PURPOSE
Calcineurin inhibitors (CNI) are standard components of graft-versus-host disease (GVHD) prophylaxis after hematopoietic cell transplantation (HCT). Prior data suggested that CNI-free approaches using donor T-cell depletion, either by ex vivo CD34 selection or in vivo post-transplant cyclophosphamide (PTCy) as a single agent, are associated with lower rates of chronic GVHD (cGVHD).
METHODS
This multicenter phase III trial randomly assigned patients with acute leukemia or myelodysplasia and an HLA-matched donor to receive CD34-selected peripheral blood stem cell, PTCy after a bone marrow (BM) graft, or tacrolimus and methotrexate after BM graft (control). The primary end point was cGVHD (moderate or severe) or relapse-free survival (CRFS).
RESULTS
Among 346 patients enrolled, 327 received HCT, 300 per protocol. Intent-to-treat rates of 2-year CRFS were 50.6% for CD34 selection (hazard ratio [HR] compared with control, 0.80; 95% CI, 0.56 to 1.15; P = .24), 48.1% for PTCy (HR, 0.86; 0.61 to 1.23; P = .41), and 41.0% for control. Corresponding rates of overall survival were 60.1% (HR, 1.74; 1.09 to 2.80; P = .02), 76.2% (HR, 1.02; 0.60 to 1.72; P = .95), and 76.1%. CD34 selection was associated with lower moderate to severe cGVHD (HR, 0.25; 0.12 to 0.52; P = .02) but higher transplant-related mortality (HR, 2.76; 1.26 to 6.06; P = .01). PTCy was associated with comparable cGVHD and survival outcomes to control, and a trend toward lower disease relapse (HR, 0.52; 0.28 to 0.96; P = .037).
CONCLUSION
CNI-free interventions as performed herein did not result in superior CRFS compared with tacrolimus and methotrexate with BM. Lower rates of moderate and severe cGVHD did not translate into improved survival.
INTRODUCTION
Graft-versus-host disease (GVHD) is a common complication after allogeneic hematopoietic cell transplantation (HCT) and remains a challenge for broader application of this curative therapy.1 The combination of a calcineurin inhibitor (CNI) such as cyclosporine or tacrolimus (Tac) with methotrexate (MTX), described over three decades ago,2 provides partial protection against severe forms of acute GVHD and historically has been the basis of most HCT regimens worldwide. However, this standard approach has several limitations. CNI requires pharmacokinetic monitoring and can accentuate the risk of renal toxicity and thrombotic microangiopathy. Prolonged CNI prophylaxis predisposes patients to infections and may dampen graft-versus-tumor effects,3 and may also preclude or diminish the effectiveness of post-HCT cellular or other therapies for treatment or prevention of infections or disease relapse. Perhaps, most importantly, CNI-based GVHD prophylaxis does not effectively prevent chronic GVHD,4 the most common cause of late post-HCT morbidity and mortality, affecting 35%-55% of transplant survivors.5-7 The choice of graft source affects the incidence of chronic GVHD, with mobilized peripheral blood stem cells (PBSC) having higher rates and more severe manifestations compared with bone marrow (BM) grafts.5,8
CONTEXT
Key Objective
Are calcineurin inhibitor (CNI)-free graft-versus-host disease (GVHD) prophylaxis superior to contemporary combination of tacrolimus and methotrexate with bone marrow (BM) for matched transplants in relation to better prevention of chronic GVHD and survival?
Knowledge Generated
CD34 selection resulted in lower rates of chronic GVHD but was associated with worse survival compared with the other interventions.
Post-transplant cyclophosphamide without CNI was not superior to tacrolimus and methotrexate with BM in relation to chronic GVHD and survival.
Relevance
CNI-free interventions with post-transplant cyclophosphamide or CD34 selection were not better than tacrolimus and methotrexate with BM after HLA-match transplants. Improvement of chronic GVHD prevention did not translate to better survival.
Recognition that GVHD is mediated by donor-derived T cells led to preclinical and clinical exploration of strategies for T-cell depletion (TCD).9,10 Positive selection of CD34+ cells using immunomagnetic beads and column separation leads to consistent and reproducible broad T-cell depletion of PBSC grafts, achieving 4-5 log-reduction of T cells in the graft.11 Despite early concerns of increased disease relapse,12 more recent studies using this approach with intensified myeloablative conditioning regimens demonstrated no increased risk in disease relapse among patients with acute leukemia.13
Another approach uses cyclophosphamide post-HCT (PTCy) for in vivo purging of alloreactive T cells. This demonstrated promising GVHD control as a single agent without chronic immunosuppression in the setting of HLA-matched HCT with BM and myeloablative conditioning.14,15
The objective of this trial was to compare these two CNI-free approaches for reduction of chronic GVHD to one another and to standard CNI-based GVHD prevention in patients with HLA-matched donors. An important goal was to identify effective CNI-free GVHD prophylaxis strategies that could serve as a platform for post-HCT therapies, particularly cellular therapies, to further optimize transplant outcomes.
METHODS
Design and Patients
The Blood and Marrow Transplant Clinical Trial Network (BMT CTN) 1301 trial was a phase III randomized, multicenter unblinded study comparing three HCT approaches among recipients of an HLA-matched graft and using myeloablative conditioning (NCT02345850). It enrolled patients at 26 centers in the United States and Germany.
Eligible patients were age 65 years and younger and about to undergo HLA-matched myeloablative HCT for treatment of acute leukemia in complete morphologic remission (CR), CR without hematologic recovery, or myelodysplastic syndrome (MDS) with < 5% blasts in BM. HLA-matched related or unrelated donors were defined as 8 out of 8 matches at HLA-A, -B, -C and DRB1 (Data Supplement, online only). Patients whose donors had a preference or contraindication to donate mobilized PBSC or BM were not eligible. The study was approved by each center's institutional review board, and all patients provided written informed consent before enrollment.
Procedures
Patients were randomly assigned to one of three specified interventions: (1) ex vivo CD34 selected T-cell–depleted PBSC graft without additional immunosuppression; donors were mobilized with granulocyte colony-stimulating factor according to institutional standards with a target CD34+ cell dose of ≥ 5 × 106 cells/kg; (2) unmanipulated BM graft followed by Cy 50 mg/kg on days +3 and +4 post-HCT; or (3) unmanipulated BM graft with post-HCT Tac and MTX. Tac was started on day –3 and dosed to maintain therapeutic levels between 5 and 15 ng/mL for a minimum of 90 days post-HCT. MTX was dosed at 15 mg/m2 intravenously (IV) at day +1 and 10 mg/m2 IV days +3, +6, and +11. Conditioning regimens varied per treatment arm and are shown in the Data Supplement. Supportive care details are described in the Data Supplement.
Cell Processing
Ex vivo T-cell depletion in the CD34 selection arm was performed using the CliniMACS CD34 Reagent System (Miltenyi Biotec, Gladbach, Germany) according to standard operating procedures in place and validated at treatment sites. Cell processing occurred within 36 hours from cell collection. The release criteria for the postprocessing graft included viability ≥ 70%, negative gram stain, CD34+ cell dose ≥ 2 × 106 per kg, and CD3+ cell dose ≤ 1 × 105 per kg.
