Abstract
Objective:
The aim of this review is addressing the mechanisms of asbestos carcinogenesis, including chronic inflammation and autophagy-mediated cell survival, and propose potential innovative therapeutic targets to prevent mesothelioma development or improve drug efficacy by reducing inflammation and autophagy.
Background:
Diffuse malignant pleural mesothelioma is an aggressive cancer predominantly related to chronic inflammation caused by asbestos exposure. Millions of individuals have been exposed to asbestos or to other carcinogenic mineral fibers occupationally or environmentally, resulting in an increased risk of developing mesothelioma. Overall patient survival rates are notably low (about 8–14 months from the time of diagnosis) and mesothelioma is resistant to existing therapies. Additionally, individuals carrying inactivating germline mutations in the BRCA-associated protein 1 (BAP1) gene and other genes are predisposed to developing cancers, prevalently mesothelioma. Their risk of developing mesothelioma further increases upon exposure to asbestos. Recent studies have revealed the mechanisms and the role of inflammation in asbestos carcinogenesis. Biomarkers for asbestos exposure and malignant mesothelioma have also been identified. These findings are leading to the development of novel therapeutic approaches to prevent or delay the growth of mesothelioma.
Methods:
Review of full length manuscripts published in English from January 1980 to February 2021 gathered from PubMed.gov from the National Center of Biotechnology Information and the National Library of Medicine were used to inform this review.
Conclusion:
Key regulators of chronic inflammation mediate asbestos-driven mesothelial cell transformation and survival through autophagic pathways. Recent studies have elucidated some of the key mechanisms involved in asbestos-induced chronic inflammation, which are largely driven by extracellular high mobility group box 1 (HMGB1). Upon asbestos exposure, mesothelial cells release HMGB1 from the nucleus to the cytoplasm and extracellular space, where HMGB1 initiates an inflammatory response. HMGB1 translocation and release also activates autophagy and other pro-survival mechanisms, which promotes mesothelioma development. HMGB1 is currently being investigated as a biomarker to detect asbestos exposure and to detect mesothelioma development in its early stage when therapy is more effective. In parallel, several approaches inhibiting HMGB1 activities have been studied and have shown promising results. Moreover, additional cytokines, such as IL-1β and TNF-α are being targeted to interfere with the inflammatory process that drives mesothelioma growth. Developing early detection methods and novel therapeutic strategies is crucial to prolong overall survival of patients with mesothelioma. Novel therapies targeting regulators of asbestos-induced inflammation to reduce mesothelioma growth may lead to clinical advancements to benefit patients with mesothelioma.
Keywords: Mesothelioma, asbestos, inflammation, high mobility group box 1 (HMGB1), IL-1β, autophagy
Introduction
Malignant pleural mesothelioma, a rare and aggressive malignancy due to mesothelial cell transformation, is the most common cancer caused by prolonged asbestos exposure (1,2). At the time of diagnosis, diffuse pleural mesothelioma, which must be distinguished from the exceedingly rare, localized forms, is very often at advanced stages and median survival rates are approximately 8–14 months (3). Mesothelioma is resistant to current therapies; however, potential biomarkers for early diagnosis and combinatory therapies are being tested in clinical trials.
Studies of asbestos carcinogenesis revealed that asbestos induces cell death, mostly by necrosis in human mesothelial cells (HM), and that necrotic cells release high mobility group box 1 (HMGB1) into the cytoplasm and the extracellular space (4) (Figure 1). HMGB1 is a prototypical damaged-associated molecular pattern (DAMP) that initiates the inflammatory process. It recruits macrophages, which release pro-inflammatory cytokines interleukin-1beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) acting on neighboring HM (4). Upon asbestos exposure, active secretion and cytoplasmic accumulation of HMGB1 also activates autophagy, allowing HM to evade asbestos-induced cell death (5). Autophagy is an essential and physiological pathway that allows cells to modulate cell survival and protect against stress and nutrient deprivation. Autophagy upregulation in transformed cells is associated with resistance to chemotherapies. Therefore, inhibiting autophagy has been proposed as a possible therapeutic approach to promote drug sensitivity and reduce tumor growth (5,6). The objective of this literature review is examining the possible innovative therapeutic targets to reduce inflammation and chemoresistance to prevent or delay mesothelioma development while addressing the mechanisms of asbestos carcinogenesis, including chronic inflammation and autophagy-mediated cell survival. We present the following article in accordance with the Narrative Review Reporting Checklist (available at https://dx.doi.org/10.21037/pcm-21-12).
Figure 1.
