Abstract
Enteric fever is the only bacterial infection of humans for which bone marrow examination is routinely recommended. A prospective study of the concentrations of bacteria in the bone marrow and their relationship to clinical features was conducted with 120 Vietnamese patients with suspected enteric fever, of whom 89 had confirmed typhoid fever. Ninety-three percent of the Salmonella enterica serovar Typhi samples isolated were resistant to ampicillin, chloramphenicol, and co-trimoxazole. For 81 patients with uncomplicated typhoid and satisfactory bone marrow aspirates, the number of serovar Typhi CFU in bone marrow aspirates was a median value of 9 (interquartile range [IQR], 1 to 85; range, 0.1 to 1,580) compared to 0.3 (IQR, 0.1 to 10; range, 0.1 to 399) CFU/ml in simultaneously sampled blood. The ratio of individual blood counts to bone marrow counts was 10 (IQR, 2.3 to 97.5). The number of bacteria in blood but not bone marrow was correlated inversely with the duration of preceding fever. Thus, with increasing duration of illness the ratio of bone marrow-to-blood bacterial concentrations increased; the median ratio was 4.8 (IQR, 1 to 27.5) during the first week compared with 158 (IQR, 60 to 397) during the third week. After lysing the host cells, the median ratio of viable bone marrow to blood increased, reflecting the higher concentration of intracellular serovar Typhi in the bone marrow. Effective antibiotic pretreatment had a significantly greater effect in reducing blood counts compared to bone marrow counts (P < 0.001). Thus, bacteria in the bone marrow of typhoid patients are less affected by antibiotic treatment than bacteria in the blood. The numbers of bacteria in bone marrow correlated negatively with the white blood cell (R = −0.3, P = 0.006) and platelet counts (R = −0.32, P = 0.01) and positively with fever clearance time after treatment (R = 0.4, P < 0.001). The bacterial load in bone marrow therefore may reflect the clinical course of the infection, and high levels may suppress neutrophil proliferation.
Typhoid fever, a prolonged, debilitating illness caused by infection with Salmonella enterica serovar Typhi, continues to be a significant global problem, with an estimated 16.6 million cases annually (14). Serovar Typhi is a pathogen only of humans, and many aspects of the pathogenicity of human typhoid are not well understood (8, 15). Serovar Typhi is concentrated in lymphoid tissues. Early histopathological descriptions of the inflammatory processes in Peyer's patches, mesenteric lymph nodes, liver, and spleen from human autopsy material (12) are reminiscent of the histiocytic proliferation and granuloma formation described more recently in the bone marrow of patients with typhoid fever (4, 10, 11, 17). Bone marrow aspirates are known to yield a higher rate of positive cultures in typhoid than peripheral blood (5, 7). The bone marrow may be both an important organ of host defense and an important focus of infection for serovar Typhi. Positive correlations between the concentrations of organisms in blood and the severity of the clinical condition have been shown for many infections (9, 20, 21), but in typhoid fever blood bacterial counts do not correlate with outcome (1) or with clinical and laboratory measures of severity (19). Indeed, bacterial concentrations in the blood do not seem to reflect the total bacterial load in human typhoid fever. The aim of this study was to measure bone marrow bacterial concentrations of serovar Typhi in typhoid fever and to examine their relationship to clinical and laboratory parameters and the response to antimicrobial treatment.
MATERIALS AND METHODS
Patients.
Adults and children admitted to the infection ward of the Dong Thap Provincial Hospital, Cao Lanh City, Vietnam, were studied during two periods: February to March 1995 and April to May 1997. Informed consent was obtained from patients or their guardians. The scientific and ethical committees of both Dong Thap Provincial Hospital and the Centre for Tropical Diseases, Ho Chi Minh City, Vietnam, approved the study. Criteria for the clinical diagnosis of typhoid fever were fever for more than 4 days with no obvious focus of infection, abdominal discomfort or a characteristic change in affect, and a negative blood smear for malaria. On admission the clinical history and examination findings were recorded on a standard form. Blood, bone marrow, and up to three stools were collected for culture before treatment was started. If possible, a single specimen of urine was collected on admission for the detection of antimicrobial activity.
All patients with nonsevere typhoid fever were treated with a standard regimen of antimicrobial chemotherapy, oral ofloxacin (10 to 15 mg/kg of body weight per day for 5 days) (Oflocet, Rousell, France). Patients with typhoid fever complicated by perforation of the small bowel, cholecystitis, altered consciousness, or severe abdominal pain were treated with variable doses of cefotaxime with or without gentamicin at the discretion of the treating physician (Table 1). Fever clearance times (FCT) were calculated as the time from the beginning of treatment until the time temperature fell below 37.5°C and remained so for 48 h.
