PURPOSE
We evaluated whether combining a humanized antidisialoganglioside monoclonal antibody (hu14.18K322A) throughout therapy improves early response and outcomes in children with newly diagnosed high-risk neuroblastoma.
PATIENTS AND METHODS
We conducted a prospective, single-arm, three-stage, phase II clinical trial. Six cycles of induction chemotherapy were coadministered with hu14.18K322A, granulocyte-macrophage colony-stimulating factor (GM-CSF), and low-dose interleukin-2 (IL-2). The consolidation regimen included busulfan and melphalan. When available, an additional cycle of parent-derived natural killer cells with hu14.18K322A was administered during consolidation (n = 31). Radiation therapy was administered at the end of consolidation. Postconsolidation treatment included hu14.18K322A, GM-CSF, IL-2, and isotretinoin. Early response was assessed after the first two cycles of induction therapy. End-of-induction response, event-free survival (EFS), and overall survival (OS) were evaluated.
RESULTS
Sixty-four patients received hu14.18K322A with induction chemotherapy. This regimen was well tolerated, with continuous infusion narcotics. Partial responses (PRs) or better after the first two chemoimmunotherapy cycles occurred in 42 of 63 evaluable patients (66.7%; 95% CI, 55.0 to 78.3). Primary tumor volume decreased by a median of 75% (range, 100% [complete disappearance]-5% growth). Median peak hu14.18K322A serum levels in cycle one correlated with early response to therapy (P = .0154, one-sided t-test). Sixty of 62 patients (97%) had an end-of-induction partial response or better. No patients experienced progressive disease during induction. The 3-year EFS was 73.7% (95% CI, 60.0 to 83.4), and the OS was 86.0% (95% CI, 73.8 to 92.8), respectively.
CONCLUSION
Adding hu14.18K322A to induction chemotherapy improved early objective responses, significantly reduced tumor volumes in most patients, improved end-of-induction response rates, and yielded an encouraging 3-year EFS. These results, if validated in a larger study, may be practice changing.
INTRODUCTION
Treatment for high-risk (HR) neuroblastoma includes induction therapy (chemotherapy and surgery), consolidation with high-dose chemotherapy followed by autologous hematopoietic stem-cell transplant (ASCT), radiotherapy, and postconsolidation treatment with isotretinoin and a monoclonal antibody (mAb) that targets the disialoganglioside GD2. Administration of a chimeric anti-GD2 mAb (dinutuximab) with granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-2 (IL-2), and isotretinoin in the postconsolidation setting significantly improved 2- and 5-year event-free survival (EFS; 66% v 46%, P = .011; 56.6 ± 4.7% v 46.1 ± 5.1%, P = .042,2 respectively). However, nearly half of all patients experienced relapse and succumb to disease.
CONTEXT
Key Objective
Despite aggressive multimodal treatment, nearly half of all patients with newly diagnosed high-risk (HR) neuroblastoma still succumb to their disease. Will the combination of a humanized antidisialoganglioside monoclonal antibody (hu14.18K322A) with induction chemotherapy improve early response rates after two cycles and improve outcomes in children with newly diagnosed HR neuroblastoma?
Knowledge Generated
Early responses were significantly improved when compared with a historical group of children treated with identical chemotherapy, without hu14.18K322A. None of the patients in our trial experienced tumor progression during induction chemotherapy, and the overall outcomes are very encouraging.
Relevance
These results, if validated in a larger study, may change the standard of care for children with HR neuroblastoma.
Dinutuximab has been administered in postconsolidation to avoid chemotherapy-induced immunosuppression since it can adversely affect antibody-dependent cell-mediated cytotoxicity (ADCC). However, preclinical studies in neuroblastoma models and adult clinical studies demonstrated that concurrent chemotherapy with various monoclonal antibodies provides additive or synergistic benefits.3-10 We postulated that incorporating an anti-GD2 mAb throughout therapy for the treatment of HR neuroblastoma would improve clinical responses and outcomes. We used a unique humanized anti-GD2 mAb, hu14.18K322A, that retains the binding specificity of dinutuximab, is 98% human, has a single point mutation to reduce complement-associated pain, and is produced in a YB2/0 rat myeloma cell line to reduce fucosylation and enhance ADCC.11 We demonstrated that combining hu14.18K322A with chemotherapy is tolerable and clinically active in patients with relapsed neuroblastoma.12 Therefore, hu14.18K322A was administered concurrently with chemotherapy during induction in this phase II (NB2012), single-institution setting to assess efficacy and determine if chemoimmunotherapy warrants further evaluation in a randomized phase III study. The results of the first 42 patients, demonstrating significant improvement in early responses, were previously published.13 This report details the results of the entire cohort of 64 patients, their end-of-induction responses, estimates of event-free and overall survival (OS), and information on human antihuman antibody (HAHA) development.
In ANBL02P1, the Children's Oncology Group (COG) investigators reported a 40% partial response (PR) rate after two cycles of induction therapy (cyclophosphamide and topotecan) in HR patients14; this response was similar to the response described in ANBL0532 in which patients received the same induction regimen.15 NB2012 used the identical chemotherapy induction regimen as ANBL02P1 and ANBL0532. The primary objectives of NB2012 were to assess response after two cycles of chemoimmunotherapy compared with a previous study with chemotherapy only and to estimate the EFS when hu14.18K322A was added to all phases of treatment. Changes in Curie scores (CSs) from diagnosis to end-of-induction chemotherapy were also assessed. Postinduction CSs have been previously validated as prognostic in two large independent cohorts of patients.16-18
PATIENTS AND METHODS
Study Design
This study was an open-label, single-arm phase II trial. The primary objective was to compare the response rates of the first two cycles of chemoimmunotherapy (cyclophosphamide, topotecan, and hu14.18K322A, followed by GM-CSF and IL-2) with the response rates of two cycles of cyclophosphamide and topotecan alone reported in a previous study (ANBL02P1) in children with newly diagnosed HR neuroblastoma.14 At an interim analysis, the response rate exceeded the a priori benchmark for activity13; therefore, enrollment was expanded to estimate EFS as a primary objective.
Statistical Analysis Plan
In the COG phase III randomized trial ANBL0532,15 the chemotherapy components of the induction regimen were identical to NB2012 and the 95% CI of 3-year EFS was (46.5%, 55.3%). A Kaplan-Meier estimate-based design was used to test the null hypothesis of a 46% 3-year EFS rate, against the desired alternative 3-year EFS rate of 62%. A total of 61 patients provided 80% power to detect a 16% increase of 3-year EFS with a one-sided type I error rate of 5%. Analysis of EFS was performed in the intention-to-treat population, defined as all enrolled patients, and the efficacy-evaluable population, defined as all patients who had received at least one dose of study treatment and had at least one available postbaseline tumor assessment. EFS and OS were analyzed using the Kaplan-Meier method. ORR and 95% CIs were calculated using the Clopper-Pearson method. Summary statistics were provided for clinical and demographic characteristics and for AEs. The association between the EFS and the peak and trough level of hu14.18K322A were explored. We performed all statistical tests using SAS (version 9.4).
Patients
Children (< 19 years) with newly diagnosed HR neuroblastoma were eligible for enrollment. Diagnosis, staging, and response assessments were performed according to the 1993 International Criteria for Neuroblastoma Response,19 and HR neuroblastoma was defined by the criteria used by the COG.20
This trial (NCT01857934) was approved by our institutional review board and opened in May 2013. The last patient completed therapy in July 2019. Written informed consent was obtained from participants in accordance with institutional guidelines (Protocol, online only).