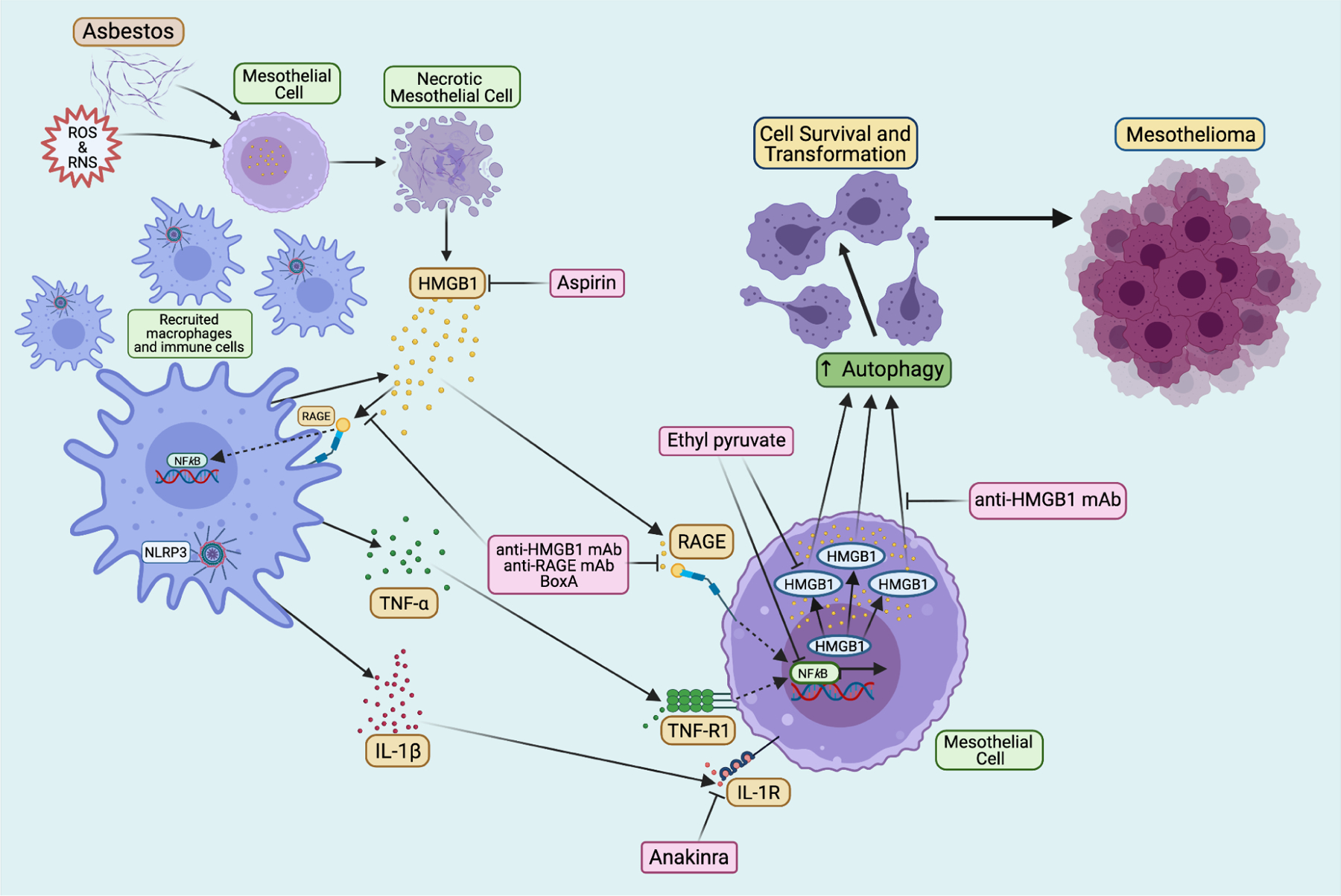
Key signaling pathways in asbestos-induced chronic inflammation and mesothelioma pathogenesis as well as related therapeutic approaches. Asbestos fibers cause necrotic mesothelial cell death and the consequent release of HMGB1 into the extracellular space. Extracellular HMGB1 initiates inflammatory response. It induces the accumulation of immune cells, as well as activates the NLRP3 inflammasome and NF-κB pathway, which causes secretion of many pro-inflammatory cytokines, including TNF-α and IL-1β. These factors bind to their respective receptors on neighboring mesothelial cells and promote the survival and transformation of mesothelial cells. Moreover, HMGB1 also induces autophagy in mesothelial cells following asbestos exposure that further promotes the cell survival and malignant transformation. The accumulation of survived mesothelial cells carrying DNA defects induced by asbestos and asbestos-generated ROS and RNS eventually leads to mesothelioma development. Many potential targets have been identified to interfere with the above carcinogenic process. For example, HMGB1 antagonist BoxA, anti-HMGB1 and anti-RAGE monoclonal antibodies can efficiently inhibit HMGB1 activities; Ethyl pyruvate reduces HMGB1 translocation and release by down-regulating the HMGB1-RAGE axis; IL-1 receptor antagonist Anakinra prevents the activity of IL-1β; and anti-inflammatory drug aspirin was also found to have anti-HMGB1 function. Created with Biorender.com.
Methods
Literature gathered to inform this review was obtained from PubMed.gov from the National Center of Biotechnology Information and the National Library of Medicine. Searches included full length manuscripts published in the English language in high impact journals between the dates of January 1980 and February 2021.
Discussion
Asbestos cytotoxicity and mesothelioma detection
Asbestos describes two families of six different fibers including chrysotile, amosite, crocidolite, anthophyllite, tremolite, and actinolite. These fibers have different carcinogenic properties. Amphiboles, including tremolite, anthophyllite, amosite, and crocidolite, are more pathogenic and remain in tissues for many years. Serpentine is mostly made up of chrysotile fibers. Its widespread use accounts for about 95% of global asbestos and is the most common fiber mined worldwide. Chrysotile was mined in the United States until 2002 (7). Although chrysotile is less carcinogenic than crocidolite asbestos due to its reduced bio-persistence as well as the dimension and aspect ratio (fiber length to diameter), which are essential factors to determine carcinogenicity, experimental evidence show that prolonged and continuous chrysotile exposure also causes transformation of HM through continuous HMGB1 release and TNF-α secretion (8).
Asbestos has been identified as a carcinogen since the 1960s, but the asbestos-driven carcinogenic process was not fully understood until recent years (3). Early studies suggested asbestos fibers mechanically interfere with chromosomal segregation during mitosis and cause DNA damage in HM, which eventually becomes mesothelioma (9). Additionally, HM susceptibility to asbestos cytotoxicity was initially proposed to be associated predominantly with apoptosis (10). However, these hypotheses have since been ruled out, and current studies suggest that asbestos-induced carcinogenesis is mainly due to the inflammatory response caused by asbestos deposits, and that the chemical structure of some types of asbestos fibers may play an important role in the malignant transformation of HM (3,11,12).
Compared to other cell types, HM are more vulnerable to asbestos cytotoxicity (13,14). Once inhaled, asbestos fibers remain in situ in the pleura and, over time, induce HM transformation that is largely ascribed to the chronic inflammatory response driven by the release of HMGB1. In efforts to remove inhaled asbestos fibers, macrophages and mesothelial cells attempt to envelop and phagocytize these fibers, which causes a wide range of cytotoxic effects including intracellular oxidation, DNA damage, cell cycle delay, cell death, and release of superoxide and cytokines (15,16). Moreover, the release of reactive oxygen and nitrogen species (ROS and RNS) (17,18) and the pro-inflammatory microenvironment induced by asbestos causes even more DNA damage, which over the course of several decades may lead to malignant transformation of HM and mesothelioma onset (19).