TABLE 1.
Patients with complications of typhoid fever
| Complicationa | n | Count (CFU/ml)
|
Treatmentb | |
|---|---|---|---|---|
| QBMC | QBC | |||
| Cholecystitis | 1 | 1,200 | 138 | Cro, CN |
| Perforation | 1 | 1 | 0.1 | Cro, CN |
| Death (GI bleeding) | 1 | 5,333 | 15 | Cro, CN |
| GI bleeding | 1 | 1 | Negative | Cro |
| Severe abdominal pain | 1 | 1 | 1 | Cro, CN |
| Nonspecific | 3 | 2; 316; 1 | 0.1; 8; 0.01 | Unknown |
GI, gastrointestinal.
Cro, cefotaxime; CN, gentamicin.
Clinical methods.
As soon as a clinical diagnosis of typhoid fever had been made, 15 ml of blood (or 7.5 ml from small children) was collected for broth culture. The blood was divided equally into three bottles, two of which contained 50 ml each of brain heart infusion broth (Oxoid, Basingstoke, United Kingdom) containing 0.05% sulfpolyanethosulfonate (SPS; Sigma, Poole, United Kingdom) (one of these also contained 0.05% saponin [Sigma]) and a third contained Oxgall broth (Difco, Detriot, Mich.). In mild-infection patients, where specific treatment was guided by the 24-h blood culture result, specimens for quantitative culture comprised of 6 ml of blood (or 3 ml from small children) and at least 1 ml of bone marrow aspirated from the iliac crest were collected on the following day in sterile heparinized tubes. Patients with more severe disease requiring immediate treatment had specimens taken for broth (5 ml) culture and quantitative culture at the same time.
Microscopy of bone marrow.
Two sets of slides were prepared from bone marrow aspirates and were fixed in acetone at the time of collection. One set was later stained with acridine orange (Difco) for 1 min and examined using 100× magnification to assess the quality of the specimen. The second set of slides was postfixed in methanol before being stained with Wrights Giemsa (BDH, Poole, United Kingdom). Differential cell counts were performed later by two experienced histopathologists, one from the hematology center at Ho Chi Minh City, Vietnam, and one by R. P. Hasserjian. Counts were performed on 200 cells from each specimen.
Bacteriological methods.
All quantitative cultures were carried out using a pour plate method. Three 1-ml aliquots of blood or a single 0.5-ml aliquot of bone marrow was mixed with 19 ml of molten (50°C) Columbia agar (Oxoid) containing 0.05% SPS in sterile petri dishes. In order to lyse all cells present, the same volume of blood or bone marrow was mixed with an equal volume of 0.1% digitonin (Sigma) and incubated at 37°C for 15 min. Pour plates were then prepared by mixing the lysed specimens with 19 ml of molten (50°C) Columbia agar (Oxoid) containing 0.05% SPS in sterile petri dishes. After being allowed to set, all plates were incubated at 37°C for 4 days and counts were recorded as CFU per milliliter. Up to five colonies were picked to the surface of the agar and after overnight incubation were identified using Kligler iron agar slants (Oxoid) and agglutination with antisera specific for 09, Vi, and 02 salmonella antigens (Wellcome Diagnostics, Dartford, United Kingdom). Antimicrobial sensitivity testing was performed by a modified Bauer-Kirby method using the following disks: ampicillin (10 μg), chloramphenicol (30 μg), trimethoprim-sulphamethoxazole (1.25 and 23.75 μg, respectively), ceftriaxone (30 μg), ofloxacin (5 μg), and nalidixic acid (30 μg) (Oxoid). Multidrug-resistant (MDR) strains were defined as those resistant simultaneously to ampicillin, chloramphenicol, and trimethoprim-sulphamethoxazole and tetracycline.
Treatment before admission to the hospital is common in Vietnam and may affect the bacterial counts. These antibiotics are often present in urine. When urine was collected from typhoid patients on admission to the hospital, the presence of antibacterial activity against Escherichia coli ATCC 25922 and/or MDR serovar Typhi was detected using a 5-mm filter-paper disk dipped into the urine and then placed onto a lawn of the organisms on an agar plate. Any zone observable after incubation at 37°C overnight was recorded as positive. Patients were assumed to have taken antimicrobial agents active against the infecting organisms if (i) the patient was culture positive for sensitive serovar Typhi and had urine activity against E. coli or (ii) the patient was culture positive for MDR serovar Typhi and had urine activity against MDR serovar Typhi.
