Abstract
Background
To summarize the incidence and management strategy of vascular lake (VL) during the treatment of hepatocellular carcinoma (HCC) using transarterial chemoembolization (TACE) with CalliSpheres drug-eluting beads (DEBs), and to analyze its relationship with tumor response rate (RR). The etiology and clinical significance of VL were also analyzed based on the available literature.
Methods
The clinical data of 92 HCC patients who were treated with chemoembolization using CalliSpheres DEBs (DEB-TACE) in two centers were retrospectively analyzed. All 92 patients were treatment-naïve and treated by DEB-TACE. The incidence of VL and its clinical treatment during the first embolization session were summarized. The lesions were divided into a VL group and a non-VL group to analyze the relationship between VL and tumor RR.
Results
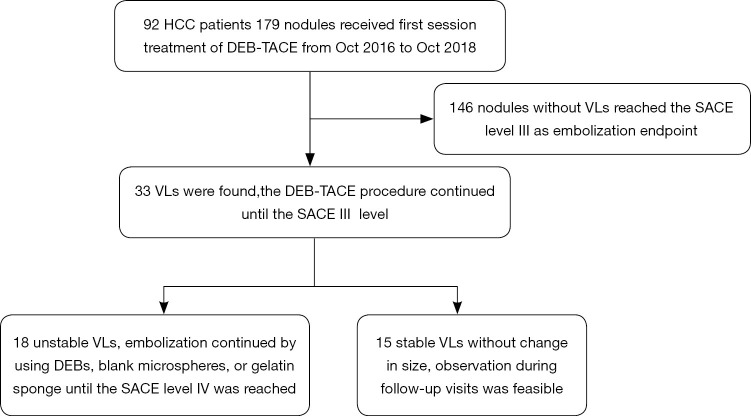
The embolization was successful in 98.9% of patients (91/92). A total of 33 VLs (18.4%; including 15 stable and 18 unstable VLs) were found among the 179 nodules treated. The unstable VLs were further embolized with embolic agent. One patient with unstable VL developed bleeding due to hepatic rupture and died. During the follow-up, residual tumors were found around 2 stable VLs, 2 lesions in 2 patients were treated with CT-guided radiofrequency ablation. The tumor RR was 84.4% in VL group, which was significantly higher than that (58.9%) in the non-VL group (P=0.007).
Conclusions
VL is a unique phenomenon during DEB-TACE. It may be accompanied by residual tumors and bleeding due to rupture. Therefore, VL should be cautiously managed in clinical practice.
Keywords: Hepatocellular carcinoma (HCC), CalliSpheres, chemoembolization, drug-eluting beads (DEBs), vascular lake
Introduction
Hepatocellular carcinoma (HCC) is a malignant tumor with high rates of recurrence and mortality. It is the sixth most common cancer and the second leading cause of cancer-related death worldwide. There were around 782,500 new cases and 745,500 deaths from liver cancer worldwide in 2012, and more than half of these new cases and deaths occurred in China (1,2). Up to 70% of HCC patients are already in the advanced stages when the disease is confirmed and thus have lost the chance to receive surgical treatment. Their 5-year survival rate is very low (1). As a local treatment, transarterial chemoembolization (TACE) has been recommended as the first-line treatment for Barcelona Clinic Liver Cancer (BCLC) stage B HCC in Europe and North America (3-5). Currently TACE is divided into conventional TACE (C-TACE) and TACE using drug-eluting beads (DEB-TACE). C-TACE is associated with high incidences of systemic and cardiac toxicities. In contrast, DEB-TACE uses DEBs loaded with chemotherapeutic agents to embolize the tumor-feeding arteries, thus achieving the effective combination of the sustained release of drugs and the embolization of arteries. Since the introduction of DEBs in clinical practice in 2004, many clinical studies and meta-analyses have demonstrated that DEB-TACE can achieve lower drug toxicities, higher tumor response rate (RR), and longer survival (6,7).
The imported DEBs in China include DCB (Biocompatibles, Farham, UK) and Hepasphere (HepaSphere; Merit Medical, South Jordan, Utah, USA); however, their clinical applications are restricted due to their high prices. CalliSpheres DEB is an independently developed DEBs product in China (Hengrui-Callisyn, Suzhou, Jiangsu Province, China). It was marketed two years ago, and therefore few studies have described its clinical applications.