Crocidolite and amosite fibers have high biopersistence and contain high amounts of iron that form iron-rich chemical structures (ferruginous bodies), and some other asbestos fibers harbor iron as a surface impurity (11). Elevated levels of iron favor excessive ROS production and DNA damage catalyzed by oxidation: common hallmarks of cancer (20). Studies suggest that asbestos-driven carcinogenesis is associated with ferroptosis, an iron-dependent cell death pathway induced by overproduction of ROS (21). Recent data show that asbestos fibers induce distinctive necrotic cell death, and the cells initially undergo lysosome-dependent cell death (LDCD) and then undergo ferroptosis (22). The necrotic macrophages induce iron-dependent oxidative damage and DNA double-strand breaks in mesothelial cells, which contribute to asbestos carcinogenesis (22). Furthermore, the tumor suppressor activity of BAP1 has been recently linked to promoting ferroptosis by modulating the repression of cystine/glutamate antiporter SLC7A11 expression (3,23,24). It was reported that cells carrying BAP1 mutations are defective in regulating SLC7A11 and ferroptosis, which uncovers a novel mechanism linking ferroptosis to tumor suppression (3,23,24).
Some recent studies indicate the Epithelial to Mesenchymal Transition (EMT) as an important event in asbestos-induced HM transformation (8,25). EMT is an essential pathogenic process in tumor progression allowing epithelial cells to acquire characteristics of mesenchymal cells (26). Data suggests that HM exposed to asbestos undergo EMT, and this transition is mediated by the increased secretion of transforming growth factor β (TGF-β) (25), HMGB1, and TNF-α (8,25), which are all driven by asbestos exposure. Moreover, asbestos induces ROS production that activates TGF-β secretion (27) and the activation of activator-protein-1 (AP-1) (28,29), an important regulator of inflammation, cell proliferation, and transformation, ultimately influencing asbestos cytotoxicity (30,31). It was demonstrated that upregulation of the AP-1 component Fra-1 in response to asbestos modulates malignant transformation of mesothelial cells (31,32). It was also found that because of the “field effect” of asbestos exposure and/or germline mutations, multiple foci of transformed cells may develop simultaneously: accordingly, mesothelioma is often a polyclonal malignancy (33).
Inflammatory factors in mesothelioma
Chronic inflammation is caused by unresolved acute inflammation and promotes the development of several diseases (34–36). Numerous findings indicate that chronic inflammation is also a hallmark of cancer (37) and is involved in the development (4,12,38) and progression (39) of mesothelioma.
Throughout this chronic inflammatory process, the pro-inflammatory cytokine TNF-α is secreted and activates pro-tumoral transcription factors (such as NF-κB), which promotes HM survival and contributes to the pathogenesis of asbestos (12). Knocking out TNF-α receptors was sufficient to protect against asbestos-induced fibroproliferative lesions (40), which underscores the importance of TNF-α signaling in asbestos-induced inflammation. Early studies in asbestos-driven carcinogenesis suggest that HMGB1 is released from the nucleus to the cytoplasm and to the extracellular space where HMGB1 induces an inflammatory response (4,12). Once HMGB1 is released into the extracellular space, it binds directly with IL-1β and increases the production of TNF-α and macrophage inflammatory protein 2 (MIP-2 or CXCL2), which have pro-inflammatory activity (41). Together these factors contribute to the chronic inflammatory process resulting in survival of HM that can undergo transformation and give rise to mesothelioma.
At the site of accumulated asbestos deposits the chronic inflammatory response is also driven by the activation and formation of the NOD-like receptor family member containing a pyrin domain (NLRP3) inflammasome. The active NLRP3 inflammasome primed by asbestos promotes a pro-inflammatory microenvironment in the pleura by recruiting macrophages and releasing IL-1β, TNF-α, and HMGB1 (Figure 1). TNF-α binds to its receptor and activates NF-κB, which promotes the survival of HM and also induces the transcription of inflammatory cytokine genes (42). Activation of the NLRP3 inflammasome in macrophages and HM after asbestos exposure initiates the downstream inflammatory response by secretion of pro-inflammatory cytokines (43). In addition to NLRP3 inflammasome activation by asbestos sensing, the presence of ROS modulates inflammasome formation. Asbestos exposure causes oxidation of the antioxidant thioredoxin-1 (Trx1) and thioredoxin interacting protein (TXNIP) release: both contribute to the activation of the NLRP3 inflammasome (42,44). Moreover, the inflammasome is also activated and regulated by DAMP including HMGB1 (19,45–49). The activated NLRP3 inflammasome controls and promotes the secretion of IL-1β and IL-18 by caspase-1 cleaving pro-IL-1β and pro-IL-18 to their mature forms (50,51). IL-1β secretion is essential to inflammation-induced mesothelioma because IL-1β regulates other cytokines contributing to mesothelioma development (52,53). Since IL-1β levels regulate the secretion of other pro-inflammatory cytokines, IL-1 receptor antagonists have been proposed as a therapeutic strategy to reduce inflammation in mesothelioma (38,54,55).
It was shown that following asbestos exposure, IL-1β secretion was reduced in NLRP3-deficient mice compared to wild type mice. However, there was no significant difference regarding the incidence of mesothelioma between the two groups (56), which suggested that asbestos-induced inflammation is initially inflammasome-dependent, but NLRP3 may not be essential during the later stage of chronic inflammation that causes mesothelioma development following asbestos exposure. Instead, the chronic inflammation at the site of asbestos deposits is supported by the active release of HMGB1, initially by dying HM, and over time by accumulating macrophages that actively secrete HMGB1 extracellularly. Accordingly, asbestos-exposed individuals have elevated HMGB1 serum levels and its levels further increased during mesothelioma development, suggesting that HMGB1 can be a potential biomarker for asbestos exposure and early detection of mesothelioma (4,39,57,58).
Genetic susceptibility and mesothelioma
During the past decade, numerous studies have confirmed and expanded upon our discovery that mesothelioma is frequent in families carrying germline mutations of BAP1 and other tumor suppressor genes (59,60). BAP1 heterozygous inactivating mutations reduce the ability of the cells to repair the DNA and reduce the ability of cells to undergo cell death in response to genetic damage i.e., haploinsufficiency. These dual effects on DNA repair and cell death favor further accumulation of genetic damage that can ensue in malignant transformation and mesothelioma development, a process that can be enhanced by asbestos exposure (61–63). Additionally, reduced levels of BAP1 induce aerobic glycolysis, which favors tumor growth (64). About 30% of carriers of germline BAP1 mutations have developed diffuse malignant mesothelioma (62). The critical role of BAP1 in mesothelioma is supported by the frequent somatic biallelic inactivation of this gene in sporadic mesothelioma, which account for more than 60% of mesotheliomas (65,66), a malignancy in which recurrent genetic mutations are rare (67–69). Moreover, mouse models carrying heterozygous inactivating germline BAP1 mutations are more susceptible to asbestos-induced mesothelioma, i.e., Gene X Environment interaction (38,63), and BAP1 germline mutations interact with other tumor suppressor gene mutations to favor mesothelioma development, i.e., Gene X Gene interaction (70).