Outcomes
The primary end point of this trial was a composite of moderate to severe chronic GVHD, disease relapse, and survival (CRFS).16 Secondary end points included overall survival (OS), acute and chronic GVHD, disease relapse, relapse-free survival (RFS), transplant-related mortality (TRM), immunosuppression-free survival, hematologic recovery (neutrophil and platelets, and delayed engraftment), toxicities, infections (overall, cytomegalovirus [CMV] and Epstein-Barr virus [EBV]), and health-related quality of life (QOL). Outcome definitions are in the Data Supplement.
Statistical Analysis
This study was designed as a three-arm randomized phase III multicenter trial comparing the two CNI-free strategies for GVHD prophylaxis to one another and to standard Tac and MTX in patients with acute leukemia or MDS undergoing a myeloablative conditioning HCT. Patients were randomly assigned using permuted blocks of random sizes and stratified by donor type (sibling v unrelated) and age (< 18, 18-40, and > 40 years). For each pairwise comparison, a P value of .05/3 (.017) was considered statistically significant. A stopping rule for 100-day mortality after random assignment was monitored separately in each of the three treatment arms using sequential probability ratio test for censored exponential data. An independent medical monitor and a data and safety monitoring board oversaw trial safety. The primary analysis was planned for when 155 events were observed for each comparison to control or after all patients completed 2-year follow-up per protocol, whichever came first. The trial design assumed that the CRFS probabilities in the control group were 46%, 28%, and 22% at 6 months, 1 year, and 2 years, respectively, on the basis of data from the Center for International Blood and Marrow Transplant Research (CIBMTR), used a piecewise exponential survival function to fit those CRFS probabilities, and ensured approximately 85% power to detect a hazard ratio (HR) of 0.576 (corresponding to a 20% difference in CRFS at 1 year) with a target enrollment of 346 patients. The primary analysis was performed using intent-to-treat population, with Kaplan-Meier (KM) estimates of CRFS described for each group and pairwise comparisons conducted using log-rank tests, with a type I error of 0.05/3 to account for multiple testing. HRs and CIs from a Cox model with treatment as a covariate are also provided. OS was a key secondary end point, with explicit control of type I error rate through a gatekeeper approach17 for formal significance testing when the CRFS comparison was significant. OS and RFS were estimated using KM method for each group and compared using log-rank tests. Acute and chronic GVHD, disease relapse, TRM, hematologic recovery, and infections were described using cumulative incidence and compared using Gray's test.18 Secondary analyses of CRFS, OS, RFS, relapse, TRM, and GVHD were conducted using Cox regression, adjusting for donor type, age, performance status, disease, and disease risk index (DRI).19 Post hoc as-treated analysis was performed based on the number of patients who did not receive the intervention on the basis of the random assignment results, mostly observed in the CD34 selection group.
All components of CRFS were reviewed by an independent end point review committee that was blinded to treatment allocation. SAS and STAT software program, version 9.4, was used for all analyses.
RESULTS
Patients
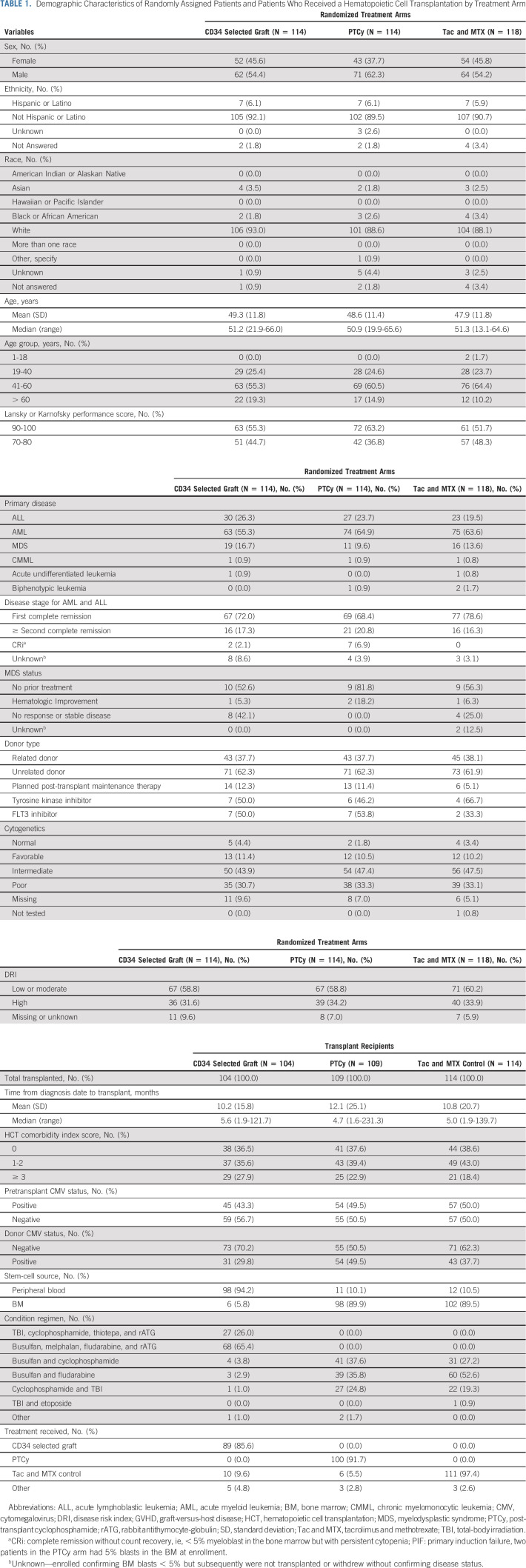
From September 2015 through June 2018, a total of 346 patients from 26 centers in the United States and Germany were enrolled, 327 patients were transplanted, and 300 treated per protocol (Fig 1). Noncompliance with treatment arm allocation rates was 14.4%, 8.2%, and 2.6% for the CD34+ selection, PTCy, and Tac and MTX control, respectively. Median age was 51 years (range, 13-66 years), 61.3% of patients had acute myeloid leukemia, 33.2% had high DRI, 62.1% received an unrelated donor graft, and the median time from diagnosis to HCT was 5 months (range, 1.3-231 months); all variables were balanced across treatment arms (Table 1).
FIG 1.
