (7.5) |
Not reported |
| Rodrigues et al14 | 1 | Shoulder pain Limited ROM |
100 | Ice packs Physical therapy Oral steroids |
1(100) | Not reported |
| Veera et al20 | 1 | Shoulder pain Decreased range of motion Paresthesia |
100 | Osteopathic manipulative medicine under general anesthesia Acupuncture treatments Nonanesthetic osteopathic manipulations Physical therapy |
1 (100) | 1 (100) |
| Wong et al30 | 1 | Shoulder pain Rotator cuff bursitis Impingement syndrome |
100 | Arthroscopic shoulder debridement Subacromial bursectomy |
1 (100) | 1 (100) |
| Wright et al23 | 1 | Shoulder pain Subacromial or subdeltoid bursitis |
100 | Steroid injection | 1 (100) | 1 (100) |
NSAID, nonsteroidal anti-inflammatory drug; ROM, range of motion.
Ultimately, presentation will be related to the site of needle entry and the anatomic site of injury (Table 1). Systemic symptoms are uncommonly reported. However, they may rarely present secondarily to a disseminated infectious cause or in conjunction with an inflammatory reaction. Complex regional pain syndrome, though unlikely, may also be considered in patients where an underlying injury is not found and symptoms persist. Caution should be practiced when diagnosing complex regional pain syndrome. As an example, it was initially suspected that the human papillomavirus vaccine was associated with an increased risk of acquiring complex regional pain syndrome. This led to a temporary suspension of the vaccine in certain countries. It was later found that evidence supporting the association lacked significance, and the vaccine suspension was removed.21
Clinical Evaluation
For cases of persistent or worsening shoulder pain beyond 48 hours of vaccination, clinical evaluation may be warranted. The initial evaluation should consist of taking a history to correlate the symptoms to the injection, as well as to rule out other comorbidities or preexisting shoulder pathology. A physical examination of the shoulder should begin with inspection of the injection site. This is best done with full exposure of both shoulders, free of clothing, to maximize visualization and facilitate the evaluation of asymmetry, skin changes, or edema. Next, palpation of the site should be done gently, seeking to identify potential hematoma, fluctuance, abscess, or crepitus. Range of motion is initiated by first soliciting active range of motion by the patient in all planes of the shoulder. Typical range of motion of the shoulder includes forward flexion of 150° to 180°, extension of 40° to 60°, abduction of 150° to 180°, internal rotation to the thoracic spine, and external rotation of 60° to 90°.22 If active range of motion of the patient is abnormal, the shoulder can be passively manipulated to assess for maintenance of the full passive arc of shoulder motion. Next, strength testing can be undertaken, with particular attention being paid to the integrity of the deltoid function. This is achieved by testing the arm against resisted abduction (middle deltoid) and forward flexion (anterior deltoid). However, differentiating diminished strength from pain can be difficult and may warrant further evaluation with imaging.22 Finally, a thorough neurovascular examination should be done. Vascular injuries are not a recognized SIRVA complication and can be readily ruled out by palpating the brachial artery at mid-arm medially or the radial artery at the wrist volar-radially.
Diagnostic Studies
Depending on the clinical concerns, a number of diagnostic studies are available to evaluate SIRVA. Radiographs (x-rays) are most readily available but will unlikely be positive or diagnostic early in the clinical course. If available, the imaging of choice would be magnetic resonance imaging (MRI), as it would provide the greatest detail of the typical pathologies associated with SIRVA, including hematoma, infection, subacromial bursitis, joint synovitis, adhesive capsulitis, and rotator cuff injury.23 In the majority of these injuries, contrast is unnecessary. However, MRI with contrast is recommended if an infection is suspected, as this will more clearly portray the amount of osseous and nonosseous involvement, and will establish the presence of tissue necrosis.24 If an MRI is not available, an ultrasound evaluation can also be considered and could provide clinical data for the same clinical diagnoses related to SIRVA.23
In cases of neurologic complaints consisting of pain, numbness, tingling, paresthesias, and weakness, an electrodiagnostic evaluation may also be warranted.11 However, such an evaluation—consisting of a nerve conduction study and electromyogram performed in tandem—is best performed at least 2 to 3 weeks after vaccination in order for any potential nerve injury to begin its cascade of myelin sheath and axonal sheath changes, for the study to be able to identify it.