Mesothelioma also develops in carriers of germline mutations of other tumor suppressor genes known to cause other tumor predisposition syndromes, such as BLM, BRCA1 and BRCA2, TP53, and ATM. Mesotheliomas developing in carriers of germline BAP1 mutations, or of other tumor suppressor genes, have a significantly improved prognosis compared to sporadic (not genetically linked) and, most often, asbestos-induced mesotheliomas (71–74). We have recently reviewed the role of genetics in causing mesothelioma (3), the BAP1 cancer syndrome (75), and the role and mechanisms of Gene X Environment interaction in human cancer including mesothelioma (62) in detail, and therefore we will not further discuss these issues here.
Autophagy as a mechanism for pre-cancer cell survival in response to chronic inflammation in mesothelioma
A recent study by Xue et al. (5) identified a key mechanism by which HMGB1 favors mesothelioma development. It was found that asbestos induces HMGB1 translocation from the nucleus to the cytoplasm, which leads to the activation of autophagy in HM (5). It was also shown previously that asbestos exposure causes DNA damage, such as double-strand breaks (76,77), which are associated with the loss of nuclear HMGB1 (78). Therefore, following asbestos exposure, increased cytoplasmic HMGB1 induces autophagy, which allows the cells to escape cell death despite DNA damage (79). In contrast, when HM were silenced for HMGB1, autophagy activation was reduced and HMGB1-silenced HM exhibited increased cell death by apoptosis and necrosis upon asbestos exposure (5). Similarly, murine mesothelial cells from Hmgb1 conditional knockout mice exposed to asbestos demonstrated lower autophagy activation and increased apoptosis and necrosis compared to primary mesothelial cells harvested from Hmgb1 wild-type mice (5). Moreover, asbestos-induced autophagy was completely inhibited by treating HM with BoxA (5), an HMGB1 antagonist (5,39,80,81). These findings demonstrated that HMGB1-driven autophagy contributes to mesothelial cell transformation upon asbestos exposure, which further suggest that targeting HMGB1 or inhibiting autophagy might help prevent or delay mesothelioma.
Possible therapeutic approaches to reduce chronic inflammation and cancer cell survival in mesothelioma
Sporadic mesothelioma is resistant to current therapies, and depending on histology, overall patient survival is about 8–14 months from diagnosis. In contrast, median survival for mesothelioma patients carrying germline mutations is 5–7 years (3,82–84). The standard first-line therapy for sporadic mesothelioma consists of cisplatin and pemetrexed combination chemotherapy which may increase median survival by about 3 months, whereas the role and type of surgery continues to be investigated (3,85–89). The addition of bevacizumab to cisplatin-pemetrexed further extends median survival to about 18 months, while immunotherapy may help a fraction of patients with pleural mesothelioma (90–92). Novel therapies are being investigated. For example, several reagents including HMGB1 inhibitors, IL-1 receptor antagonists, anti-inflammatory drugs, and autophagy inhibitors have been identified and demonstrated their effects in reducing mesothelioma in vitro (Figure 1) and in vivo animal models. Current understandings of these developing therapies are discussed below.
Since the HMGB1 release appears necessary for mesothelioma progression, targeting HMGB1 has been explored as a potential therapy for mesothelioma (19,39,93,94). As discussed, HMGB1 is released into the extracellular space by HM exposed to asbestos fibers and the consequent HMGB1-RAGE interaction activates NF-κB. Ethyl pyruvate (EP), a derivative of pyruvic acid, has shown effective to reduce inflammation (95,96). The main effect of EP is the down-regulation of the HMGB1-RAGE axis, with the consequent reduced expression of inflammatory cytokines and of NF-κB. Pellegrini et al. showed that EP may act as an HMGB1-RAGE axis inhibitor in mesothelioma and that EP inhibited the translocation of HMGB1 from the nucleus to the cytoplasm, the release into the extracellular space, and led to reduced RAGE expression as well, resulting in the inhibition of NF-κB activity (93). In mouse mesothelioma xenografts, EP therapy resulted in both smaller tumor volume and reduced HMGB1 serum levels compared to untreated mice (93). Furthermore, pre-treatment with EP prior to the exposure to asbestos in both HM and in mice resulted in a reduced number of transformed foci in vitro and decreased HMGB1 serum levels in vivo (93). EP has been included in some food products and used as therapeutics in various diseases other than cancer; thus, its use is considered to be safe for human health (97,98). These findings of HMGB1 inhibition by EP in cancer, and especially in mesothelioma, point to EP as a potential novel therapy that could be tested in clinical trials. Additionally, a recent study showed that heparan sulfate octadecasaccharide (18-mer-HP) can neutralize the pro-inflammatory activity of the HMGB1-RAGE axis and protect against acetaminophen/paracetamol-induced acute liver failure in a mouse model (99), which makes 18-mer-HP a potential candidate to target HMGB1 release induced by asbestos exposure that is worth investigating in future studies.