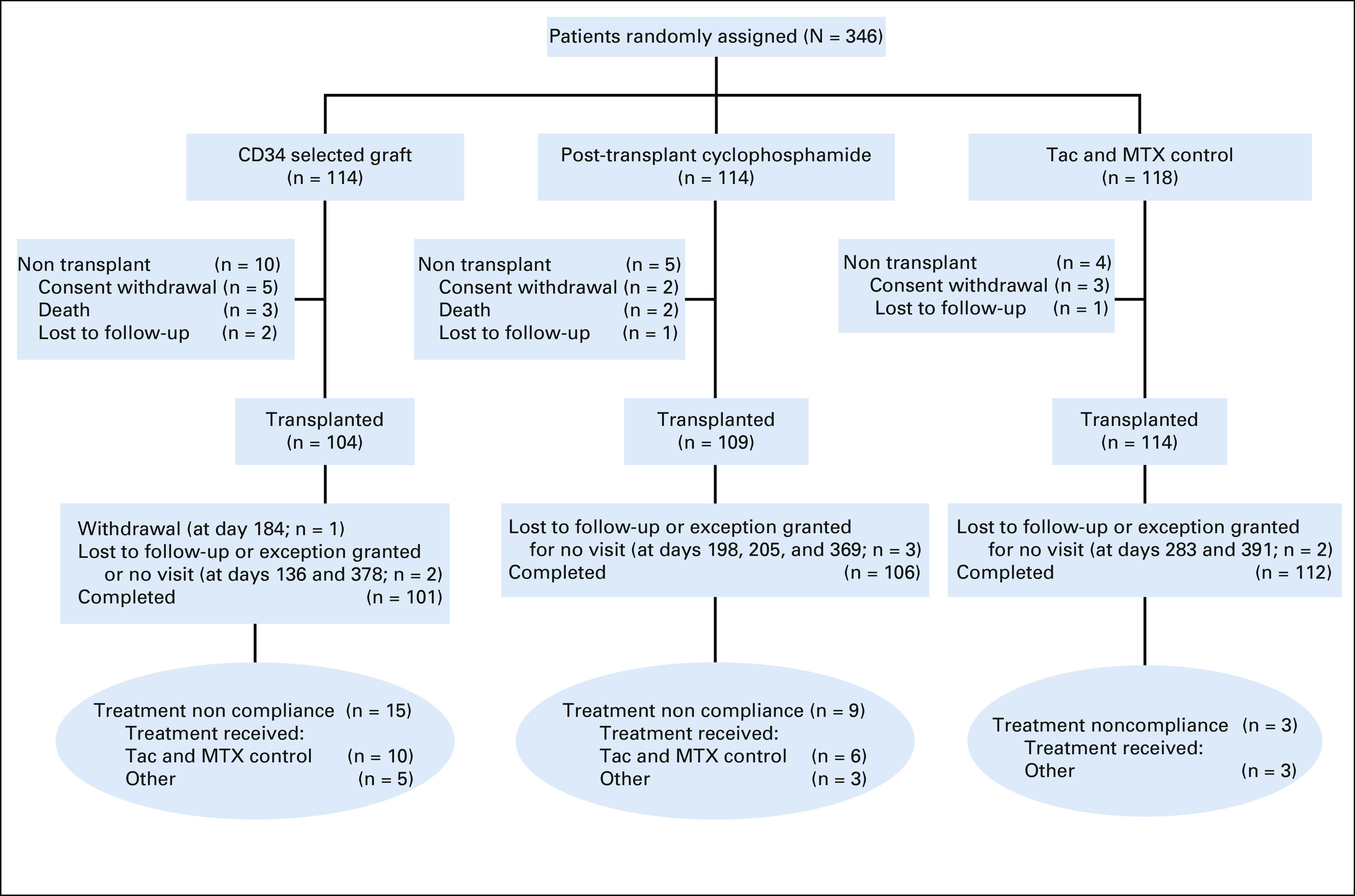
CONSORT diagram. Completed indicates participants has completed the 2-year follow-up per protocol or died during the study. Tac and MTX, tacrolimus and methotrexate.
TABLE 1.
Demographic Characteristics of Randomly Assigned Patients and Patients Who Received a Hematopoietic Cell Transplantation by Treatment Arm
CRFS
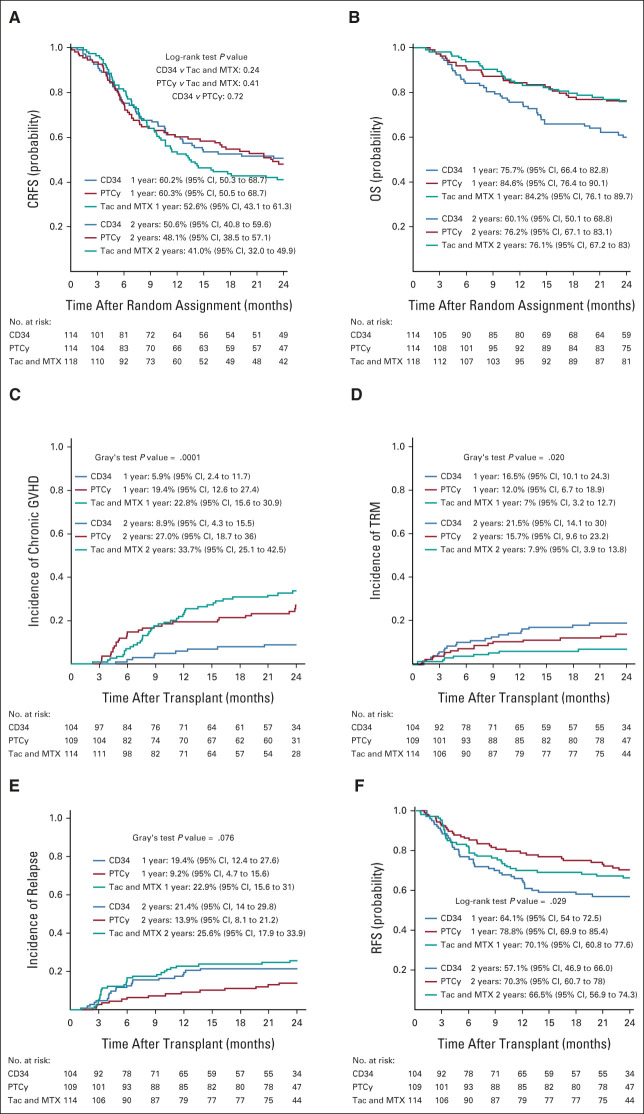
Two-year KM estimates for CRFS were 50.6% (95% CIs, 40.8 to 59.6), 48.1% (95% CI, 38.5 to 57.1), and 41.0% (95% CI, 32 to 49.9) for CD34+ selection, PTCy, and Tac and MTX, respectively (Fig 2A). The HRs for CRFS were 0.80 (95% CI, 0.56 to 1.15; P = .24) for CD34+ selection and 0.86 (95% CI, 0.61 to 1.23; P = .41) for PTCy compared with controls, and 0.93 (95% CI, 0.64 to 1.35; P = .72) comparing CD34+ selection with PTCy. Among the covariates tested in a preplanned multivariate Cox model of CRFS, patients age ≥ 50 years had an HR of 1.43 (P = .026) compared with younger patients (Data Supplement).
FIG 2.
(A) Probabilities of CRFS by treatment arm. (B) Probabilities OS by treatment arm. (C) Cumulative incidences of moderate to severe chronic GVHD by treatment arm. (D) Cumulative incidences of TRM by treatment arm. (E) Cumulative incidence of disease relapse by treatment arm. (F) Probabilities of RFS by treatment arm. CRFS, chronic relapse-free survival; GVHD, graft-versus-host disease; OS, overall survival; PTCy, post-transplant cyclophosphamide; RFS, relapse-free survival; Tac and MTX, tacrolimus and methotrexate; TRM, transplant-related morality.
Secondary Outcomes
Two-year KM estimates for OS were 60.1% (95% CI, 50.1 to 68.8), 76.2% (95% CI, 67.1 to 83.1), and 76.1 (95% CI, 67.1 to 83) for CD34+ selection, PTCy, and Tac and MTX, respectively (Table 2, Fig 2B). HRs for overall mortality were 1.74 (P = .02) for CD34+ selection and 1.016 (P = .95) for PTCy compared with Tac and MTX, and 1.77 (P = .02) comparing CD34+ selection with PTCy. Among covariates tested in the overall mortality model in addition to treatment arm, patients age ≥50 years compared with younger had an HR of 1.75 (P = .01) and high DRI compared with low or moderate was associated with an HR of 1.80 (P = .01; Data Supplement).
