Abstract
A mutation (CCG→CTG [Arg→Leu]) in codon 463 of katG (catalase peroxidase) of Mycobacterium tuberculosis has been found in isoniazid (INH)-resistant strains. A PCR restriction endonuclease analysis to detect this mutation was applied to 395 M. tuberculosis isolates from patients in The Netherlands. The proportion of isolates with a detectable mutation was 32% (32 out of 100) and 29% (85 out of 295) among INH-susceptible isolates and INH-resistant or -intermediate isolates, respectively. Sequencing of five INH-susceptible isolates with such mutations showed that all five had the Arg463Leu mutation. We conclude that the Arg463Leu mutation of katG of M. tuberculosis is not a reliable indicator of INH resistance.
Tuberculosis is the leading cause of death due to infectious diseases worldwide (4), although various drugs against Mycobacterium tuberculosis are available. One of the mainstay drugs for the treatment of tuberculosis is isoniazid (INH). Its effectiveness against M. tuberculosis was initially reported in 1952 (3, 15). Today, INH-resistant M. tuberculosis organisms are not rare anymore; prevalence was reported to be 7% in a recent study performed in The Netherlands (25). The emergence of multidrug-resistant strains (resistant to at least INH and rifampin) (7, 11, 12, 20) has further complicated the treatment of tuberculosis. Therefore, and because of the organism's slow growth rate, rapid methods for detecting drug resistance in clinical isolates of M. tuberculosis are required. The primary mechanism of resistance in M. tuberculosis is the accumulation of mutations in genes coding for drug targets or drug-converting enzymes (16).
In the last decade, mutations in katG (24, 26) and inhA (2) have been found to account for 60 to 70% and 10 to 15% of INH resistance cases among M. tuberculosis isolates, respectively (11). The two predominant mutations of katG, and those most referred to, are found within codons 315 and 463 (17).
The mutation at codon 315 has been found to be an important indicator for INH resistance as well as for multidrug resistance among isolates of M. tuberculosis organisms recovered from patients in The Netherlands (25).
The aims of this study were to assess whether the Arg463Leu mutation is also predictive of INH resistance and, if so, to develop a diagnostic PCR-based screening method for this type of INH resistance.
(This work was presented in part at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada, 17 to 20 September 2000.)
M. tuberculosis isolates and assessment of INH resistance.
M. tuberculosis isolates from 395 patients who were diagnosed with tuberculosis in The Netherlands in the period of 1993 to 1997 were used in this study. The isolates were sent by medical microbiology laboratories in The Netherlands to the National Institute of Public Health and the Environment (RIVM, Bilthoven, The Netherlands) for routine typing and susceptibility tests. Susceptibility to INH was measured with the MIC method using 0.1, 0.2, 0.5, 1, 2, 5, 10, 20, and 50 μg of INH/ml in Middlebrook 7H10 medium (8). Isolates were considered resistant if more than 1% of the bacteria in the inoculum grew in the presence of INH concentrations of ≥1 μg/ml. If growth of more than 1% of the inoculum in the presence of 0.5 μg of INH/ml occurred, then the isolates were classified as having intermediate susceptibility.
DNA isolation.
M. tuberculosis isolates were grown on Löwenstein-Jensen solid medium or Middlebrook 7H9 liquid medium for 7 days to an optical density corresponding to 108 bacteria/ml and were harvested by centrifugation (4,500 × g for 15 min). Chromosomal DNA was isolated as described by Ausubel et al. (1). Briefly, the bacteria were killed by heating at 80°C for 20 min and then incubated with 1 mg of lysozyme/ml at 37°C for 1 h. The bacterial suspension was further incubated with 1% sodium dodecyl sulfate and 0.1 mg of proteinase K/ml at 65°C for 10 min. Lysis was completed by incubating the suspension with 1% N-cetyl-N,N,N-trimethyl ammonium bromide at 65°C for 10 min. DNA was extracted from the lysed bacteria by chloroform-isoamyl alcohol and subsequently precipitated with isopropanol.
PCR.
