Abstract
Previous studies found that high red cell distribution width (RDW) value is associated with poor outcomes among out-of-hospital cardiac arrest survivors. The aim of this study was to investigate whether post-ROSC RDW value was associated with survival and neurological outcomes of in-hospital cardiac arrest (IHCA) patients achieving return of spontaneous circulation (ROSC) but remaining critically ill.
This retrospective single-center observational study included IHCA adults with sustained ROSC between January 1, 2017 and January 1, 2021 at an academic medical center in China. PostROSC RDW values were measured within 1 hour after sustained ROSC. The primary outcome was survival to hospital discharge and the secondary outcome was favorable neurological outcome at hospital discharge. The associations between postROSC RDW value and outcomes among IHCA patients with ROSC were evaluated by using multivariate logistic regression.
A total of 730 patients with sustained ROSC following IHCA were ultimately included in this study. Of whom 194 (26.6%) survived to hospital discharge and 116 (15.9%) had a favorable neurological outcome at hospital discharge. In multivariable logistic regression analysis, lower postROSC RDW value was independently associated with survival to hospital discharge (odds ratio 0.19, 95% confidence interval 0.15–0.63, P = .017, cut-off value: 15.5%) and favorable neurological outcome at hospital discharge (odds ratio 0.23, 95% confidence interval 0.07–0.87, P < .001, cut-off value: 14.6%). Other independent factors including younger age, initial shockable rhythm, shorter total cardiopulmonary resuscitation duration and post-ROSC percutaneous coronary intervention were also associated with survival to hospital discharge. Regarding favorable neurological outcome at hospital discharge, significant variables other than the aforementioned factors included postROSC targeted temperature management and absence of pre-existing neurological insufficiency.
Low postROSC RDW value was associated with survival to hospital discharge and favorable neurological outcome at hospital discharge.
Keywords: in-hospital cardiac arrest, neurological outcome at hospital discharge, prognostic factors, red cell distribution width, survival to hospital discharge
1. Introduction
In-hospital cardiac arrest (IHCA) is a life-threatening emergency that can potentially affect any hospitalized patients. Annually, more than 290,000 adults are affected by IHCA in the United States,[19] over 70% of those affected die and only 25% patients survive to hospital discharge.[6,9] Despite continuing efforts to improve the “chain of survival,” IHCA is still a major clinical problem and remains the leading cause of death with an associated severe cost and medical burdens.[3] It is in fact noteworthy that as high as 28% survivors suffer from a poor long-term outcome with risk of cognitive and physical impairments.[16] With the ageing of population increasing dramatically, the incidence of IHCA is increasing over time. In such a context, the early and accurate prediction of survival and neurological outcomes would be useful to both physicians and families to aid in medical decision making for IHCA patients who obtain return of spontaneous circulation (ROSC) but remain critically ill.
Red cell distribution width (RDW) is a measure of size variability and heterogeneity of circulating erythrocytes, and is routinely measured with complete blood count, which is a standard routine laboratory test in any illness. Given the relative inexpensive price and speed with which it can be obtained, RDW has lately grown in popularity and has been used to assess the severity and prognosis of diverse diseases including, but not limited to, hypertension,[7] sepsis,[35] acute kidney injury,[26] stroke[4,11] and heart failure[18,23] and cancer.[25] The clinical usefulness of RDW has also been established in patients with out-of-hospital cardiac arrest (OHCA). Kim et al[24] measured RDW values in 409 OHCA victims, 219 of whom with ROSC, and found that the highest RDW (>15.4%) quartile was independently associated with all-cause mortality during 30-day postresuscitation period. With the exception of mortality, RDW was also proven to be as a predictor of neurological outcome in OHCA survivors. Woo et al[37] reported that an initial RDW (≥15.0%) in the highest quartile as of the emergency department (ED) visit was independently associated with poor neurological outcome at hospital discharge. In addition, high RDW level also was related to unfavorable outcome at 3 months.[12] However, IHCA remains a somewhat neglected event compared with OHCA. To the best of our knowledge, it is unknown whether that the prognostic value of RDW applies to IHCA patients as well.
In the present study, we aimed to investigate whether post-ROSC RDW (measured within 1 hour after sustained ROSC) value was associated with outcomes among IHCA patients achieving ROSC but remaining critically ill, which is essential to the development of focused IHCA prognostication tools.
2. Methods
2.1. Study design and setting
This was a retrospective single-center observational study performed in a tertiary academic medical center with about 5000 beds. An approximate 1000 IHCA events occur per year. When an IHCA emergency happens, junior residents will perform cardiopulmonary resuscitation (CPR) immediately. Experienced senior residents and attendants will participate in the subsequent CPR process, including the endotracheal intubation and epinephrine administration. Targeted temperature management (TTM), percutaneous coronary intervention (PCI) and extracorporeal membrane oxygenation will be performed according to the standardized institutional protocols, if patients with ROSC needed further treatment. The present study was in compliance with the Declaration of Helsinki and was approved by the Human Ethical Committee of Henan Provincial People's Hospital (Reference number: 2021-49). The requirement for informed consent was waived before data collection.