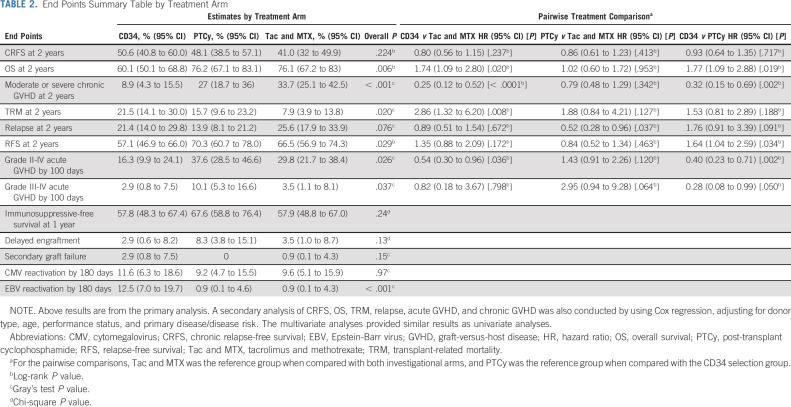
TABLE 2.
End Points Summary Table by Treatment Arm
Cumulative incidences of moderate to severe chronic GVHD at 2 years were 8.9% (95% CI, 4.3 to 15.5), 27% (95% CI, 18.7 to 36), and 33.7% (95% CI, 25.1 to 42.5) for CD34+ selection, PTCy, and Tac and MTX, respectively (Fig 2C). Corresponding HRs for moderate to severe chronic GVHD were 0.25 (95% CI, 0.12 to 0.52, P < .001) and 0.79 (95% CI, 0.48 to 1.29, P = .342) compared with Tac and MTX, and 0.32 (95% CI, 0.15 to 0.69, P = .02) comparing CD34+ selection with PTCy.
Cumulative incidences of TRM at 2 years were 21.5% (95% CI, 14.1 to 30), 15.7% (95% CI, 9.6 to 23.2), and 7.9% (95% CI, 3.9 to 13.8) for CD34+ selection, PTCy, and Tac and MTX, respectively (Fig 2D). Corresponding HRs for TRM compared with controls were 2.76 (95% CI, 1.32 to 6.20, P = .008) and 1.88 (95% CI, 0.84 to 4.21, P = .127), and 1.53 (95% CI, 0.81 to 2.89, P = .188) for CD34+ selection compared with PTCy. Among the covariates tested in the TRM model in addition to treatment arm, patients age ≥ 50 years compared with younger patients had an HR of 3.29 (P < .01).
Disease relapse (Fig 2E), acute GVHD, RFS (Fig 2F), immunosuppression-free survival, delayed engraftment, secondary graft failure, CMV, and EBV activation by treatment are shown in Table 2. The proportion of patients who required CNI for treatment of acute GVHD was 1.9% and 5.5% for CD34+ selection and PTCy, respectively.
Hematologic recovery is shown in Figures 3A and 3B. Median times to neutrophil recovery were 11, 19, and 17 days for CD34+ selection, PTCy, and Tac and MTX respectively. Corresponding median times to platelet recovery were 17, 28 and 21 days. The proportion of grades 3-5 nonhematologic toxicities was 76.9%, 80.7%, and 87.7% for CD34+ selection, PTCy, and Tac and MTX (P = .11; Data Supplement). Cumulative incidences of grade III infections at 2 years were 29.8% (95% CI, 21.8 to 39.2), 20.2% (13.7 to 28.7), and 14% (95% CI, 8.7 to 21.7) for CD34+ selection, PTCy, and Tac and MTX, respectively (P = .014; Data Supplement).
FIG 3.
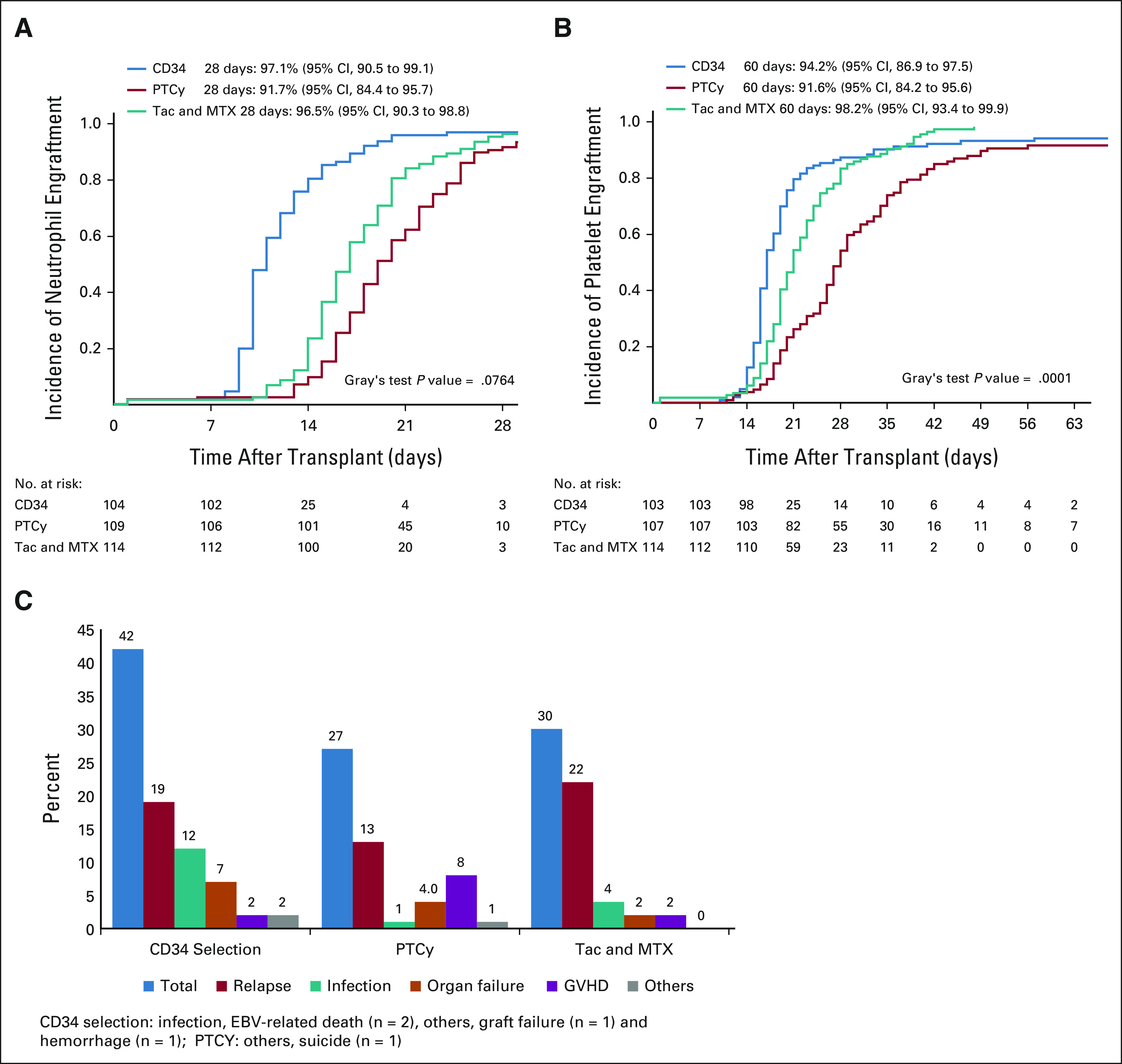
(A) Cumulative incidences of neutrophil recovery by treatment arm. (B) Cumulative incidences of platelet recovery by treatment arm. (C) Summary of causes of death by treatment arm. EBV, Epstein-Barr virus; GVHD, graft-versus-host disease; PTCy, post-transplant cyclophosphamide; Tac and MTX, tacrolimus and methotrexate.