Treatment
The treatment of SIRVA is based on a clinical and diagnostic evaluation of the patient’s shoulder complaints. The rarity of cases and incomplete understanding of the pathophysiology has led to varying patient-centered treatment plans focused on individualized symptom relief. Currently, there is no algorithmic approach to treating SIRVA patients. However, there are certain principles that should be followed.
For noninfectious and nonneurologic joint-related complaints, the initial treatment strategy should be nonsurgical management using a multimodal approach consisting of rest, activity modification, anti-inflammatory medication, and physical therapy. These approaches were demonstrated in varying orders of predominance among studies (Table 2). Atanasoff et al,19 Hesse et al,9 and Hibbs et al4 reported that nonsteroidal anti-inflammatory drugs or other nonnarcotic analgesics were used by 61.5%, 50.4%, and 46.5% of patients, respectively, and physical therapy was used by 46.2%, 80%, and 39.4% of patients, respectively. For cases of advanced or recalcitrant inflammatory complaints, such as subacromial bursitis, joint synovitis, and adhesive capsulitis, corticosteroid treatment either orally or injected locally may be of value. Steroid injections were used by 61.5%, 61.1%, or 20% of patients in the studies by Atanasoff et al,19 Hesse et al,9 and Hibbs et al,4 respectively.
For advanced noninfectious and nonneurologic SIRVA shoulder complaints that are unresponsive to formal and diligent initial nonsurgical management, or in cases of rotator cuff injury, surgical intervention to repair damaged tissue may be warranted. Atanasoff et al,19 Hesse et al,9 and Hibbs et al4 reported that surgery was performed in 30.8%, 32.5%, and 2.9% of patients, respectively. This decision should be made collaboratively between the patient and physician and should be considered only when all other treatment options have been exhausted.
Nerve injury can result from needle-associated trauma, chemical irritation, or neuritis from inflammation.25 The anterior branch of the axillary nerve winds around the surgical neck of the humerus and is in close proximity to the suggested intramuscular injection site, placing it at risk with an errant injection.25 Most patients with nerve injury will report immediate pain following vaccine injection. They may additionally report paresthesia, muscle weakness, and possible muscle atrophy over time.26 For neurologic complaints, the initial treatment strategy is also nonsurgical management using a multimodal approach consisting of rest, activity modification, anti-inflammatory medication, nerve modulating medication, and physical therapy. Medications include nonsteroidal anti-inflammatories, as well as nerve modulating agents such as gabapentin and pregabalin. Therapy should focus on joint mobilization, deltoid and rotator cuff muscle strengthening, and nerve gliding and desensitization. In cases with prolonged documented nerve injury or secondary nerve impingement, surgical intervention such as nerve decompression may be warranted.
Local infection is a rare phenomenon that can occur secondary to vaccine injection and arises when pathogens gain entry into the system through breaks in the skin.27,28 Presentation is typically acute and consists of pain, erythema, swelling, and warmth to the area.28 Treatment may be initially surgical or nonsurgical and is ultimately driven by the acuity and severity of infection symptoms. Uncomplicated infections, without systemic complaints, should be treated with oral antistreptococcal antimicrobial agents.28 In persistent infection, if systemic symptoms are present, or if abscess or necrotizing fasciitis is suspected, further workup and more aggressive treatment is necessary. Blood cultures, needle aspiration, or punch biopsy may help to identify the bacteria. However, these cultures often yield negative or inconclusive results.28 Basic infectious workup should be done, including white blood cell count and procalcitonin. Magnetic resonance imaging can help to identify or rule out necrotizing fasciitis or osteomyelitis. Ultrasound or MRI can also rule out or identify abscess.28 Patients with systemic symptoms should be admitted for intravenous antibiotic treatment. In cases with abscess or necrotizing fasciitis, the patient should be admitted for urgent surgical debridement and administration of bacterial specific antibiotics.28
Outcomes
The long-term outcome of SIRVA is not well-documented. Data sources are often incomplete, and many patients are lost to follow-up. The data that are available show that the majority of SIRVA patients report residual pain with permanent decreased range of motion after treatment. Hesse et al9 reported that only 24% of the 476 patients who petitioned claims to the VICP between 2010 and 2016 indicated that their symptoms had fully resolved by the final reported clinical appointment. Similarly, Atanasoff et al19 reported that only 31% of the 13 patients in their study reported complete recovery. Finally, in a study evaluating 16 patients that experienced subdeltoid bursitis, only 12.5% experienced full recovery (Table 2).10