Hu14.18K322A
The hu14.18K322A production cell line was provided by Merck Serono (Darmstadt, Germany) and manufactured for clinical use by the Children's GMP, LLC (Memphis, TN). With each cycle of therapy, hu14.18 K322A levels were measured by ELISA in serum obtained 1 hour after the first antibody infusion, as previously described.12,21 Patients were monitored for the development of HAHA before first dose of hu14.18K322A with every treatment cycle (induction, consolidation, and postconsolidation phase) with an ELISA as previously described.22
Treatment
The schedule and dosages of induction chemotherapy agents cyclophosphamide, topotecan, cisplatin, etoposide, doxorubicin, and vincristine were identical to those used in ANBL02P1 and ANBL0532.14,15 Four daily doses of hu14.18K322A (days 2-5; administered over 4 hours, when possible) at 40 mg/m2/dose (once daily) were given with each cycle of induction chemotherapy. All patients received continuous infusions of opioids at standard dosages, approximately 30 minutes before antibody infusions. Systemic steroids were not allowed. Each cycle was followed by daily subcutaneous GM-CSF (250 μg/m2 once per day) through the nadir until an absolute neutrophil count of ≥ 2,000/mm3 and subcutaneous IL-2 (106 units/m2), once every other day for six doses (Fig 1).
FIG 1.
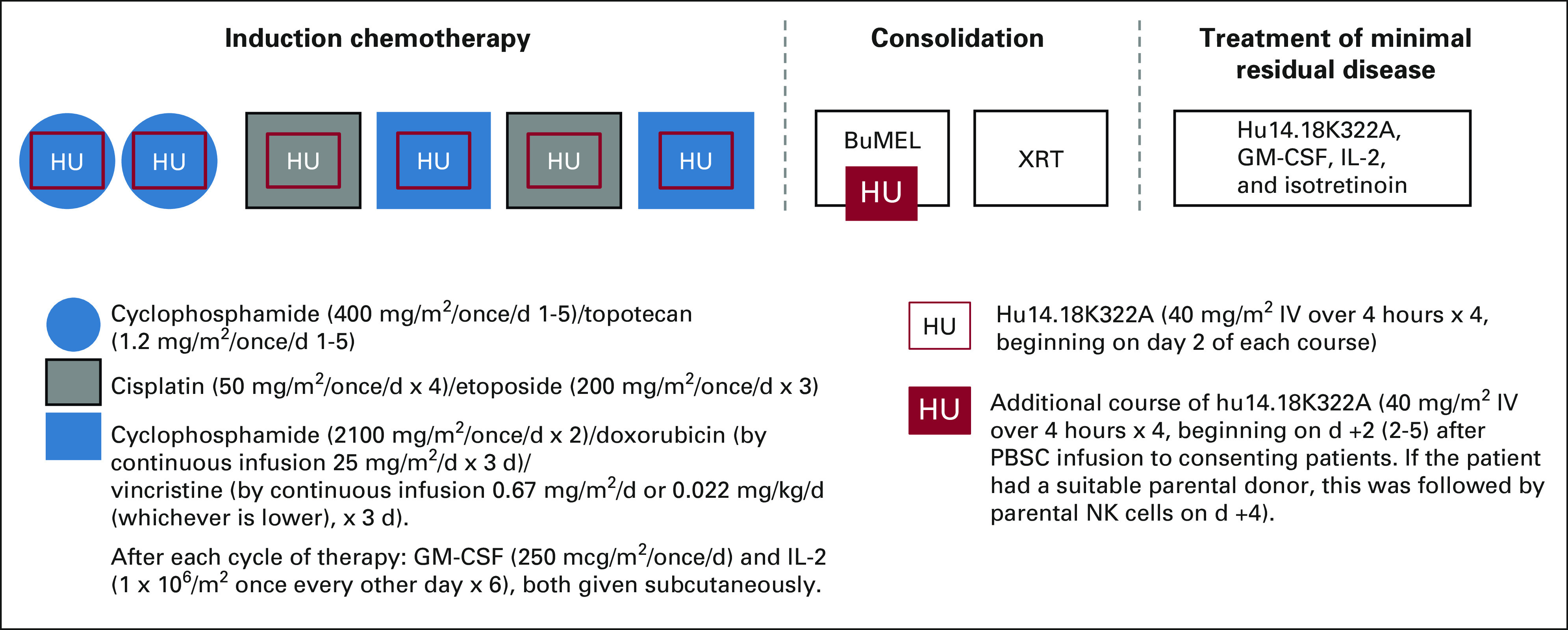
Study schema. BuMel, busulfan and melphalan; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL-2, interleukin-2; NK, natural killer; PBSC, peripheral blood stem cell; XRT, radiation therapy.
Hematopoietic progenitor cells were collected after induction cycle 2 or 4. Primary tumors were resected at any time point after induction cycle 2, when the surgeon determined that a maximum resection could be safely achieved. Consolidation therapy included ASCT with a Busulfan and Melphalan (BuMel)–conditioning regimen23 and, for consenting patients, experimental therapy with an additional cycle of daily hu14.18K322A administration beginning 2 days (days 2-5) after ASCT (given on day 0) and parental natural killer (NK) cell infusions (when available), derived as previously described.23 Tolerability of hu14.18K322A during recovery from consolidation therapy was evaluated in two dosage cohorts (25 mg/m2 once per day for 4 days, n = 16 [with NK cells, n = 10]; 40 mg/m2 once per day for 4 days, n = 26; with NK cells [n = 21]). The NK cells were infused 4 days after ASCT. When two of the first 42 patients developed a hemophagocytic lymphohistiocytosis-like syndrome,24 the additional cycle of hu14.18K322A during consolidation was eliminated in the subsequent cohort (n = 17). After recovering from ASCT (typically within 43 days of stem-cell reinfusion), patients received intensity-modulated radiation therapy or scanned proton beam radiation therapy (2,340 cGy in 180 cGy fractions). Those with macroscopic residual disease after induction chemotherapy received an additional 720 cGy to those sites.