Twenty-four patients received post-HCT donor lymphocyte infusions (DLI): CD34+ selection, n = 12; PT-Cy, n = 4; and Tac and MTX, n = 8. In 22 patients, DLI was given for treatment of disease relapse; two patients in the CD34+ selection arm received DLI because of poor T-cell chimerism.
Causes of death after the CD34+ selection arm were disease relapse (45.2%), infection (28.6%), organ failure (16.6%), GVHD (4.8%), and other causes (4.8%); after PTCy, disease relapse (48.1%), infection (3.7%), organ failure (14.8%), GVHD (29.7%), and other causes (3.7%); and, after Tac and MTX, disease relapse (73.4%), infection (13.3%), organ failure (6.6%), and GVHD (6.7%; Fig 3C).
QOL
Compliance with QOL evaluation was 80% or greater at all timepoints. Profiles of median scores during the transplant course, measured by SF36 physical and mental components, FACT-BMT, and MDASI, were similar across treatment arms (Data Supplement).
As-Treated Analysis
Among 300 patients who received therapy per protocol, the KM estimates of CRFS at 2 years were 53.8% (95% CI, 42.9 to 63.5), 51.1% (95% CI, 41.2 to 60.8), and 41.2% (95% CI, 32 to 50.2) for CD34+ selection, PTCy, and Tac and MTX, respectively (Data Supplement). Corresponding HRs for CRFS compared with controls were 0.73 (P = .12) and 0.80 (P = .23) for CD34 selection and PTCy, respectively, and 0.92 (P = .71) comparing CD34+ selection with PTCy. Two-year OS were 60.3% (95% CI, 49.3 to 69.7), 78.7% (95% CI, 69.2 to 85.6), and 77.3 (95% CI, 68.3 to 84.1) for CD34+ selection, PTCy, and Tac and MTX, respectively (Data Supplement). Corresponding HR for overall mortality compared with controls were 1.82 (P = .02) and 0.97 (P = .92) for CD34 selection and PTCy, respectively, and 1.93 (P = .01) comparing CD34+ selection with PTCy.
DISCUSSION
This randomized phase III trial compared three approaches for chronic GVHD prevention and specifically investigated whether two CNI-free approaches, ex vivo TCD using CD34 selection of PBSC grafts or in vivo TCD of marrow grafts using PTCy alone, could lead to superior control of chronic GVHD without excess disease relapse and mortality. Our study demonstrated that both experimental arms resulted in similar but not better outcomes compared with HCT using standard Tac and MTX with BM graft as measured by CRFS; however, mortality was higher with CD34 selection compared with other strategies.
An important finding of this trial is that the outcomes of the control arm using standard Tac and MTX with a BM graft were better than anticipated, with 2-year cumulative incidence of TRM only 7.9% and 2-year OS, 76.1%. Although Tac and MTX was associated with the highest rate of chronic GVHD (cGVHD; 33.7% at 2 years), the low TRM and relapse rates associated with this approach rendered it comparable to the CNI-free arms, with similar QOL as assessed by standard, validated QOL instruments. Additionally, the rates of grades 3-5 kidney injury were not higher with CNI in this trial. These results affirm that Tac and MTX with a BM graft should still be considered one of the standards of care for patients with acute leukemia or MDS receiving myeloablative HLA-matched transplantation. Although the results suggest that patients receiving PTCy without CNI have similar outcomes, the trial was not sufficiently powered to conclude that they were comparable.
Our results also confirmed previous studies, including the BMT CTN 0303 trial,13 which showed that ex vivo TCD using CD34+ selection significantly reduces moderate-to-severe chronic GVHD without increasing disease relapse. Unfortunately, patients undergoing CD34+ selection in this trial experienced inferior OS mainly driven by excess TRM compared with the other arms, predominantly from infection and organ failure, offsetting its potential GVHD control benefits. Relapse rates were not higher with CD34+ selection. It is possible that the infection and organ failure risks associated with the CD34+ selection arm were driven by the intensity of the conditioning regimens using this approach, with the addition of thiotepa to high-dose Cy and TBI 1375 cGy, and addition of melphalan to high-dose busulfan and fludarabine. In fact, the previous BMT CTN 0303 trial, which used the same TBI conditioning for CD34+ selection, observed the same TRM rates.20 The field continues to move away from very high intensity toward less toxic (although still myeloablative) regimens including fludarabine and busulfan for HCT using Tac and MTX and PTCy. Exploring less-intensive regimens with CD34+ selection may be warranted.
BMT using PTCy without CNI demonstrated similar rates of all components of CRFS compared with BMT using Tac and MTX. PTCy was associated with increased grade II-IV acute GVHD compared with CD34+ selection, but there was also a trend toward lower disease relapse compared with controls. Moderate-to-severe cGVHD and OS in the PTCy arm was also similar to standard Tac and MTX. Engraftment after PTCy was also slower compared with the other interventions, and nine patients experienced delayed engrafted compared with four and three patients in the Tac and MTX and CD34 selection, respectively. Among these nine patients, eight subsequently engrafted. Acute GVHD rates were comparable with single-center experience in the setting of HLA-matched donors.14,15 PTCy platform is associated with lower rates of cGVHD in studies of both haploidentical and HLA-matched marrow transplantation.14,21,22 However, it should be noted that in the current trial, PTCy was used alone, without tacrolimus and mycophenolate mofetil (MMF) as is used in haploidentical and most HLA-matched settings, since the purpose of this trial was to explore regimens that were CNI-free. It is likely that Tac and MMF is important as an adjunct for preventing cGVHD and that GVHD control after PCTy could be further improved with their addition. The study suggests that BMT with PTCy alone could be a viable CNI-free option for matched donor HCT. Additionally, cardiac effects related to PTCy need to be considered although studies demonstrate that this association requires continued investigation.23,24
The results in the Tac and MTX control arm observed in this trial were much better than expected. The trial design estimated a CRFS to be 22% for the control arm and the observed CRFS was 44%. The trial modeling used registry data to calculate CRFS using a population where the majority received a PBSC graft and the CRFS used overall chronic GVHD instead of moderate-to-severe chronic GVHD. Both contributed to higher rate of chronic GVHD events and a lower CRFS in the registry cohort. Also, all chronic GVHD events on the trial were reviewed by an independent committee blinded to treatment assignment to determine moderate-to-severe cases, and only cases that met predefined criteria were considered an event for CRFS. Overperformance of the Tac and MTX arm also affected the number of events detected in the first 2 years resulting in decreased power of pairwise comparisons, which needs to be taken into account when interpreting the results.
