Abstract
Highly active antiretroviral therapy has been effective in lowering viral loads in the peripheral blood, restoring immune function and reducing the incidence of opportunistic infections and dementia in human immunodeficiency virus (HIV)-infected individuals. However, motor and cognitive deficits and peripheral neuropathy continue, with some studies reporting an increase in prevalence of nervous system disease. The authors developed an accelerated, consistent simian model of HIV infection in which pigtailed macaques are dual inoculated with a neurovirulent simian immunodeficiency virus (SIV) clone and an immunosuppressive SIV strain. Infected animals invariably develop acquired immunodeficiency syndrome (AIDS) and over 90% develop central nervous system disease as well as peripheral nervous system disease with neurodegeneration by 3 months post inoculation. This model provides outstanding opportunities to delineate the pathogenesis of infection, to study the regulation of virus gene expression, and to identify host immune responses throughout the acute, clinically silent and late stages of infection. Using this model, the authors have demonstrated that the virus enters the brain within days after inoculation, that CCL2 (monocyte chemoattractant protein [MCP]-1) plays a major role in recruiting monocytes/macrophages to the brain, and that type I interferons are critical in suppressing early virus replication and inducing viral latency. This model provides a rigorous platform for the testing of potential antiretroviral, immune reconstituting, and/or neuroprotective agents and already has been used to confirm the neuroprotective properties of minocycline, which now is being tested in clinical trials of HIV-infected individuals.
Keywords: animal model, dementia, HIV, nervous system, SIV
Introduction
Treatment of human immunodeficiency virus (HIV)-infected individuals with highly active antiretroviral therapy (HAART) has significantly changed the clinical course of HIV infection and the development of HIV-associated dementia. Although the incidence of HIV-associated dementia has declined, the cumulative prevalence has continued to increase (Maschke et al, 2000; McArthur et al, 2003; Sacktor et al, 2002). HIV-associated minor cognitive impairment is now the most prevalent form of HIV-associated central nervous system disease and there also is an increasing prevalence of HIV-associated peripheral neuropathy in HIV-infected individuals (Simpson et al, 2006; Smyth et al, 2007). Thus, it continues to be important to understand the pathogenesis of HIV infections of both the central (CNS) and peripheral (PNS) nervous systems. Simian immunodeficiency virus (SIV) infection of macaques recapitulates many aspects of HIV infection and disease progression including infection of both lymphocytes and macrophages as well as the development of immunosuppression and CNS and PNS disease. Infection of macaques with SIV, similar to infection of humans with HIV, is characterized by highly variable periods of time between acute infection and the development of immunosuppression as well as variation in the incidence of CNS disease. To investigate the cellular and molecular mechanisms that contribute to infection and the development of disease in the nervous system, it was necessary to examine the central and peripheral nervous systems at various stages throughout infection with the confidence that the animals were destined to develop neurological disease. Thus it was critical to develop a SIV model in which infected macaques predictably develop CNS disease and preferably with a shortened time course (Clements et al, 2002; Zink et al, 1997, 1999; Zink and Clements, 2002).
Our laboratory developed an accelerated, consistent SIV/macaque model that requires simultaneous inoculation with two viruses: a neurovirulent molecular clone (SIV/17E-Fr) and an uncloned strain of SIV that is immunosuppressive (SIV/DeltaB670). This combination of viruses results in high levels of acute virus replication with plasma RNA levels, reaching 108 copy equivalents per milliliter, continued high plasma viral load thoughout infection, rapid loss of CD4+ lymphocytes beginning as early as 35 days postinoculation, and development of CNS and PNS diseases in the majority of animals by 84 days post inoculation (Zink et al, 1999). In addition to the combination of viruses, our model uses pigtailed macaques (Macaca nemestrina), which develop immunosuppression more rapidly than rhesus macaques inoculated with the same viruses and develop CNS lesions with a higher frequency than either rhesus macaques or cynomologous monkeys (Zink et al, 1997). Thus, both viral and host genetic components contribute to the accelerated development of acquired immunodeficiency syndrome (AIDS) and the high frequency of nervous system pathology in this SIV/macaque model. Here we review the model and describe how it has been used to study the selection of viral genotypes in the brain, mechanisms by which viral latency is established in the brain, markers of CNS disease in cerebrospinal fluid (CSF) and plasma, development of encephalitis and peripheral neuropathy, and novel treatment approaches.
Viral components of the model
In developing the model, infection of the brain with constant development of CNS disease was a primary goal. However, it was clear from studies in humans with both AIDS dementia and other HIV-associated neurologic diseases that immunosuppression accompanied the clinical development of these diseases (Gibbs et al, 1990). Consequently, both a neurovirulent SIV and an SIV strain that caused immunosuppression were used for this model. A neurovirulent strain of SIV was derived by the passage of the cloned, lymphocyte-tropic SIVmac239 four times in the brains of rhesus macaques. SIV was isolated from the brain of a macaque that developed CNS lesions (passage 3), and that virus was used to infect another macaque. This macaque developed CNS disease more rapidly and the virus isolated from this macaque brain, SIV17E-Br, was macrophage-tropic, in contrast to the lymphocyte-tropic original virus used for the original inoculation. SIV/17E-Br was used to construct molecular clones to identify the genetic basis for neurovirulence (Anderson et al, 1993). A recombinant virus, SIV/17E-Fr, that contained the entire envelope, nef, and long terminal repeat (LTR) from SIV/17E-Br was macrophage-tropic and neurovirulent in macaques (Flaherty et al, 1997; Mankowski et al, 1997). Studies of this and other recombinant macrophage-tropic SIV clones demonstrated that although macrophage tropism is necessary for neurovirulence of SIV, it is not sufficient to cause CNS lesions.