Acetylsalicylic acid (ASA), or aspirin, is well known for its anti-inflammatory effects and, after 5 years of continuous therapy, aspirin reduced the incidence of colorectal cancer (CRC) by 30% and reduced the risk of metastases in early stage CRC (100). Treatment with ASA and its deacetylated metabolite salicylic acid (SA) demonstrated specific modulation of HMGB1 in vitro and in vivo, and effectively lowered HMGB1 serum levels and reduced tumor growth in mouse mesothelioma xenografts compared to untreated mice (101). In malignant mesothelioma cells secreting HMGB1, ASA and SA suppressed anchorage-independent cell growth in tissue culture (101). HMGB1 is a chemoattractant, therefore when HMGB1 is secreted into the extracellular space it induces the migration and recruitment of granulocytes, macrophages, and other cell types (102,103). ASA inhibited the chemoattractant activity of HMGB1 and, consequently, reduced cell migration (101). Thus, it appears possible that individuals exposed to asbestos, and those carrying germline BAP1 mutations, would benefit from aspirin treatment given reducing HMGB1 secretion should delay the onset or progression of mesothelioma (101). When cells secrete HMGB1 into the extracellular space, HMGB1 binds to the surface of macrophages (104). BoxA, the DNA-binding domain for HMGB1, competes with HMGB1 for these binding sites. By inhibiting HMGB1 from binding to the surface of the macrophages, less HMGB1-induced proinflammatory cytokines are released from the macrophages (104). In studies of HMGB1 inhibition as treatment for sepsis, BoxA attenuated HMGB1-driven release of IL-1β and TNF-α from macrophages by more than 75% (104). In asbestos-exposed HM treated with BoxA, macrophages secreted less TNF-α than untreated controls (4). Similarly to aspirin, BoxA also reduced mesothelioma growth in vitro and in vivo (39,101,105). In a comparative study of aspirin and BoxA as HMGB1 antagonists, both treatments inhibited the chemoattractant activity of HMGB1 and therefore reduced cell migration in vitro (101). In mouse mesothelioma xenografts, both treatments reduced tumor growth, however, BoxA-treated mice survived longer than ASA-treated mice (101).
Monoclonal antibodies (mAb) for HMGB1 and for its RAGE receptor inhibited HMGB1 activity in vitro and in vivo (39). In mesothelioma cell lines, both anti-HMGB1 and anti-RAGE antibodies induced cytotoxicity and reduced viability (39). Targeting HMGB1 and RAGE significantly reduced cell viability, underscoring the key role of the HMGB1-RAGE axis in cell growth and thus its potential value as a therapeutic target (39). Similar to aspirin and BoxA, anti-HMGB1 and anti-RAGE antibodies reduced anchorage-independent cell growth in vitro (39). Additionally, HM exposed to asbestos and treated with either BoxA or anti-HMGB1 mAbs exhibited a two-week delay in foci formation, an in vitro measure of cell transformation, and an overall reduced number of foci compared to controls exposed to asbestos and not treated with any anti-HMGB1 therapy (39). Also, in mouse mesothelioma xenografts, treatment with anti-HMGB1 antibody reduced tumor growth (39).
The NLRP3 inflammasome is responsible for the secretion of several pro-inflammatory factors, which contribute to HM transformation upon asbestos exposure. Since IL-1β, which is released by the NLRP3 inflammasome, initiates the secretion of many other cytokines, several research groups have studied IL-1 receptor antagonists to inhibit cytokine transcription and activity (106,107). Pre-treatment with Anakinra, an IL-1 receptor antagonist, followed by asbestos-exposure reduced the levels of NLRP3, IL-1β, IL-6, IL-8, HMGB1, vascular endothelial growth factor (VEGF), and granulocyte-colony stimulating factor (G-CSF) (43). Moreover, Anakinra treatment increased Nf2+/− and Cdkna2+/− mice survival exposed to asbestos (38,68), suggesting that Anakinra may mitigate mesothelioma progression by reducing inflammation.
Conclusions
Chronic inflammation caused by asbestos drives mesothelial cell transformation. Developing early detection methods and novel therapeutic approaches is essential to promote overall survival of patients with mesothelioma. Recent studies have elucidated some of the key mechanisms involved in asbestos-induced chronic inflammation, which are largely driven by extracellular HMGB1 (Figure 1). HMGB1 is initially released by HM undergoing asbestos-induced necrosis, and later it is released by macrophages which are recruited at the site of asbestos deposits in tissues by the presence of extracellular HMGB1. These macrophages actively secrete HMGB1, thus propagating the inflammatory process and the secretion of IL-1β and other pro-inflammatory cytokines, such as TNF-α, which leads to the subsequent activation of NF-κB, a pro-survival pathway. HMGB1 activates autophagy and additional mechanisms that promote cell survival. These pro-survival mechanisms allow some of the mesothelial cells exposed to asbestos to escape cell death despite asbestos-induced DNA damage. This information is leading to the design of novel therapeutic approaches. HMGB1 is being investigated as a biomarker to detect asbestos exposure and to detect mesothelioma development in its early stage. In parallel, several approaches are being investigated to target and inhibit HMGB1 activities, a strategy that both in vitro and in mice has shown promising results to reduce mesothelial cell transformation and mesothelioma growth. Moreover, additional cytokines, such as IL-1β and TNF-α are being targeted to interfere with the inflammatory process that drives mesothelioma growth. It is hoped that these mechanistically driven novel therapies will result in clinical improvements for patients with mesothelioma in the near future.
Acknowledgments
Funding:
MC and HY have grants from the National Institutes of Health, National Cancer Institute, the US Department of Defense, and the UH Foundation through donations to support research on “Pathogenesis of Malignant Mesothelioma” from Honeywell International Inc, Riviera United-4-a Cure, and the Maurice and Joanna Sullivan Family Foundation. HIP has funding from the National Cancer Institute, the Department of Defense, the Center for Disease Control, Genentech, and Belluck and Fox.