In our current study, we summarized the experience of two clinical centers in treating HCC with DEB-TACE using CalliSpheres and analyzed the incidence of vascular lake (VL), a unique complication during DEB-TACE, and its clinical treatment strategy. In addition, the etiology and clinical significance of VL were also investigated.
Methods
Patients
This was a multi-center retrospective study. All the data were collected from our department and the Sun Yat-sen Memorial Hospital, Sun Yat-sen University. The inclusion criteria were as follows: (I) with clinically or pathologically confirmed HCC; (II) with a Child-Pugh class of A or B; (III) with an ECOG PS score of 0 or 1; (IV) in the BCLC stage A or B; (V) without surgical indication or unwilling to receive surgical resection; and (VI) with normal cognitive and physical abilities. The exclusion criteria included the following: (I) with a Child-Pugh class of C; (II) in the BCLC stage C or D; (III) accompanied by acute infection or with poorly controlled chronic infectious disease; (IV) with heart, lung, and/or kidney dysfunction; and (V) with ascites or spontaneous peritonitis. A total of 92 HCC patients treated with DEB-TACE using CalliSpheres from October 2016 to October 2018 were enrolled in this study (Table 1). The disease was pathologically or clinically diagnosed and met the diagnostic criteria of the American Association for the Study of Liver Diseases (AASLD) (8).
Table 1. Baseline data of patients.
| Parameters | Patients (n=92) |
|---|---|
| Age (years) | 43.5±16.2 |
| Gender (male/female) | 75/17 |
| History of hepatitis, n (%) | 90 (97.8) |
| HBV | 83 (90.2) |
| HCV | 6 (6.5) |
| HBV and HCV, n (%) | 1 (1.0) |
| No history of hepatitis, n (%) | 2 (2.1) |
| Child-Pugh stage, n (%) | |
| A | 62 (67.4) |
| B | 30 (32.6) |
| ECOG performance status, n (%) | |
| 0 | 75 (81.5) |
| 1 | 17 (18.4) |
| BCLC clinical stages, n (%) | |
| A | 45 (48.9) |
| B | 47 (51.0) |
| Tumor distribution, n (%) | |
| Single lesion | 43 (46.7) |
| Multiple lesions | 49 (53.3) |
| Tumor size (cm) | 6.4±2.3 |
| Previous treatments, n (%) | |
| Surgery | 21 (22.8) |
| No treatment | 71 (77.1) |
Data are presented as mean ± standard deviation, median (25th–75th) or count (%). ECOG, Eastern Cooperative Oncology Group; BCLC, Barcelona Clinic Liver Cancer BCLC, Barcelona Clinic Liver Cancer.
Routine examinations before chemoembolization procedure
After admission, the patient underwent routine plain and contrast-enhanced abdominal CT scans and routine electrocardiography (ECG). Blood biochemical tests were performed for blood cell counts, liver and kidney functions, bleeding and clotting time, hepatitis B virus DNA, tumor marker, and other indicators. Meanwhile, hepatoprotective symptomatic treatments and anti-hepatitis B virus therapy were provided as appropriate. After the examination results became available, the liver function was assessed according to Child–Pugh classification and the clinical staging was performed based on the BCLC guidelines. All patients and/or their families signed an informed consent form and a sheet of implantable medical materials before the procedure.
Tramadol 100 mg was intra-muscularly injected for analgesia 30 min before the procedure. The venous route was placed, and its patency was maintained with normal saline.