Radiation therapy was delivered to the presurgical gross tumor volume and sites of residual disease on the basis of imaging performed at the end of induction. Postconsolidation therapy with hu14.18K322A, GM-CSF, IL-2, and isotretinoin (doses and schedules identical to those reported by Yu et al1) was started within 100 days from ASCT. Hu14.18K322A (40 mg/m2 once per day for 4 days) was administered in place of dinutuximab.1 Adverse events were assessed by the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). If narcotic infusions required increased dosages, pain was designated grade 3. Diagnosis, staging, and response assessments were performed according to the 1993 International Criteria for Neuroblastoma Response.19 Metastatic complete response was defined as no skeletal uptake on nuclear imaging and complete response in bone marrow and other metastatic sites. Responses were evaluated after induction cycles 2 and 6, after consolidation, and at the end of therapy. Anatomic imaging was assessed by one study radiologist (M.B.M.). Semiquantitative MIBG scoring (ie, CS) was assessed by one nuclear medicine physician (B.S.) at diagnosis and after cycles 2 and 6 of induction chemotherapy as described by Yanik et al.18
RESULTS
Patient Characteristics
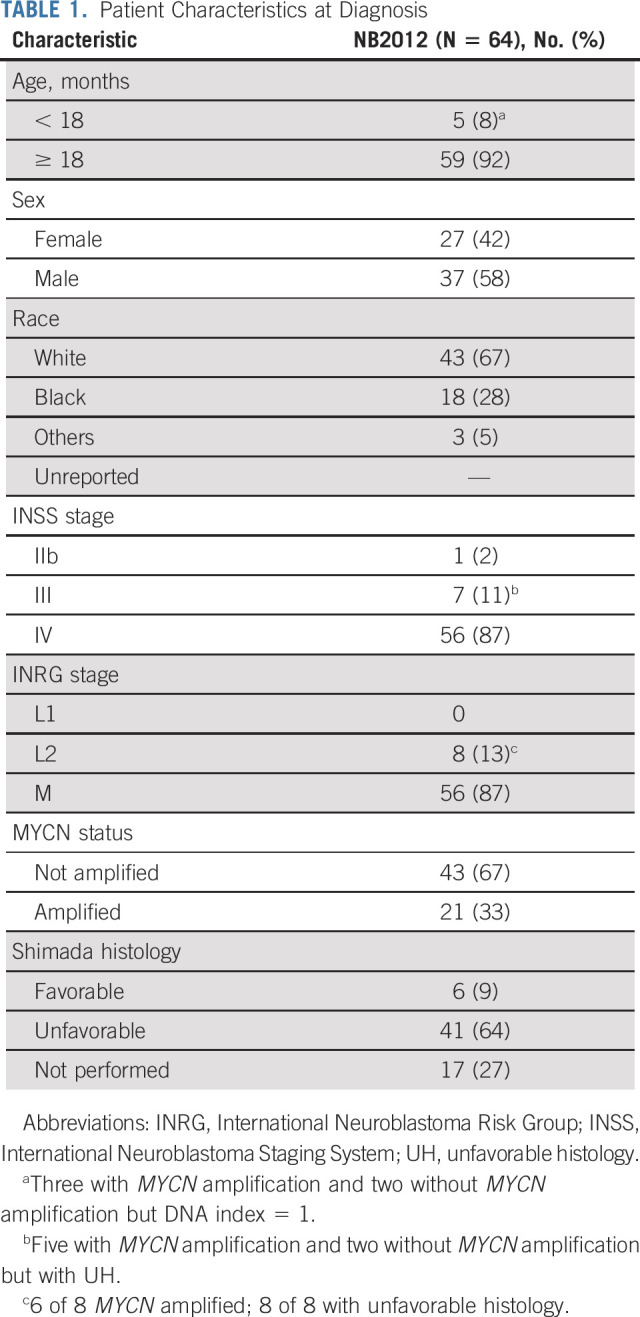
Sixty-three of the 64 patients had measurable or evaluable disease at enrollment. Patient characteristics are described in Table 1. Most patients were male (n = 37), White (n = 43), and ≥ 18 months (n = 59), with a median age of 3.1 years (range, 0.5-15.2 years), and had International Neuroblastoma Staging System (INSS) stage IV or International Neuroblastoma Risk Group stage M disease (n = 56). These characteristics were similar to patients enrolled on COG studies ANBL02P1,14 ANBL0532,15 and ANBL12P125 (Appendix Table A1, online only). During consolidation, 25 patients received proton beam radiation therapy and 34 patients received intensity-modulated radiation therapy.
TABLE 1.
Patient Characteristics at Diagnosis
Safety and Toxicity
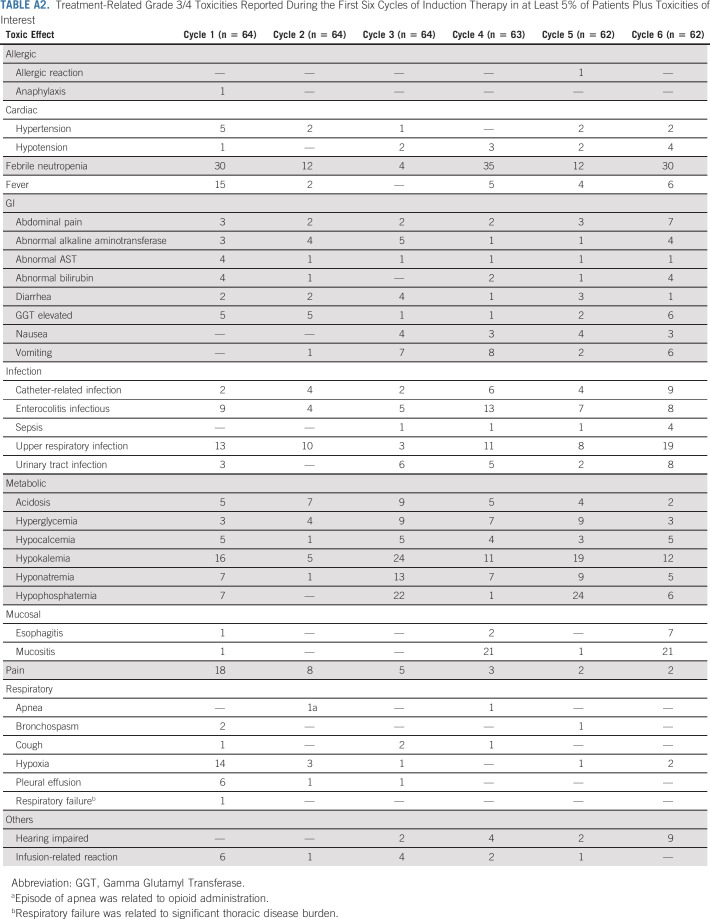
The addition of hu14.18K322A to induction chemotherapy was well tolerated, with continuous infusion narcotics adjusted to patient tolerance. Therapy-related grade 3 of 4 toxicities during induction chemotherapy-hu14.18K322A coadministration are described in Appendix Table A2 (online only). Toxicities attributed to antibody infusion included pain (10%; 38 patient episodes in 379 cycles), hypotension (3%; 12 of 379), cough (1%; 4 of 379), hypoxia (6%; 21 of 379), and infusion-related reactions (4%; 14 of 379).1,26 Events related to myelosuppression, liver function abnormalities, enterocolitis, and fever with or without infection were similar to those reported previously.13,14
Tumor Response
Responses were evaluated using the International Neuroblastoma Response Criteria (INRC)19 after two cycles of therapy (early response) and at the end of induction (Table 2). Two patients had resection of their primary tumors before beginning therapy but had other sites of evaluable disease. Of the 63 children evaluable for early response, 42 (66.7%; 95% CI, 55.0 to 78.3%) experienced PR or better. Figure 2 demonstrates the INRC components of response for individual patients after induction cycle two and the end of induction. All but one patient had a decrease in their primary tumor volume at cycle 2 with 42 of 63 achieving reductions of at least 50%. Fourteen had a metastatic very good partial response after induction cycle 2, and 3 had metastatic site complete responses. At the end of induction, 60 of 62 evaluable patients had PRs or better (96.8%; 95% CI, 88.8.0 to 99.6) including 40 with mCRs (65%).
TABLE 2.
Responses and Curie Scores During Induction Chemoimmunotherapy
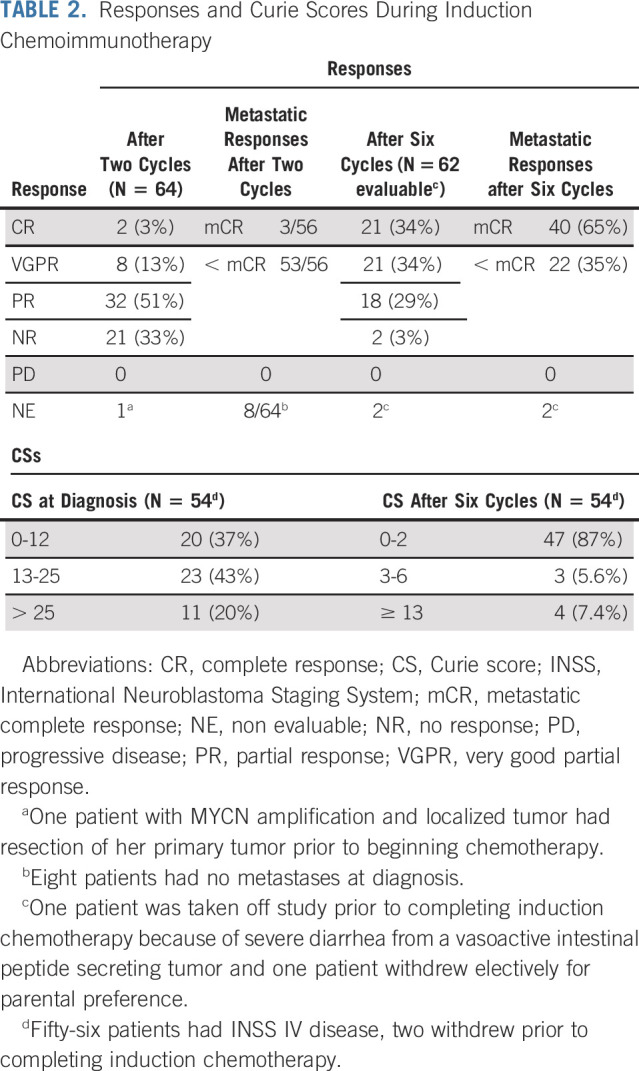
FIG 2.