Footnotes
Reporting Checklist: The authors have completed the Narrative Review Reporting Checklist. Available at https://dx.doi.org/10.21037/pcm-21-12
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/pcm-21-12). The series “Malignant Pleural Mesothelioma” was commissioned by the editorial office without any funding or sponsorship. MC and HY have patents issued for HMGB1. MC is a board-certified pathologist who provides consultation for mesothelioma expertise and diagnosis. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1.Carbone M, Ly BH, Dodson RF, et al. Malignant mesothelioma: facts, myths, and hypotheses. J Cell Physiol 2012;227:44–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaudino G, Xue J, Yang H. How asbestos and other fibers cause mesothelioma. Transl Lung Cancer Res 2020;9:S39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carbone M, Adusumilli PS, Alexander HR Jr, et al. Mesothelioma: Scientific clues for prevention, diagnosis, and therapy. CA Cancer J Clin 2019;69:402–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang H, Rivera Z, Jube S, et al. Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation. Proc Natl Acad Sci U S A 2010;107:12611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xue J, Patergnani S, Giorgi C, et al. Asbestos induces mesothelial cell transformation via HMGB1-driven autophagy. Proc Natl Acad Sci U S A 2020;117:25543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Echeverry N, Ziltener G, Barbone D, et al. Inhibition of autophagy sensitizes malignant pleural mesothelioma cells to dual PI3K/mTOR inhibitors. Cell Death Dis 2015;6:e1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumann F, Ambrosi JP, Carbone M. Asbestos is not just asbestos: an unrecognised health hazard. Lancet Oncol 2013;14:576–8. [DOI] [PubMed] [Google Scholar]
- 8.Qi F, Okimoto G, Jube S, et al. Continuous exposure to chrysotile asbestos can cause transformation of human mesothelial cells via HMGB1 and TNF-α signaling. Am J Pathol 2013;183:1654–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olofsson K, Mark J. Specificity of asbestos-induced chromosomal aberrations in short-term cultured human mesothelial cells. Cancer Genet Cytogenet 1989;41:33–9. [DOI] [PubMed] [Google Scholar]
- 10.Broaddus VC, Yang L, Scavo LM, et al. Asbestos induces apoptosis of human and rabbit pleural mesothelial cells via reactive oxygen species. J Clin Invest 1996;98:2050–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagai H, Ishihara T, Lee WH, et al. Asbestos surface provides a niche for oxidative modification. Cancer Sci 2011;102:2118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang H, Bocchetta M, Kroczynska B, et al. TNF-alpha inhibits asbestos-induced cytotoxicity via a NF-kappaB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis. Proc Natl Acad Sci U S A 2006;103:10397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bocchetta M, Di Resta I, Powers A, et al. Human mesothelial cells are unusually susceptible to simian virus 40-mediated transformation and asbestos cocarcinogenicity. Proc Natl Acad Sci U S A 2000;97:10214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang H, Testa JR, Carbone M. Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Curr Treat Options Oncol 2008;9:147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang L, Nagai H, Ohara H, et al. Characteristics and modifying factors of asbestos-induced oxidative DNA damage. Cancer Sci 2008;99:2142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu W, Ernst JD, Broaddus VC. Phagocytosis of crocidolite asbestos induces oxidative stress, DNA damage, and apoptosis in mesothelial cells. Am J Respir Cell Mol Biol 2000;23:371–8. [DOI] [PubMed] [Google Scholar]
- 17.Huang SX, Jaurand MC, Kamp DW, et al. Role of mutagenicity in asbestos fiber-induced carcinogenicity and other diseases. J Toxicol Environ Health B Crit Rev 2011;14:179–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang SX, Partridge MA, Ghandhi SA, et al. Mitochondria-derived reactive intermediate species mediate asbestos-induced genotoxicity and oxidative stress-responsive signaling pathways. Environ Health Perspect 2012;120:840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carbone M, Yang H. Molecular pathways: targeting mechanisms of asbestos and erionite carcinogenesis in mesothelioma. Clin Cancer Res 2012;18:598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toyokuni S Iron-induced carcinogenesis: the role of redox regulation. Free Radic Biol Med 1996;20:553–66. [DOI] [PubMed] [Google Scholar]
- 21.Toyokuni S Iron addiction with ferroptosis-resistance in asbestos-induced mesothelial carcinogenesis: Toward the era of mesothelioma prevention. Free Radic Biol Med 2019;133:206–15. [DOI] [PubMed] [Google Scholar]
- 22.Ito F, Yanatori I, Maeda Y, et al. Asbestos conceives Fe(II)-dependent mutagenic stromal milieu through ceaseless macrophage ferroptosis and β-catenin induction in mesothelium. Redox Biol 2020;36:101616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Affar EB, Carbone M. BAP1 regulates different mechanisms of cell death. Cell Death Dis 2018;9:1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Shi J, Liu X, et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol 2018;20:1181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turini S, Bergandi L, Gazzano E, et al. Epithelial to Mesenchymal Transition in Human Mesothelial Cells Exposed to Asbestos Fibers: Role of TGF-β as Mediator of Malignant Mesothelioma Development or Metastasis via EMT Event. Int J Mol Sci 2019;20:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014;15:178–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pociask DA, Sime PJ, Brody AR. Asbestos-derived reactive oxygen species activate TGF-beta1. Lab Invest 2004;84:1013–23. [DOI] [PubMed] [Google Scholar]
- 28.Ding M, Dong Z, Chen F, et al. Asbestos induces activator protein-1 transactivation in transgenic mice. Cancer Res 1999;59:1884–9. [PubMed] [Google Scholar]
- 29.Vallyathan V, Ding M, Shi X, et al. Molecular Activation of Activator Protein-1 In Silica and Asbestos-Induced Carcinogenesis. Inhal Toxicol 2000;12 Suppl 3:353–7. [DOI] [PubMed] [Google Scholar]
- 30.Ramos-Nino ME, Blumen SR, Sabo-Attwood T, et al. HGF mediates cell proliferation of human mesothelioma cells through a PI3K/MEK5/Fra-1 pathway. Am J Respir Cell Mol Biol 2008;38:209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramos-Nino ME, Timblin CR, Mossman BT. Mesothelial cell transformation requires increased AP-1 binding activity and ERK-dependent Fra-1 expression. Cancer Res 2002;62:6065–9. [PubMed] [Google Scholar]
- 32.Heintz NH, Janssen-Heininger YM, Mossman BT. Asbestos, lung cancers, and mesotheliomas: from molecular approaches to targeting tumor survival pathways. Am J Respir Cell Mol Biol 2010;42:133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Comertpay S, Pastorino S, Tanji M, et al. Evaluation of clonal origin of malignant mesothelioma. J Transl Med 2014;12:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Del Campo JA, Gallego P, Grande L. Role of inflammatory response in liver diseases: Therapeutic strategies. World J Hepatol 2018;10:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yi G, Liang M, Li M, et al. A large lung gene expression study identifying IL1B as a novel player in airway inflammation in COPD airway epithelial cells. Inflamm Res 2018;67:539–51. [DOI] [PubMed] [Google Scholar]