In summary, the BMT CTN 1301 trial demonstrated similar but not better CRFS after two CNI-free GVHD prophylaxis strategies to standard Tac and MTX after HLA-matched transplantation with myeloablative conditioning. CD34 selection was associated with significant reduction in cGVHD, although this benefit was offset by an excess of TRM. Future CD34 selection studies should thus focus on reduction of TRM and infections. Although BMT using PTCy alone was associated with more acute GVHD compared with Tac and MTX, TRM, cGVHD CRFS, and OS were similar. Interestingly, there was a trend toward better relapse rates and RFS for PTCy in pairwise comparisons to Tac and MTX and CD34+ depletion, respectively. Consequently, the PTCy regimen could potentially be offered as a transplant option for patients with contraindication to CNI or who need a CNI-free approach for planned post-transplant interventions. An alternative approach to cGVHD reduction has been the addition of in vivo T-cell depletion with ATG or anti–T-lymphocyte globulin to CNI and MTX. Several randomized trials demonstrated lower cGVHD rates without compromising relapse rates or survival, although one randomized study suggested inferior survival despite lower cGVHD rates.25-29
This trial focused on strategies to improve transplant outcomes by decreasing moderate-to-severe chronic GVHD without increasing disease relapse and the only strategy that significantly reduced cGVHD was associated with higher mortality. Further improvements of Tac and MTX with BM need to focus on chronic GVHD and disease relapse. Fortunately, there are emerging post-transplant interventions that offer promise in this area,30 and designing trials to maximize improvement of PTCy by adding Tac and MMF or to add ATG to Tac and MTX similar to European trials may further optimize transplant outcomes. Finally, it should be emphasized that HLA-matched HCT with contemporary myeloablative conditioning, with either Tac and MTX or PTCy and a BM graft, administered as postremission therapy, results in two-year survival rates > 75% and should be considered the new benchmark for patients with hematologic malignancies.
Leo Luznik
Consulting or Advisory Role: Gilead Sciences, Talaris Therapheutics, Precision BioSciences, Rubius Therapheutics, WindMiL Therapheutics
Research Funding: Genentech, Amgen (I)
Patents, Royalties, Other Intellectual Property: Patent holder WindMIL therapheutics
Uncompensated Relationships: WindMIL therapheutics
Marcelo C. Pasquini
Honoraria: Honoraria
Consulting or Advisory Role: Pfizer, Medigene (Inst), Amgen¸ Bristol Myers Squibb
Research Funding: Kite/Gilead (Inst), Novartis (Inst), Bristol Myers Squibb (Inst)
Brent Logan
Consulting or Advisory Role: Daiichi Sankyo, Enlivex Therapeutics Ltd, Gamida Cell
Robert J. Soiffer
Leadership: Kiadis Pharma, Be the Match/NMDP
Consulting or Advisory Role: Juno Therapeutics, Gilead Sciences, Rheos Medicines, Cugene, Jazz Pharmaceuticals, Precision Biosciences, Takeda, Jasper Therapeutics, Alexion Pharmaceuticals
Expert Testimony: Pfizer
Travel, Accommodations, Expenses: Gilead Sciences
Steven M. Devine
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Kiadis Pharma
Consulting or Advisory Role: Bristol Myers Squibb
Research Funding: Orca Bio (Inst), Kiadis Pharma (Inst)
Travel, Accommodations, Expenses: Orca Bio
Sergio Giralt
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Celgene, Takeda, Amgen, Jazz Pharmaceuticals, Sanofi
Consulting or Advisory Role: Celgene, Takeda, Sanofi, Jazz Pharmaceuticals, Amgen, Janssen, Actinuum, Bristol Myers Squibb, Johnson & Johnson, Pfizer
Research Funding: Celgene (Inst), Takeda, Miltenyi Biotec (Inst), Johnson & Johnson, Amgen, Actinuum, Sanofi
Travel, Accommodations, Expenses: Celgene, Sanofi, Amgen, Jazz Pharmaceuticals
Helen E. Heslop
Stock and Other Ownership Interests: Marker Therapeutics, Allovir, Fresh Wind Biotechnologies
Consulting or Advisory Role: Gilead Sciences, Novartis, Kiadis Pharma, Tessa Therapeutics, Marker Therapeutics, PACT Pharma, Mesoblast, GlaxoSmithKline, Takara Bio
Research Funding: Tessa Therapeutics (Inst)
Mary M. Horowitz
Consulting or Advisory Role: Medac
Research Funding: Biovitrum (Inst), Jazz Pharmaceuticals (Inst), Magenta Therapeutics (Inst), Novartis (Inst), Kite/Gilead (Inst), Actinium Pharmaceuticals (Inst), Amgen (Inst), Bluebird Bio (Inst), Bristol Myers Squibb (Inst), Chimerix (Inst), CSL Behring¸ Daiichi Sankyo (Inst), Gamida Cell (Inst), GlaxoSmithKline (Inst), Mesoblast (Inst), Miltenyi Biotec (Inst), Oncoimmune (Inst), Pfizer (Inst), Pharmacyclics (Inst), Regeneron (Inst), Sanofi (Inst), Seattle Genetics (Inst), Shire (Inst), Astellas Pharma, Xenikos (Inst)
Mark R. Litzow
Consulting or Advisory Role: Omeros, Jazz Pharmaceuticals
Research Funding: Amgen, Astellas Pharma, Actinium Pharmaceuticals, Pluristem Therapeutic, AbbVie/Genentech, Tolero Pharmaceuticals, AbbVie
Other Relationship: Biosight
Lori Muffly
Stock and Other Ownership Interests: Corvus Pharmaceuticals
Honoraria: UpToDate
Consulting or Advisory Role: Pfizer, Amgen, medexus, Jazz Pharmaceuticals
Speakers' Bureau: Adaptive Biotechnologies
Research Funding: Shire, Adaptive Biotechnologies, Astellas Pharma, Jasper Therapeutics
Eneida R. Nemecek
Consulting or Advisory Role: Medexus, Medac, Novartis, Atara Biotherapeutics
Richard J. O'Reilly
Consulting or Advisory Role: Atara Biotherapeutics
Research Funding: Atara Biotherapeutics
Patents, Royalties, Other Intellectual Property: I have received royalties following licensure of the EBV-specific T-cell bank by Atara Biotherapeutics
Johannes Schetelig
Honoraria: Roche, AbbVie, Janssen, Bristol Myers Squibb/Sanofi, Gilead Sciences, AstraZeneca
Consulting or Advisory Role: AstraZeneca, AbbVie, Janssen
Sumithira Vasu
Consulting or Advisory Role: Omeros, Johnson and Johnson
Research Funding: Sanofi
Open Payments Link: https://openpaymentsdata.cms.gov/physician/725618
Vincent T. Ho
Consulting or Advisory Role: Jazz Pharmaceuticals, Janssen, Alexion Pharmaceuticals, Omeros
Research Funding: Jazz Pharmaceuticals
Miguel-Angel Perales
Stock and Other Ownership Interests: NexImmune
Honoraria: MorphoSys
Consulting or Advisory Role: Incyte, Merck, Servier/Pfizer, NexImmune, Novartis, MolMed¸ Medigene, Takeda, Nektar, AbbVie, Cidara Therapeutics, Celgene, Kite/Gilead, Bristol Myers Squibb, Omeros, Vor Biopharma
Research Funding: Incyte (Inst), Miltenyi Biotec (Inst), Novartis (Inst), Kite, a Gilead company (Inst), Nektar (Inst)
No other potential conflicts of interest were reported.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
PRIOR PRESENTATION
Presented in part at the 2021 Transplant and Cell Therapy Virtual Meeting, February 8-12, 2021 and the 2021 European Blood and Marrow Transplant Virtual Meeting, March 14-17, 2021.