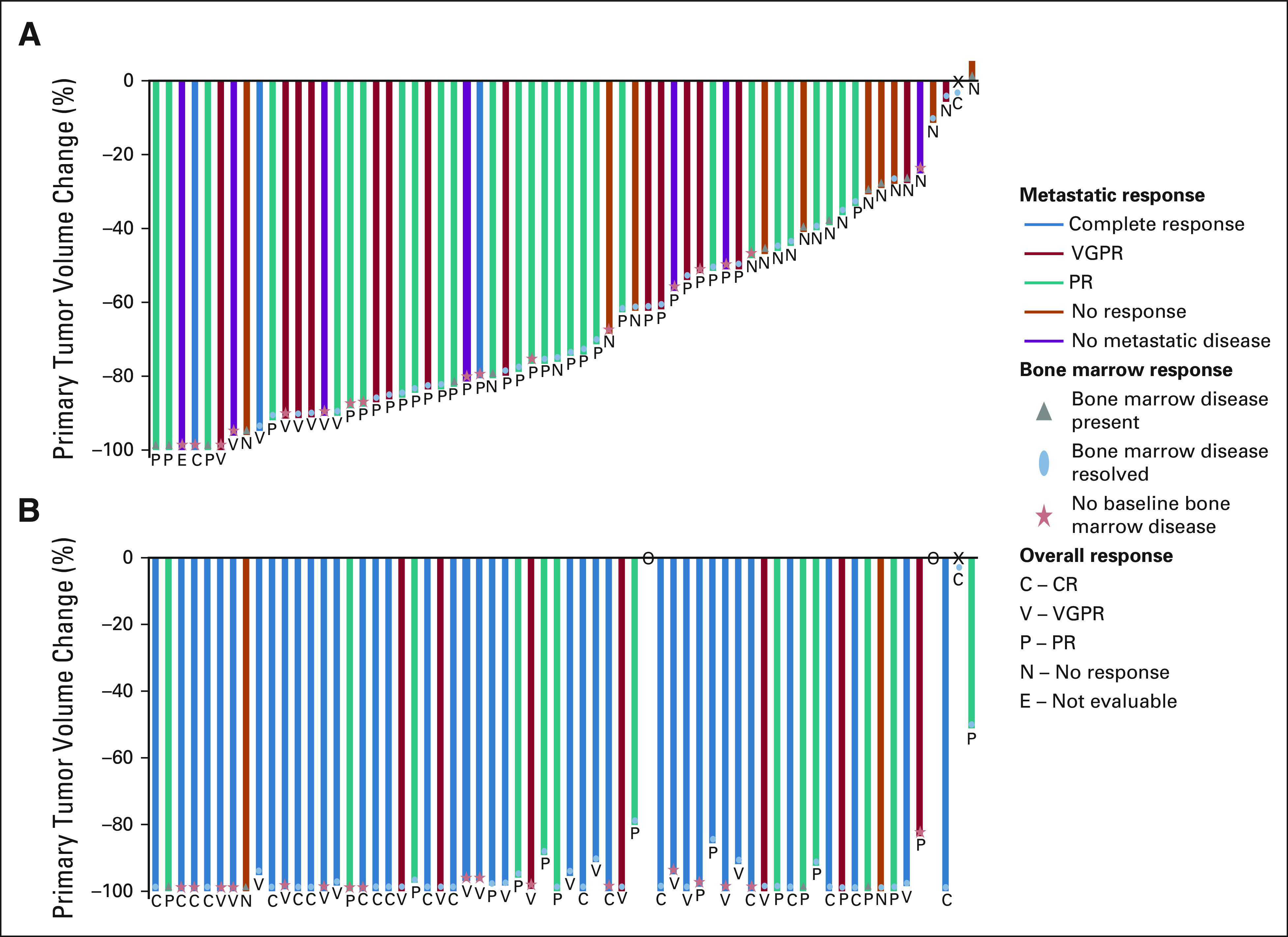
Disease response assessed by INRC for patients (A) after induction cycle 2 and (B) at the end of induction. Each bar represents an individual patient. The primary tumor response is demonstrated as a percentile change in volume of the primary tumor compared with the primary tumor volume at baseline. The color of the individual bars signifies the metastatic response including anatomic imaging, functional imaging (MIBG or PET), and bone marrow assessment. The blue bar represents a complete metastatic response. The red bar represents a metastatic VGPR. The teal bar represents a metastatic partial response. The orange bar represents no metastatic response. The purple bar signifies patients without metastatic disease at diagnosis. Bone marrow response is depicted by the following shapes: gray triangle represents the presence of bone marrow disease at the time of the assessment, blue oval represents bone marrow disease present at baseline that resolved at the time of the assessment, and the red star represents no bone marrow disease at baseline. Together, the primary tumor response, metastatic response, and bone marrow response are used to inform the INRC overall response. The overall response of the individual patient is presented by the letter below each bar. The letter C signifies a CR; V is a VGPR; P is a PR; N is a no response; and E means that the patient was not evaluable at that time point because of the primary tumor resection performed before therapy. X represents a stage IV patient who did not have a primary tumor at diagnosis. O represents two patients who discontinued protocol therapy before completing induction. CR, complete response; INRC, International Neuroblastoma Response Criteria; MIBG, [125I]metaiodobenzylguanidine; PET, positron emission tomography; PR, partial response; VGPR, very good partial response.
MIBG scoring (CS) was performed at every evaluation time point. CSs at diagnosis for the 56 patients with INSS stage IV disease ranged from 1 to 28 (median, 15) and from 0 to 23 (median, 0) at the end-of-induction chemotherapy. For the 54 patients with INSS stage IV disease who completed induction, 47 (87%) had end-of-induction CSs of two or less, as compared with a median CS of 15 at diagnosis (Table 2).