Due to their nature, case reports and case series tend to present increased rates of recovery in comparison to larger, retrospective studies. Cross et al29 reported on 2 cases. The first an 82-year-old female who experienced severe shoulder pain 2 hours after receiving the pneumococcal polysaccharide vaccine. The patient was diagnosed via ultrasound with a complete supraspinatus tear accompanied by a moderate subdeltoid bursa collection. After failing conservative treatment, the patient underwent a surgical joint washout in addition to an extended course of empirical intravenous flucloxacillin. She was pain free and had full range of shoulder motion 1 month after the procedure. The second patient was a 23-year-old woman who experienced left shoulder pain 24 hours after receiving an intramuscular deltoid injection of the diphtheria, tetanus, and whooping cough vaccine. She was treated with a corticosteroid injection and was pain free with full range of motion at her 3-month follow-up appointment.29
Wong et al30 presented another case in which a patient experienced increased shoulder pain 48 hours after receiving the influenza vaccination. The patient was diagnosed via MRI with rotator cuff bursitis and bursal foreign body reaction. After 3 months of unsuccessful treatment with conservative management, including nonsteroidal anti-inflammatory drugs, a single cortisone subacromial injection, and physical therapy, the patient opted for arthroscopic debridement of the inflamed bone and tissue. This patient reported significant relief at her 3-month postoperative visit.30
Recently, there has been a growing number of case reports describing COVID-19 vaccination–related SIRVA. Chuaychoosakoon et al13 reported on a 52-year-old male with no prior shoulder injury who experienced shoulder pain, limited range of motion, and fever 3 days after receiving the Sinovac COVID-19 injection. An ultrasound showed subacromial-subcorocoid-subdeltoid bursitis, and aspiration removed 5 mL of serosanguinous fluid. Joint fluid analysis showed 45,500 cells/mm3 in the white blood cell count with no organisms. The patient was admitted to the hospital for intravenous antibiotic treatment. His fever resolved and he started to regain shoulder range of motion over the next few days.13
Rodrigues et al14 also reported on a patient that experienced shoulder pain following COVID-19 vaccine injection. The patient, a 61-year-old female, experienced shoulder pain within 30 minutes of receiving her first shot. She initially treated the injury with ice packs and topical diclofenac with no resolution. At 8 weeks, she continued to experience pain and decreased range of motion. Magnetic resonance imaging and an ultrasound lead to a diagnosis of subacromial-subdeltoid bursitis and rotator cuff tendinopathy. The patient was started on oral prednisone, vitamin D supplements, and a physical therapy regimen. It is unclear whether the patient has undergone full recovery.14
Discussion
The US Department of Health and Human Services has an annual goal of vaccinating 70% of the population for both influenza and COVID-19. With this increase in vaccine administration, there will likely also be an increase in SIRVA cases. Beyond better understanding and treating SIRVA, the initial priority should continue to focus on prevention through sound injection technique. Emphasis should be placed on choosing the correct syringe, needle gauge, and needle length. The syringe choice may vary from 1 mL to 3 mL, the needle gauge may vary from 22 gauge to 25 gauge, and the length may vary from 0.625 inches to 1.5 inches, based on age and body habitus.3,31 Increased attention should be placed on needle length, as a needle that is either too short or too long may lead to improper administration and possible complications if the needle penetrates too deeply. Needle placement during intramuscular administration is into the thickest portion of the deltoid muscle. It is recommended that the needle should be injected at a 90° angle, directly into the center of the deltoid muscle, approximately 2 inches (5.08 cm) below the acromion process, and just below the level of the armpit (Figure 1, Figure 2).3 This does, however, place the axillary nerve at minor risk, particularly in women who have a shorter acromion-to-nerve distance of 5.72 ± 0.75 cm.15 It may be advisable to reevaluate this recommendation, as the placement puts the axillary nerve at risk and places the insertion site at a location where the deltoid muscle is thinner than its slightly more proximal location. Further research would benefit this clinical procedure.