SUPPORT
Supported by grants U10HL069294 and U24HL138660 to the Blood and Marrow Transplant Clinical Trials Network from the National Heart, Lung, and Blood Institute and the National Cancer Institute, and from Miltenyi Biotec.
CLINICAL TRIAL INFORMATION
L.L. and M.C.P. are co-first authors and equal contributors.
V.T.H. and M.-A.P. are co-final authors and equal contributors.
DATA SHARING STATEMENT
Deidentified participant data for BMT CTN 1301 will be deposited in the NHLBI Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC; https://biolincc.nhlbi.nih.gov/home/, a publicly available database. Study documents including the study Protocol (online only), informed consent form, data dictionary, and case report forms for data collection are also available via the repository. Data will become accessible 3 years after the end of clinical activity and 2 years after the primary publication. BMT CTN Data and Coordinating Center (DCC) will complete the submission to BioLINCC in 2022. Instructions on specimen or data requests and contact information for BioLINCC are also available. Study results were submitted to ClinicalTrials.gov in October 2021 and will be posted after approval by Protocol Registration and Results System.
AUTHOR CONTRIBUTIONS
Conception and design: Leo Luznik, Marcelo C. Pasquini, Brent Logan, Robert J. Soiffer, Steven M. Devine, Nancy Geller, Sergio Giralt, Helen E. Heslop, Mary M. Horowitz, Richard J. Jones, Adam Mendizabal, Eneida R. Nemecek, Leyla Shune, Scott R. Solomon, Miguel-Angel Perales
Administrative support: Marcelo C. Pasquini, Raquel Palencia
Provision of study materials or patients: Marcelo C. Pasquini, Robert J. Soiffer, Steven M. Devine, Sergio Giralt, Richard J. Jones, Mark R. Litzow, Eneida R. Nemecek, Richard J. O'Reilly, Johannes Schetelig, Sumithira Vasu, Vincent T. Ho, Miguel-Angel Perales
Collection and assembly of data: Leo Luznik, Marcelo C. Pasquini, Brent Logan, Robert J. Soiffer, Juan Wu, Mary M. Horowitz, Mark R. Litzow, Adam Mendizabal, Lori Muffly, Raquel Palencia, Johannes Schetelig, Leyla Shune, Sumithira Vasu, Vincent T. Ho, Miguel-Angel Perales
Data analysis and interpretation: Leo Luznik, Marcelo C. Pasquini, Brent Logan, Robert J. Soiffer, Juan Wu, Nancy Geller, Mary M. Horowitz, Richard J. Jones, Mark R. Litzow, Adam Mendizabal, Lori Muffly, Eneida R. Nemecek, Lynn O'Donnell, Richard J. O'Reilly, Johannes Schetelig, Leyla Shune, Scott R. Solomon, Sumithira Vasu, Vincent T. Ho, Miguel-Angel Perales
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Phase III BMT CTN Trial of Calcineurin Inhibitor–Free Chronic Graft-Versus-Host Disease Interventions in Myeloablative Hematopoietic Cell Transplantation for Hematologic Malignancies
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Leo Luznik
Consulting or Advisory Role: Gilead Sciences, Talaris Therapheutics, Precision BioSciences, Rubius Therapheutics, WindMiL Therapheutics
Research Funding: Genentech, Amgen (I)
Patents, Royalties, Other Intellectual Property: Patent holder WindMIL therapheutics
Uncompensated Relationships: WindMIL therapheutics
Marcelo C. Pasquini
Honoraria: Honoraria
Consulting or Advisory Role: Pfizer, Medigene (Inst), Amgen¸ Bristol Myers Squibb
Research Funding: Kite/Gilead (Inst), Novartis (Inst), Bristol Myers Squibb (Inst)
Brent Logan
Consulting or Advisory Role: Daiichi Sankyo, Enlivex Therapeutics Ltd, Gamida Cell
Robert J. Soiffer
Leadership: Kiadis Pharma, Be the Match/NMDP
Consulting or Advisory Role: Juno Therapeutics, Gilead Sciences, Rheos Medicines, Cugene, Jazz Pharmaceuticals, Precision Biosciences, Takeda, Jasper Therapeutics, Alexion Pharmaceuticals
Expert Testimony: Pfizer
Travel, Accommodations, Expenses: Gilead Sciences
Steven M. Devine
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Kiadis Pharma
Consulting or Advisory Role: Bristol Myers Squibb
Research Funding: Orca Bio (Inst), Kiadis Pharma (Inst)
Travel, Accommodations, Expenses: Orca Bio
Sergio Giralt
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Celgene, Takeda, Amgen, Jazz Pharmaceuticals, Sanofi
Consulting or Advisory Role: Celgene, Takeda, Sanofi, Jazz Pharmaceuticals, Amgen, Janssen, Actinuum, Bristol Myers Squibb, Johnson & Johnson, Pfizer
Research Funding: Celgene (Inst), Takeda, Miltenyi Biotec (Inst), Johnson & Johnson, Amgen, Actinuum, Sanofi
Travel, Accommodations, Expenses: Celgene, Sanofi, Amgen, Jazz Pharmaceuticals
Helen E. Heslop
Stock and Other Ownership Interests: Marker Therapeutics, Allovir, Fresh Wind Biotechnologies
Consulting or Advisory Role: Gilead Sciences, Novartis, Kiadis Pharma, Tessa Therapeutics, Marker Therapeutics, PACT Pharma, Mesoblast, GlaxoSmithKline, Takara Bio
Research Funding: Tessa Therapeutics (Inst)
Mary M. Horowitz
Consulting or Advisory Role: Medac
Research Funding: Biovitrum (Inst), Jazz Pharmaceuticals (Inst), Magenta Therapeutics (Inst), Novartis (Inst), Kite/Gilead (Inst), Actinium Pharmaceuticals (Inst), Amgen (Inst), Bluebird Bio (Inst), Bristol Myers Squibb (Inst), Chimerix (Inst), CSL Behring¸ Daiichi Sankyo (Inst), Gamida Cell (Inst), GlaxoSmithKline (Inst), Mesoblast (Inst), Miltenyi Biotec (Inst), Oncoimmune (Inst), Pfizer (Inst), Pharmacyclics (Inst), Regeneron (Inst), Sanofi (Inst), Seattle Genetics (Inst), Shire (Inst), Astellas Pharma, Xenikos (Inst)
Mark R. Litzow
Consulting or Advisory Role: Omeros, Jazz Pharmaceuticals
Research Funding: Amgen, Astellas Pharma, Actinium Pharmaceuticals, Pluristem Therapeutic, AbbVie/Genentech, Tolero Pharmaceuticals, AbbVie
Other Relationship: Biosight
Lori Muffly
Stock and Other Ownership Interests: Corvus Pharmaceuticals
Honoraria: UpToDate
Consulting or Advisory Role: Pfizer, Amgen, medexus, Jazz Pharmaceuticals
Speakers' Bureau: Adaptive Biotechnologies
Research Funding: Shire, Adaptive Biotechnologies, Astellas Pharma, Jasper Therapeutics
Eneida R. Nemecek
Consulting or Advisory Role: Medexus, Medac, Novartis, Atara Biotherapeutics
Richard J. O'Reilly
Consulting or Advisory Role: Atara Biotherapeutics
Research Funding: Atara Biotherapeutics
Patents, Royalties, Other Intellectual Property: I have received royalties following licensure of the EBV-specific T-cell bank by Atara Biotherapeutics
Johannes Schetelig
Honoraria: Roche, AbbVie, Janssen, Bristol Myers Squibb/Sanofi, Gilead Sciences, AstraZeneca
Consulting or Advisory Role: AstraZeneca, AbbVie, Janssen
Sumithira Vasu
Consulting or Advisory Role: Omeros, Johnson and Johnson
Research Funding: Sanofi
Open Payments Link: https://openpaymentsdata.cms.gov/physician/725618
Vincent T. Ho
Consulting or Advisory Role: Jazz Pharmaceuticals, Janssen, Alexion Pharmaceuticals, Omeros
Research Funding: Jazz Pharmaceuticals
Miguel-Angel Perales
Stock and Other Ownership Interests: NexImmune
Honoraria: MorphoSys
Consulting or Advisory Role: Incyte, Merck, Servier/Pfizer, NexImmune, Novartis, MolMed¸ Medigene, Takeda, Nektar, AbbVie, Cidara Therapeutics, Celgene, Kite/Gilead, Bristol Myers Squibb, Omeros, Vor Biopharma
Research Funding: Incyte (Inst), Miltenyi Biotec (Inst), Novartis (Inst), Kite, a Gilead company (Inst), Nektar (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Appelbaum FR: Hematopoietic-cell transplantation at 50. N Engl J Med 357:1472-1475, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Storb R, Deeg HJ, Whitehead J, et al. : Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med 314:729-735, 1986 [DOI] [PubMed] [Google Scholar]