Statistical analysis.
SPSS for Microsoft Windows release 7.5.1 (SPSS Benelux, Inc., Gorinchem, The Netherlands) was used for all statistical analyses. Abnormally distributed data are described using medians and interquartile ranges (IQR). Correlation was assessed by measurement of the Spearman correlation coefficient. Confounding factors were controlled for by using partial correlation coefficients on the ranked data. Differences in bacterial counts between patient groups defined by dichotomous variables were tested by using the Mann-Whitney U test. Differences in bacterial counts between two specimens were tested by the Wilcoxon signed-rank test. Differences in bacterial counts by week of illness were tested by the Kruskal-Wallis test.
RESULTS
Clinical results.
Clinical and laboratory parameters on admission for mild- and severe-disease patients are compared in Table 2. Quantitative blood and bone marrow cultures were performed on 120 patients; serovar Typhi was isolated from 89 patients, 81 nonsevere and 8 severe cases. Salmonella enterica serovar Paratyphi A was isolated from one patient and E. coli from one other patient. The results of the blood culture media comparison will be reported elsewhere. Patients with severe typhoid have not been used to analyze the relationship between FCT and bacterial load, because the treatment given was not standardized. All 81 nonsevere typhoid patients recovered. One of the eight severe typhoid patients died from acute gastrointestinal bleeding.
TABLE 2.
Clinical and laboratory variables for patients with typhoid fever
| Variable | Value for:
|
|
|---|---|---|
| Patients with mild typhoid fever (n = 81) | Patients with severe typhoid fever (n = 8) | |
| Age (yr)a | 10 (7–20) (4–42) | 9.5 (7–18.5) (6–21) |
| Duration of fever (days)a | 7 (5–12) (2–30) | 5.5 (3.5–7) (3–15) |
| FCT (h)a | 99 (83–123) (36–300) | 75 (45–150) (36–168) |
| Admission temp (°C)a | 39.5 (39–40) (37–41) | 39 (38.5–40) (37.5–40.5) |
| No. of femalesb | 41/81 (50%) | 5/8 (62.5%) |
| Abdominal painb | 38/80 (47%) | 7/8 (87.5%) |
| Confusionb | 0/80 | 0/8 |
| Diarrheab | 57/80 (70%) | 5/8 (62.5%) |
| Gastrointestinal bleedingb | 0/81 | 3/7 (43%) |
| Clinical jaundiceb | 2/79 (2.5%) | 0/8 |
| Splenomegalyb | 14/80 (17.5%) | 2/8 (25%) |
| Rashb | 0/81 | 0/8 |
| Hepatomegalyb | 49/81 (60.5%) | 6/8 (75%) |
| White blood cell count/μla | 7,400 (5,700–9,400) (2,700–1,6700) | 7700 (5,275–13,900) (3,600–16,000) |
| Platelet count/μla | 214,000 (137,500–277,000) (66,000–490,000) | 163,500 (98,250–261,000) (78,000–300,000) |
| Hematocrit %a | 32 (30–35) (21–44) | 31 (28–36) (25–37) |
Results are given as median (IQR) (range) for abnormally distributed data.
Results are given as number of patients positive (percentage).
Bone marrow results.
There were 60 cases where sufficient bone marrow aspirate was received for the preparation of smears for histological examination. A microscopic picture indicating predominantly blood was observed in 12 out of 60 cases. This was taken to mean excessive blood contamination of the bone marrow sample. Nevertheless, the 12 cases with excessive blood contamination had higher bacterial counts in bone marrow (quantitative bone marrow bacterial cultures [QBMC]): median value, 23.5 (IQR, 2 to 88.3), compared with blood (quantitative blood bacterial cultures [QBC]): median value, 0.3 (IQR, 1 to 5.6) (P = 0.001). This was not significantly different from the 48 “satisfactory” specimens: QBMC median value, 8 (IQR, 1 to 131), and QBC median value, 0.1 (IQR, 0.1 to 14.3). All specimens were therefore included in the analysis. The overall median ratio of the number of bacteria in all 89 bone marrow specimens compared to the number of bacteria in the simultaneous venous blood specimen (QBMC/QBC) was 13.5 (IQR, 7 to 68). To investigate this further, the nucleated cells in 48 specimens of bone marrow were counted using standard flow cytometry, and the number of each cell type present in each specimen was then calculated from the differential cell count of the bone marrow. There was no correlation between bacterial counts and either the total number of nucleated cells or the absolute numbers or proportions of any or all cell types of myeloid origin.
Histological features.