2.2. Study population
Patients who achieved sustained ROSC following an IHCA event over a 4-year period from January 1, 2017 to January 1, 2021 were retrospectively screened. Sustained ROSC was defined as patients with a persistent circulation for at least 20 consecutive minutes without resumption of chest compressions.[21] Patients with age younger than 18 years, pregnant, major trauma etiology, with missing data on RDW measured within 1 hour after sustained ROSC (postROSC RDW) were excluded from this study. Patients were also excluded if family members withhold aggressive treatment after ROSC, as were patients with an unfavorable pre-arrest neurological status. A Cerebral Performance Category (CPC) score of 3 to 5 was considered an unfavorable neurological status.[17]
2.3. Data collection and outcomes
The following data were obtained from electronic medical records: age, gender, comorbidities, other variables in accordance with the Ustein template,[21] and postROSC interventions, postROSC RDW data. The normal laboratory range of RDW in our hospital is 10.9- 13.4%. A high RDW was defined as >13.4%. A senior resident checked all data to ensure accuracy and checked that data had been anonymized.
The primary outcome was survival to hospital discharge, which was defined as discharged from the hospital regardless of the neurological status. The secondary outcome was favorable neurological outcome (CPC 1–2) at hospital discharge. An investigator blinded to the study hypothesis determined the CPC score by medical record review.
2.4. Statistical analysis
Continuous data were expressed as mean ± standard deviation or median and inter-quarter range. The Kolmogorov-Smirnov test was used to assess normality distributions of continuous variables. Normally distributed continuous variables were compared using Student t test, while the continuous variables that were not normally distributed were compared using Mann–Whitney U test. Categorical data were presented as counts and percentage and were analyzed with Chi-Squared test or Fisher exact test. Logistic regression analysis was used to evaluate outcomes based on variables (odds ratio [OR],[1] 95% confidence interval [CI]). After univariate analysis, variables with P value <.10 were considered for multivariate regression analysis to determine the independent factor for outcomes. Finally, a receiver-operating characteristic (ROC) curve was generated for predictive accuracy of the RDW value, and the area under the curve (AUC) was calculated. A two-sided P value <.05 was considered statistically significant. All analyses were conducted using SPSS statistical software version 24.0 (IBM Corporation, Armonk, NY).
3. Results
3.1. Baseline and arrest characteristics of the study population
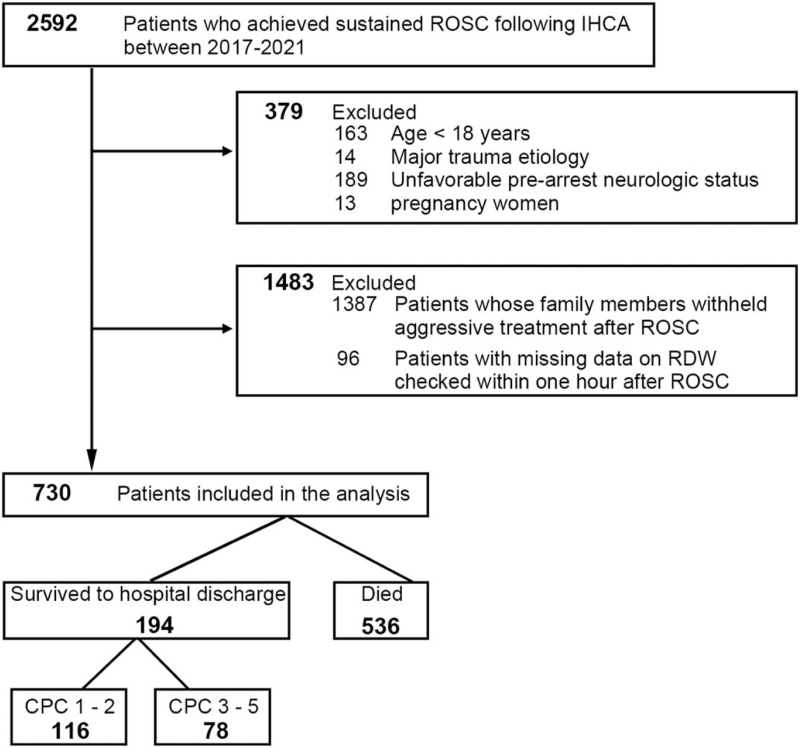
A total of 2592 patients between 2017 and 2021 who obtained sustained ROSC following IHCA were screened. Of these, 379 patients were excluded due to various combinations of age (<18 years), major trauma etiology, pregnancy, and presenting with an unfavorable prearrest neurological status (CPC 3–5). Notably, 1387 postROSC patients were further excluded as their family members withheld aggressive treatment. Ninety six patients with missing data on post-ROSC RDW checked within 1 hour after ROSC were excluded. Finally, 730 patients were enrolled in this study (Fig. 1).
Figure 1.
Flowchart of the study. CPC = cerebral performance category, IHCA = in-hospital cardiac arrest, RDW = red blood cell distribution width, ROSC = return of spontaneous circulation.
