Abstract
Background
It is known that organ transplant recipients have a significantly higher risk for developing cancers, but the association between immunosuppression in organ transplantation and the risk for prostate cancer (PCa) remains unclear. We aimed to assess the evidence regarding the association of solid organ transplantation with PCa risk.
Methods
A literature search of the PubMed, Embase, and Web of Science databases was performed up to March 2019. Combined relative risks (RRs) and 95% confidence intervals (CIs) were calculated by using a fixed-effect or random-effect model.
Results
In total, 26 articles including 33 independent population-based cohort studies with 556,812 recipients and 2,438 PCa cases were identified and included in this meta-analysis. PCa risk in the solid organ transplant recipients did not increase compared with the general population (RR=1.04; 95% CI: 0.90–1.18). Independent analysis of different kinds of organ replacements further indicated immune inhibition in the transplantation of kidney, liver, heart, and lung, and was not associated with elevated PCa risk (RR=0.89; 95% CI: 0.83–0.95; RR=0.61, 95% CI: 0.21–1.02; RR=1.70, 95% CI: 0.88–2.52; RR=0.87, 95% CI: 0.57–1.16, respectively).
Conclusions
This study demonstrated that immunosuppression in solid organ transplant recipients was not associated with higher PCa risk.
Keywords: Prostate cancer (PCa), immunosuppression, solid organ transplantation, standardized incidence ratio, meta-analysis
Introduction
Prostate cancer (PCa) is one of the most common male malignancies in many developed countries globally and accounts for the second most common cause of death in males (1). Aging is a known risk factor of PCa (2), which may be due to the age-related decreased immunosurveillance (3). It is believed that active immunosurveillance in the young body can effectively eliminate neoplastic cells (4,5). Nevertheless, the relationship between PCa risk and either immunodeficiency or immunosuppression remains unclear.
Solid organ transplantation is considered to be the best therapeutic option for patients with end-stage organ failure. Successful outcomes of solid organ transplantation can be achieved by applying strong immunosuppressive drugs that are expected to decrease the incidence of acute graft rejection (6). Notably, solid organ transplant recipients under such treatment are at a higher risk of developing certain malignancies compared with the general population (7). The long duration of immunosuppression in solid organ recipients impairs the immunosurveillance of the body and results in the outgrowth of neoplastic cells (8). To date, organ transplant recipients have a higher risk of developing bladder cancer (9), head and neck cancer (10), thyroid cancer (11), and colorectal carcinoma (12). Based on its 5-years mortality, cancer is now the second, third, and fourth most common cause of death in the transplantation of liver, kidney, and lung recipients, respectively (13).
Although the association between immunosuppression in solid organ transplantation and PCa risk has received much attention lately, the data is still controversial because most studies were carried out from a single center or small sample set; consequently, we were motivated to investigate this association at a meta-analytical level.
Understanding the potential risks of any specific cancer in solid organ transplantation recipients will not only help in fully informing the patient before transplantation, but also allow for a rational approach to monitoring patients during the postoperative period. Currently, there are no conclusive recommendations for the screening of PCa in the solid-organ-transplanted population. Therefore, we conducted the first meta-analysis to investigate the relative risk (RR) of PCa associated with overall and different subsets of solid organ transplant recipients compared with the general male population based on eligible cohort studies.
Methods
Literature search strategy
We performed a systematic search of literature published from 1990 to March 2019 in the PubMed, Embase, and Web of Science databases, using the following keywords: “cancer” or “malignancy” or “neoplasms” or “carcinoma” or “tumor transplantation” or “transplant recipients” or “cohort” and the combination of these phrases. Additional reports were collected from the cross-references within both the original and review articles. No language restrictions were applied.
Inclusion and exclusion criteria
Studies were included in this meta-analysis if they met these criteria: (I) it was published between 1990 and March 2019; (II) it was an original cohort study in humans; (III) it examined the association of solid organ transplantation with PCa risk; (IV) it provided enough information to calculate the RR with 95% confidence interval (CI).
Studies were excluded if they met any of the following criteria: (I) they were not cohort studies that evaluated the association between immune inhibition in solid organ transplantation and PCa risk; (II) they were case reports, letters, reviews, editorials, or correspondence articles; (III) they were studies based on incomplete raw data; (IV) the study only contained duplicate data. Also, in cases of multiple publications of the same or overlapping populations, only the most recently published studies or the studies with a large sample size were included.