- 36.Zhang XY, Zhang PY, Aboul-Soud MA. From inflammation to gastric cancer: Role of Helicobacter pylori. Oncol Lett 2017;13:543–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 38.Kadariya Y, Menges CW, Talarchek J, et al. Inflammation-Related IL1β/IL1R Signaling Promotes the Development of Asbestos-Induced Malignant Mesothelioma. Cancer Prev Res (Phila) 2016;9:406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jube S, Rivera ZS, Bianchi ME, et al. Cancer cell secretion of the DAMP protein HMGB1 supports progression in malignant mesothelioma. Cancer Res 2012;72:3290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu JY, Brass DM, Hoyle GW, et al. TNF-alpha receptor knockout mice are protected from the fibroproliferative effects of inhaled asbestos fibers. Am J Pathol 1998;153:1839–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sha Y, Zmijewski J, Xu Z, et al. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol 2008;180:2531–7. [DOI] [PubMed] [Google Scholar]
- 42.Dostert C, Pétrilli V, Van Bruggen R, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008;320:674–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hillegass JM, Miller JM, MacPherson MB, et al. Asbestos and erionite prime and activate the NLRP3 inflammasome that stimulates autocrine cytokine release in human mesothelial cells. Part Fibre Toxicol 2013;10:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson JK, Westbom CM, MacPherson MB, et al. Asbestos modulates thioredoxin-thioredoxin interacting protein interaction to regulate inflammasome activation. Part Fibre Toxicol 2014;11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jia C, Zhang J, Chen H, et al. Endothelial cell pyroptosis plays an important role in Kawasaki disease via HMGB1/RAGE/cathespin B signaling pathway and NLRP3 inflammasome activation. Cell Death Dis 2019;10:778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jo EK, Kim JK, Shin DM, et al. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol 2016;13:148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim EJ, Park SY, Baek SE, et al. HMGB1 Increases IL-1β Production in Vascular Smooth Muscle Cells via NLRP3 Inflammasome. Front Physiol 2018;9:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rubartelli A DAMP-Mediated Activation of NLRP3-Inflammasome in Brain Sterile Inflammation: The Fine Line between Healing and Neurodegeneration. Front Immunol 2014;5:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng D, Liwinski T, Elinav E. Inflammasome activation and regulation: toward a better understanding of complex mechanisms. Cell Discov 2020;6:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell 2004;117:561–74. [DOI] [PubMed] [Google Scholar]
- 51.Thornberry NA, Bull HG, Calaycay JR, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 1992;356:768–74. [DOI] [PubMed] [Google Scholar]
- 52.Bent R, Moll L, Grabbe S, et al. Interleukin-1 Beta-A Friend or Foe in Malignancies? Int J Mol Sci 2018;19:2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Griffith DE, Miller EJ, Gray LD, et al. Interleukin-1-mediated release of interleukin-8 by asbestos-stimulated human pleural mesothelial cells. Am J Respir Cell Mol Biol 1994;10:245–52. [DOI] [PubMed] [Google Scholar]
- 54.Matsushita A, Sato T, Mukai S, et al. TAZ activation by Hippo pathway dysregulation induces cytokine gene expression and promotes mesothelial cell transformation. Oncogene 2019;38:1966–78. [DOI] [PubMed] [Google Scholar]
- 55.Westbom C, Thompson JK, Leggett A, et al. Inflammasome Modulation by Chemotherapeutics in Malignant Mesothelioma. PLoS One 2015;10:e0145404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chow MT, Tschopp J, Möller A, et al. NLRP3 promotes inflammation-induced skin cancer but is dispensable for asbestos-induced mesothelioma. Immunol Cell Biol 2012;90:983–6. [DOI] [PubMed] [Google Scholar]
- 57.Tabata C, Shibata E, Tabata R, et al. Serum HMGB1 as a prognostic marker for malignant pleural mesothelioma. BMC Cancer 2013;13:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ying S, Jiang Z, He X, et al. Serum HMGB1 as a Potential Biomarker for Patients with Asbestos-Related Diseases. Dis Markers 2017;2017:5756102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bononi A, Goto K, Ak G, et al. Heterozygous germline BLM mutations increase susceptibility to asbestos and mesothelioma. Proc Natl Acad Sci U S A 2020;117:33466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Testa JR, Cheung M, Pei J, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet 2011;43:1022–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bononi A, Giorgi C, Patergnani S, et al. BAP1 regulates IP3R3-mediated Ca2+ flux to mitochondria suppressing cell transformation. Nature 2017;546:549–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carbone M, Arron ST, Beutler B, et al. Tumour predisposition and cancer syndromes as models to study gene-environment interactions. Nat Rev Cancer 2020;20:533–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Napolitano A, Pellegrini L, Dey A, et al. Minimal asbestos exposure in germline BAP1 heterozygous mice is associated with deregulated inflammatory response and increased risk of mesothelioma. Oncogene 2016;35:1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bononi A, Yang H, Giorgi C, et al. Germline BAP1 mutations induce a Warburg effect. Cell Death Differ 2017;24:1694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kobrinski DA, Yang H, Kittaneh M. BAP1: role in carcinogenesis and clinical implications. Transl Lung Cancer Res 2020;9:S60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nasu M, Emi M, Pastorino S, et al. High Incidence of Somatic BAP1 alterations in sporadic malignant mesothelioma. J Thorac Oncol 2015;10:565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bueno R, Stawiski EW, Goldstein LD, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet 2016;48:407–16. [DOI] [PubMed] [Google Scholar]
- 68.Guo G, Chmielecki J, Goparaju C, et al. Whole-exome sequencing reveals frequent genetic alterations in BAP1, NF2, CDKN2A, and CUL1 in malignant pleural mesothelioma. Cancer Res 2015;75:264–9. [DOI] [PubMed] [Google Scholar]