Most SIV strains utilize CCR5 as the coreceptor for cell entry despite their cellular tropism, although at least one monkey species can be infected with a strain of SIV that uses CCR2b (Chen et al, 1998; Marx and Chen, 1998). Like HIV-2, some strains of SIV are able to infect cells without CD4. Receptor and coreceptor usage of the macrophage-tropic, neurovirulent, uncloned SIV/17E-Br and cloned SIV/17E-Fr revealed that they both were CD4-independent, CCR5-dependent viruses (Edinger et al, 1997a, 1997b). In contrast, the macrophage-tropic, non-neurovirulent virus SIV/17E-Cl, which was constructed by inserting the gp120 portion of the env gene of SIV/17E-Fr into the backbone of SIVmac239, required both CD4 and CCR5 for entry into cells. Thus, the CD4-independent SIV strains appear to be more pathogenic in the CNS.
The immunosuppressive strain used in this model is an uncloned virus, SIV/DeltaB670. This was isolated from lymph node of an SIV-infected rhesus macaque and has been shown to contain over 20 viral strains distinguished by sequences in the V1 region of the envelope gene (Amedee et al, 1995). The viruses in the SIV/DeltaB670 swarm are a mixture of lymphocyte-tropic and macrophage-tropic strains. SIV/DeltaB670 infection causes AIDS and CNS disease in approximately 30% of infected rhesus macaques, with a time course of 6 to 45 months (Trichel et al, 2002). Infection of rhesus macaques with SIV/17E-Fr also causes AIDS and CNS disease in 3 to 15 months and 67% develop CNS lesions, with replicating virus detected in the brains of 80% of the animals (Mankowski et al, 1997). Thus, dual infection of pigtailed macaques with SIV/17E-Fr and SIV/DeltaB670 has a consistently short time course of 3 months and a high probability of CNS disease in comparison to infection of macaques with other viruses.
SIV encephalitis: parallels with HIV-associated neurological disease
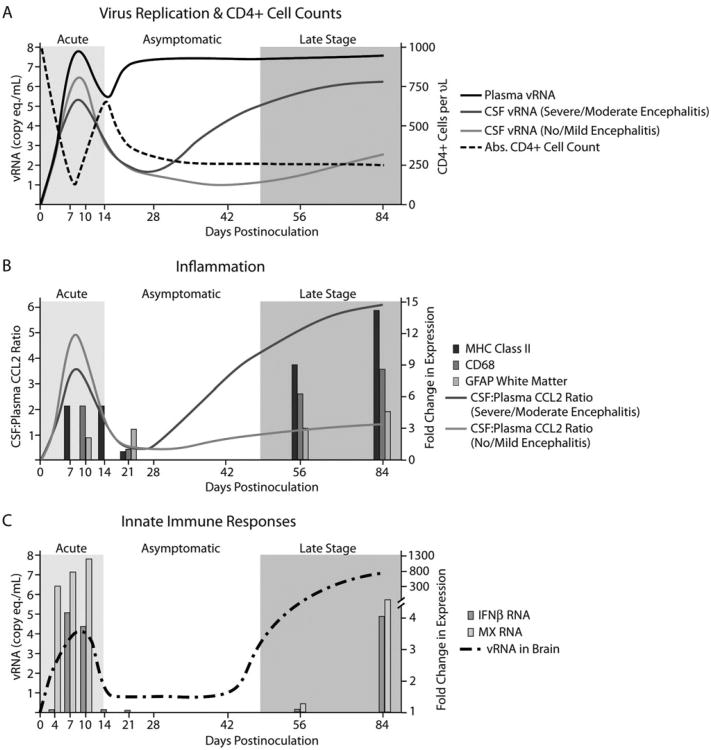
Over the last decade, 29 pigtailed macaques have been dual inoculated with SIV/17E-Fr and SIV/DeltaB670, their plasma and CSF has been sampled at regular intervals throughout infection, and they have been euthanized at 84 days post inoculation (p.i.). In addition, significant numbers of macaques have been euthanized at each of 4, 7, 10, 14, 21, 42, and 56 days p.i. This has provided us with a detailed picture of virological, inflammatory, immunological, and neurological events throughout the course of infection and the development of CNS and PNS disease. Immediately after inoculation, there is a consistent rapid rise in viral load in plasma and CSF, peaking at 7 to 10 days, at a concentration of approximately 108 and 106-7 viral RNA copy equivalents per milliliter, respectively (Figure 1A) (Zink et al, 1999). By days 14 to 21, plasma viral loads have declined by 1 to 2 logs and CSF viral loads have declined more precipitously. By approximately 28 days p.i., plasma viral loads have risen again and remain at 107 to 108 copy equivalent/ml throughout infection. CSF viral loads also begin to increase at this point, and animals that ultimately develop severe or moderate encephalitis develop significantly higher CSF viral RNA levels. Twenty-eight days p.i., there is a dramatic decline in absolute CD4+ cell counts in the peripheral blood, with development of AIDS in 100% of macaques by day 84 p.i.
Figure 1.