DEB-TACE procedure
The DEB-TACE procedure for all patients was performed under digital subtraction angiography (DSA) in the interventional operating room of two centers. The procedure was performed by a deputy chief physician or an attending physician with more than 10 years of experience under the guidance of a chief physician. All 92 patients were treatment-naïve and received DEB-TACE for the first time. (I) Local anesthesia was obtained with 1% lidocaine. After femoral artery puncture under local anesthesia, the guide wire and catheter were inserted using the Seldinger technique. (II) After a pigtail angiographic catheter (Cook, USA) was placed for abdominal aortography, a Yashiro catheter (Terumo, Japan) was introduced into the celiac artery and superior mesenteric artery for angiography, so as to identify the site, number, and target vessels of tumors. (III) Callispheres DEBs were loaded with pirarubicin 50 mg (Shenzhen Main Luck Pharmaceuticals, Shenzhen, China). More specifically, a vial of Callispheres DEBs was drawn with a 20 mL syringe and left at room temperature for 5 minutes before the supernatants were discarded. Then, 5 mL of pirarubicin solution was added and let stand for 30 minutes, during which the mixture was gently shaken every 5 minutes. After 30 minutes, 12 mL of non-ionic contrast agent and 3 mL of water for injection were added. Then, the syringe was connected to a T-tube and a 3 mL syringe. (IV) Superselective arterial catheterization into the tumor-feeding vessel was performed using a microcatheter (Progreat; Terumo, Tokyo, Japan), during which any injury to the gallbladder artery, right gastric artery, and non-targeted blood vessels should be avoided. (V) The microcatheter was connected into a T-tube. A 3 mL syringe was used to draw 3 mL of the mixture of DEBs and contrast agent, which was slowly injected into the microcatheter at a rate of 1 mL/min. Meanwhile, fluoroscopy was used to monitor the flow and distribution of the mixture. The microcatheter was intermittently and slowly rinsed with normal saline. (VI) A subjective angiographic chemoembolization endpoint (SACE) level III (9) was used as the embolization endpoint; that is, the tumor stain diminished and the tumor feeding vessels decreased. If unstable VL was found, DEBs and/or blank microspheres (large particle size) and gelatin sponge were used for embolization until the SACE IV level (i.e., both tumor stain and tumor feeding vessels disappeared) was reached (Figure 1). (VII) After reaching the embolization endpoint, a second angiography was performed after waiting 5 minutes. If the embolization endpoint was found to be degraded, further embolization sessions were performed until the embolization endpoint reached a steady state. (VIII) If the embolization endpoint was still not reached after one vial of DEBs was used up, additional embolization sessions with 300–500 µm of blank microspheres were performed until the endpoint was reached. Embolization of the main tumor feeding artery with a gelatin sponge was not performed in patients without unstable VL.
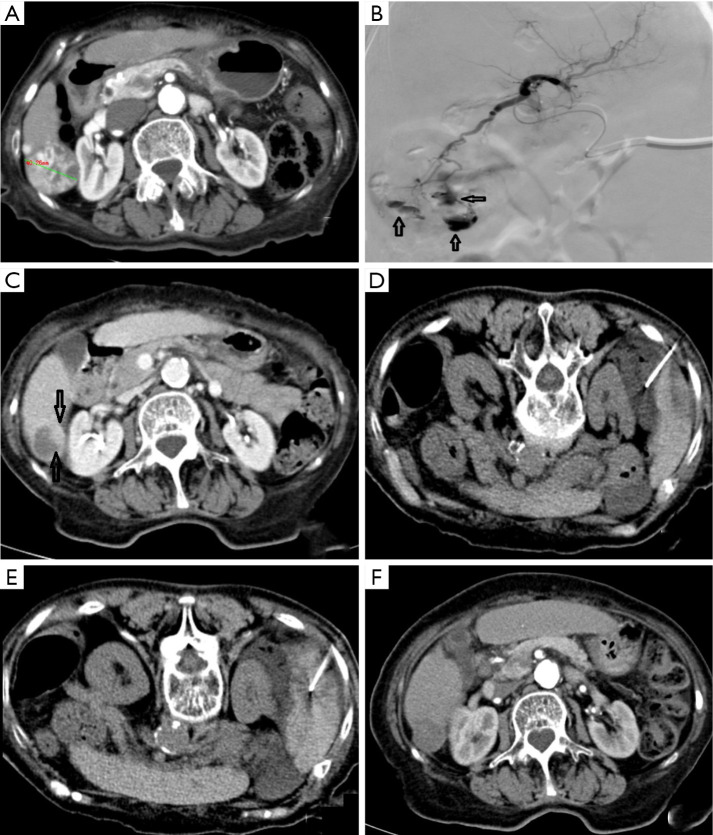
Figure 1.