Baseline and arrest characteristics can be viewed in Table 1. Of the included population, 496 (67.9%) were male and the median age was 66 years. Total of 536 (73.4%) patients died, 194 (26.6%) survived to hospital discharge and 116 (15.9%) had a favorable neurological outcome (CPC 1–2) at hospital discharge. Younger age and initial shockable rhythm were associated with better outcomes, as was the post-ROSC PCI intervention, while patients with longer total CPR duration and higher RDW value were related to worse outcomes.
Table 1.
Baseline characteristics of patients stratified by outcomes.
| Survival to hospital discharge | Neurological outcome at hospital discharge | ||||||
| Overall (n = 730) | Yes (n = 194) | No (n = 536) | P value | CPC 1–2 (n = 116) | CPC 3–5 (n = 78) | P value | |
| Age, yr, median (IQR) | 66 (54, 76) | 60 (47, 68) | 67 (57, 77) | <.001 | 56 (46, 65) | 65 (47, 77) | <.001 |
| Male, n (%) | 496 (67.9) | 135 (69.6) | 361 (67.4) | .567 | 83 (71.6) | 52 (66.7) | .468 |
| Comorbidities, n (%) | |||||||
| Heart disease | 204 (27.9) | 53 (27.3) | 151 (28.2) | .821 | 34 (29.3) | 20 (25.6) | .576 |
| Heart failure, this admission | 89 (12.2) | 25 (12.9) | 64 (11.9) | .73 | 13 (11.2) | 12 (15.4) | .395 |
| Heart failure, prior admission | 72 (9.9) | 26 (13.4) | 46 (8.6) | .054 | 17 (14.7) | 9 (11.5) | .532 |
| Myocardial infarction, this admission | 25 (3.4) | 6 (3.1) | 19 (3.5) | .767 | 3 (2.6) | 3 (3.9) | .619 |
| Myocardial infarction, prior admission | 93 (12.7) | 21 (10.8) | 72 (13.4) | .319 | 9 (7.8) | 12 (15.4) | .094 |
| Arrhythmia | 105 (14.4) | 23 (11.9) | 82 (15.3) | .242 | 15 (12.9) | 8 (10.3) | .572 |
| Coronary artery disease | 248 (34.0) | 66 (34.0) | 182 (34.0) | .987 | 38 (32.3) | 28 (35.9) | .651 |
| Hypertension | 328 (44.9) | 86 (44.3) | 242 (45.1) | .844 | 47 (40.5) | 39 (50.0) | .192 |
| Hypotension | 10 (1.4) | 4 (2.1) | 6 (1.1) | .333 | 2 (1.7) | 2 (2.6) | .687 |
| Neurological insufficiency | 189 (25.9) | 48 (24.7) | 141 (26.3) | .670 | 19 (16.4) | 29 (37.2) | <.001 |
| Respiratory insufficiency | 407 (55.8) | 101 (52.1) | 306 (57.1) | .227 | 62 (53.5) | 39 (50.0) | .637 |
| Hepatic insufficiency | 109 (14.9) | 27 (13.9) | 82 (15.3) | .644 | 12 (10.3) | 15 (19.2) | .08 |
| Renal insufficiency | 153 (20.9) | 36 (18.6) | 117 (21.8) | .337 | 20 (17.2) | 16 (20.5) | .565 |
| Diabetes | 198 (27.1) | 52 (26.8) | 146 (27.2) | .907 | 31 (26.7) | 21 (26.9) | .976 |
| Sepsis | 112 (15.3) | 23 (11.9) | 89 (16.0) | .116 | 12 (10.3) | 11 (14.1) | .427 |
| Cancer | 87 (11.9) | 17 (8.7) | 69 (13.1) | .114 | 7 (6.0) | 10 (12.8) | .101 |
| Other∗ | 233 (31.9) | 56 (28.9) | 177 (33.0) | .287 | 32 (27.6) | 24 (30.8) | .631 |
| Alcohol abuse | 9 (1.2) | 2 (1.0) | 7 (1.3) | .766 | 1 (0.9) | 1 (1.3) | .776 |
| Blood transfusion over the last 30 days | 31 (4.2) | 9 (4.6) | 22 (4.1) | .752 | 3 (2.6) | 6 (7.7) | .097 |
| Arrest at night, n (%) | 197 (27.0) | 46 (23.7) | 151 (28.2) | .23 | 25 (21.6) | 21 (26.9) | .388 |
| Arrest on weekend, n (%) | 71 (9.7) | 12 (6.2) | 59 (11.0) | .62 | 8 (6.9) | 4 (5.1) | .616 |
| Arrest location, n (%) | .232 | .336 | |||||
| Emergency department | 223 (30.5) | 60 (30.9) | 163 (30.4) | 33 (28.4) | 27 (34.6) | ||
| ICU | 216 (29.6) | 46 (23.7) | 170 (31.7) | 25 (21.6) | 21 (26.9) | ||
| General ward | 238 (32.6) | 71 (36.6) | 167 (31.2) | 49 (42.2) | 22 (28.2) | ||
| Outpatient clinic | 25 (3.4) | 9 (4.6) | 16 (3.0) | 4 (3.4) | 5 (6.4) | ||