The 25-μl reaction mixture for PCR contained 100 ng of chromosomal DNA as template, 0.2 mM concentrations of each deoxynucleoside triphosphate (Amersham Pharmacia Biotech, Piscataway, N.J.), 0.5 pM primer 1.1 (5′-CTGCTCCGCTGGAGCAGATG-3′), 0.5 pM primer 1.2 (5′-CCGACTTGGGCTGCAGGCG-3′), 1.25 U of Taq polymerase (Perkin-Elmer, Norwalk, Conn.), and 2 mM MgCl2 in PCR buffer B (Promega, Madison, Wis.), with final concentrations of 10 mM Tris-HCl (pH 9.0), 50 mM KCl, and 0.1% Triton X-100. The thermocycling protocol was 95°C for 1 min, 66°C for 1 min and 72°C for 1 min for 8 cycles, followed by 32 cycles of 95°C for 1 min, 58°C for 1 min, and 72°C for 1 min.
Restriction endonuclease analysis (REA).
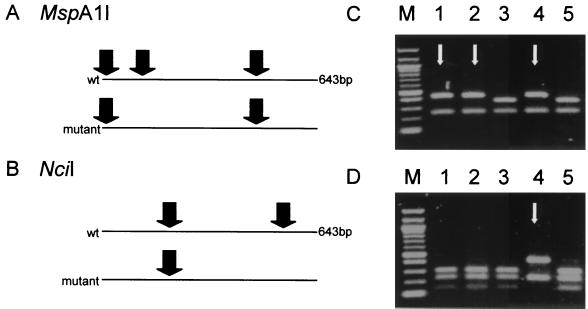
To detect the Arg463Leu mutation of katG (463-REA), PCR products were digested with NciI according to the instructions of the manufacturer (New England Biolabs, Beverly, Mass.). NciI cut the wild-type amplicon at two positions but cut an amplicon with the Arg463Leu (CGG→CTG) mutation at only one position (Fig. 1). In theory, an Arg463Pro (CGG→CCG) mutation would remain undetected with this assay. Furthermore, it is possible that a mutation outside codon 463, but within the recognition site of NciI comprising codon 463, would prevent NciI from cutting. However, in the literature, no mention of such mutations was found.
FIG. 1.
(A and B) REA of a 643-bp amplicon of M. tuberculosis katG. (A) In order to detect a mutation at codon 315, the amplicon was digested by MspA1I. A wild-type (wt) amplicon was cut at three positions, but an amplicon with a mutation in the recognition sequence encompassing codon 315 was cut at only two. (B) NciI was used to detect the mutation at codon 463. NciI cut a wild-type amplicon at two positions, but an amplicon with a mutation in the recognition sequence containing codon 463 was cut at only one. (C and D) Gel electrophoresis of digested fragments derived from five different isolates (1 to 5) of M. tuberculosis allows discrimination of wild-type and mutant isolates for codons 315 (C) and 463 (D). Arrows indicate mutant DNA fragments. Lane M, 100-bp ladder with bands at 0.1-kb intervals, starting at 0.1 kb.
The mutation at codon 315 was detected by the digestion of the PCR product with MspA1I (315-REA). MspA1I cuts the wild-type amplicon at three positions, but it cuts an amplicon with a mutation at codon 315 (AGC→ACC or ACA [Ser315Thr], AGC→AAC [Ser315Asn], AGC→ATC [Ser315Ile] [9, 10, 17]) at two positions (Fig. 1). An amplicon with an AGC→CGC (Ser315Arg) mutation, which has been described once (9), was cut where the wild type was cut and was therefore not detected with this assay.
The DNA restriction fragments were analyzed on 1% agarose, as described earlier (19).
Fluorescence-based sequencing and analysis.
The katG region comprising the mutation at codon 463 was amplified using primers 1.12 (5′-CAAGCAGACCCTGCTGTGGC-3′) and 2.0 (5′-TGCTGCTTTCTCTATGGCGG-3′). The DNA sequences of these amplicons were determined by a PCR-based sequence reaction using the ABI PRISM Dye Terminator Cycle Sequencing Core Kit (Perkin-Elmer, Gouda, The Netherlands) according to the instructions supplied by Applied Biosystems Incorporated (Foster City, Calif.). The sequences were analyzed on an automatic sequenator (model 370A; Applied Biosystems Incorporated).
Prevalence of mutations at codon 315 and 463 of katG in the Netherlands. In total, 395 patient isolates of M. tuberculosis were tested for INH susceptibility and analyzed with 463-REA. Of these isolates, 225 were resistant and 70 were of intermediate susceptibility, while 100 were INH susceptible.