- 69.Ivanov SV, Miller J, Lucito R, et al. Genomic events associated with progression of pleural malignant mesothelioma. Int J Cancer 2009;124:589–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Badhai J, Pandey GK, Song JY, et al. Combined deletion of Bap1, Nf2, and Cdkn2ab causes rapid onset of malignant mesothelioma in mice. J Exp Med 2020;217:e20191257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baumann F, Flores E, Napolitano A, et al. Mesothelioma patients with germline BAP1 mutations have 7-fold improved long-term survival. Carcinogenesis 2015;36:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hassan R, Morrow B, Thomas A, et al. Inherited predisposition to malignant mesothelioma and overall survival following platinum chemotherapy. Proc Natl Acad Sci U S A 2019;116:9008–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Panou V, Gadiraju M, Wolin A, et al. Frequency of Germline Mutations in Cancer Susceptibility Genes in Malignant Mesothelioma. J Clin Oncol 2018;36:2863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pastorino S, Yoshikawa Y, Pass HI, et al. A Subset of Mesotheliomas With Improved Survival Occurring in Carriers of BAP1 and Other Germline Mutations. J Clin Oncol 2018. [Epub ahead of print]. doi: 10.1200/JCO.2018.79.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carbone M, Harbour JW, Brugarolas J, et al. Biological Mechanisms and Clinical Significance of BAP1 Mutations in Human Cancer. Cancer Discov 2020;10:1103–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Both K, Henderson DW, Turner DR. Asbestos and erionite fibres can induce mutations in human lymphocytes that result in loss of heterozygosity. Int J Cancer 1994;59:538–42. [DOI] [PubMed] [Google Scholar]
- 77.Pietruska JR, Johnston T, Zhitkovich A, et al. XRCC1 deficiency sensitizes human lung epithelial cells to genotoxicity by crocidolite asbestos and Libby amphibole. Environ Health Perspect 2010;118:1707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.White RR, Milholland B, de Bruin A, et al. Controlled induction of DNA double-strand breaks in the mouse liver induces features of tissue ageing. Nat Commun 2015;6:6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.White E The role for autophagy in cancer. J Clin Invest 2015;125:42–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jube SL, Rivera Z, Casalgrandi M, et al. BoxA and ethyl pyruvate offer novel therapeutic approaches for human malignant mesothelioma. Cancer Res 2013;73:5557. [Google Scholar]
- 81.Sitia G, Iannacone M, Müller S, et al. Treatment with HMGB1 inhibitors diminishes CTL-induced liver disease in HBV transgenic mice. J Leukoc Biol 2007;81:100–7. [DOI] [PubMed] [Google Scholar]
- 82.McCambridge AJ, Napolitano A, Mansfield AS, et al. Progress in the Management of Malignant Pleural Mesothelioma in 2017. J Thorac Oncol 2018;13:606–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mutti L, Peikert T, Robinson BWS, et al. Scientific Advances and New Frontiers in Mesothelioma Therapeutics. J Thorac Oncol 2018;13:1269–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsao AS, Lindwasser OW, Adjei AA, et al. Current and Future Management of Malignant Mesothelioma: A Consensus Report from the National Cancer Institute Thoracic Malignancy Steering Committee, International Association for the Study of Lung Cancer, and Mesothelioma Applied Research Foundation. J Thorac Oncol 2018;13:1655–67. [DOI] [PubMed] [Google Scholar]
- 85.Goparaju CM, Blasberg JD, Volinia S, et al. Onconase mediated NFKβ downregulation in malignant pleural mesothelioma. Oncogene 2011;30:2767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nasu M, Carbone M, Gaudino G, et al. Ranpirnase Interferes with NF-κB Pathway and MMP9 Activity, Inhibiting Malignant Mesothelioma Cell Invasiveness and Xenograft Growth. Genes Cancer 2011;2:576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pass HI, Brewer GJ, Dick R, et al. A phase II trial of tetrathiomolybdate after surgery for malignant mesothelioma: final results. Ann Thorac Surg 2008;86:383–9; discussion 390. [DOI] [PubMed] [Google Scholar]
- 88.Pass HI, Mew DJ, Carbone M, et al. Inhibition of hamster mesothelioma tumorigenesis by an antisense expression plasmid to the insulin-like growth factor-1 receptor. Cancer Res 1996;56:4044–8. [PubMed] [Google Scholar]
- 89.Rivera Z, Ferrone S, Wang X, et al. CSPG4 as a target of antibody-based immunotherapy for malignant mesothelioma. Clin Cancer Res 2012;18:5352–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baas P, Scherpereel A, Nowak AK, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 2021;397:375–86. [DOI] [PubMed] [Google Scholar]
- 91.Disselhorst MJ, Baas P. Chemotherapy options versus “novel” therapies: how should we treat patients with malignant pleural mesothelioma. Transl Lung Cancer Res 2020;9:S77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gray SG, Mutti L. Immunotherapy for mesothelioma: a critical review of current clinical trials and future perspectives. Transl Lung Cancer Res 2020;9:S100–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pellegrini L, Xue J, Larson D, et al. HMGB1 targeting by ethyl pyruvate suppresses malignant phenotype of human mesothelioma. Oncotarget 2017;8:22649–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Musumeci D, Roviello GN, Montesarchio D. An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies. Pharmacol Ther 2014;141:347–57. [DOI] [PubMed] [Google Scholar]
- 95.Fink MP. Ethyl pyruvate: a novel anti-inflammatory agent. Crit Care Med 2003;31:S51–6. [DOI] [PubMed] [Google Scholar]
- 96.Yang R, Zhu S, Tonnessen TI. Ethyl pyruvate is a novel anti-inflammatory agent to treat multiple inflammatory organ injuries. J Inflamm (Lond) 2016;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Andersen PH, Jensen NJ. Mutagenic investigation of flavourings: dimethyl succinate, ethyl pyruvate and aconitic acid are negative in the Salmonella/mammalian-microsome test. Food Addit Contam 1984;1:283–8. [DOI] [PubMed] [Google Scholar]
- 98.Bennett-Guerrero E, Swaminathan M, Grigore AM, et al. A phase II multicenter double-blind placebo-controlled study of ethyl pyruvate in high-risk patients undergoing cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2009;23:324–9. [DOI] [PubMed] [Google Scholar]
- 99.Arnold K, Xu Y, Sparkenbaugh EM, et al. Design of anti-inflammatory heparan sulfate to protect against acetaminophen-induced acute liver failure. Sci Transl Med 2020;12:eaav8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rothwell PM, Wilson M, Price JF, et al. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet 2012;379:1591–601. [DOI] [PubMed] [Google Scholar]
- 101.Yang H, Pellegrini L, Napolitano A, et al. Aspirin delays mesothelioma growth by inhibiting HMGB1-mediated tumor progression. Cell Death Dis 2015;6:e1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schiraldi M, Raucci A, Muñoz LM, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med 2012;209:551–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Venereau E, Casalgrandi M, Schiraldi M, et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med 2012;209:1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang H, Ochani M, Li J, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A 2004;101:296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mezzapelle R, Rrapaj E, Gatti E, et al. Human malignant mesothelioma is recapitulated in immunocompetent BALB/c mice injected with murine AB cells. Sci Rep 2016;6:22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov 2012;11:633–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schwarznau A, Hanson MS, Sperger JM, et al. IL-1beta receptor blockade protects islets against pro-inflammatory cytokine induced necrosis and apoptosis. J Cell Physiol 2009;220:341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