Thirty-seven bone marrow smears chosen at random were described histologically. There was a variable degree of myeloid hyperplasia. Lymphocytes were increased above normal reference values in 6 of 37 cases, up to a maximum of 40% of the bone marrow cells. Most of the lymphocytes in these cases were small, but some large nucleolated lymphoid cells were also seen. Monocytes were increased above normal reference ranges in 20 of 37 of the cases, up to a maximum of 15% of the cellularity; many had vacuolated cytoplasm, but hemophagocytosis was not seen. Granulomas were not identified in any of the 37 cases.
Quantitative culture results.
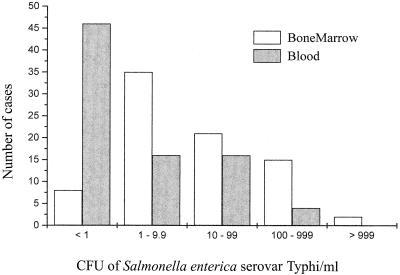
The distribution of bacterial counts in the blood and bone marrow of patients with noncomplicated typhoid fever is graphically described in Fig. 1. Lysing of bone marrow cells increased the number of CFU/milliliter significantly, indicating that bacteria were concentrated within cells. In 18 cases where sufficient bone marrow aspirate had been collected, a viable count before and after lysis of the bone marrow was performed. The average increase in the yield was 3.3-fold. In 53 cases viable counts were performed before and after lysis of blood, and the observed increase in yield was 1.9-fold (Table 3). There was a median of 36 (IQR, 3.7 to 1,335) times as many viable bacteria in the lysed bone marrow as in the lysed blood of the 81 patients with uncomplicated typhoid fever studied (Table 3). This was higher than the ratio of 10:1 (IQR, 2.3 to 97.5) seen with unlysed samples (P = 0.004), indicating that the proportion of bacteria intracellular in the bone marrow is higher than that in the blood (Fig. 2). Not all patients had greater numbers of organisms in the bone marrow. In six patients exactly the same bacterial count was recorded from blood and bone marrow. These patients all had very low numbers of bacteria (0.1 CFU/ml) in both bone marrow and blood. In seven patients there were fewer bacteria in the bone marrow than in the blood (QMBC to QBC median value of 0.14 [IQR, 0.1 to 0.5]). This was not an effect of sampling, as there was no difference in the cellular content of the specimens of the two groups. When comparing absolute numbers or proportions of any or all cell types of myeloid origin found in the bone marrow or peripheral white blood cell counts, there was no significant difference (P = 0.3). There was also no difference in previous antimicrobial therapy (P = 0.18). One of these patients had 399 serovar Typhi CFU/milliliter of blood and only 1 CFU/milliliter in bone marrow.
FIG. 1.
The distribution of blood and bone marrow bacterial counts in 81 patients with mild typhoid fever.
TABLE 3.
Quantitative culture results from 81 patients with mild typhoid fever
| Specimen (n = 81 unless otherwise stated) | Count (CFU/ml)
|
||
|---|---|---|---|
| Median | IQR | Range | |
| Bone marrow (QBMC) | 9 | 1.0–85 | 0.1–1580 |
| Blood (QBC) | 0.3 | 0.1–10 | 0.1–399 |
| QBMC/QBC | 10 | 2.3–97.5 | 0.01–4,160 |
| Lysed/nonlysed QBMC (n = 18)a | 3.3 | 1.2–5.6 | 0.1–120 |
| Lysed/nonlysed QBC (n = 53) | 1.9 | 1.0–6.6 | 0.4–310 |
| Lysed QBMC/lysed QBC (n = 17) | 36 | 3.7–1,335 | 0.5–25,440 |
An equal volume of sample was lysed with digitonin before culture. This was only possible if sufficient bone marrow aspirate was available.
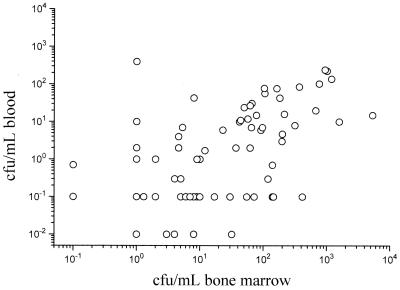
FIG. 2.
Relationship between serovar Typhi viable counts in 15 ml of whole blood and 1 ml of whole bone marrow in 89 patients.
QBMC and QBC were correlated positively (R = 0.6, P = <0.001), but the correlations with clinical parameters were different for QBMC and QBC. There was no statistically significant correlation between QBC and white blood cell count, platelet count, or hematocrit. There was a weak negative correlation between QBMC and the white blood cell count (R = −0.3, P = 0.006) and QBMC and circulating platelets (R = −0.32, P = 0.01).