| Other† | 28 (3.8) | 8 (4.1) | 20 (3.7) | 5 (4.3) | 3 (3.8) | ||
| Witnessed arrest, n (%) | 704 (96.4) | 189 (97.4) | 515 (96.1) | .389 | 112 (96.6) | 77 (98.7) | .351 |
| Initial shockable rhythm, n (%) | 91 (12.5) | 41 (21.1) | 50 (9.3) | <.001 | 32 (27.6) | 9 (11.5) | <.001 |
| Total CPR duration, mins, median (IQR) | 12 (4,24) | 4 (3, 7) | 21 (12, 30) | <.001 | 3 (2, 4) | 8 (7,13) | <.001 |
| PostROSC interventions, n (%) | |||||||
| TTM | 82 (11.2) | 23 (11.9) | 59 (11.0) | .7485 | 20 (17.2) | 3 (3.8) | .004 |
| PCI | 62 (8.5) | 44 (22.7) | 18 (3.4) | <.001 | 33 (28.5) | 11 (14.1) | .019 |
| ECMO | 34 (4.7) | 6 (3.1) | 28 (5.2) | .2274 | 2 (1.7) | 4 (5.1) | .179 |
| Blood sample wthin 1h after sustained ROSC | |||||||
| WBC, 109/L, median (IQR) | 12.3 (6.4, 11.7) | 9.8 (5.3, 21.4) | 11.7 (6.8, 17.6) | .793 | 9.3 (4.7, 19.8) | 12.4 (7.4, 17.3) | .503 |
| RBC, 1012/L, median (IQR) | 2.8 (2.3, 4.1) | 3.0 (2.7, 3.9) | 2.8 (2.2, 3.5) | .401 | 3.1 (2.7, 4.1) | 2.7 (2.1, 3.6) | .627 |
| Hemoglobin, g/L, median (IQR) | 92 (73, 116) | 92 (76, 118) | 88 (64, 112) | .225 | 91 (79, 127) | 91 (64, 110) | .284 |
| RDW, %, median (IQR) | 14.9 (13.7, 16.8) | 14.0 (13.5, 15.6) | 15.7 (14.2, 17.4) | .032 | 13.9 (13.5, 14.9) | 15.8 (14.1, 17.2) | .014 |
| CRP, mg/L, median (IQR) | 64.3 (18.3, 138.5) | 61.4 (28.6, 153.1) | 65.3 (23.1, 145.8) | .671 | 79.8 (33.4, 157.3) | 52.4 (14.5, 131.6) | .291 |
| Glucose, mmol/L, median (IQR) | 8.2 (5.7, 11.2) | 8.8 (6.5, 13.2) | 8.3 (5.9, 12.1) | .388 | 8.9 (6.8, 12.9) | 8 (5.7, 11.4) | .243 |
| Sodium, mmol/L, median (IQR) | 141 (136, 145) | 141 (137, 144) | 141 (136.,145) | .812 | 141 (135, 145) | 141 (136, 144) | .981 |
| Potassium, mmol/L, median (IQR) | 4.3 (3.6, 4.9) | 4.3 (3.6, 5.1) | 4.2 (3.8, 4.8) | .935 | 4.3 (3.5, 4.9) | 4.3 (3.6, 4.9) | .964 |
| Calcium, mmol/L, mean (SD) | 2.06 (0.21) | 2.07 (0.25) | 2.09 (0.22) | .633 | 2.05 (0.25) | 2.06 (0.23) | .616 |
CPC = cerebral performance category, CPR = cardiopulmonary resuscitation, CRP = C-reactive protein, ECMO = extracorporeal membrane oxygenation, ICU = intensive care unit, IQR = inter-quartile range, PCI = percutaneous coronary intervention, RBC = red blood cell, RDW = red blood cell distribution width, ROSC = return of spontaneous circulation, SD = standard deviation, TTM = targeted temperature management, WBC = white blood cell.
Including but not limited to electrolyte disturbances, hyperlipidemia, and alcohol use disorder, drug overdose/side effect.
Including ambulatory or floor, diagnostic or interventional areas, examination room, operating room, postanesthesia recovery room, rehabilitation unit, and delivery room.
3.2. RDW and survival to hospital discharge
Total of 194 (26.6%) survived to hospital discharge. Compared with non-survivors, survivors were younger (median 60 vs 67 years, P < .001). They had more initial shockable rhythm (21.1% vs 9.3%, P < .001), shorter total CPR duration (median 4 vs 21 mins, P < .001), and a higher proportion of population underwent postROSC PCI intervention (22.7% vs 3.4%, P < .001). Moreover, survivors more likely to present with lower postROSC RDW values (median 14.0% vs 15.7%, P = .032) (Table 1). After relevant confounders adjustment using multivariate regression model, postROSC RDW value (OR 0.19, 95% confidence interval 0.15–0.63, P = .017) was an independent variable for survival to hospital discharge (Table 2).