Data extraction
All data were extracted independently by two investigators (JM Bao and HL Zhu) according to the described selection criteria. Any discrepancy was resolved by the third investigator (GS Yang). The following data were extracted: the name of the first author, publication year, study location, type of transplantation, number of participants and PCa cases, follow-up period, RR and 95% CIs. If one article examined the associations of multiple organ transplantation with PCa risk and provided corresponding independent RR and 95% CIs, we considered this article to contain multiple independent studies.
Quality assessment
Two investigators (JM Bao and HL Zhu) independently evaluated the quality of all included studies using the criteria adapted from the Cochrane handbook for systematic reviews of interventions (14) and the Newcastle-Ottawa scale (NOS) (15). The NOS ranges from 0 to 9 stars, and study with a score of 7 stars or greater was regarded as high quality. Discrepancies were resolved as described above.
Statistical analysis
RR and 95% CI evaluated the strength of the association between immunosuppression in solid organ transplantation and PCa risk.
The heterogeneity among studies in terms of the degree of association was assessed using the Chi2 test. The I2 statistic was used to estimate the percentage of variation between the results occurring because of heterogeneity rather than sampling error (I2<50% was considered as no significant heterogeneity). When heterogeneity was detected, the RR was pooled according to a random-effect model using inverse variance heterogeneity method (16); otherwise, the fixed-effect model using the inverse variance method (17) was chosen. The Z-test determined the significance of the pooled RR.
Sensitivity analyses were carried out to assess the stability of the results of this study. The impact of each study was evaluated by calculating the combined RRs in the absence of every single study (18).
Potential publication bias was estimated by the Begg’s rank correlation (19), Egger’s linear regression test (20), and visual inspection of the Begg’s funnel plots. For all analyses, a P value <0.05 was considered to represent statistical significance for all comparisons. All statistical analyses were performed using Stata statistical software, version 12.0 (Stata Corp. College Station, TX, USA).
Results
Characteristics of eligible studies
We initially identified 26,836 results relevant to the search terms in the selected databases. After reading the titles and abstracts, 49 articles were included for full-text review. Of these, 22 articles were excluded as their data could not be merged. After further screening, 1 article (21) was excluded for duplicate data. Finally, a total of 26 articles totaling 556,812 solid organ transplant recipients, 2,438 PCa cases, and comprising 33 independent cohort studies that examined the association between solid organ transplantation and PCa risk, were selected for meta-analysis. The specific search workflow is shown in Figure 1.
Figure 1.
Flow diagram of the study selection process.
Of all the included cohort studies, there were 3 single-center cohort studies (22-24), 2 multicenter cohort study (25,26), and the rest were population-based cohort studies (27-47); 4 cohort studies (37,38,40,46) were conducted with Asians, and the remaining 29 studies were conducted with Europeans. In these studies, the observed number of PCa cases in the solid-organ-transplanted population was compared with the expected number of PCa case based on the standardized incidence rate of PCa, so the control group for each cohort consisted of all the males in a specific country or area. The characteristics of these studies are presented in Table 1.
Table 1. Characteristics of included cohort studies.