Quantitative and qualitative differences in NK cells on a subset of these children were also examined and previously reported.27
Hu14.18K322A Levels and HAHA Response
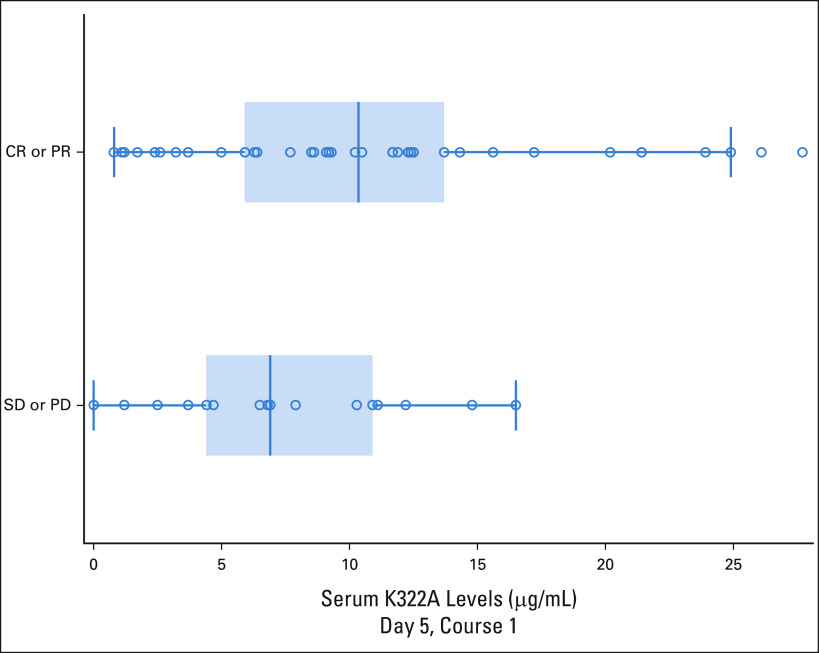
Median peak hu14.18K322A serum levels on cycle 1 of induction correlated with the clinical responses that were performed after the first two cycles of induction chemoimmunotherapy (PR or greater; n = 42) versus those who had stable disease (n = 17; P = .054, one-sided Wilcoxon rank-sum test; Fig 3). There was no association of EFS with the peak level of hu14.18K322A in cycle 1 (peak levels were dichotomized, above median or below median, and compared using the log-rank test [P = .682], and there was no association of early response after two cycles [PR or greater] with the trough level of hu14.18K322 A at the start of cycle 2 [P = .336, two-sided Wilcoxon rank-sum test]).
FIG 3.
Association of hu14.18K322A peak serum levels (µg/mL) and response after two cycles of chemoimmunotherapy. Relationship between response (CR or PR [n = 42] v SD or PD [n = 17]) and peak hu14.18K322A levels, measured on first day of mAb infusion, 1 hour after a 4-hour infusion. P = .0154 (one-tailed t-test). CR, complete response; mAb, monoclonal antibody; PD, progressive disease; PR, partial response; SD, stable disease.
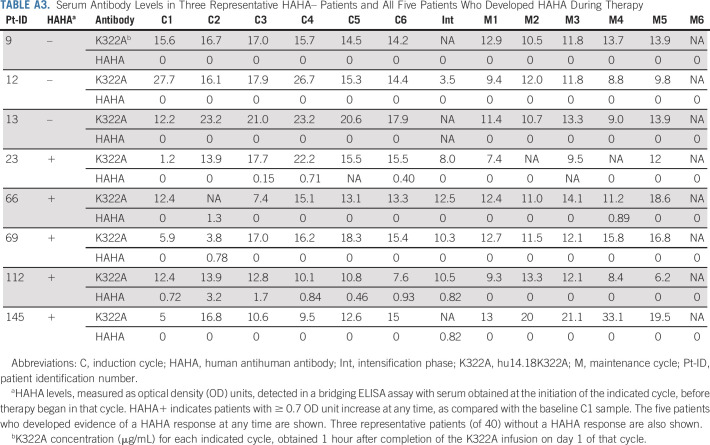
Five of 64 evaluable patients (7.8%) developed significant HAHA responses to hu14.18K322A after chemoimmunotherapy. None had a HAHA response associated with a drop in the peak hu14.18K322A level in cycles after the HAHA development (Appendix Table A3, online only). The five HAHA+ patients did not show any significant difference in EFS (P = .75, log-rank test) or noticeable differences in tolerance to antibody than the 59 HAHA– patients.
Effect of an Additional Cycle of hu14.18K322A With or Without Parentally Derived NK Cells During Consolidation
Fifty-nine of 64 patients received consolidation with BuMel (Fig 4) as previously described.23 Forty-two of these received an additional cycle of hu14.18K322A (25 mg/m2 once per day × 4 [n = 16] or 40 mg/m2 once per day × 4 [n = 26]), and 31 also received parentally derived NK cells. Seventeen patients received BuMel without the additional cycle of hu14.18K322A with or without NK cells. There was no difference in EFS between patients who received ASCT with BuMel and those who received ASCT with BuMel or hu14.18K322A with or without NK cells (data not shown).
FIG 4.
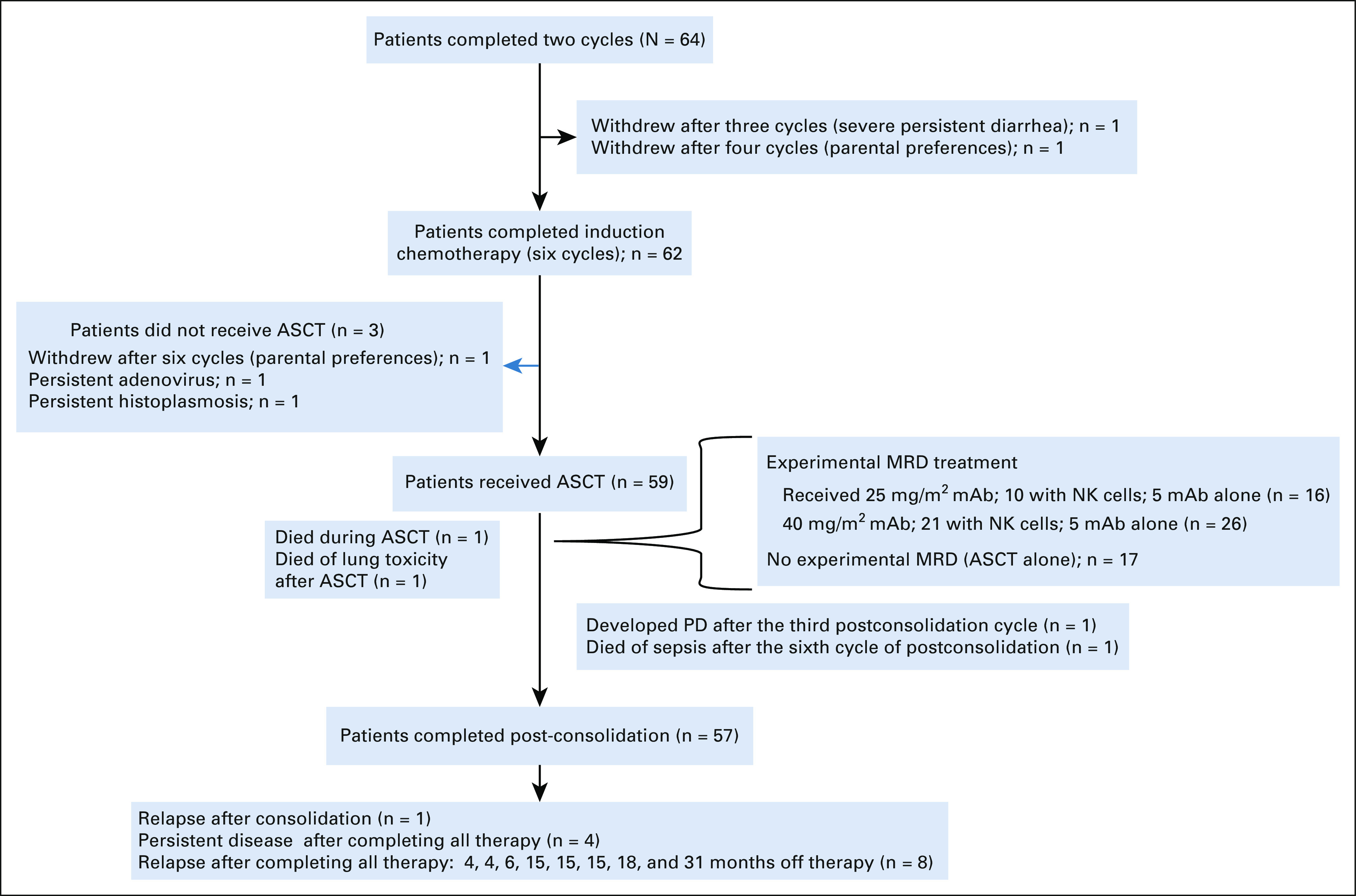
Flow diagram. ASCT, autologous hematopoietic stem-cell transplant; mAb, monoclonal antibody; MRD, minimal residual disease; PD, progressive disease.