Table 2.
Logistic regression models of variables associated with survival to hospital discharge.
| Univariate regression model | Multivariate regression model | |||||
| Odds ratio | 95% Confidence interval | P value | Odds ratio | 95% Confidence interval | P value | |
| Age | 0.96 | 0.94–0.97 | <.001 | 0.96 | 0.94–0.97 | <.001 |
| Initial shockable rhythm | 2.23 | 1.23–4.53 | <.001 | 1.76 | 1.03–3.26 | .007 |
| Total CPR duration | 0.93 | 0.92–0.94 | <.001 | 0.91 | 0.89–0.93 | <.001 |
| Post-ROSC PCI intervention | 6.05 | 3.08–13.18 | <.001 | 6.72 | 3.77–12.51 | .019 |
| RDW | 0.26 | 0.07–0.80 | .023 | 0.19 | 0.15–0.63 | .017 |
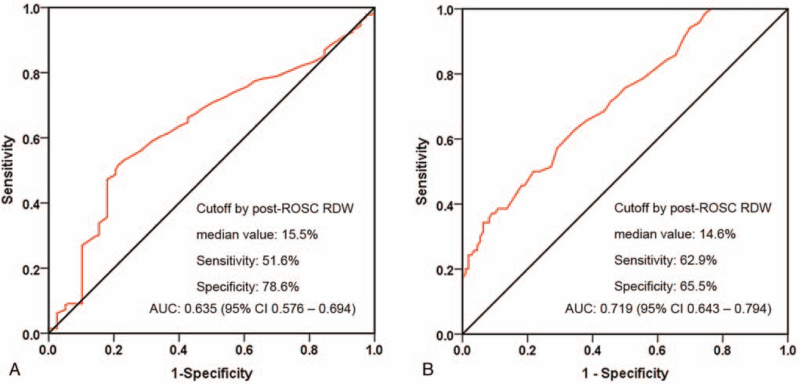
The AUC of postROSC RDW value in predicting survival to hospital discharge was 0.635 (95% confidence interval 0.576–0.694, P = .012) (Fig. 2A). Using a cut-off value of 15.5% for predicting survival to hospital discharge in IHCA patients with sustained ROSC, the sensitivity was 51.6%, and the specificity was 78.6%.
Figure 2.
ROC curves of post-ROSC RDW for the prediction of survival to hospital discharge (A) and neurological outcome at hospital discharge (B). RDW = red blood cell distribution width, ROC = receiver operating characteristic, ROSC = return of spontaneous circulation.
3.3. RDW and neurological outcome
Total of 116 (59.8%) survivors presented a favorable neurological outcome (CPC 1–2) at hospital discharge. Patients with younger age (median 56 vs 65 years, P < .001), lower incidence of pre-existing neurological insufficiency (16.4% vs 37.2%, P < .001), more initial shockable rhythm (27.6% vs 11.5%, P < .001), shorter CPR duration (median 3 vs 8 minutes, P < .001), more postROSC TTM (17.2% vs 3.8%, P = .004) and PCI (28.5% vs 14.1%, P = .019) interventions were more possible to present a favorable neurological outcome at hospital discharge. Additionally, patients with a favorable neurological outcome had lower postROSC RDW values compared to those with an unfavorable outcome (median 13.9% vs 15.8%, P = .014) (Table 1). After adjustment for age, initial rhythm, total CPR duration, postROSC PCI and TTM, and neurological insufficiency, low post-ROSC RDW value was independently associated with favorable neurological outcome at hospital discharge (OR 0.23, 95% CI 0.07–0.87, P < .001) (Table 3).
Table 3.
Logistic regression models of variables associated with neurological outcome at hospital discharge.
| Univariate regression model | Multivariate regression model | |||||
| Odds ratio | 95% Confidence interval | P value | Odds ratio | 95% Confidence interval | P value | |
| Age | 0.93 | 0.81–1.0 | <.001 | 0.88 | 0.84–0.91 | <.001 |
| Neurological insufficiency | 5.53 | 2.54–15.44 | <.001 | 3.29 | 2.38–19.83 | <.001 |
| Initial shockable rhythm | 1.82 | 1.36–2.72 | <.001 | 1.69 | 1.13–2.59 | <.001 |
| Total CPR duration | 0.92 | 0.84–0.93 | <.001 | 0.92 | 0.88–0.93 | <.001 |
| Post-ROSC TTM intervention | 4.09 | 1.93–7.72 | <.001 | 3.56 | 1.80–8.06 | .005 |
| PostROSC PCI intervention | 8.44 | 2.97–16.25 | .037 | 8.05 | 3.15–14.90 | .011 |
| RDW | 0.17 | 0.09–0.84 | .002 | 0.23 | 0.07–0.87 | <.001 |
CPR = cardiopulmonary resuscitation, PCI = percutaneous coronary intervention, RDW = red blood cell distribution width, ROSC = return of spontaneous circulation, TTM = targeted temperature management.