Viral, inflammatory, and immune parameters in the accelerated, consistent SIV/macaque model of HIV-associated neurological disease. (A) Viral RNA levels in plasma increase rapidly during the first 7 to 10 days after virus inoculation, decline by approximately 1 log, then increase again and remain at 107 to 108 for the remainder of the infection period. Viral RNA levels in CSF increase during acute infection, decline by several logs during the next 2 weeks, then increase again only in macaques that will develop moderate to severe encephalitis. CD4+ cell counts in peripheral blood decline during acute infection, recover briefly, then begin to decline again during the asymptomatic stage of infection. (B) CCL2 production in the CNS, expressed as the ratio of CCL2 in CSF:plasma rises during acute infection, declines by day 21 p.i., then increases again in macaques that will develop moderate to severe encephalitis. Markers of macrophage infiltration and activation (CD68 and MHC class II) are elevated in brain tissue during acute and terminal infection. In contrast, activation of astrocytes as measured by expression of GFAP in brain tissue increases gradually throughout infection. (C) During acute infection, when there is an increase in viral RNA in the brain, IFNβ and MX RNA are elevated in the brain. Viral RNA declines rapidly after expression of IFNβ and MX and rises again late in infection in conjunction with immunosuppression. IFNβ and MX RNA are again expressed at high levels during late-stage infection.
Cytokine and chemokine expression in CSF also follows a biphasic pattern throughout infection. During the first 7 to 10 days of infection, CSF CCL2 levels rise to several times the level in plasma, implying that a gradient of CCL2 develops that would recruit monocytes into the brain during acute infection (Figure 1B) (Zink et al, 2001). By days 14 to 21, CSF CCL2 levels have declined to normal levels. After day 28 p.i., CSF CCL2 levels rise again and CSF:plasma ratios of CCL2 that are higher than 2.65 predict macaques that will develop moderate or severe encephalitis. Like CCL-2, CSF interleukin (IL)-6 levels also precede and predict the subsequent development of SIV encephalitis, although CSF IL-6 levels often lag CCL-2 levels by approximately 2 weeks (Mankowski et al, 2004).
Upon immunohistochemical staining of brain tissue at various stages of infection, it was noted that expression of the macrophage activation markers CD68 and major histocompatibility complex (MHC) class II were increased in brain tissue during acute and late-stage infection (Figure 1B), following the same biphasic pattern as expression of the macrophage chemoattractant protein CCL2 in CSF. Expression of the macrophage and microglial activation marker CD68 corresponded with SIV encephalitis severity, illustrating that macrophage activation plays a central role in SIV CNS disease, like HIV CNS disease (Zink et al, 1997). When there were increased numbers and activation of macrophages in the brain parenchyma, viral RNA in brain homogenates also was elevated. These findings are consistent with the expression of CCL2 recruiting virus-containing macrophages to the brain during acute and late-stage infection. Interestingly, expression of glial fibrillary acidic protein (GFAP) in white matter increases gradually throughout infection, reaching significant levels as early as day 21. This suggests that activation of astrocytes is initiated during early infection and continues unabated throughout infection, with the potential to irrevocably alter the neural environment of infected individuals.
Of the 29 macaques that were euthanized at 84 days p.i., approximately 90% developed neurological disease. Twenty-one percent were classified pathologically as mild encephalitis, 28% as moderate encephalitis, and 41% as severe encephalitis. SIV encephalitis, characterized by numerous perivascular cuffs of epithelioid macrophages and multinucleated giant cells, as well as multifocal nests of activated macrophages and astrocytes in the parenchyma, is very similar to HIV encephalitis except that the lesions in SIV-infected macaques often contain more lymphocytes, perhaps because macaques are euthanized when the first show clinical signs of disease, so the CNS likely is examined at an earlier stage of infection (Czub et al, 1996). Immunohistochemical staining and in situ hybridization consistently reveal viral antigen and viral RNA almost exclusively in cells of macrophage lineage in the brain parenchyma and in perivascular cuffs (Zink et al, 1997, 2001). As in HIV encephalitis, a small percentage of astrocytes are positive for antigens of early viral genes such as nef throughout the disease process (Guillemin et al, 2000; Overholser et al, 2003; Saito et al, 1994; Tornatore et al, 1994).
In addition to CNS inflammation, neuronal dysfunction in SIV-infected macaques can be detected by immunostaining for amyloid precursor protein (APP) in white matter tracts such as the corpus callosum (Mankowski et al, 2002). Animals that developed SIV encephalitis had a high likelihood of containing elevated APP in white matter as compared to SIV-infected animals that did not develop encephalitis. The amount of APP accumulating within axons correlated strongly with the amount of SIV protein in the CNS and the extent of decline in bimanual motor performance on neurobehavioral testing (Weed et al, 2003).
Given that neuronal dysfunction developed in this SIV/macaque model, we next examined CSF collected at various times during infection to determine whether we could detect evidence of neuronal damage markers prior to onset of overt SIV encephalitis. 14–3–3 proteins were detected in the CSF by immunoblotting beginning during asymptomatic infection and then persisting until terminal stages of disease in animals that developed SIV encephalitis. Levels of CSF 14–3–3 proteins closely correlated with the extent of SIV replication in the CNS (basal ganglia, P = .001), suggesting that CNS virus replication induces sustained neuronal damage even during asymptomatic stages of infection when monocyte/macrophage activation represented by CD68 immunostaining approximates preinfection levels in subcortical white matter (Helke et al, 2005). This finding corresponds with earlier studies showing that the extent of APP accumulation in the corpus callosum is highly correlated with SIV protein levels in the CNS (Mankowski et al, 2002).