A 48-year-old male received DEB-TACE for HCC, and the VL was managed. (A,B) CT scan before surgery; (C) an angiography after superselective microcatheterization in the left hepatic artery revealed a tumor with rich blood supply in the left liver lobe; (D) multiple unstable VLs (indicated by hollow arrows) were found after DEB-TACE, and tumor stain decreased remarkably; (E) a second angiography after embolization using gelatin sponge revealed that both the tumor-feeding vessels and unstable VLs had disappeared, with the embolization endpoint being SACE level IV. DEB, drug-eluting beads; TACE, transarterial chemoembolization; VL, vascular lake; SACE, subjective angiographic chemoembolization endpoint.
Treatments after embolization
After the patient returned to the ward, ECG monitoring was applied for 24 hours; if VL was identified, the duration of monitoring was extended to 72 hours. Meanwhile, symptomatic and supportive treatments (including liver-protective, antiemetic, and antiviral treatments) were applied.
Definition, classification, and statistical methods of VL
The VL was defined as a localized pooling of contrast agent within the tumor in any step of embolization, which persists in the venous phase of angiography, in different forms of lumps or patches. It can be focal, with clear boundary, and is quite different from tumor stain. VL can occur at different stages of embolization, and its shape and size can vary with the injection of DEBs (10-13). In our current study, VLs were divided into two types according to their morphological enlargement and adjacent position: stable and unstable. Stable VLs had no morphological or size changes after multiple angiography scans and were not treated. In contrast, the unstable VLs gradually increased in size on angiographic scans or they were more adjacent to the liver surface; thus, DEBs or large particle-sized microspheres and gelatin sponge (1,000–1,400 µm) were applied for embolization until the VL disappeared and the main tumor-feeding artery was occluded. The embolization endpoint was SACE level IV.
Follow-up plan
Patients were asked to return for a follow-up visit 6–8 weeks after the procedure. The follow-up evaluation included upper abdominal CT/MRI plain and contrast-enhanced scans, measurements of tumor markers (e.g., AFP), and liver and kidney function tests. Based on the evaluation results, patients received further treatment or were arranged to receive the next follow-up visits.
Data analysis
The number of tumor nodules in these 92 patients was counted. The number of LVs detected in the first DEB-TACE session and their treatment methods were also summarized. Multiple VLs found in one single tumor nodule were counted as 1 VL.
Successful operation
During a successful procedure, the catheter introduced into the feeding vessel and delivers DEBs to the target area, reaching the expected endpoint of embolization. No procedure-related death occurs during the hospitalization.
Evaluation methods
The treatment response was evaluated with the Modified Response Evaluation Criteria in Solid Tumors (mRECIST) (14). The response rate (RR) was defined as complete response rate (CR) + partial response rate (PR), and the result was analyzed and recorded for each lesion. Patients were divided into a VL group and a non-VL group, and the difference in RR after the first DEB-TACE session was compared between these two groups.
Statistical analysis
Statistical analysis was performed in the SPSS 13.0 software (IBM, USA). The difference in RR value between these two groups was compared using the chi square test. P value of <0.05 was considered statistically significant, and P value of <0.01 was considered highly statistically significant.
Results
All the 92 HCC patients were treatment-naïve, and DEB-TACE was applied in the first interventional therapy. The success rate was 98.9%. Among them, 43 patients had a single nodule while 49 (64%) had multiple nodules. A total of 179 nodules were found, among which 33 VLs (18.4%) (15 stable VLs and 18 unstable VLs) were detected. For unstable VLs, embolic agents were used for subsequent embolization sessions until both VL and tumor-feeding vessels disappeared. In one patient with unstable VL, the diameter of the lesion was 3.0 cm. Embolization using DEBs continued until SACE level IV was achieved. Abdominal distension and decreased blood pressure occurred 6 hours later. The uncoagulated blood was extracted by diagnostic abdominal puncture. The clinical diagnosis was hemorrhage due to hepatic rupture. A second angiography revealed that the hepatic artery and its branches remarkably contracted and thus the superselective catheterization could not be completed. The main hepatic artery was embolized with gelatin sponge. The patient died of abdominal hemorrhage and multiple organ failure 48 hours after the operation. In the VL group, 2 patients (2 nodules) with stable VL were found to have residual lesions near the VLs. After CT-guided radio frequency ablation (RFA), the residual lesions disappeared (Figure 2). The nodules evaluated as CR were followed up regularly and the remaining nodules were treated with a second DEB-TACE or CT-guided RFA.