| First author [year] | Study location | Type of transplant | No. of patients | No. of PCa cases | Follow-up period | RR (95% CI) | Quality score |
|---|---|---|---|---|---|---|---|
| Birkeland [1995] | Nordic countries | Kidney | 5,692 | 11 | 1964–1986 | 2.10 (1.10–3.80) | 7 |
| Kasiske [2004] | USA | Kidney | 35,765 | NR | 1995–2001 | 0.79 (0.62–1.00) | 7 |
| Vajdic [2006] | Australia and New Zealand | Kidney | 28,855 | 41 | 1982–2003 | 0.95 (0.68–1.29) | 7 |
| Villeneuve [2007] | Canada | Kidney | 11,155 | 37 | 1981–1998 | 0.9 (0.6–1.3) | 7 |
| Vegso [2007] | Hungary | Kidney | 2,535 | 3 | 1973–2007 | 0.65 (0.28–1.59) | 7 |
| Webster [2007] | Australia and New Zealand | Kidney | 15,183 | 43 | 1963–2004 | 0.9 (0.64–1.2) | 7 |
| Aberg [2008] | Finland | Liver | 540 | 2 | 1982–2005 | 1.24 (0.15–4.47) | 7 |
| Jiang [2008] | Canada | Liver | 2,034 | 5 | 1983–1998 | 1.0 (0.3–2.4) | 7 |
| Kellerman [2009] | USA | Heart | 851 | 22 | 1994–2007 | 1.2 (0.76–1.8) | 7 |
| Jiang [2010] | Canada | Heart | 1,703 | 15 | 1981–1998 | 1.3 (0.7–2.2) | 7 |
| Collett [2010] | UK | Kidney | 37,617 | 112 | 1980–2007 | 1.1 (0.9–1.4) | 7 |
| Wisgerhof [2011] | Netherlands | Kidney | 1,906 | 8 | 1966–2006 | 0.77 (0.38–1.5) | 7 |
| Engels [2011] | USA | Kidney/lung/liver/heart | 175,732 | 1039 | 1987–2008 | 0.92 (0.87–0.98) | 7 |
| Cheung [2012] | China | Kidney | 4,674 | 6 | 1972–2011 | 0.88 (0.39–1.95) | 7 |
| Li [2012] | China | Kidney | 4,716 | 4 | 1997–2008 | 1.79 (0.67–4.76) | 7 |
| Sampaio (K) [2012] | USA | Kidney | 123,380 | 446 | 1999–2008 | 0.82 (0.75–0.91) | 7 |
| Sampaio (L) [2012] | USA | Liver | 43,106 | 155 | 1999–2008 | 0.88 (0.75–1.03) | 7 |
| Sampaio (H) [2012] | USA | Heart | 16,511 | 235 | 1999–2008 | 3.07 (2.7–3.49) | 7 |
| Sampaio (Lu) [2012] | USA | Lung | 10,908 | 33 | 1999–2008 | 0.88 (0.62–1.24) | 7 |
| Kaneko [2013] | Japan | Liver | 360 | 2 | NR | 2.2 (0.6–8.9) | 6 |
| Piselli [2013] | Italy | Kidney | 7,217 | 35 | 1997–2009 | 1.7 (1.2–2.3) | 7 |
| Krynitz (K) [2013] | Sweden | Kidney | 7,952 | 86 | 1970–2008 | 1.1 (0.9–1.3) | 7 |
| Krynitz (L) [2013] | Sweden | Liver | 1,221 | 4 | 1970–2008 | 0.5 (0.1–1.2) | 7 |
| Krynitz (H/Lu) [2013] | Sweden | Heart/Lung | 1,012 | 10 | 1970–2008 | 1.3 (0.6–2.3) | 7 |
| Tessari [2013] | Italy | Kidney | 3,537 | 19 | 1980–NR | 1.3 (0.8–2.1) | 6 |
| Na (L) [2013] | Australia | Liver | 1,926 | 7 | 1984–2006 | 0.62 (0.27–1.19) | 7 |
| Na (H) [2013] | Australia | Heart | 1,518 | 24 | 1984–2006 | 1.10 (0.71–1.60) | 7 |
| Na (Lu) [2013] | Australia | Lung | 1,200 | 3 | 1984–2006 | 0.73 (0.15–2.14) | 7 |
| Maisonneuve [2013] | USA | Lung/Liver | 2,749 | 1 | 1990–2009 | 1.8 (0.1–8.7) | 7 |
| Secnikova [2015] | Czech | Heart | 603 | 10 | 1993–2010 | 2.03 (1.05–3.62) | 7 |
| Taborelli [2018] | Italy | Liver | 2,832 | 2 | 1985–2014 | 0.1 (0.0–0.5) | 7 |
| Heo [2018] | South Korea | Kidney | 1,343 | 3 | 2010–2014 | 3.49 (0.70–10.19) | 7 |
| Jäämaa-Holmberg [2019] | Finland | Heart | 479 | 15 | 1985–2014 | 1.5 (0.8–2.4) | 7 |
No., number; RR, relative risks; 95% CI, 95% confidence interval; NR, not reported; K, kidney; L, liver; H, heart; Lu, lung; PCa, prostate cancer.