- 3.Bacigalupo A, Van Lint MT, Occhini D, et al. : Increased risk of leukemia relapse with high-dose cyclosporine A after allogeneic marrow transplantation for acute leukemia. Blood 77:1423-1428, 1991 [PubMed] [Google Scholar]
- 4.Sorror ML, Leisenring W, Deeg HJ, et al. : Twenty-year follow-up of a controlled trial comparing a combination of methotrexate plus cyclosporine with cyclosporine alone for prophylaxis of graft-versus-host disease in patients administered HLA-identical marrow grafts for leukemia. Biol Blood Marrow Transplant 11:814-815, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Anasetti C, Logan BR, Lee SJ, et al. : Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med 367:1487-1496, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couban S, Simpson DR, Barnett MJ, et al. : A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood 100:1525-1531, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Schmitz N, Eapen M, Horowitz MM, et al. : Long-term outcome of patients given transplants of mobilized blood or bone marrow: A report from the International bone marrow transplant registry and the European group for blood and marrow transplantation. Blood 108:4288-4290, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SJ, Logan B, Westervelt P, et al. : Comparison of patient-reported outcomes in 5-year survivors who received bone marrow vs peripheral blood unrelated donor transplantation: Long-term follow-up of a randomized clinical trial. JAMA Oncol 2:1583-1589, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jakubowski AA, Small TN, Young JW, et al. : T cell depleted stem-cell transplantation for adults with hematologic malignancies: Sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood 110:4552-4559, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papadopoulos EB, Carabasi MH, Castro-Malaspina H, et al. : T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: Freedom from relapse in the absence of graft-versus-host disease. Blood 91:1083-1090, 1998 [PubMed] [Google Scholar]
- 11.Keever-Taylor CA, Devine SM, Soiffer RJ, et al. : Characteristics of CliniMACS® System CD34-enriched T cell-depleted grafts in a multicenter trial for acute myeloid leukemia-Blood and Marrow Transplant Clinical Trials Network (BMT CTN) protocol 0303. Biol Blood Marrow Transplant 18:690-697, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner JE, Thompson JS, Carter SL, et al. : Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell depletion trial): A multi-centre, randomised phase II-III trial. Lancet 366:733-741, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Devine SM, Carter S, Soiffer RJ, et al. : Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: Results of the blood and marrow transplant clinical trials network protocol 0303. Biol Blood Marrow Transplant 17:1343-1351, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanakry CG, Tsai HL, Bolanos-Meade J, et al. : Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS. Blood 124:3817-3827, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luznik L, Bolanos-Meade J, Zahurak M, et al. : High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood 115:3224-3230, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasquini MC, Logan B, Jones RJ, et al. : Blood and marrow transplant clinical trials network report on the development of novel endpoints and selection of promising approaches for graft-versus-host disease prevention trials. Biol Blood Marrow Transplant 24:1274-1280, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dmitrienko A, Tamhane AC: Gatekeeping procedures with clinical trial applications. Pharm Stat 6:171-180, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Gray RJ: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141-1154, 1988 [Google Scholar]
- 19.Armand P, Kim HT, Logan BR, et al. : Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood 123:3664-3671, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasquini MC, Devine S, Mendizabal A, et al. : Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transplantation. J Clin Oncol 30:3194-3201, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luznik L, Fuchs EJ: High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol Res 47:65-77, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luznik L, O'Donnell PV, Symons HJ, et al. : HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 14:641-650, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh JC, Whited LK, Saliba RM, et al. : Cardiac toxicity after matched allogeneic hematopoietic cell transplantation in the post-transplant cyclophosphamide era. Blood Adv 5:5599-5607, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dulery R, Mohty R, Labopin M, et al. : Early cardiac toxicity associated with post-transplant cyclophosphamide in allogeneic stem cell transplantation. JACC CardioOncol 3:250-259, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finke J, Bethge WA, Schmoor C, et al. : Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: A randomised, open-label, multicentre phase 3 trial. Lancet Oncol 10:855-864, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Finke J, Schmoor C, Bethge WA, et al. : Long-term outcomes after standard graft-versus-host disease prophylaxis with or without anti-human-T-lymphocyte immunoglobulin in haemopoietic cell transplantation from matched unrelated donors: Final results of a randomised controlled trial. Lancet Haematol 4:e293-e301, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Soiffer RJ, Kim HT, McGuirk J, et al. : Prospective, randomized, double-blind, phase III clinical trial of anti-T-lymphocyte globulin to assess impact on chronic graft-versus-host disease-free survival in patients undergoing HLA-matched unrelated myeloablative hematopoietic cell transplantation. J Clin Oncol 35:4003-4011, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker I, Panzarella T, Couban S, et al. : Addition of anti-thymocyte globulin to standard graft-versus-host disease prophylaxis versus standard treatment alone in patients with haematological malignancies undergoing transplantation from unrelated donors: Final analysis of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol 7:e100-e111, 2020 [DOI] [PubMed] [Google Scholar]
- 29.Kroger N, Solano C, Wolschke C, et al. : Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N Engl J Med 374:43-53, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Webster JA, Luznik L, Tsai HL, et al. : Allogeneic transplantation for Ph+ acute lymphoblastic leukemia with posttransplantation cyclophosphamide. Blood Adv 4:5078-5088, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified participant data for BMT CTN 1301 will be deposited in the NHLBI Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC; https://biolincc.nhlbi.nih.gov/home/, a publicly available database. Study documents including the study Protocol (online only), informed consent form, data dictionary, and case report forms for data collection are also available via the repository. Data will become accessible 3 years after the end of clinical activity and 2 years after the primary publication. BMT CTN Data and Coordinating Center (DCC) will complete the submission to BioLINCC in 2022. Instructions on specimen or data requests and contact information for BioLINCC are also available. Study results were submitted to ClinicalTrials.gov in October 2021 and will be posted after approval by Protocol Registration and Results System.