Antibiotic usage.
Urine was collected from 50 patients before antimicrobial therapy was started in the hospital. Active antimicrobial agents were detected in 30 of these patients (60%). Patients with active antimicrobial agents in their urine had slightly lower concentrations (in CFU/milliliter) of bacteria in their bone marrow (QBMC median, 8.5 [IQR, 1 to 74]) than did patients without active antimicrobial agents (median, 29.5 [IQR, 1.5 to 128]), but this difference was not significant (P = 0.7). A bigger difference was seen in the QBC concentration (median, 0.1 [IQR, 0.1 to 3.2]) in the presence of antimicrobial activity when compared with the QBC concentration where no activity was detected (median, 2.5 [IQR, 0.1 to 14.5]) (P = 0.07). There was a considerable difference in the ratio of bone marrow bacterial counts to blood bacterial counts for patients in whom antibiotic activity was present in their urine to patients without activity in their urine (median, 55.5 [IQR, 6 to 193.5] versus 4.6 [IQR, 1 to 30]) (P = 0.03).
Duration of illness.
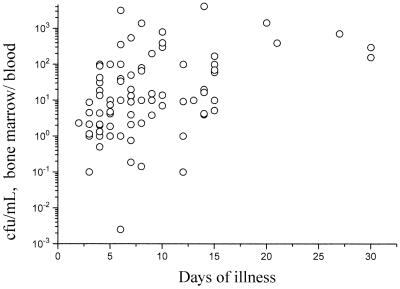
Of the 81 nonsevere typhoid patients studied, 42 were in the first week of illness when admitted to the hospital, 28 were in the second week, and 11 were in the third or subsequent week. The viable serovar Typhi counts per milliliter of whole blood (QBC) declined with week of illness: first week, median value of 2 (IQR, 0.1 to 27.5); second week, median of 0.1 (IQR, 0.1 to 5.2); third and subsequent weeks, median of 0.1 (IQR, 0.1 to 0.3) (P = 0.02), whereas the bone marrow counts did not change: first week, median value of 9 (IQR, 1 to 75); second week, median of 8 (1 to 85); third and subsequent weeks, median value of 30 (IQR, 6 to 119) (P = 0.5). This was reflected in the trend for the ratio of bone marrow viable bacterial count to blood count to increase over time (Fig. 3): first week, median value of 4.8 (IQR, 1.6 to 25); second week, median of 10 (IQR, 4 to 95); and third and subsequent weeks, median of 158 (60 to 397) (P = 0.001)
FIG. 3.
The effect of duration of illness of typhoid fever patients on the ratio of viable organisms in the bone marrow/blood.
Antimicrobial resistance and bacterial counts.
Susceptibility testing showed that 83 of 89 (93.2%) of the serovar Typhi isolates from both severe and nonsevere cases of typhoid fever were MDR; that is, resistant to ampicillin, co-trimoxazole, and chloramphenicol. No resistance was seen to ofloxacin, but 14 of 89 isolates (16%) were resistant to nalidixic acid. Of the 81 nonsevere typhoid patients, 75 were infected with MDR serovar Typhi (93%), and these patients had more viable bacteria in their bone marrow than did 6 patients infected with non-MDR serovar Typhi, with a median CFU/milliliter value of 10 (IQR, 1 to 97), compared with a median CFU/milliliter value of 1.5 (IQR, 0.8 to 11) (P = 0.045. There were also higher numbers of serovar Typhi bacteria in the blood of patients infected with MDR organisms, but this did not reach statistical significance: median CFU/milliliter value of 0.7 (IQR, 0.1 to 10) compared with a median CFU/milliliter value of 0.1 (IQR, 0.1 to 0.3) (P = 0.2). Patients infected with MDR typhoid fever came to the hospital earlier than patients with sensitive typhoid fever; history of illness at 7 days (IQR, 5 to 10 days) compared with 14 days (IQR, 10 to 18 days) (P = 0.047). There were no differences in bacterial counts (in CFU/milliliter) from either the blood or the bone marrow for 14 patients infected with nalidixic acid-resistant disease compared to 75 patients infected with nalidixic acid-sensitive serovar Typhi: bone marrow median, 9 (IQR, 1 to 233) compared with a median of 9.5 (IQR, 1 to 88.5) (P = 0.9) and a blood median value of 0.3 (IQR, 0.1 to 10) compared with a median of 0.7 (IQR, 0.1 to 10.5) (P = 0.7).
Stool culture results.