Figure 2.
A 75-year-old female received DEB-TACE for HCC. VL was found but not treated. Local residual tumors were treated with radiofrequency ablation. (A) Pre-operative CT scan revealed a 4.0-cm HCC lesion with rich blood supply in hepatic segment 6 (S6). (B) VLs (indicated by hollow arrows) were found during D-RACE. Stable VLs were considered, and no further treatment was applied. The embolization endpoint was SACE level III. (C) Local residual lesions (indicated by hollow arrows) around VL, along with necrotic tissues around the tumors, were found 2 months after operation. (D) After CT-guided puncture of the hepatorenal recess with a 21 G puncture needle, 500 mL of normal saline was injected to induce artificial ascites, which increased the distance between liver and kidney. (E) CT-guided radiofrequency ablation of residual lesions was performed using a 17 G radiofrequency needle, assisted by artificial ascites technique. (F) Two months after radiofrequency ablation, no residual tumors were found, the lesion area was not enhanced, and the lesion was reduced to 2 cm in diameter. DEB, drug-eluting beads; TACE, transarterial chemoembolization; HCC, hepatocellular carcinoma; SACE, subjective angiographic chemoembolization endpoint.
Postoperative follow-up was completed in the remaining 91 patients (178 nodules) (Table 2). Statistical analysis showed that there was a highly significant difference in RR between the non-VL group and VL group after the first DEB-TACE session (P<0.01).
Table 2. Local response rate in the two groups.
| Group | CR | PR | SD | PD | Response rate (CR+PR) |
|---|---|---|---|---|---|
| Non-VL group (n=146) | 40 | 46 | 40 | 20 | 58.9% (86/146) |
| VL group* (n=32) | 10 | 17 | 5 | 0 | 84.4% (27/32) |
χ2=7.345, P=0.007. *, one patient died of liver rupture and was not enrolled in the follow-up.
Discussion
While VL occurs occasionally during DEB-TACE for HCC, few systematic reports have described this condition. The incidence of VL ranged from 12.1% to 25.5% (calculated by nodules) as reported in the literature (10-12). In our current retrospective analysis, the clinical data from two medical centers showed that the incidence of VL was 18.4%, which was similar to the data reported in the literature. However, the DEBs used in the literatures were DCB or Hepasphere, and no article has reported VLs found after the application of CalliSpheres for the treatment of HCC.
CalliSpheres is the first DEBs with independent intellectual property rights in China. Its material and drug-loading principle are basically similar to DCB. CalliSphere carries negatively charged sulfonic acid groups, which can be loaded with adriamycin through ion exchange. It is characterized by high compressibility and high throughput. Animal experiments and pharmacological experiments have verified its drug-loading performance (15), and a number of clinical studies have confirmed its safety and efficiency (16,17). Since CalliSpheres DEBs have just been marketed, few clinical studies have investigated this product; to our knowledge, no clinical report on VL and its management has been published.
VL is a unique complication found only in HCC patients treated with DEB-TACE; this has not been found in C-TACE cases. Yamanaka et al. (18) reported the local retention of contrast agent following the invasion of vascular wall by tumors and the necrosis of tumors; however, this phenomenon could be found during angiography. In contrast, VL was not found during angiography; rather, it occurred only after the initiation of embolization. Furthermore, it could occur in all phases of embolization, and the timing of its occurrence was irregular. VL was not found during the treatment of liver metastases from colorectal cancer and other types of liver metastases by using DEBs loaded with irinotecan in two medical centers, which might be explained by the different characteristics of the feeding vessels of primary and metastatic liver tumors.
The etiology of VL remains unclear. Seki et al. (10) discussed the formation of VL as follows: when the DEBs slowly flew into the tumor vessels along with the arterial blood stream, they were distributed along with the dominant blood stream rather than evenly entering all the tumor vessels. As a result, some tumor vessels were occluded while others remained patent, resulting in the change in intratumoral blood pressure and the re-distribution of the blood stream. Due to an aberrant intratumoral space newly developing during embolization, the intratumoral blood pressure gradually increased with the injection of DEBs, leading to the rupture of unoccluded tumor vessels; as a result, the contrast material extravasation into tumor parenchyma caused the rupture and bleeding of the tumor, which is similar to the “tumor apoplexy” in patients with liver cancer rupture and bleeding. Under the angiography, the size and shape of the contrast material vary after entering VLs and may change or remain unchanged with the injection of contrast material.