Survival Outcomes
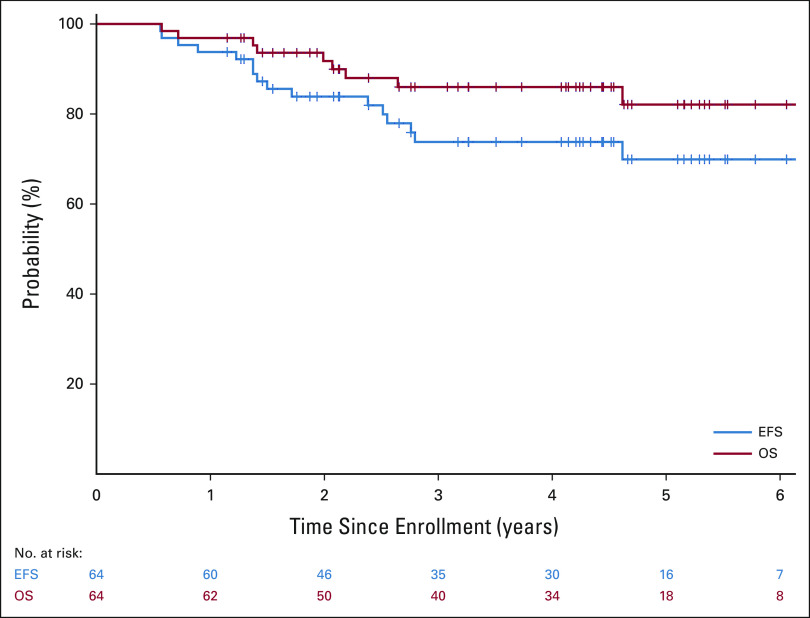
As of November 4, 2020, 54 of the 64 patients were alive, with a median follow-up time for the 48 patients who did not experience an event of 4.3 years (range, 1.1-6.7 years). Two patients died of complications from therapy without evidence of disease (one because of transplant-related complications and the other because of non-neutropenic sepsis); one progressed after consolidation, four had persistent disease after completing all therapy; one patient experienced disease recurrence after postconsolidation minimal residual disease cycle 3; and eight patients experienced relapse at 4, 4, 6, 15, 15, 15, 18, and 31 months, respectively, after completing all therapy. Four of these patients, one with persistent disease and three with relapsed disease, also died of disease progression. The overall 3-year EFS and OS rates were 73.7% (95% CI, 60.0 to 83.4) and 86% (95% CI, 73.8 to 92.8), respectively (Fig 5).
FIG 5.
EFS and OS for all patients enrolled. EFS, event-free survival; OS, overall survival.
DISCUSSION
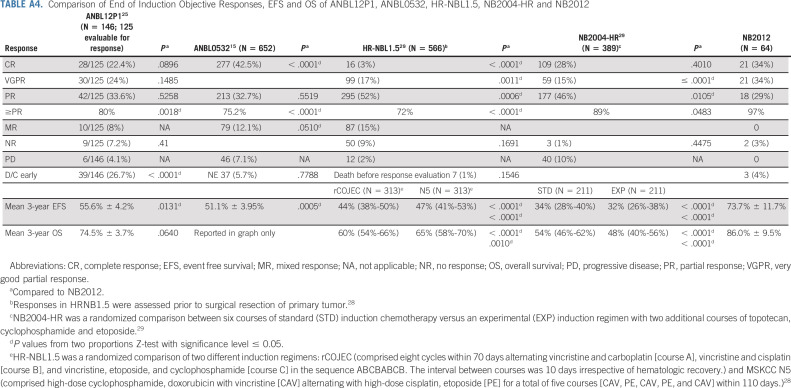
We have demonstrated that the addition of hu14.18K322A to induction chemotherapy significantly improves early responses in patients with newly diagnosed HR neuroblastoma (66.7% v 39.1%; 95% CI 55.0 to 78.3 v 35.3 to 43.0; P < .0001) compared with ANBL0532.15 Additionally, our chemoimmunotherapy induction regimen resulted in CSs ≤ 2 in 47 of 54 patients with INSS IV disease (87%), a finding that has been correlated with improved outcomes in both COG and SIOPEN trials.17,18 The significant improvement in early responses and postinduction CSs suggests that hu14.18K322A, GM-CSF, and IL-2 added to induction chemotherapy could significantly affect outcome. Although our sample size was small, our encouraging 3-year EFS of 73.7% (95% CI, 60 to 83.4) compares favorably with recent COG, GPOH, and SIOPEN trials (Appendix Table A4, online only).15,25,28,29 Park et al15 reported an end-of-induction (EoI) Objective Response (≥ PR) of 75.2% and a 3-year EFS from the time of random assignment for tandem transplant of 51.1% (95% CI, 47.1 to 55.0) for all 652 eligible patients.15 In our trial, the EoI Objective Response was 97%. More recently, Granger et al25 evaluated BuMel ASCT after a five-cycle COG induction regimen and reported an EoI Objective Response of 80% and a 3-year EFS of 55.6% ± 4.2% for all 146 patients enrolled (Appendix Table A4).25 Comparing NB2012 results with ANBL0032,1,15,25 ANBL053215 and ANBL12P125 are complicated by the facts that in the study by Yu et al,1 only patients who achieved a PR or better to induction therapy were included, Park et al15 only randomly assigned patients at end of induction and only 355 of 652 patients originally enrolled were randomly assigned, and in Granger's report, 39 of 146 patients (26.7%) discontinued treatment early.25 The NB2012 EFS estimate begins at the time of enrollment and includes all patients. Importantly, we did not observe disease progression in any patients during induction, whereas 7%-14% of patients enrolled in previous COG trials experienced disease progression before receiving ASCT.15,30
Peak serum levels of hu14.18K322A during the induction and postconsolidation cycles generally exceeded the level of antibody expected to facilitate ADCC in vivo.31 Higher levels of serum hu14.18K322A in cycle 1 were associated with more rapid response to treatment (PR or better after two cycles), consistent with dinutuximab plus temozolomide and irinotecan in relapsed neuroblastoma.32 These findings may suggest that greater in vivo exposure to an anti-GD2 mAb might be associated with improved outcomes. Although there was no significant association of hu14.18K322A levels with EFS, this might reflect the overall favorable EFS for the patients in this trial, which limits the statistical power to associate differences in hu14.18K322A levels with favorable versus unfavorable outcomes. The incidence of HAHA was substantially less than that seen in our phase I trial (5 of 64 v 15 of 37 patients [40.5%; P < .0001, Fisher's exact test]).11 Furthermore, the HAHA response here seemed weaker even in the five patients who developed HAHA (on the basis of the absence of any drop in peak hu14.18K322A levels for these five HAHA+ patients) compared with that observed in our previous phase I trial.11 We think that this difference likely reflects the immunosuppressive effect of concurrent chemotherapy on the induction of an antidrug-antibody response (like HAHA or HACA), as also seen recently in a COG trial of dinutuximab plus temozolomide and irinotecan.32
Although promising, these results must be interpreted within the context of a single-institution phase II study. Although the chemotherapy backbone was identical and the major prognostic factors of our patient populations were comparable with those of ANBL02P1,14 ANBL0532,15 and ANBL12P125 (Appendix Table A1), several other differences in therapy might have contributed to the excellent NB2012 response rates. For example, GM-CSF was used during induction chemoimmunotherapy, rather than filgrastim, both for its ability to enhance ADCC33,34 and for primary prophylaxis of febrile neutropenia.35 Additionally, low-dose IL-2 was used for its ability to enhance ADCC.36 The BuMel preparative regimen and additional cycle of hu14.18K322A (n = 42) with parental-derived NK cells (n = 31 of 42) during consolidation might have also affected EFS. Although BuMel was found to be a superior consolidation regimen within the context of rapid COJEC induction of SIOPEN,37 an arm evaluating its efficacy within the COG induction on the current open COG HR trial ANBL1531 was recently closed because of inferior outcomes. NB2012 was not designed to evaluate the effects of these modifications on the overall outcome.