The ROC curve of post-ROSC RDW value yielded an AUC of 0.719 (95% CI 0.643–0.794, P = .009) for predicting favorable neurological outcome at discharge (Fig. 2B). Using a cut-off value of 14.6% for predicting favorable neurological outcome at hospital discharge in patients who achieved sustained ROSC following an IHCA event, the sensitivity was 62.9%, and the specificity was 65.5%.
4. Discussion
In this retrospective study, we explored if the prognostic ability of post-ROSC RDW in OHCA went for IHCA patients who achieved ROSC but remained critically ill. Our results showed that post-ROSC RDW was an independent predictor of survival to hospital discharge and neurological outcome at hospital discharge in IHCA after adjusting for multiple confounding factors. The cutoff points of post-ROSC RDW value for survival and neurological outcomes were 15.5% and 14.6% respectively, which were similar with the thresholds used in OHCA patients.[24,37] The ROC curves showed that postROSC RDW had moderate discriminative power for survival to hospital discharge and neurological outcome at hospital discharge.
Among the laboratory parameters with easily available and rapidly confirmable result, the promising prognostic factor of RDW has been used to predict the severity and mortality in many diseases, including hypertension,[7] sepsis,[35] acute kidney injury,[26] stroke,[4,11] heart failure[18,23] and cancer.[25] Few studies have focused on the associations between RDW and outcomes in patients with ROSC following an OHCA event. In previous studies, high RDW values were shown to be associated with mortality in OHCA patients.[24,37] The authors found that the highest RDW quartile (>15.4%) in OHCA patients admitted to the ED was independently associated with 30-day all-cause mortality. Although OHCA differs from IHCA in patient and arrest characteristics,[30,32] our study yielded similar results. Among the 194 IHCA patients who achieved ROSC, the postROSC RDW was independently associated with survival to hospital discharge. In addition, prior studies have shown that RDW was an effective prognostic factor for neurological outcome in OHCA patients. Woo et al[37] analyzed 1008 OHCA patients who were admitted to the ED, the RDW value of ≥15.0% at the time of the ED visit had a sensitivity of 29.8% and a specificity of 88.2% for predicting unfavorable outcome at hospital discharge. Fontana et al[12] also found that RDW levels on admission were associated with neurological outcome among cardiac arrest survivors admitted to the intensive care unit. The RDW threshold of 13.4% had a sensitivity of 64% and a specificity of 43% for predicting poor neurological outcome after 3 months of survival. The present study showed that a postROSC RDW threshold of 14.6% had a sensitivity of 62.9% and a specificity of 65.5% for predicting neurological outcome at hospital discharge in IHCA patients.
Among the laboratory parameters obtainable in the early hours after ROSC, lactate level has been used to predict outcomes after cardiac arrest. Prior investigators found that postROSC elevation in lactate was highly predictive of mortality after OHCA.[10,36] Recently, this model was proven to be applicable in IHCA patients obtaining ROSC but remaining critically ill.[20] Our results also showed that postROSC RDW had a similar predictive power for outcomes between IHCA and OHCA patients. The potential mechanism underlying the associations between high RDW value and poor outcomes shown in our study remain unclear. However, several explanations based on previous studies are possible. Well-known causes of increased RDW such as iron, vitamin B12 or folate deficiency and decreased RBC life span are common findings in severe illnesses. RDW has also been noted to be associated with renal dysfunction, inflammation, and oxidative stress. Therefore, it seems that RDW represents an integrative measure of underlying pathologic conditions. It is conceivable that RDW are specifically related to certain pathophysiologic components of post cardiac-arrest syndrome. Myocardial dysfunction is one of the main pathophysiologic processes characterizing the immediate post-resuscitation period.[8,22] RDW shows close correlation with brain natriuretic peptide,[14] and is a strong predictor of cardiac dysfunction[2] and mortality in congestive heart failure[13] and acute coronary syndrome.[29] Meanwhile, high RDW is associated with increased inflammation[28,31] and decreased levels of antioxidants,[15,33] which can reduce red blood cell deformability and survival.[27] Reduced RBC deformability impairs the cerebral microcirculation and may result in tissue hypoxia.[5,34] Taken together, postROSC RDW alone or combined with other prognostic factors may be useful in predicting the outcomes of IHCA survivors.
The present study has several limitations. First, given the retrospective, observational nature of our study, the results regarding the associations between postRDW and outcomes need to be interpreted with caution. Second, we could not collect the entire variables potentially influencing survival and neurological outcomes, and lack other variables that could affect RDW value. Third, the accuracy of postROSC RDW value to predict outcomes were relatively poor which may significantly limit its application in predicting patients at higher risk of poor outcomes. Fourth, we could not analyze the associations between postROSC RDW and long-term survival and neurological outcomes. Further studies should examine 3 or 6-month outcomes, activities of daily living, and return to work as elements of health-related quality of life in IHCA survivors. Fifth, we did not specifically compare postROSC RDW with other systemic biomarkers that have already been identified in previous studies. Further prospective studies on IHCA survivors are needed to validate the predictive associations between postROSC and poor outcomes. However, our study is meaningful because it suggested that post-ROSC RDW could be used as an early prognostic factor for poor outcomes in IHCA survivors.