The risk factors and clinical significance of VLs also need to be further clarified. In their multivariate regression analysis, Seki et al. (10) found that the maximum tumor diameter ≥3 cm and a dosage of beads used of more than 10 mg were two independent risk factors for VLs. Cavalcante et al. (11) found that the predictors of VL included pseudocapsule, tumor diameter greater than 3 cm, and increased AFP. Both of these studies also analyzed the clinical significance of VL and concluded that patients in the VL group could obtain higher RR. Similarly, our current study also demonstrated that the RR in the VL group was significantly higher than that in the non-VL group.
Unfortunately, no consensus has been reached on VL management. In Seki et al.’s study, the stable or growing VLs were embolized with gelatin sponge particles until the VLs disappeared, and the disappearance or remarkable shrinkage of the VLs during the DEB procedure and achievement of the DEB embolic endpoint, the operation was finished (10). In Cavalcante et al.’s practice, if VL was detected during the treatment, the TACE using DEBs or blank microspheres continued until the VL disappeared on a second angiography (11). According to Chang et al., if VL was found, embolization with gelatin sponge particles was performed until the VL disappeared, so as to achieve better efficiency (19). In our current study, VLs were divided into stable or unstable types, and different treatments were applied. The unstable VL is characterized by its large size, gradual enlargement over time on multiple angiographic scans, and risk of rupture near the liver surface. The features of stable VL are as follows: located in the deep layer of the tumor; without a trend of growth on repeated angiographic scans; and without any vital structure around it. The specific management strategies were as follows (Figure 3): if VL was detected during the procedure, the DEB-TACE continued as planned; when the DEBs were used up, a larger number of large-particle blank microspheres were used for embolization until SACE level III was reached. For the unstable VLs, embolization continued by using DEBs, blank microspheres, or gelatin sponge until the SACE level IV was reached; for the stable VLs without change in size, observation during follow-up visits was feasible. Patients with VL returned to the ward after the operation, and their blood pressure was monitored for 72 hours by ECG.
Figure 3.
Treatment strategies flow.
During the follow-up, two stable VLs were found to have residual tumors in the VL region, along with obvious necrosis in other regions. CT-guided local ablation was then applied. The possible etiology might be as follows: the extravascular shunt of DEBs leads to incomplete embolization of tumor-feeding vessels, resulting in incomplete tumor necrosis. In one patient with one unstable VL, embolization using DEBs alone was applied because the lesion was small. It might because the microspheres re-distributed along with blood stream over time and then re-located, leading to incomplete embolization; VL gradually grew with the increased intratumoral blood pressure and finally became ruptured. Neither case has been reported in the literature.
There are some limitations to the present study. (I) Due to the small sample size of our study and the low incidence of VL, there were only a few patients in the VL group and non-VL group, and the numbers were too small to support further stratification; (II) since only the VLs found during the first DEB-TACE session were analyzed, we did not collect survival-related data and thus could not analyze the relationship between VL and survival; (III) the difference in VL incidence between CalliSphere and other imported DEBs was not compared; and (IV) whether the inconsistent endpoint of embolization between the unstable VL subgroup and the non-VL group affects the RR of tumors could not be explored in our current study due to the small sample size of the subgroup.
Conclusions
CalliSpheres is China’s first drug-eluting microsphere product, with independent intellectual property rights. Embolization using CalliSpheres DEBs for HCC can also cause VL, which is a very unique complication of this procedure. Although it has been reported in the literature that VL is a predictor of higher RR after DEB-TACE for HCC, it can also be associated with local residual tumor or tumor rupture, which should be actively recognized and carefully dealt with.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committee of Shenzhen People’s Hospital (IRB number: LL-KY-201610001) and written informed consent was obtained from all patients. Informed consent was obtained from all individual participants included in the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.65). The authors have no conflicts of interest to declare.