In conclusion, the addition of hu14.18K322A to induction chemotherapy resulted in a near doubling of early responses, major reductions in tumor volume, improved responses, and a very encouraging 3-year EFS of 73.7% (95% CI, 60 to 83.4). These results, if validated in a larger study, may change the standard of care for children with HR neuroblastoma.
ACKNOWLEDGMENT
The authors thank Gwen Anthony, Deanna Welsh, clinical and laboratory personnel, operations staff, and all the patients and their families who participated in this study.
APPENDIX
TABLE A1.
Comparison of Patient Characteristics
TABLE A2.
Treatment-Related Grade 3/4 Toxicities Reported During the First Six Cycles of Induction Therapy in at Least 5% of Patients Plus Toxicities of Interest
TABLE A3.
Serum Antibody Levels in Three Representative HAHA– Patients and All Five Patients Who Developed HAHA During Therapy
TABLE A4.
Comparison of End of Induction Objective Responses, EFS and OS of ANBL12P1, ANBL0532, HR-NBL1.5, NB2004-HR and NB2012
Barry L. Shulkin
Consulting or Advisory Role: Navidea
Matthew Krasin
Consulting or Advisory Role: Debiopharm Group
Michael W. Bishop
Consulting or Advisory Role: Fennec Pharma
Research Funding: Pfizer
Fariba Navid
Research Funding: Bayer (Inst)
Brandon Triplett
Travel, Accommodations, Expenses: Miltenyi Biotec
Stephen D. Gillies
Employment: LinkedUp Bioscience
Leadership: LinkedUp Bioscience
Stock and Other Ownership Interests: Provenance Biopharmaceuticals
Consulting or Advisory Role: Delos Capital Partners
Patents, Royalties, Other Intellectual Property: Patent owner not related to this paper
Alice Yu
Leadership: OPKO Health
Stock and Other Ownership Interests: OPKO Health/GeneDx
Honoraria: EUSA Pharma
Consulting or Advisory Role: OBI Pharma
Speakers' Bureau: EUSA Pharma
Research Funding: United Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: Globo H-Diphtheria toxoid vaccine for cancer therapy, NKT stimulatory phenyl-glycolipids for cancer therapy and vaccine adjuvant, Cancer targeting peptides, Methods for suppressing cancer by inhibition of TMCC3
Travel, Accommodations, Expenses: EUSA Pharma
Paul M. Sondel
Research Funding: Invenra Inc (Inst)
Patents, Royalties, Other Intellectual Property: I have partial interest in patents related to my work at the University of Wisconsin-Madison WI, which are held by and managed by the University of Wisconsin Foundation. I am an unpaid medical advisor to Invenra Inc, a monoclonal antibody biotech firm in Madison WI. My UW laboratory is collaborating with Invenra and receiving research reagents from them for mutual research
Uncompensated Relationships: Invenra
Wing H. Leung
Employment: Miltenyi Biotec
Alberto Pappo
Honoraria: Bayer, Roche
Consulting or Advisory Role: Merck, Loxo/Bayer, EUSA Pharma, Debbio
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the ASCO Annual Meeting, June 3-7, 2016, Chicago, IL; ASCO Annual Meeting, June 1-5, 2017, Chicago, IL; and at Advances in Neuroblastoma Research virtual meeting, January 25-27, 2021.
SUPPORT
Supported by St Jude Children's Research Hospital Comprehensive Cancer Center Support Grant (2 P30 CA021765), American Lebanese Syrian Associated Charities, and Cookies for Kids' Cancer and Cure Childhood Cancer Foundation.
CLINICAL TRIAL INFORMATION
NCT01857934 (NB2012)
AUTHOR CONTRIBUTIONS
Conception and design: Wayne L. Furman, Beth McCarville, Matthew Krasin, Jianrong Wu, Fariba Navid, Victor Santana, Stephen D. Gillies, Alice Yu, Paul M. Sondel, Wing H. Leung, Alberto Pappo, Sara M. Federico
Administrative support: Jacquelyn A. Hank
Provision of study materials or patients: Wayne L. Furman, Beth McCarville, Andrew Davidoff, Matthew Krasin, Rachel Brennan, Elizabeth Stewart, Brandon Triplett, Stephen D. Gillies, Alberto Pappo, Sara M. Federico
Collection and assembly of data: Wayne L. Furman, Beth McCarville, Barry L. Shulkin, Matthew Krasin, Rachel Brennan, Sara Helmig, Elizabeth Stewart, Jacquelyn A. Hank, Paul M. Sondel, Alberto Pappo, Sara M. Federico
Data analysis and interpretation: Wayne L. Furman, Beth McCarville, Barry L. Shulkin, Andrew Davidoff, Matthew Krasin, Chia-Wei Hsu, Haitao Pan, Jianrong Wu, Michael W. Bishop, Brandon Triplett, Victor Santana, Teresa Santiago, Jacquelyn A. Hank, Paul M. Sondel, Wing H. Leung, Alberto Pappo, Sara M. Federico
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Improved Outcome in Children With Newly Diagnosed High-Risk Neuroblastoma Treated With Chemoimmunotherapy: Updated Results of a Phase II Study Using hu14.18K322A
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Barry L. Shulkin
Consulting or Advisory Role: Navidea
Matthew Krasin
Consulting or Advisory Role: Debiopharm Group
Michael W. Bishop
Consulting or Advisory Role: Fennec Pharma
Research Funding: Pfizer
Fariba Navid
Research Funding: Bayer (Inst)
Brandon Triplett
Travel, Accommodations, Expenses: Miltenyi Biotec
Stephen D. Gillies
Employment: LinkedUp Bioscience
Leadership: LinkedUp Bioscience
Stock and Other Ownership Interests: Provenance Biopharmaceuticals
Consulting or Advisory Role: Delos Capital Partners
Patents, Royalties, Other Intellectual Property: Patent owner not related to this paper
Alice Yu
Leadership: OPKO Health
Stock and Other Ownership Interests: OPKO Health/GeneDx
Honoraria: EUSA Pharma
Consulting or Advisory Role: OBI Pharma
Speakers' Bureau: EUSA Pharma
Research Funding: United Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: Globo H-Diphtheria toxoid vaccine for cancer therapy, NKT stimulatory phenyl-glycolipids for cancer therapy and vaccine adjuvant, Cancer targeting peptides, Methods for suppressing cancer by inhibition of TMCC3
Travel, Accommodations, Expenses: EUSA Pharma
Paul M. Sondel
Research Funding: Invenra Inc (Inst)
Patents, Royalties, Other Intellectual Property: I have partial interest in patents related to my work at the University of Wisconsin-Madison WI, which are held by and managed by the University of Wisconsin Foundation. I am an unpaid medical advisor to Invenra Inc, a monoclonal antibody biotech firm in Madison WI. My UW laboratory is collaborating with Invenra and receiving research reagents from them for mutual research
Uncompensated Relationships: Invenra
Wing H. Leung
Employment: Miltenyi Biotec
Alberto Pappo
Honoraria: Bayer, Roche
Consulting or Advisory Role: Merck, Loxo/Bayer, EUSA Pharma, Debbio
No other potential conflicts of interest were reported.