5. Conclusions
PostROSC RDW was an independent predictor of outcomes of adults with sustained ROSC following an IHCA event. Low postROSC RDW values were associated with survival to hospital discharge and favorable neurological outcome at hospital discharge. PostROSC RDW could be useful for physicians and families to help make medical decisions for IHCA patients.
Acknowledgment
I would like to express my gratitude to my colleagues who helped and supported during the writing of this manuscript and special thanks to all the peer reviewers and editors for their sincere concerns and suggestions.
Author contributions
Conceptualization: Yanwei Cheng.
Data curation: Hailin Peng.
Formal analysis: Juan Zhu.
Validation: Jiange Zhang, Lijun Xu.
Writing – original draft: Yanwei Cheng.
Writing – review & editing: Yanwei Cheng, Xue Cao, Lijie Qin.
Footnotes
Abbreviations: AUC = area under the curve, CPC = cerebral performance category, CPR = cardiopulmonary resuscitation, ED = emergency department, IHCA = in-hospital cardiac arrest, OR = odds ratio, PCI = percutaneous coronary intervention, RDW = high red cell distribution width, ROC = receiver-operating characteristic, ROSC = return of spontaneous circulation, TTM = targeted temperature management.
How to cite this article: Cheng Y, Peng H, Zhang J, Zhu J, Xu L, Cao X, Qin L. Associations between red cell distribution width and outcomes of adults with in-hospital cardiac arrest: a retrospective study. Medicine. 2022;101:4(e28750).
YC, HP, and JZ contributed equally to this work.
The present work was supported by the 23456 Talent Project of Henan Provincial People's Hospital to LQ, Research Startup fund of Henan Provincial People's Hospital to YC and XC, and Joint project of medical teaching and research to JZ (Wjlx2020044).
The authors have no conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr 1994;50:760–3. [DOI] [PubMed] [Google Scholar]
- [2].Al-Najjar Y, Goode KM, Zhang J, Cleland JG, Clark AL. Red cell distribution width: an inexpensive and powerful prognostic marker in heart failure. Eur J Heart Fail 2009;11:1155–62. [DOI] [PubMed] [Google Scholar]
- [3].Andersen LW, Holmberg MJ, Berg KM, Donnino MW, Granfeldt A. In-hospital cardiac arrest: a review. JAMA 2019;321:1200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ani C, Ovbiagele B. Elevated red blood cell distribution width predicts mortality in persons with known stroke. J Neurol Sci 2009;277:103–8. [DOI] [PubMed] [Google Scholar]
- [5].Bailey DM, Rasmussen P, Overgaard M, et al. Nitrite and S-Nitrosohemoglobin exchange across the human cerebral and femoral circulation: relationship to basal and exercise blood flow responses to hypoxia. Circulation 2017;135:166–76. [DOI] [PubMed] [Google Scholar]
- [6].Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 2018;137:e67–492. [DOI] [PubMed] [Google Scholar]
- [7].Bilal A, Farooq JH, Kiani I, Assad S, Ghazanfar H, Ahmed I. Importance of mean red cell distribution width in hypertensive patients. Cureus 2016;8:e902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chalkias A, Xanthos T. Redox-mediated programed death of myocardial cells after cardiac arrest and cardiopulmonary resuscitation. Redox Rep 2012;17:80–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chan PS, Nallamothu BK, Krumholz HM, et al. Long-term outcomes in elderly survivors of in-hospital cardiac arrest. N Engl J Med 2013;368:1019–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cocchi MN, Salciccioli J, Yankama T, et al. Predicting outcome after out-of-hospital cardiac arrest: lactate, need for vasopressors, and cytochrome c. J Intensive Care Med 2020;35:1483–9. [DOI] [PubMed] [Google Scholar]
- [11].Feng GH, Li HP, Li QL, Fu Y, Huang RB. Red blood cell distribution width and ischaemic stroke. Stroke Vasc Neurol 2017;2:172–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fontana V, Spadaro S, Villois P, et al. Can red blood cell distribution width predict outcome after cardiac arrest? Minerva Anestesiol 2018;84:693–702. [DOI] [PubMed] [Google Scholar]
- [13].Förhécz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohászka Z, Jánoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J 2009;158:659–66. [DOI] [PubMed] [Google Scholar]
- [14].Fukuta H, Ohte N, Mukai S, et al. Elevated plasma levels of B-type natriuretic Peptide but not C-reactive protein are associated with higher red cell distribution width in patients with coronary artery disease. International heart journal 2009;50:301–12. [DOI] [PubMed] [Google Scholar]