SIV-induced PNS disease
The most common neurologic condition currently associated with HIV infection is peripheral neuropathy, frequently manifested clinically as a debilitating distal sensory neuropathy. Study of HIV PNS disease is complicated by the potential for various highly active antiretroviral therapy (HAART) regimens to potentiate HIV-induced PNS damage (Keswani et al, 2006; Pettersen et al, 2006). Given the high prevalence of CNS lesions in the accelerated SIV/macaque model, we examined whether lesions also developed in the PNS by examining somatosensory ganglia for evidence of virus replication and host immune responses. Initial studies that focused on the trigeminal ganglia to address these questions identified several parallels between SIV PNS disease and SIV CNS disease, including (1) replication of SIV predominantly within macrophages in ganglia; (2) the presence of SIV RNA in ganglia during acute infection, with reduction of SIV replication after acute infection; and (3) increases in CD68 immunostaining in the ganglia of SIV-infected animals, likely representing both recruitment of monocytes as well as activation of endogenous macrophage populations (Laast et al, 2007). However, severity of ganglionitis and magnitude of CD68 immunostaining was not strongly linked to the severity of SIV encephalitis. In contrast, the level of SIV replication in PNS and CNS were strongly correlated (P = .036, r = .8). Additional studies targeting pathologic and corresponding physiologic alterations in the PNS of SIV-infected pigtailed macaques will further our understanding of the pathogenesis of HIV PNS disease.
Viral genotypes in the brain
The viral inoculum used in our accelerated SIV/macaque model provided us with the opportunity to examine the viral strains that were present in DNA and RNA from the brains of macaques during the acute, clinically silent and late stages of infection and with CNS lesions of various severities and compare them to the viral strains replicating in the peripheral blood. Viral sequences present in brain DNA provided us with an archive of the viral strains that had passed through the brain, some of which remain as latent virus (Clements et al, 2002), whereas viral sequences in brain and peripheral blood mononuclear cell (PBMC) RNA indicated strains that were actively replicating at the time the samples were obtained. We used the V1 portion of env as our marker sequence because it is the most variable portion of the envelope gene and because previous studies identified over 20 V1 genotypes in our inoculum viruses (SIV/17E-Fr and SIV/DeltaB670), including both lymphocyte-tropic, presumably immunosuppressive, strains and macrophage-tropic strains, some of which were expected to be neurovirulent (Amedee et al, 1995).
A wide variety of lymphocyte-tropic and macrophage-tropic viral genotypes were identified in DNA and RNA isolated from brain and the same genotypes were identified in RNA isolated from PBMCs during acute infection. This suggested that there was no apparent viral selection during early seeding of the brain (Babas et al, 2006). Starting at 21 days p.i. (early asymptomatic infection) and continuing through late-stage infection, there was a gradually increasing predominance of two macrophage-tropic, neurovirulent viral genotypes, SIV/17E-Fr and SIV/DeltaB670 Cl-2, in RNA isolated from brain. During late-stage infection, these genotypes comprised 95% of the viral genotypes detected in brain. There was a significantly greater number of viral genotypes in DNA isolated from brain and in RNA from PBMC than in RNA isolated from the brain (P = .004). Both macrophage- and lymphocyte-tropic viral genotypes were identified in DNA from brain and RNA from PBMCs. The viral genotype that predominated in RNA from brain, however, frequently was different from that which predominated in the PBMCs of the same animal. This suggested that although many genotypes have access to the brain via trafficking PBMCs, only some of them remain and replicate in the brain microenvironment. These data further suggest that the selection of macrophage-tropic, neurovirulent viruses occurs not at the level of the blood-brain barrier, but at a stage after entry of virus-infected cells to the CNS.
An interesting observation suggested that during terminal infection, when there is abundant virus replication in the brain, the brain may seed the periphery with virus. At 56 days p.i., over half of the genotypes detected in RNA from brain consisted of the two neurovirulent viral genotypes SIV/17E-Fr and SIV/DeltaB670 (Cl-2) these genotypes were not present in PBMC RNA at that time. However, these genotypes were strongly represented in PBMCs of those same macaques at 84 days p.i., a time when there was abundant replication of these viruses in the brain (Babas et al, 2006). That replication of these genotypes in the brain preceded their appearance in the peripheral blood suggests that the brain might be a source of virus in the periphery. This is important because most antiretroviral drugs do not reach effective levels in the CNS, leaving virus potentially able to replicate in the brain and seed the periphery.
Control of virus replication in the brain and establishment of viral latency
Early HIV entry into the CNS during acute infection has been demonstrated in a small number of cases (Davis et al, 1992; Gray et al, 1996; An et al, 1999; Gray et al, 1996). However, it is not clear whether early CNS infection occurs consistently or whether virus persists in the brain after acute infection or is cleared by immune responses. Because of the consistent development of CNS lesions in this SIV model, such studies were possible. We showed that virus replication in the brain measured by real-time reverse transcriptase–polymerase chain reaction (RT-PCR) occurred as early as 7 days p.i. (Figure 1C) and in CSF at 3 days. Viral RNA in CSF reached peak levels at 10 days p.i. However, viral RNA levels were down regulated at 21 days p.i., suggesting that virus replication in the brain is controlled. This control of virus replication was independent of virus replication in the peripheral blood, which continued at a high level (Barber et al, 2006; Clements et al, 2002). Although brain viral RNA levels were greatly reduced, there was no reduction in the levels of viral DNA detected by real-time PCR from acute throughout the asymptomatic infection period, suggesting that there was control of virus replication but not elimination of infected cells. This suggested that non-cytolytic mechanisms down-regulated virus replication at the transcriptional level (Clements et al, 2002).