At least one stool sample was collected from each of 52 patients before treatment; 16 of these were positive for serovar Typhi and 36 were negative for Salmonella and Shigella species. The stool culture-positive group had significantly higher bacterial counts (in CFU/milliliter) in the blood than the culture-negative group, with a median value of 2 (IQR, 0.25 to 10) versus a median of 0.1 (IQR, 0.1 to 6) (P = 0.04) and also generally higher counts in the bone marrow: median of 70 (IQR, 3 to 144) versus a median of 8 (IQR, 1 to 59) (P = 0.14). Results for stool culture-positive and stool culture-negative patients presented to the hospital after a similar history of illness are as follows: median, 7.5 (IQR, 5 to 8.75) days versus median, 7 (IQR, 5.25 to 11.5) days. Three out of six patients infected with non-MDR serovar Typhi had stool culture analyses; all were negative.
FCT.
Patients with higher bacterial counts from the bone marrow had longer FCT, after treatment. Five patients with FCT of greater than 156 h were all infected with nalidixic acid-resistant serovar Typhi (n = 5). There was a positive correlation between QBMC and FCT (R = 0.4, P < 0.001). This correlation remained significant when nalidixic acid resistance of the isolate, age, and length of illness were controlled for (R = 0.38, P = 0.001) and became stronger (R = 0.5) when patients infected with nalidixic acid-resistant serovar Typhi were removed from the analysis. A significant correlation between the blood bacterial count and FCT (R = 0.3, P = 0.02) was seen only when patients infected with nalidixic acid-resistant serovar Typhi were removed from the analysis.
DISCUSSION
Enteric fever is the only bacterial infection of humans for which bone marrow examination is routinely recommended. It is generally believed that bone marrow cultures will increase the diagnostic yield by about one-third compared with those from blood, despite the considerably lower volume of bone marrow culture (0.5 to 1 ml) compared to that of routine blood culture (5 to 30 ml). This study provides formal mathematical proof that the concentrations of serovar Typhi in the bone marrow are considerably higher than in peripheral blood and that the probability of a positive culture, in part, depends on the volume of sample processed. If there are 9 CFU/ml, as in bone marrow, there is a 90% chance of isolating serovar Typhi from 1 ml of specimen. Given a median bacterial count in blood of 0.3 CFU/ml, there is only a 30% chance of isolating serovar Typhi from the same volume of blood (1 ml). However, 7 ml of blood should be sufficient for a 90% chance of isolating a single bacterium. Looking at the ratio of individual bone marrow bacterial counts to blood bacterial counts suggests that even higher volumes of blood may be necessary. In the bone marrow there were over 10 times more bacteria than in peripheral blood. It seems likely therefore that a large-volume blood culture (>10 ml) would be needed to match the positivity rate of a 1-ml bone marrow culture, and the necessity of taking a bone marrow aspirate may be reduced if blood cultures of greater than 10 ml are collected. This should be true particularly for patients who have been previously treated or who present late in the illness. It may, however, be difficult to collect this volume of blood from some patients.
Lysing the blood cells to release intracellular organisms (which would otherwise count as one colony in a pour plate even if a single cell contained many organisms) yielded an average bone-marrow-to-blood bacterial concentration ratio of 36. Whether this concentration of serovar Typhi in human bone marrow results from local intracellular replication or the phagocytosis of multiple bacteria is not known. Indeed, of typhoid fever in humans very little is known even about the site of replication of the bacterial pathogen. In the widely used rodent models of typhoid fever, Salmonella enterica serovar Typhimurium has been shown to invade and multiply in mouse hepatocytes (2) and neutrophils (3) and in a mononuclear Kupfer cell preparation from rat livers (13). In cell culture experiments serovar Typhi can replicate in human macrophages to reach an average of 14 organisms per cell (18). In contrast, the peripheral blood monocytes from patients infected with serovar Typhi contain an average of only 1.3 CFU/cell (16, 19). The exact location of serovar Typhi in human bone marrow has not been characterized either, but assuming a predominantly intracellular location, the threefold increase in the number of bacteria found in lysed compared to unlysed bone marrow suggests that there is a median of at most three bacteria per cell in the bone marrow during human typhoid fever.
FCT were correlated positively with the bone marrow counts in this series, suggesting that numbers of bacteria in the bone marrow reflect the bacterial load in typhoid fever patients. Although many different factors determine the therapeutic response in typhoid fever, including age, immune status, susceptibility of the infecting organism, and antibiotic regimen, antimicrobial treatment was not a confounder in this series, as all patients with uncomplicated typhoid received the same treatment.