The methodological quality of the included studies
The scores of the included studies ranged from 6 to 7 (Table 1). Thus, the quality of these studies was generally high.
PCa risk in all solid organ transplant recipients
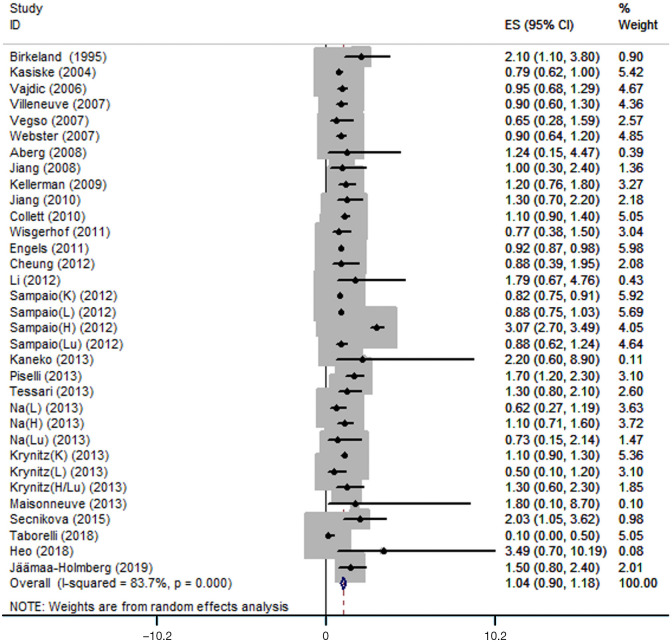
A total of 26 articles reporting associations of immunosuppression in solid organ transplantation with PCa risk were identified and included in this meta-analysis. These comprised 33 independent cohort studies with 556,812 solid organ transplant recipients and 2,438 PCa cases. Significant heterogeneity was detected, and the analysis was therefore conducted using a random-effect model. PCa risk in solid organ transplant recipients did not increase compared with that in the general population (RR=1.04, 95% CI: 0.90–1.18) (Figure 2).
Figure 2.
Forest plot of pooled RR with 95% CI for the association between immunosuppression in solid organ transplantation and PCa risk. ES, effect size; 95%CI, 95% confidence interval; K, kidney; L, liver; H, heart; Lu, lung; PCa, prostate cancer.
PCa risk in different subsets of the transplanted population
PCa risk in the renal-transplanted population
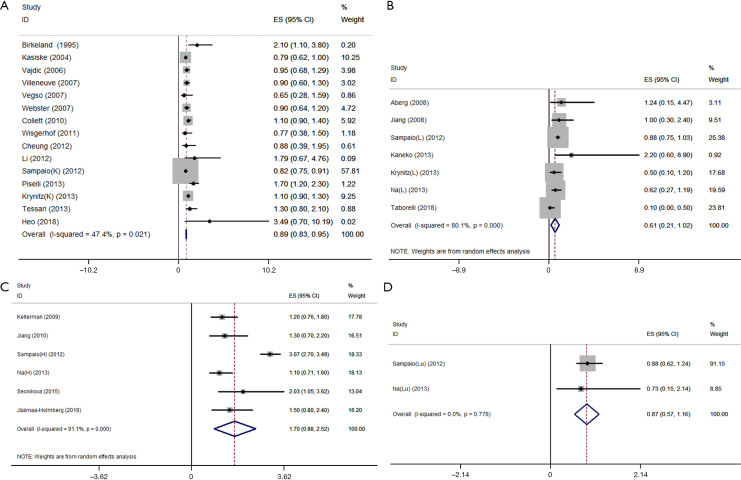
The association of immunosuppression in renal transplantation with the risk of PCa was investigated in 15 independent studies including a total of 291,527 renal transplant recipients. There was no significance between-study heterogeneity by Q-test, and a fixed effect model was used to conduct the analysis. The results showed an absence of a positive association between kidney transplantation and PCa risk (RR=0.89, 95% CI: 0.83–0.95) (Figure 3A).
Figure 3.
Forest plot of pooled RR with 95% CI for the association between PCa risk and immune inhibition in the transplantation of the kidney (A), liver (B), heart (C), and lung (D). RR, relative risks; ES, effect size; 95%CI, 95% confidence interval; K, kidney; L, liver; H, heart; Lu, lung; PCa, prostate cancer.