Innate immune responses, in particular type I interferons (IFNs), are important for early antiviral responses in the brain and periphery (Griffin, 2003). IFNβ is the first type I IFN induced and it has been shown to inhibit HIV replication in macrophages, in part at the transcriptional level (Honda et al, 1998; Kornbluth et al, 1990). We demonstrated that IFNβ inhibited SIV replication in vitro in macrophages by the same transcriptional mechanism (Figure 1C). IFNβ induces the expression of a dominant-negative form of the transcription factor CCAAT/enhancer-binding protein-β (C/EBPβ), also called LIP (Barber et al, 2004, 2006). We then examined IFNβ expression in the brain and found that both mRNA and protein were detected at 7 days p.i. and increased expression of IFNβ and the inhibitory form of C/EBPβ, LIP, correlated temporally with the suppression of acute SIV replication.
Further studies using chromatin immunoprecipitation (ChIP) analyses of SIV-infected brain or SIV LTR expression in macrophages in culture demonstrated that IFNβ induced increased levels of LIP, which led to decreased levels of acetyted histone H4 on the SIV LTR and transcriptional silencing of the SIV LTR (Barber et al, 2006). We recently have demonstrated a novel downstream signaling mechanism for IFNβ, the phosphorylation of an RNA binding protein (CUGBP1) that binds the C/EBP mRNA and increases translation of LIP (Dudaronek et al, 2007). These studies demonstrated that IFNβ and LIP expression leads to transcriptional silencing of SIV and provides another mechanism for establishing latency of HIV and SIV in infected individuals. This is particularly important in the brain where many antiretroviral drugs fail to penetrate to effective levels (Letendre et al, 2008; Wynn et al, 2002) and long-lived infected macrophages harbor latent virus providing a reservoir of HIV.
Treatment paradigms and the strength of the model
The accelerated SIV/macaque model is ideal for testing potentially neuroprotective therapeutics because over 90% of inoculated macaques develop neurological disease (and all macaques develop AIDS). The high levels of virus replication in the periphery and the CNS provide a very rigorous testing platform for novel therapeutics. Further, the short time required for establishment of SIV encephalitis in inoculated macaques makes testing of therapeutics in the accelerated SIV/macaque timely and efficient. SIV infection of macaques has the potential to play a significant role in determining whether drugs that are already Food and Drug Administration (FDA) approved for other purposes might also have an ameliorative effect on HIV-associated neurological disease. Although such drugs can be tested in HIV-infected individuals, establishing and obtaining approval for protocols for clinical trials, garnering funding, recruiting patients, and collecting and analyzing the data make the process costly and extremely time consuming. In addition, the fact that the majority of patients in developed countries are undergoing highly active antiretroviral therapy (HAART) is a significant confounder to such studies. This is particularly true when there is no single ideal combination of antiretroviral drugs for every individual. An examination of antiretroviral drug use in one clinical cohort revealed that 265 different antiretroviral regimens were in use (Ellis, 2007).
The feasibility of using the accelerated SIV/macaque model to test a new use for an established drug was established by studies examining the ability of the tetracycline derivative minocycline to protect macaques from SIV-induced inflammation and neurodegeneration. This study was inspired by previous reports showing that minocycline was effective in reducing joint inflammation in people with rheumatoid arthritis (O'Dell et al, 2001), that it appeared to reduce the number of relapses in individuals with remitting and relapsing multiple sclerosis (Metz et al, 2004), and that it was neuroprotective in animal models of several neurological conditions, including Parkinson's disease, Huntington's disease, stroke, and cerebral trauma (Arvin et al, 2002; Chen et al, 2000; Du et al, 2001; Popovic et al, 2002; Thomas et al, 2003; Zhu et al, 2002). Macaques were inoculated with SIV/17E-Fr and SIV/DeltaB670 and oral minocycline treatment was initiated once the initial burst of virus replication in the CNS during acute infection had begun to decline in response to host immune responses (between 12 and 21 days post inoculation) (Zink et al, 2005). Macaques were euthanized at 84 days post inoculation and their brains were examined for markers of macrophage activation and infiltration (CD68 and MHC class II), for GFAP as a marker of astrocyte activation, for activation of the neurodegenerative mitogen-activated protein (MAP) kinases JNK and p38, and for APP, a marker of neuronal degeneration. In addition, viral RNA was quantitated by real-time RT-PCR and viral antigen by quantitative immunohistochemistry. All of these parameters were significantly lower in the minocycline-treated macaques as compared to untreated controls, demonstrating that minocycline was anti-inflammatory and neuroprotective in the CNS of SIV-infected macaques. This study demonstrated that the accelerated SIV/macaque model could successfully be used for preclinical testing of candidate drugs to treat neuroAIDS. The efficacy of minocycline in ameliorating neurological disease in HIV-infected individuals is now being tested in clinical trials in the United States and Africa. Thus, this model has not only proven its facility in helping reveal the mechanisms by which HIV causes neurological disease, but it also has shown significant utility in testing drugs to fight neuroAIDS.
Acknowledgments
The authors thank Suzanne Queen and Brandon Bullock for their expert technical assistance in the development of the accelerated, consistent SIV/macaque model. These studies were funded by NIH grants NS055648, NS047984, MH070306, NS36911, MH61189, NS44815, MH069116, and NS055651.
Dedicated to the memory of Opendra “Bill” Narayan.