Bone marrow counts of serovar Typhi were significantly higher in patients presenting with MDR organisms. We have shown previously that these organisms reach higher concentrations in blood and have speculated that this may reflect greater intrinsic virulence of MDR serovar Typhi in this area (19). Stool carriage, and therefore presumably transmissibility, was also greater in patients with higher bone marrow and blood bacteria counts. There were insufficient data in this study to relate MDR infection with stool carriage, although in previous studies of quantitative blood cultures a positive relationship was apparent (19).
The viable organism counts in the bone marrow were considerably less affected by antibiotic treatment than the quantitative blood counts. Indeed, despite successful treatment bone marrow samples may remain positive for up to 5 days or longer after starting effective treatment with fluoroquinolones (6), and this contributes to the superiority of bone marrow isolation over blood culture in diagnosis. As in this series, many patients in tropical countries are treated with antibiotics before presenting to the hospital. In the context of previous treatment, bone marrow culture is particularly useful and should be performed if typhoid is suspected. How serovar Typhi survives in the bone marrow despite the presence of high concentrations of a bactericidal antibiotic which reaches high intracellular concentration is not clear, although the compartment of the cell occupied by serovar Typhi is not known.
Typhoid and brucellosis share several pathological features in common. Both are characterized by persistent infection and leukopenia, in contrast to the more usual leukocytosis of other bacterial infections. Brucellosis is characterized by granuloma formation, including in the bone marrow, whereas in this series of patients with typhoid fever, despite infiltration of monocytes into the bone marrow in over half the patients and lymphocytic infiltration in 16% of the cases, no granulomata were seen.
Only five (6.2%) of the patients with uncomplicated typhoid fever were leukopenic (less than 4,000 leukocytes per ml of blood), but the white blood cell count was related inversely to the number of bacteria in the bone marrow. Although this does not exclude a systemic explanation, it does suggest that the high concentrations of serovar Typhi within cells of the bone marrow directly inhibit neutrophil proliferation. There was no evidence for neutrophil accumulation in the bone marrow to suggest any maturation abnormality. The mediators involved in suppressing neutrophil proliferation remain to the determined.
Although it is tempting to compare the complicated and uncomplicated cases in this study, the small number and wide variety of complications prohibits this. It is, however, of interest to note that the complicated cases showed a great deal of variation in bacterial counts. It is also our experience that isolation of serovar Typhi from the blood and bone marrow of patients suffering certain complications, such as perforation of the gut, is far more difficult than for patients suffering from other complications. In order to investigate the relationship between bacterial count and the complications of typhoid fever, a larger study is under way at the Dong Thap Hospital.
These data provide insights into the pathology of typhoid and an explanation for the superiority of bone marrow over blood in diagnosis. Serovar Typhi accumulates preferentially within cells of the bone marrow and appears relatively refractory to antibiotic effects there, despite, in this series, the use of fluoroquinolones which have excellent cellular penetration. These data also provide support to observations from earlier studies which suggested that MDR serovar Typhi is intrinsically more virulent than antibiotic-sensitive organisms (19). Further study to determine whether active serovar Typhi replication occurs in human bone marrow will be of interest.
ACKNOWLEDGMENTS
We are very grateful to the director and staff of the Dong Thap Provincial Hospital and The Centre for Tropical Diseases, Ho Chi Minh City, Vietnam, and to Thomas Butler and John Robbins for advice at the beginning of the study.
This study was supported by the Wellcome Trust of Great Britain.