In order to detect whether the Arg463Leu mutation could be present, a 643-bp region of katG was amplified by PCR using primers 1.1 and 1.2. For detection of the mutation at codon 315, the same amplicon was used but was digested with MspA1I.
463-REA showed that among the 225 resistant isolates, 64 (28%) had a mutation in the NciI recognition sequence that includes codon 463. Of the 70 isolates with an intermediate susceptibility to INH and the 100 INH-susceptible isolates, 21 (30%) and 32 (32%) isolates also carried a mutation in that recognition sequence, respectively (Table 1).
TABLE 1.
Distribution of katG mutations at the NciI recognition site including codon 463 over INH susceptibility status of M. tuberculosis organisms taken from patients in The Netherlands
| INH susceptibility | No. (%) of isolates
|
Total no. of isolates | ||
|---|---|---|---|---|
| Mutant katG | Wild-type katG | PCR negative | ||
| Susceptible | 32 (32) | 62 (62) | 6 (6) | 100 |
| Intermediate | 21 (30) | 44 (63) | 5 (7) | 70 |
| Resistant | 64 (28) | 143 (64) | 18 (8) | 225 |
| Total | 117 (30) | 249 (63) | 29 (7) | 395 |
From the 100 INH-susceptible isolates, five 463-REA-positive and five 463-REA-negative isolates were randomly selected and the katG region comprising codon 463 was sequenced. The five mutation-negative isolates had the wild-type sequence at codon 463, while the five mutation-positive isolates had the G→T mutation at the second base pair position of codon 463, resulting in a putative Arg→Leu change.
315-REA showed that among 100 INH-susceptible isolates, none were found to have a mutation in the MspA1I recognition sequence that includes codon 315.
INH resistance of M. tuberculosis organisms is associated with mutations in or deletions of katG (60 to 70%) or mutations in inhA (10 to 20%). The genetic mechanism of INH resistance remains unknown for 10 to 15% of the INH-resistant isolates. The mutations in katG occur in 50 to 60% of the INH-resistant isolates at codon 315 and in 25 to 45% of these isolates at codon 463 (5, 16, 17).
Our results, in conjunction with those of an earlier study (25), show that there is a strict relationship between the presence of a mutation at codon 315 and INH resistance of M. tuberculosis isolates.
In contrast, the Arg463Leu mutation and INH resistance are not as strictly associated. katG encoding Leu at codon 463, either as the prevalent allele or as a polymorphism, is also present in Mycobacterium intracellulare, Mycobacterium bovis, M. bovis BCG, Mycobacterium africanum, and Mycobacterium microti isolates. These mycobacterial species are in general less susceptible to INH (9, 10). For M. bovis BCG, there is a strong association between MICs of INH and the presence of the CGG→CTG mutation at codon 463 (for 463R, MIC < 0.05 μg/ml, for 463L, MIC > 2 μg/ml).
However, in previous studies, the mutation at codon 463 was found in 3 to 61% of INH-susceptible M. tuberculosis isolates (5, 6, 13, 17, 18, 23). Also, it was found that the activity of catalase, a katG-encoded enzyme, did not differ among isolates having either Arg or Leu at codon 463 (21, 22). Furthermore, complementation of katG-negative INH-resistant M. tuberculosis strains with katG having the CGG→CTG (Arg463Leu) mutation fully restored the virulence and catalase activity of these strains (14, 21). Hence, there was no biochemical support for the observation that CGG→CTG (Arg463Leu) was associated with resistance to INH. The results of our study support this observation since the distribution of a mutation in the recognition site of NciI comprising codon 463 among INH-resistant isolates, isolates with intermediate susceptibility, and INH-susceptible isolates was similar. Sequencing of five randomly selected INH-susceptible isolates that had a mutation in this recognition site confirmed the presence of the CGG→CTG (Arg463Leu) mutation. These results show that the Arg463Leu mutation in katG of M. tuberculosis does not, at least not by itself, confer resistance to INH.
In addition, we assessed whether the presence or absence of the CGG→CTG (Arg463Leu) mutation was associated with differences in the distribution of MICs, resistance to drugs other than INH, or the probability of being in a restriction fragment length polymorphism cluster, similarly as reported by van Soolingen et al. (25). However, virtually no differences were found (data not shown).