References
- Amedee AM, Lacour N, Gierman JL, Martin LN, Clements JE, Bohn R, Harrison RM, Murphey-Corb M. Genotypic selection of simian immunodeficiency virus in macaque infants infected transplacentally. J Virol. 1995;69:7982–7990. doi: 10.1128/jvi.69.12.7982-7990.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An SF, Groves M, Gray F, Scaravilli F. Early entry and widespread cellular involvement of HIV-1 DNA in brains of HIV-1 positive asymptomatic individuals. J Neuropathol Exp Neurol. 1999;58:1156–1162. doi: 10.1097/00005072-199911000-00005. [DOI] [PubMed] [Google Scholar]
- Anderson MG, Hauer D, Sharma DP, Joag SV, Narayan O, Zink MC, Clements JE. Analysis of envelope changes acquired by SIVmac239 during neuroadaption in rhesus macaques. Virology. 1993;195:616–626. doi: 10.1006/viro.1993.1413. [DOI] [PubMed] [Google Scholar]
- Arvin KL, Han BH, Du Y, Lin SZ, Paul SM, Holtzman DM. Minocycline markedly protects the neonatal brain against hypoxic-ischemic injury. Ann Neurol. 2002;52:54–61. doi: 10.1002/ana.10242. [DOI] [PubMed] [Google Scholar]
- Babas T, Dewitt JB, Mankowski JL, Tarwater PM, Clements JE, Zink MC. Progressive selection for neurovirulent genotypes in the brain of SIV-infected macaques. AIDS. 2006;20:197–205. doi: 10.1097/01.aids.0000198078.24584.21. [DOI] [PubMed] [Google Scholar]
- Barber SA, Gama L, Dudaronek JM, Voelker T, Tarwater PM, Clements JE. Mechanism for the establishment of transcriptional HIV latency in the brain in a simian immunodeficiency virus-macaque model. J Infect Dis. 2006;193:963–970. doi: 10.1086/500983. [DOI] [PubMed] [Google Scholar]
- Barber SA, Herbst DS, Bullock BT, Gama L, Clements JE. Innate immune responses and control of acute simian immunodeficiency virus replication in the central nervous system. J Neuro Virol. 2004;10(Suppl 1):15–20. doi: 10.1080/753312747. [DOI] [PubMed] [Google Scholar]
- Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH, Friedlander RM. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- Chen Z, Kwon D, Jin Z, Monard S, Telfer P, Jones MS, Lu CY, Aguilar RF, Ho DD, Marx PA. Natural infection of a homozygous delta24 CCR5 red-capped mangabey with an R2b-tropic simian immunodeficiency virus. J Exp Med. 1998;188:2057–2065. doi: 10.1084/jem.188.11.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JE, Babas T, Mankowski JL, Suryanarayana K, Piatak M, Jr, Tarwater PM, Lifson JD, Zink MC. The central nervous system as a reservoir for simian immunodeficiency virus (SIV): steady-state levels of SIV DNA in brain from acute through asymptomatic infection. J Infect Dis. 2002;186:905–913. doi: 10.1086/343768. [DOI] [PubMed] [Google Scholar]
- Czub S, Muller JG, Czub M, Muller-Hermelink HK. Nature and sequence of simian immunodeficiency virus-induced central nervous system lesions: a kinetic study. Acta Neuropathol. 1996;92:487–498. doi: 10.1007/s004010050551. [DOI] [PubMed] [Google Scholar]
- Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, Young SA, Mills RG, Wachsman W, Wiley CA. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DL, Luecke S, Phebus LA, Bymaster FP, Paul SM. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc Natl Acad Sci U S A. 2001;98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudaronek JM, Barber SA, Clements JE. CUGBP1 is required for IFNbeta-mediated induction of dominant-negative CEBPbeta and suppression of SIV replication in macrophages. J Immunol. 2007;179:7262–7269. doi: 10.4049/jimmunol.179.11.7262. [DOI] [PubMed] [Google Scholar]
- Edinger AL, Amedee A, Miller K, Doranz BJ, Endres M, Sharron M, Samson M, Lu ZH, Clements JE, Murphy-Corb M, Peiper SC, Parmentier M, Broder CC, Doms RW. Differential utilization of CCR5 by macrophage and T cell tropic simian immunodeficiency virus strains. Proc Natl Acad Sci U S A. 1997a;94:4005–4010. doi: 10.1073/pnas.94.8.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger AL, Mankowski JL, Doranz BJ, Margulies BJ, Lee B, Rucker J, Sharron M, Hoffman TL, Berson JF, Zink MC, Hirsch VM, Clements JE, Doms RW. CD4-independent, CCR5-dependent infection of brain capillary endothelial cells by a neurovirulent simian immunodeficiency virus strain. Proc Natl Acad Sci USA. 1997b;94:14742–14747. doi: 10.1073/pnas.94.26.14742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. ISNV Meeting; San Diego, CA. November 2007.2007. [Google Scholar]
- Flaherty MT, Hauer DA, Mankowski JL, Zink MC, Clements JE. Molecular and biological characterization of a neurovirulent molecular clone of simian immunodeficiency virus. J Virol. 1997;71:5790–5798. doi: 10.1128/jvi.71.8.5790-5798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs A, Andrewes DG, Szmukler G, Mulhall B, Bowden SC. Early HIV-related neuropsychological impairment: relationship to stage of viral infection. J Clin Exp Neuropsychol. 1990;12:766–780. doi: 10.1080/01688639008401018. [DOI] [PubMed] [Google Scholar]