References
- 1.Nenu I, Breaban I, Pascalau S, et al. The future is now: beyond first line systemic therapy in hepatocellular carcinoma. Transl Cancer Res 2019;8:S261-74. 10.21037/tcr.2018.11.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 3.Chen P, Yuan P, Chen B, et al. Evaluation of drug-eluting beads versus conventional transcatheter arterial chemoembolization in patients with unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol 2017;41:75-85. 10.1016/j.clinre.2016.05.013 [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Feng LH, Qian YW, et al. Does microvascular invasion in Barcelona Clinic Liver Cancer stage A multinodular hepatocellular carcinoma indicate early-stage behavior? Ann Transl Med 2019;7:428. 10.21037/atm.2019.08.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005;42:1208-36. 10.1002/hep.20933 [DOI] [PubMed] [Google Scholar]
- 6.Gorodetski B, Chapiro J, Schernthaner R, et al. Advanced-stage hepatocellular carcinoma with portal vein thrombosis: conventional versus drug-eluting beads transcatheter arterial chemoembolization. Eur Radiol 2017;27:526-35. 10.1007/s00330-016-4445-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ni JY, Xu LF, Wang WD, et al. Conventional transarterial chemoembolization vs microsphere embolization in hepatocellular carcinoma: a meta-analysis. World J Gastroenterol 2014;20:17206-17. 10.3748/wjg.v20.i45.17206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin B, Wang DX, Lewandowski RJ, et al. The Impact of Chemoembolization Endpoints on Survival in Hepatocellular Carcinoma Patients. AJR Am J Roentgenol 2011;196:919-28. 10.2214/AJR.10.4770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seki A, Hori S, Shimono C. Management of vascular lake phenomenon on angiography during chemoembolizational with superabsorbent polymer microspheres. JPN J Radiol 2015;33:741-8. 10.1007/s11604-015-0486-2 [DOI] [PubMed] [Google Scholar]
- 11.Cavalcante RN, Nasser F, Motta JM, et al. Occurrence of vascular lake phenomenon as a predictor of Improved tumor response in HCC patients that underwent DEB-TACE. Cardiovasc Intervent Radiol 2017; 40:1044-51. 10.1007/s00270-017-1678-1 [DOI] [PubMed] [Google Scholar]
- 12.Crespi S, Martinetti L, Nicolini A. Outcome of HCC patients Presenting pooling phenomenom during TACE. Cardiovasc Intervent Radiol 2013;36:S323-8. [Google Scholar]
- 13.Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 2010;33:41-52. 10.1007/s00270-009-9711-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. 10.1055/s-0030-1247132 [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Huang C, Li Z, et al. Comparison of pharmacokinetics and drug release in tissues after transarterial chemoembolization with doxorubicin using diverse lipiodol emulsions and CalliSpheres Beads in rabbit livers. Drug Deliv 2017;24:1011-7. 10.1080/10717544.2017.1344336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Huang W, He M, et al. Efficacy and safety of CalliSpheres® drug-eluting beads transarterial chemoembolization in Barcelona Clinic Liver Cancer stage C patients. Oncol Res 2019;27:565-73. 10.3727/096504018X15313896322888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Zhou J, Zhu DD, et al. CalliSpheres® drug-eluting beads (DEB) transarterial chemoembolization (TACE) is equally efficient and safe in liver cancer patients with different times of previous conventional TACE treatments: a result from CTILC study. Clin Transl Oncol 2019;21:167-77. 10.1007/s12094-018-1902-8 [DOI] [PubMed] [Google Scholar]
- 18.Yamanaka J, Yamanaka N, Nakasho K, et al. Clinicopathologic analysis of stage II-III hepatocellular carcinoma showing early massive recurrence after liver resection. J Gastroenterol Hepatol 2000;15:1192-8. 10.1046/j.1440-1746.2000.02323.x [DOI] [PubMed] [Google Scholar]
- 19.Chang PY, Huang CC, Hung CH, et al. Multidisciplinary Taiwan Consensus Recommendations for the Use of DEBDOX-TACE in Hepatocellular Carcinoma Treatment. Liver Cancer 2018;7:312-22. 10.1159/000487608 [DOI] [PMC free article] [PubMed] [Google Scholar]