REFERENCES
- 1.Yu AL, Gilman AL, Ozkaynak MF, et al. : Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 363:1324-1334, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu AL, Gilman AL, Ozkaynak MF, et al. : Long-term follow-up of a phase III study of ch14.18 (dinutuximab) + cytokine immunotherapy in children with high-risk neuroblastoma: COG study ANBL0032. Clin Cancer Res 27:2179-2189, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nowak AK, Robinson BW, Lake RA: Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res 63:4490-4496, 2003 [PubMed] [Google Scholar]
- 4.Lake RA, Robinson BW: Immunotherapy and chemotherapy—A practical partnership. Nat Rev Cancer 5:397-405, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Hiddemann W, Kneba M, Dreyling M, et al. : Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 106:3725-3732, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Coiffier B, Lepage E, Briere J, et al. : CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346:235-242, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Hurwitz H, Fehrenbacher L, Novotny W, et al. : Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335-2342, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Slamon DJ, Leyland-Jones B, Shak S, et al. : Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783-792, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Yoshida S, Kawaguchi H, Sato S, et al. : An anti-GD2 monoclonal antibody enhances apoptotic effects of anti-cancer drugs against small cell lung cancer cells via JNK (c-Jun terminal kinase) activation. Jpn J Cancer Res 93:816-824, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kowalczyk A, Gil M, Horwacik I, et al. : The GD2-specific 14G2a monoclonal antibody induces apoptosis and enhances cytotoxicity of chemotherapeutic drugs in IMR-32 human neuroblastoma cells. Cancer Lett 281:171-182, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Navid F, Sondel PM, Barfield R, et al. : Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. J Clin Oncol 32:1445-1452, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Federico SM, McCarville MB, Shulkin BL, et al. : A pilot trial of humanized anti-GD2 monoclonal antibody (hu14.18K322A) with chemotherapy and natural killer cells in children with recurrent/refractory neuroblastoma. Clin Cancer Res 23:6441-6449, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furman WL, Federico SM, McCarville MB, et al. : A phase II trial of Hu14.18K322A in combination with induction chemotherapy in children with newly diagnosed high-risk neuroblastoma. Clin Cancer Res 25:6320-6328, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park JR, Scott JR, Stewart CF, et al. : Pilot induction regimen incorporating pharmacokinetically guided topotecan for treatment of newly diagnosed high-risk neuroblastoma: A Children's Oncology Group study. J Clin Oncol 29:4351-4357, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park JR, Kreissman SG, London WB, et al. : Effect of tandem autologous stem cell transplant vs single transplant on event-free survival in patients with high-risk neuroblastoma: A randomized clinical trial. JAMA 322:746-755, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yanik G, Naranjo A, Parisi MT, et al. : Impact of post-induction Curie scores in high-risk neuroblastoma. Biol Blood Marrow Transplant 21:S107, 2015 [Google Scholar]
- 17.Yanik GA, Parisi MT, Naranjo A, et al. : Validation of postinduction Curie scores in high-risk neuroblastoma: A Children's Oncology Group and SIOPEN Group report on SIOPEN/HR-NBL1. J Nucl Med 59:502-508, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yanik GA, Parisi MT, Shulkin BL, et al. : Semiquantitative mIBG scoring as a prognostic indicator in patients with stage 4 neuroblastoma: A report from the Children's Oncology Group. J Nucl Med 54:541-548, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brodeur GM, Pritchard J, Berthold F, et al. : Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol 11:1466-1477, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Maris JM: The biologic basis for neuroblastoma heterogeneity and risk stratification. Curr Opin Pediatr 17:7-13, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Hank JA, Gan J, Ryu H, et al. : Immunogenicity of the hu14.18-IL2 immunocytokine molecule in adults with melanoma and children with neuroblastoma. Clin Cancer Res 15:5923-5930, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albertini MR, Gan J, Jaeger P, et al. : Systemic interleukin-2 modulates the anti-idiotypic response to chimeric anti-GD2 antibody in patients with melanoma. J Immunother Emphasis Tumor Immunol 19:278-295, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Talleur AC, Triplett BM, Federico S, et al. : Consolidation therapy for newly diagnosed pediatric patients with high-risk neuroblastoma using busulfan/melphalan, autologous hematopoietic cell transplantation, anti-GD2 antibody, granulocyte-macrophage colony-stimulating factor, interleukin-2, and haploidentical natural killer cells. Biol Blood Marrow Transplant 23:1910-1917, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epperly R, Furman W, Hines M, et al. : Secondary hemophagocytic syndrome after autologous hematopoietic cell transplant and immune therapy for neuroblastoma. Pediatr Blood Cancer 66:e27964, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Granger MM, Naranjo A, Bagatell R, et al. : Myeloablative busulfan/melphalan consolidation following induction chemotherapy for patients with newly diagnosed high-risk neuroblastoma: Children's Oncology Group trial ANBL12P1. Transpl Cell Ther 27:490.e1-490.e8, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozkaynak MF, Gilman AL, London WB, et al. : A comprehensive safety trial of chimeric antibody 14.18 with GM-CSF, IL-2, and isotretinoin in high-risk neuroblastoma patients following myeloablative therapy: Children's Oncology Group study ANBL0931. Front Immunol 9:1355, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen R, Sahr N, Sykes A, et al. : Longitudinal NK cell kinetics and cytotoxicity in children with neuroblastoma enrolled in a clinical phase II trial. J Immunother Cancer 88:e000176, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garaventa A, Poetschger U, Valteau-Couanet D, et al. : Randomized trial of two induction therapy regimens for high-risk neuroblastoma: HR-NBL1.5 International Society of Pediatric Oncology European Neuroblastoma Group study. J Clin Oncol 39:2552-2563, 2021 [DOI] [PubMed] [Google Scholar]
- 29.Berthold F, Faldum A, Ernst A, et al. : Extended induction chemotherapy does not improve the outcome for high-risk neuroblastoma patients: Results of the randomized open-label GPOH trial NB2004-HR. Ann Oncol 31:422-429, 2020 [DOI] [PubMed] [Google Scholar]
- 30.Kreissman SG, Seeger RC, Matthay KK, et al. : Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): A randomised phase 3 trial. Lancet Oncol 14:999-1008, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albertini MR, Hank JA, Schiller JH, et al. : Phase IB trial of chimeric antidisialoganglioside antibody plus interleukin 2 for melanoma patients. Clin Cancer Res 3:1277-1288, 1997 [PubMed] [Google Scholar]
- 32.Mody R, Yu AL, Naranjo A, et al. : Irinotecan, temozolomide, and dinutuximab with GM-CSF in children with refractory or relapsed neuroblastoma: A report from the Children's Oncology Group. J Clin Oncol 38:2160-2169, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kushner BH, Cheung NK: GM-CSF enhances 3F8 monoclonal antibody-dependent cellular cytotoxicity against human melanoma and neuroblastoma. Blood 73:1936-1941, 1989 [PubMed] [Google Scholar]
- 34.Mueller BM, Romerdahl CA, Gillies SD, et al. : Enhancement of antibody-dependent cytotoxicity with a chimeric anti-GD2 antibody. J Immunol 144:1382-1386, 1990 [PubMed] [Google Scholar]
- 35.Furman WL, Fairclough DL, Huhn RD, et al. : Therapeutic effects and pharmacokinetics of recombinant human granulocyte-macrophage colony-stimulating factor in childhood cancer patients receiving myelosuppressive chemotherapy. J Clin Oncol 9:1022-1028, 1991 [DOI] [PubMed] [Google Scholar]
- 36.Meropol NJ, Porter M, Blumenson LE, et al. : Daily subcutaneous injection of low-dose interleukin 2 expands natural killer cells in vivo without significant toxicity. Clin Cancer Res 2:669-677, 1996 [PubMed] [Google Scholar]
- 37.Ladenstein R, Potschger U, Pearson AD, et al. : Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): An international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol 18:500-514, 2017 [DOI] [PubMed] [Google Scholar]