Our findings have three implications. First, the presence of the CGG→CTG (Arg463Leu) mutation in katG in M. tuberculosis is neither biochemically nor epidemiologically associated with INH resistance, with intermediate INH susceptibility, or with multidrug resistance in M. tuberculosis in The Netherlands. Therefore, this mutation should be considered a polymorphism unrelated to the selective pressure of drug treatment in M. tuberculosis. Second, the percentage of isolates with INH resistance which is attributable to mutations in katG has been overestimated. Third, the CGG→CTG (Arg463Leu) mutation is not indicative of INH resistance of an M. tuberculosis isolate. Therefore, the development of a diagnostic PCR for detection of INH resistance should not be based upon the katG CGG→CTG (Arg463Leu) mutation.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1994. [Google Scholar]
- 2.Banerjee A, Dubnau E, Quemard A, Balasubramanian V, Um K S, Wilson T, Collins D, de Lisle G, Jacobs W R., Jr inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science. 1994;263:227–230. doi: 10.1126/science.8284673. [DOI] [PubMed] [Google Scholar]
- 3.Berstein J, Lott W A, Steinberg B A, Yale H L. Chemotherapy of experimental tuberculosis. Am Rev Tuberc. 1952;65:357–364. doi: 10.1164/art.1952.65.4.357. [DOI] [PubMed] [Google Scholar]
- 4.Bloom B R, Murray C J. Tuberculosis: commentary on a reemergent killer. Science. 1992;257:1055–1064. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- 5.Cockerill F R, III, Uhl J R, Temesgen Z, Zhang Y, Stockman L, Roberts G D, Williams D L, Kline B C. Rapid identification of a point mutation of the Mycobacterium tuberculosis catalase-peroxidase (katG) gene associated with isoniazid resistance. J Infect Dis. 1995;171:240–245. doi: 10.1093/infdis/171.1.240. [DOI] [PubMed] [Google Scholar]
- 6.Dobner P, Rusch-Gerdes S, Bretzel G, Feldmann K, Rifai M, Loscher T, Rinder H. Usefulness of Mycobacterium tuberculosis genomic mutations in the genes katG and inhA for the prediction of isoniazid resistance. Int J Tuberc Lung Dis. 1997;1:365–369. [PubMed] [Google Scholar]
- 7.Frieden T R, Sterling T, Pablos-Mendez A, Kilburn J O, Cauthen G M, Dooley S W. The emergence of drug-resistant tuberculosis in New York City. N Engl J Med. 1993;328:521–526. doi: 10.1056/NEJM199302253280801. [DOI] [PubMed] [Google Scholar]
- 8.Gangadharam P R J. Drug resistance in mycobacteria. Boca Raton, Fla: CRC Press; 1984. [Google Scholar]
- 9.Haas W H, Schilke K, Brand J, Amthor B, Weyer K, Fourie P B, Bretzel G, Sticht-Groh V, Bremer H J. Molecular analysis of katG gene mutations in strains of Mycobacterium tuberculosis complex from Africa. Antimicrob Agents Chemother. 1997;41:1601–1603. doi: 10.1128/aac.41.7.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heym B, Alzari P M, Honore N, Cole S T. Missense mutations in the catalase-peroxidase gene, katG, are associated with isoniazid resistance in Mycobacterium tuberculosis. Mol Microbiol. 1995;15:235–245. doi: 10.1111/j.1365-2958.1995.tb02238.x. [DOI] [PubMed] [Google Scholar]
- 11.Heym B, Honore N, Truffot-Pernot C, Banerjee A, Schurra C, Jacobs W R, Jr, van Embden J D, Grosset J H, Cole S T. Implications of multidrug resistance for the future of short-course chemotherapy of tuberculosis: a molecular study. Lancet. 1994;344:293–298. doi: 10.1016/s0140-6736(94)91338-2. [DOI] [PubMed] [Google Scholar]
- 12.Kritski A L, Marques M J, Rabahl M F, Vieira M A, Werneck-Barroso E, Carvalho C E, Andrade G d N, Bravo-de-Souza R, Andrade L M, Gontijo P P, Riley L W. Transmission of tuberculosis to close contacts of patients with multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 1996;153:331–335. doi: 10.1164/ajrccm.153.1.8542139. [DOI] [PubMed] [Google Scholar]