- Gray F, Scaravilli F, Everall I, Chretien F, An S, Boche D, Adle-Biassette H, Wingertsmann L, Durigon M, Hurtrel B, Chiodi F, Bell J, Lantos P. Neuropathology of early HIV-1 infection. Brain Pathol. 1996;6:1–15. doi: 10.1111/j.1750-3639.1996.tb00775.x. [DOI] [PubMed] [Google Scholar]
- Griffin DE. Immune responses to RNA-virus infections of the CNS. Nat Rev Immunol. 2003;3:493–502. doi: 10.1038/nri1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin G, Croitoru J, Le Grand RL, Franck-Duchenne M, Dormont D, Boussin FD. Simian immunodeficiency virus mac251 infection of astrocytes. J Neuro Virol. 2000;6:173–186. doi: 10.3109/13550280009015821. [DOI] [PubMed] [Google Scholar]
- Helke KL, Queen SE, Tarwater PM, Turchan-Cholewo J, Nath A, Zink MC, Irani DN, Mankowski JL. 14–3–3 protein in CSF: an early predictor of SIV CNS disease. J Neuropathol Exp Neurol. 2005;64:202–208. doi: 10.1093/jnen/64.3.202. [DOI] [PubMed] [Google Scholar]
- Honda Y, Rogers L, Nakata K, Zhao BY, Pine R, Nakai Y, Kurosu K, Rom WN, Weiden M. Type I interferon induces inhibitory 16-kD CCAAT/enhancer binding protein (C/EBP)beta, repressing the HIV-1 long terminal repeat in macrophages: pulmonary tuberculosis alters C/EBP expression, enhancing HIV-1 replication. J Exp Med. 1998;188:1255–1265. doi: 10.1084/jem.188.7.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keswani SC, Jack C, Zhou C, Hoke A. Establishment of a rodent model of HIV-associated sensory neuropathy. J Neurosci. 2006;26:10299–10304. doi: 10.1523/JNEUROSCI.3135-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth RS, Oh PS, Munis JR, Cleveland PH, Richman DD. The role of interferons in the control of HIV replication in macrophages. Clin Immunol Immunopathol. 1990;54:200–219. doi: 10.1016/0090-1229(90)90082-2. [DOI] [PubMed] [Google Scholar]
- Laast VA, Pardo CA, Tarwater PM, Queen SE, Reinhart TA, Ghosh M, Adams RJ, Zink MC, Mankowski JL. Pathogenesis of simian immunodeficiency virus-induced alterations in macaque trigeminal ganglia. J Neuropathol Exp Neurol. 2007;66:26–34. doi: 10.1097/nen.0b013e31802c398d. [DOI] [PubMed] [Google Scholar]
- Lane JH, Sasseville VG, Smith MO, Vogel P, Pauley DR, Heyes MP, Lackner AA. Neuroinvasion by simian immunodeficiency virus coincides with increased numbers of perivascular macrophages/ microglia and intrathecal immune activation. J Neuro Virol. 1996a;2:423–432. doi: 10.3109/13550289609146909. [DOI] [PubMed] [Google Scholar]
- Lane JH, Tarantal AF, Pauley D, Marthas M, Miller CJ, Lackner AA. Localization of simian immunodeficiency virus nucleic acid and antigen in brains of fetal macaques inoculated in utero. Am J Pathol. 1996b;149:1097–1104. [PMC free article] [PubMed] [Google Scholar]
- Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, Simpson D, Grant I, Ellis RJ. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankowski JL, Flaherty MT, Spelman JP, Hauer DA, Didier PJ, Amedee AM, Murphey-Corb M, Kirstein LM, Munoz A, Clements JE, Zink MC. Pathogenesis of simian immunodeficiency virus encephalitis: viral determinants of neurovirulence. J Virol. 1997;71:6055–6060. doi: 10.1128/jvi.71.8.6055-6060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankowski JL, Queen SE, Clements JE, Zink MC. Cerebrospinal fluid markers that predict SIV CNS disease. J Neuroimmunol. 2004;157:66–70. doi: 10.1016/j.jneuroim.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Mankowski JL, Queen SE, Tarwater PM, Fox KJ, Perry VH. Accumulation of beta-amyloid precursor protein in axons correlates with CNS expression of SIV gp41. J Neuropathol Exp Neurol. 2002;61:85–90. doi: 10.1093/jnen/61.1.85. [DOI] [PubMed] [Google Scholar]
- Marx PA, Chen Z. The function of simian chemokine receptors in the replication of SIV. Semin Immunol. 1998;10:215–223. doi: 10.1006/smim.1998.0135. [DOI] [PubMed] [Google Scholar]
- Maschke M, Kastrup O, Esser S, Ross B, Hengge U, Hufnagel A. Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART) J Neurol Neurosurg Psychiatry. 2000;69:376–380. doi: 10.1136/jnnp.69.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: an evolving disease. J Neuro Virol. 2003;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- Metz LM, Zhang Y, Yeung M, Patry DG, Bell RB, Stoian CA, Yong VW, Patten SB, Duquette P, Antel JP, Mitchell JR. Minocycline reduces gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann Neurol. 2004;55:756–758. doi: 10.1002/ana.20111. [DOI] [PubMed] [Google Scholar]