Reports of SIRVA to the VICP have progressively increased each year for decades. Even with an emphasis on proper training in vaccine administration, claims of SIRVA continue to multiply.9 The VICP was developed in 1980 as a no-fault alternative to eliminate the financial risk of developing and administrating vaccines, thus protecting vaccination companies and health care providers. This helps to avoid vaccine shortages by protecting vaccine manufactures from financial risk, and therefore decreases the risk of the return of preventable diseases. In addition, it provides a platform to petition compensation for any individual that believes they have suffered harm due to SIRVA.2 This is generally beneficial; however, as Gonzalez et al12 suggests, it is difficult to isolate causative injury from chronological association. Though a handful of studies have attempted to track the incidence of actual SIRVA cases, it is probable that many cases go under- or unreported and that the true incidence is still unknown. It would be beneficial to develop tracking systems that allow for follow-up within the Vaccine Adverse Event Reporting System.
Conclusion
Published data show that the number of adult SIRVA cases reported to the VICP continues to increase each year as the number of immunizations administered to the public increases.9 Incidence of SIRVA in children is still unclear. Of the 476 identified petitions reported to the VICP from 2010 to 2016, only 1 was <18 years old.9 Current literature emphasizes the mechanism of SIRVA injury, as well as present-day clinically endorsed preventative strategies. Still, there remains a lack of literature exploring diagnosis and treatment as it relates to clinical outcomes. Large-scale studies that emphasize long-term treatment outcomes are necessary.
Footnotes
Declaration of interests: No benefits in any form have been received or will be received related directly or indirectly to the subject of this article.
References
- 1.Centers for Disease Control and Prevention Possible Side Effects From Vaccines. https://www.cdc.gov/vaccines/vac-gen/side-effects.htm Accessed December 11, 2021.
- 2.US Health Resources & Services Administration National Vaccine Injury Compensation Program. http://www.hrsa.gov/Vaccinecompensation/ Accessed March 28, 2021.
- 3.Gabler L., Staubli J., Hayney M.S. Preventing shoulder injury related to vaccine administration. J Am Pharm Assoc (2003) 2019;59(4):599–600. doi: 10.1016/j.japh.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Hibbs B.F., Ng C.S., Museru O., et al. Reports of atypical shoulder pain and dysfunction following inactivated influenza vaccine, Vaccine Adverse Event Reporting System (VAERS), 2010–2017. Vaccine. 2020;38(5):1137–1143. doi: 10.1016/j.vaccine.2019.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Flu Vaccination Coverage, United States, 2019–20 Influenza Season. https://www.cdc.gov/flu/fluvaxview/coverage-1920estimates.htm Accessed March 29, 2021.
- 6.Centers for Disease Control and Prevention COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/index.html Accessed July 21, 2021.
- 7.Centers for Disease Control and Prevention COVID-19 Vaccine Booster Shots. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html?s_cid=11706:mrna%20covid%20booster:sem.ga:p:RG:GM:gen:PTN:FY22 Accessed December 11, 2021. [PubMed]
- 8.Centers for Disease Control and Prevention COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker/#vaccinations Accessed July 22, 2021.
- 9.Hesse E.M., Atanasoff S., Hibbs B.F., et al. Shoulder injury related to vaccine administration (SIRVA): petitioner claims to the National Vaccine Injury Compensation Program, 2010–2016. Vaccine. 2020;38(5):1076–1083. doi: 10.1016/j.vaccine.2019.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hesse E.M., Navarro R.A., Daley M.F., et al. Risk for subdeltoid bursitis after influenza vaccination: a population-based cohort study. Ann Intern Med. 2020;173(4):253–261. doi: 10.7326/M19-3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martín Arias L.H., Sanz Fadrique R., Sáinz Gil M., Salgueiro-Vazquez M.E. Risk of bursitis and other injuries and dysfunctions of the shoulder following vaccinations. Vaccine. 2017;35(37):4870–4876. doi: 10.1016/j.vaccine.2017.07.055. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez A.I., Kortlever J.T.P., Moore M.G., Ring D.C. Influenza vaccination is not associated with increased number of visits for shoulder pain. Clin Orthop Relat Res. 2020;478(10):2343–2348. doi: 10.1097/CORR.0000000000001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuaychoosakoon C., Parinyakhup W., Tanutit P., Maliwankul K., Klabklay P. Shoulder injury related to Sinovac COVID-19 vaccine: a case report. Ann Med Surg (Lond) 2021;68:102622. doi: 10.1016/j.amsu.2021.102622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodrigues T.C., Hidalgo P.F., Skaf A.Y., Serfaty A. Subacromial-subdeltoid bursitis following COVID-19 vaccination: a case of shoulder injury related to vaccine administration (SIRVA) Skeletal Radiol. 2021;50(11):2293–2297. doi: 10.1007/s00256-021-03803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwarz A.M., Weiglein A.H., Schwarz U.M., et al. Definition of a risk zone for the axillary nerve based on superficial landmarks. Plast Reconstr Surg. 2021;147(6):1361–1367. doi: 10.1097/PRS.0000000000007950. [DOI] [PubMed] [Google Scholar]
- 16.Chung E.H. Vaccine allergies. Clin Exp Vaccine Res. 2014;3(1):50–57. doi: 10.7774/cevr.2014.3.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiesel B.B., Keeling L.E. Shoulder injury related to vaccine administration. J Am Acad Orthop Surg. 2021;29(17):732–739. doi: 10.5435/JAAOS-D-21-00021. [DOI] [PubMed] [Google Scholar]
- 18.Abraham A.C., Shah S.A., Thomopoulos S. Targeting inflammation in rotator cuff tendon degeneration and repair. Tech Shoulder Elb Surg. 2017;18(3):84–90. doi: 10.1097/BTE.0000000000000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atanasoff S., Ryan T., Lightfoot R., Johann-Liang R. Shoulder injury related to vaccine administration (SIRVA) Vaccine. 2010;28(51):8049–8052. doi: 10.1016/j.vaccine.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Veera S., Chin J., Kleyn L., Spinelli S., Tafler L. Use of osteopathic manipulation for treatment of chronic shoulder injury related to vaccine administration. Cureus. 2020;12(7) doi: 10.7759/cureus.9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huygen F., Verschueren K., McCabe C., et al. Investigating reports of complex regional pain syndrome: an analysis of HPV-16/18-adjuvanted vaccine post-licensure data. EBiomedicine. 2015;2(9):1114–1121. doi: 10.1016/j.ebiom.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakhsh W., Nicandri G. Anatomy and physical examination of the shoulder. Sports Med Arthrosc Rev. 2018;26(3):e10–e22. doi: 10.1097/JSA.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 23.Wright A., Patel R., Motamedi D. Influenza vaccine-related subacromial/subdeltoid bursitis: a case report. J Radiol Case Rep. 2019;13(6):24–31. doi: 10.3941/jrcr.v13i6.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Towers J.D. The use of intravenous contrast in MRI of extremity infection. Semin Ultrasound CT MR. 1997;18(4):269–275. doi: 10.1016/s0887-2171(97)80017-0. [DOI] [PubMed] [Google Scholar]
- 25.Choi H.R., Kondo S., Mishima S., et al. Axillary nerve injury caused by intradeltoid muscular injection: a case report. J Shoulder Elbow Surg. 2001;10(5):493–495. doi: 10.1067/mse.2001.114682. [DOI] [PubMed] [Google Scholar]
- 26.Cook I.F. Best vaccination practice and medically attended injection site events following deltoid intramuscular injection. Hum Vaccin Immunother. 2015;11(5):1184–1191. doi: 10.1080/21645515.2015.1017694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohl K.S., Ball L., Gidudu J., et al. Abscess at injection site: case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5821–5838. doi: 10.1016/j.vaccine.2007.04.057. [DOI] [PubMed] [Google Scholar]
- 28.Raff A.B., Kroshinsky D. Cellulitis: a review. JAMA. 2016;316(3):325–337. doi: 10.1001/jama.2016.8825. [DOI] [PubMed] [Google Scholar]
- 29.Cross G.B., Moghaddas J., Buttery J., Ayoub S., Korman T.M. Don’t aim too high: avoiding shoulder injury related to vaccine administration. Aust Fam Physician. 2016;45(5):303–306. [PubMed] [Google Scholar]
- 30.Wong W., Okafor C., Belay E., Klifto C.S., Anakwenze O. Arthroscopic surgical management of shoulder secondary to shoulder injury related to vaccine administration (SIRVA): a case report. J Shoulder Elbow Surg. 2021;30(6):e334–e337. doi: 10.1016/j.jse.2021.01.027. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention Vaccine administration: needle gauge and length. https://www.cdc.gov/vaccines/hcp/admin/downloads/vaccine-administration-needle-length.pdf Accessed December 12, 2021.