- 13.Lee A S, Tang L L, Lim I H, Ling M L, Tay L, Wong S Y. Lack of clinical significance for the common arginine-to-leucine substitution at codon 463 of the katG gene in isoniazid-resistant Mycobacterium tuberculosis in Singapore. J Infect Dis. 1997;176:1125–1127. doi: 10.1086/517320. [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Kelley C, Collins F, Rouse D, Morris S. Expression of katG in Mycobacterium tuberculosis is associated with its growth and persistence in mice and guinea pigs. J Infect Dis. 1998;177:1030–1035. doi: 10.1086/515254. [DOI] [PubMed] [Google Scholar]
- 15.Middlebrook G. Sterilization of tubercle bacilli by isonicotinic acid hydrazide and the incidence of variants resistant to the drug. Am Rev Tuberc. 1952;65:765–767. [PubMed] [Google Scholar]
- 16.Morris S, Bai G H, Suffys P, Portillo-Gomez L, Fairchok M, Rouse D. Molecular mechanisms of multiple drug resistance in clinical isolates of Mycobacterium tuberculosis. J Infect Dis. 1995;171:954–960. doi: 10.1093/infdis/171.4.954. [DOI] [PubMed] [Google Scholar]
- 17.Musser J M, Kapur V, Williams D L, Kreiswirth B N, van Soolingen D, van Embden J D. Characterization of the catalase-peroxidase gene (katG) and inhA locus in isoniazid-resistant and -susceptible strains of Mycobacterium tuberculosis by automated DNA sequencing: restricted array of mutations associated with drug resistance. J Infect Dis. 1996;173:196–202. doi: 10.1093/infdis/173.1.196. [DOI] [PubMed] [Google Scholar]
- 18.Nachamkin I, Kang C, Weinstein M P. Detection of resistance to isoniazid, rifampin, and streptomycin in clinical isolates of Mycobacterium tuberculosis by molecular methods. Clin Infect Dis. 1997;24:894–900. doi: 10.1093/clinids/24.5.894. [DOI] [PubMed] [Google Scholar]
- 19.Oudbier J H, Langenberg W, Rauws E A, Bruin-Mosch C. Genotypical variation of Campylobacter pylori from gastric mucosa. J Clin Microbiol. 1990;28:559–565. doi: 10.1128/jcm.28.3.559-565.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ristow M, Mohlig M, Rifai M, Schatz H, Feldmann K, Pfeiffer A. New isoniazid/ethionamide resistance gene mutation and screening for multidrug-resistant Mycobacterium tuberculosis strains. Lancet. 1995;346:502–503. doi: 10.1016/s0140-6736(95)91351-3. [DOI] [PubMed] [Google Scholar]
- 21.Rouse D A, Li Z, Bai G H, Morris S L. Characterization of the katG and inhA genes of isoniazid-resistant clinical isolates of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1995;39:2472–2477. doi: 10.1128/aac.39.11.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rouse D A, Morris S L. Molecular mechanisms of isoniazid resistance in Mycobacterium tuberculosis and Mycobacterium bovis. Infect Immun. 1995;63:1427–1433. doi: 10.1128/iai.63.4.1427-1433.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shim T S, Yoo C G, Han S K, Shim Y S, Kim Y W. Isoniazid resistance and the point mutation of codon 463 of katG gene of Mycobacterium tuberculosis. J Korean Med Sci. 1997;12:92–98. doi: 10.3346/jkms.1997.12.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoeckle M Y, Guan L, Riegler N, Weitzman I, Kreiswirth B, Kornblum J, Laraque F, Riley L W. Catalase-peroxidase gene sequences in isoniazid-sensitive and -resistant strains of Mycobacterium tuberculosis from New York City. J Infect Dis. 1993;168:1063–1065. doi: 10.1093/infdis/168.4.1063. [DOI] [PubMed] [Google Scholar]
- 25.van Soolingen D, de Haas P E, van Doorn H R, Kuijper E, Rinder H, Borgdorff M W. Mutations at amino acid position 315 of the katG gene are associated with high-level resistance to isoniazid, other drug resistance, and successful transmission of Mycobacterium tuberculosis in The Netherlands. J Infect Dis. 2000;182:1788–1790. doi: 10.1086/317598. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Heym B, Allen B, Young D, Cole S. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature. 1992;358:591–593. doi: 10.1038/358591a0. [DOI] [PubMed] [Google Scholar]