- [15].Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal 2008;10:1923–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Girotra S, Nallamothu BK, Spertus JA, Li Y, Krumholz HM, Chan PS. Trends in survival after in-hospital cardiac arrest. N Engl J Med 2012;367:1912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Grossestreuer AV, Abella BS, Sheak KR, et al. Inter-rater reliability of post-arrest cerebral performance category (CPC) scores. Resuscitation 2016;109:21–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gul M, Uyarel H, Ergelen M, et al. The relationship between red blood cell distribution width and the clinical outcomes in non-ST elevation myocardial infarction and unstable angina pectoris: a 3-year follow-up. Coronary Artery Dis 2012;23:330–6. [DOI] [PubMed] [Google Scholar]
- [19].Holmberg MJ, Ross CE, Fitzmaurice GM, et al. Annual incidence of adult and pediatric in-hospital cardiac arrest in the United States. Circ Cardiovasc Qual Outcomes 2019;12:e005580. [PMC free article] [PubMed] [Google Scholar]
- [20].Issa MS, Grossestreuer AV, Patel H, et al. Lactate and hypotension as predictors of mortality after in-hospital cardiac arrest. Resuscitation 2021;158:208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jacobs I, Nadkarni V, Bahr J, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries. A statement for healthcare professionals from a task force of the international liaison committee on resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa). Resuscitation 2004;63:233–49. [DOI] [PubMed] [Google Scholar]
- [22].Ji XF, Shuo W, Yang L, Li CS. Impaired β-adrenergic receptor signalling in post-resuscitation myocardial dysfunction. Resuscitation 2012;83:640–4. [DOI] [PubMed] [Google Scholar]
- [23].Kaya A, Isik T, Kaya Y, et al. Relationship between red cell distribution width and stroke in patients with stable chronic heart failure: a propensity score matching analysis. Clin Appl Thromb Hemost 2015;21:160–5. [DOI] [PubMed] [Google Scholar]
- [24].Kim J, Kim K, Lee JH, et al. Red blood cell distribution width as an independent predictor of all-cause mortality in out of hospital cardiac arrest. Resuscitation 2012;83:1248–52. [DOI] [PubMed] [Google Scholar]
- [25].Koma Y, Onishi A, Matsuoka H, et al. Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer. PLoS One 2013;8:e80240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Oh HJ, Park JT, Kim JK, et al. Red blood cell distribution width is an independent predictor of mortality in acute kidney injury patients treated with continuous renal replacement therapy. Nephrol Dial Transplant 2012;27:589–94. [DOI] [PubMed] [Google Scholar]
- [27].Patel KV, Mohanty JG, Kanapuru B, Hesdorffer C, Ershler WB, Rifkind JM. Association of the red cell distribution width with red blood cell deformability. Adv Exp Med Biol 2013;765:211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pierce CN, Larson DF. Inflammatory cytokine inhibition of erythropoiesis in patients implanted with a mechanical circulatory assist device. Perfusion 2005;20:83–90. [DOI] [PubMed] [Google Scholar]
- [29].Poludasu S, Marmur JD, Weedon J, Khan W, Cavusoglu E. Red cell distribution width (RDW) as a predictor of long-term mortality in patients undergoing percutaneous coronary intervention. Thromb Haemost 2009;102:581–7. [DOI] [PubMed] [Google Scholar]
- [30].Porzer M, Mrazkova E, Homza M, Janout V. Out-of-hospital cardiac arrest. Biomedical papers of the Medical Faculty of the University Palacky, Olomouc, Czechoslovakia. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2017;161:348–53. [DOI] [PubMed] [Google Scholar]
- [31].Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci 2015;52:86–105. [DOI] [PubMed] [Google Scholar]
- [32].Sandroni C, Nolan J, Cavallaro F, Antonelli M. In-hospital cardiac arrest: incidence, prognosis and possible measures to improve survival. Intensive Care Med 2007;33:237–45. [DOI] [PubMed] [Google Scholar]
- [33].Semba RD, Patel KV, Ferrucci L, et al. Serum antioxidants and inflammation predict red cell distribution width in older women: the Women's Health and Aging Study I. Clin Nutr 2010;29:600–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Simchon S, Jan K M, Chien S. Influence of reduced red cell deformability on regional blood flow. Am J Physiol 1987;253(4 Pt 2):H898–903. [DOI] [PubMed] [Google Scholar]
- [35].Wang AY, Ma HP, Kao WF, Tsai SH, Chang CK. Red blood cell distribution width is associated with mortality in elderly patients with sepsis. Am J Emerg Med 2018;36:949–53. [DOI] [PubMed] [Google Scholar]
- [36].Williams TA, Martin R, Celenza A, et al. Use of serum lactate levels to predict survival for patients with out-of-hospital cardiac arrest: a cohort study. Emerg Med Aust 2016;28:171–8. [DOI] [PubMed] [Google Scholar]
- [37].Woo SH, Lee WJ, Kim DH, Cho Y, Cho GC. Initial red cell distribution width as a predictor of poor neurological outcomes in out-of-hospital cardiac arrest survivors in a prospective, multicenter observational study (the KoCARC study). Sci Rep 2020;10:17549. [DOI] [PMC free article] [PubMed] [Google Scholar]