- O'Dell JR, Blakely KW, Mallek JA, Eckhoff PJ, Leff RD, Wees SJ, Sems KM, Fernandez AM, Palmer WR, Klassen LW, Paulsen GA, Haire CE, Moore GF. Treatment of early seropositive rheumatoid arthritis: a two-year, double-blind comparison of minocycline and hydroxychloroquine. Arthritis Rheum. 2001;44:2235–2241. doi: 10.1002/1529-0131(200110)44:10<2235::aid-art385>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Overholser ED, Coleman GD, Bennett JL, Casaday RJ, Zink MC, Barber SA, Clements JE. Expression of simian immunodeficiency virus (SIV) nef in astrocytes during acute and terminal infection and requirement of nef for optimal replication of neurovirulent SIV in vitro. J Virol. 2003;77:6855–6866. doi: 10.1128/JVI.77.12.6855-6866.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen JA, Jones G, Worthington C, Krentz HB, Keppler OT, Hoke A, Gill MJ, Power C. Sensory neuropathy in human immunodeficiency virus/acquired immunodeficiency syndrome patients: protease inhibitor-mediated neurotoxicity. Ann Neurol. 2006;59:816–824. doi: 10.1002/ana.20816. [DOI] [PubMed] [Google Scholar]
- Popovic N, Schubart A, Goetz BD, Zhang SC, Linington C, Duncan ID. Inhibition of autoimmune encephalomyelitis by a tetracycline. Ann Neurol. 2002;51:215–223. doi: 10.1002/ana.10092. [DOI] [PubMed] [Google Scholar]
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neuro Virol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- Saito Y, Sharer LR, Epstein LG, Michaels J, Mintz M, Louder M, Golding K, Cvetkovich TA, Blumberg BM. Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology. 1994;44:474–481. doi: 10.1212/wnl.44.3_part_1.474. [DOI] [PubMed] [Google Scholar]
- Simpson DM, Kitch D, Evans SR, McArthur JC, Asmuth DM, Cohen B, Goodkin K, Gerschenson M, So Y, Marra CM, Diaz-Arrastia R, Shriver S, Millar L, Clifford DB. HIV neuropathy natural history cohort study: assessment measures and risk factors. Neurology. 2006;66:1679–1687. doi: 10.1212/01.wnl.0000218303.48113.5d. [DOI] [PubMed] [Google Scholar]
- Smyth K, Affandi JS, McArthur JC, Bowtell-Harris C, Mijch AM, Watson K, Costello K, Woolley IJ, Price P, Wesselingh SL, Cherry CL. Prevalence of and risk factors for HIV-associated neuropathy in Melbourne, Australia 1993-2006. HIV Med. 2007;8:367–373. doi: 10.1111/j.1468-1293.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- Thomas M, Le WD, Jankovic J. Minocycline and other tetracycline derivatives: aneuroprotective strategy in Parkinson's disease and Huntington's disease. Clin Neuropharmacol. 2003;26:18–23. doi: 10.1097/00002826-200301000-00005. [DOI] [PubMed] [Google Scholar]
- Tornatore C, Chandra R, Berger JR, Major EO. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology. 1994;44:481–487. doi: 10.1212/wnl.44.3_part_1.481. [DOI] [PubMed] [Google Scholar]
- Trichel AM, Rajakumar PA, Murphey-Corb M. Species-specific variation in SIV disease progression between Chinese and Indian subspecies of rhesus macaque. J Med Primatol. 2002;31:171–178. doi: 10.1034/j.1600-0684.2002.02003.x. [DOI] [PubMed] [Google Scholar]
- Weed MR, Hienz RD, Brady JV, Adams RJ, Mankowski JL, Clements JE, Zink MC. Central nervous system correlates of behavioral deficits following simian immunodeficiency virus infection. J Neuro Virol. 2003;9:452–464. doi: 10.1080/13550280390218751. [DOI] [PubMed] [Google Scholar]
- Wynn HE, Brundage RC, Fletcher CV. Clinical implications of CNS penetration of antiretroviral drugs. CNS Drugs. 2002;16:595–609. doi: 10.2165/00023210-200216090-00002. [DOI] [PubMed] [Google Scholar]
- Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, Sarang S, Liu AS, Hartley DM, Wu du C, Gullans S, Ferrante RJ, Przedborski S, Kristal BS, Friedlander RM. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- Zink MC, Amedee AM, Mankowski JL, Craig L, Didier P, Carter DL, Munoz A, Murphey-Corb M, Clements JE. Pathogenesis of SIV encephalitis. Selection and replication of neurovirulent SIV. Am J Pathol. 1997;151:793–803. [PMC free article] [PubMed] [Google Scholar]
- Zink MC, Clements JE. A novel simian immunodeficiency virus model that provides insight into mechanisms of human immunodeficiency virus central nervous system disease. J Neuro Virol. 2002;8(Suppl 2):42–48. doi: 10.1080/13550280290101076. [DOI] [PubMed] [Google Scholar]
- Zink MC, Coleman GD, Mankowski JL, Adams RJ, Tarwater PM, Fox K, Clements JE. Increased macrophage chemoattractant protein-1 in cerebrospinal fluid precedes and predicts simian immunodeficiency virus encephalitis. J Infect Dis. 2001;184:1015–1021. doi: 10.1086/323478. [DOI] [PubMed] [Google Scholar]
- Zink MC, Suryanarayana K, Mankowski JL, Shen A, Piatak M, Jr, Spelman JP, Carter DL, Adams RJ, Lifson JD, Clements JE. High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J Virol. 1999;73:10480–10488. doi: 10.1128/jvi.73.12.10480-10488.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink MC, Uhrlaub J, DeWitt J, Voelker T, Bullock B, Mankowski J, Tarwater P, Clements J, Barber S. Neuroprotective and anti-human immunodeficiency virus activity of minocycline. JAMA. 2005;293:2003–2011. doi: 10.1001/jama.293.16.2003. [DOI] [PubMed] [Google Scholar]