REFERENCES
- 1.Butler T, Bell W R, Levin J, Linh N N, Arnold K. Typhoid fever. Studies of blood coagulation, bacteremia, and endotoxemia. Arch Intern Med. 1978;138:407–410. doi: 10.1001/archinte.138.3.407. [DOI] [PubMed] [Google Scholar]
- 2.Conlan J W, North R J. Early pathogenesis of infection in the liver with the facultative intracellular bacteria Listeria monocytogenes, Francisella tularensis, and Salmonella typhimurium involves lysis of infected hepatocytes by leukocytes. Infect Immun. 1992;60:5164–5171. doi: 10.1128/iai.60.12.5164-5171.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunlap N E, Benjamin W H J, Berry A K, Eldridge J H, Briles D E. A ‘safe-site’ for Salmonella typhimurium is within splenic polymorphonuclear cells. Microb Pathog. 1992;13:181–190. doi: 10.1016/0882-4010(92)90019-k. [DOI] [PubMed] [Google Scholar]
- 4.Fame T M, Engelhard D, Riley H D., Jr Hemophagocytosis accompanying typhoid fever. Pediatr Infect Dis. 1986;5:367–369. doi: 10.1097/00006454-198605000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Farooqui B J, Khurshid M, Ashfaq M K, Khan M A. Comparative yield of Salmonella typhi from blood and bone marrow cultures in patients with fever of unknown origin. J Clin Pathol. 1991;44:258–259. doi: 10.1136/jcp.44.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gasem M H, Dolmans W M, Isbandrio B B, Wahyono H, Keuter M, Djokomoeljanto R. Culture of Salmonella typhi and Salmonella paratyphi from blood and bone marrow in suspected typhoid fever. Trop Geogr Med. 1995;47:164–167. [PubMed] [Google Scholar]
- 7.Gilman R H, Terminel M, Levine M M, Hernandez-Mendoza P, Hornick R B. Relative efficacy of blood, urine, rectal swab, bone-marrow, and rose-spot cultures for recovery of Salmonella typhi in typhoid fever. Lancet. 1975;i:1211–1213. doi: 10.1016/s0140-6736(75)92194-7. [DOI] [PubMed] [Google Scholar]
- 8.Hsu H S. Pathogenesis and immunity in murine salmonellosis. Microbiol Rev. 1989;53:390–409. doi: 10.1128/mr.53.4.390-409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ioannidis J P, Cappelleri J C, Lau J, Sacks H S, Skolnik P R. Predictive value of viral load measurements in asymptomatic untreated HIV-1 infection: a mathematical model. AIDS. 1996;10:255–262. doi: 10.1097/00002030-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Lien-Keng K, Odang O, Tumbelaka W. Diagnostic value of bone marrow and blood picture in salmonellosis. Ann Paediatr. 1960;194:141–149. [PubMed] [Google Scholar]
- 11.Macias E G. Typhoidal cells. Lancet. 1975;ii:927–928. doi: 10.1016/s0140-6736(75)92165-0. [DOI] [PubMed] [Google Scholar]
- 12.Mallory F B. A histological study of typhoid fever. J Exp Med. 1898;3:611–638. [PMC free article] [PubMed] [Google Scholar]
- 13.Nnalue N A, Shnyra A, Hultenby K, Lindberg A A. Salmonella choleraesuis and Salmonella typhimurium associated with liver cells after intravenous inoculation of rats are localized mainly in Kupffer cells and multiply intracellularly. Infect Immun. 1992;60:2758–2768. doi: 10.1128/iai.60.7.2758-2768.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pang T, Levine M M, Ivanoff B, Wain J, Finlay B B. Typhoid fever—important issues still remain. Trends Microbiol. 1998;6:131–133. doi: 10.1016/s0966-842x(98)01236-0. [DOI] [PubMed] [Google Scholar]
- 15.Richter-Dahlfors A, Buchan A M J, Finlay B B. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med. 1997;186:569–580. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubin F A, McWhirter P D, Burr D, Punjabi N H, Lane E, Kumala S, Sudarmono P, Pulungsih S P, Lesmana M, Tjaniadi P, et al. Rapid diagnosis of typhoid fever through identification of Salmonella typhi within 18 hours of specimen acquisition by culture of the mononuclear cell-platelet fraction of blood. J Clin Microbiol. 1990;28:825–827. doi: 10.1128/jcm.28.4.825-827.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin B M, Paik I K, Cho H I. Bone marrow pathology of culture proven typhoid fever. J Korean Med Sci. 1994;9:57–63. doi: 10.3346/jkms.1994.9.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sizemore D R, Elsinghorst E A, Eck L C, Branstrom A A, Hoover D L, Warren R L, Rubin F A. Interaction of Salmonella typhi strains with cultured human monocyte-derived macrophages. Infect Immun. 1997;65:309–312. doi: 10.1128/iai.65.1.309-312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wain J, Diep T S, Ho V A, Walsh A M, Nguyen T T, Parry C M, White N J. Quantitation of bacteria in blood of typhoid fever patients and relationship between counts and clinical features, transmissibility, and antibiotic resistance. J Clin Microbiol. 1998;36:1683–1687. doi: 10.1128/jcm.36.6.1683-1687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh A M, Smith M D, Wuthiekanun V, Sputtamongkol Y, Chaowagul W, Dance D A B, Angus B, White N J. Prognostic significance of quantitative bacteraemia in seticaemic meliodeosis. Clin Infect Dis. 1995;21:1498–1500. doi: 10.1093/clinids/21.6.1498. [DOI] [PubMed] [Google Scholar]
- 21.Yaupsky P, Nolte F S. Quantitative aspects of septicemia. Clin Microbiol Rev. 1990;3:269–279. doi: 10.1128/cmr.3.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]