PCa risk in liver transplanted population
A meta-analysis of the association between immunosuppression in liver transplantation and PCa risk included 7 independent cohort studies with 52,019 liver transplant recipients. The Q-test of between-study heterogeneity was significant, and a random-effect model was used to analyze the data. The results indicated that liver transplantation was not associated with higher PCa risk (RR=0.61, 95% CI: 0.21–1.02) (Figure 3B).
PCa risk in heart transplanted population
Six independent cohort studies with a total of 21,665 heart transplant recipients were included in the meta-analysis of heart transplantation. There was significance in the between-study heterogeneity by Q-test, and the data were analyzed using a random-effect model, but no significant association between heart transplantation and PCa risk was detected (RR=1.70, 95% CI: 0.88–2.52) (Figure 3C).
PCa risk in lung transplanted population
The association between immunosuppression in lung transplantation and PCa risk was investigated in 2 independent cohort studies with 12,108 lung transplant recipients. The Q-test of heterogeneity was not significant, and a fixed effect model was used to conduct the analysis. No significant association between immunosuppression in lung transplantation and PCa risk was observed (RR=0.87, 95% CI: 0.57–1.16) (Figure 3D).
Subgroup analysis by ethnicity
The association between immunosuppression in solid organ transplantation and PCa risk in Asians
Meta-analysis of the association between immune inhibition in solid organ transplantation and PCa risk in Asians included 4 independent cohort studies with a total of 11,093 recipients. A random-effect model was selected to analyze the data. PCa risk did not increase in Asian solid organ transplant recipients (RR=1.09, 95% CI: 0.38–1.80).
The association between immunosuppression in solid organ transplantation and PCa risk in Europeans
The association of immunosuppression in solid organ transplantation and PCa risk in Europeans was investigated in 29 independent studies including a total of 545,719 solid organ transplant recipients. The Q-test of heterogeneity was significant, and a random-effect model was used. Immunosuppression in solid organ transplantation showed no significant association with PCa risk in Europeans (RR=1.03, 95% CI: 0.89–1.18). Summary of the results of this meta-analysis is listed in Table 2.
Table 2. Meta-analysis results of the association between immunosuppression in solid organ transplantation and prostate cancer risk.
| Group | No. of studies | Total patients | Total cases | Test of association | Test of heterogeneity | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pooled RR | 95% CI | Model | P | I2 | |||||
| Overall | 33 | 556,812 | 2,438 | 1.04 | 0.90–1.18 | R | 0.0 | 83.7% | |
| Type of transplantation | |||||||||
| Kidney transplantation | 15 | 291,527 | 854 | 0.89 | 0.83–0.95 | F | 0.02 | 47.4% | |
| Liver transplantation | 7 | 52,019 | 177 | 0.61 | 0.21–1.02 | R | 0.0 | 80.1% | |
| Heart transplantation | 6 | 21,665 | 321 | 1.70 | 0.88–2.52 | R | 0.0 | 91.1% | |
| Lung transplantation | 2 | 12,108 | 36 | 0.87 | 0.57–1.16 | F | 0.78 | 0.0% | |
| Ethnicity | |||||||||
| Asians | 4 | 11,093 | 15 | 1.09 | 0.38–1.80 | R | 0.58 | 0.0% | |
| Europeans | 29 | 545,719 | 2,423 | 1.03 | 0.89–1.18 | R | 0.0 | 85.5% | |
No., number; RR, relative risks; 95%CI, 95% confidence interval; R, random effect; F, fixed effect.
Sensitivity analysis and publication bias
Sensitivity analyses were done by removing every included study sequentially to evaluate the influence of every single study on the combined RRs. The results showed the pooled RRs were not significantly changed when any individual study was removed, indicating that no single study showed an excessive impact, and the results were reliable. Other results were also very stable. We conducted Begg’s and Egger’s tests to assess potential publication bias. No significant publication bias was observed (Begg, P=0.158 and Egger, P=0.437), and Begg’s funnel plots also indicated no substantial asymmetry (Figure 4). No evidence of publication bias was detected in other results.
Figure 4.
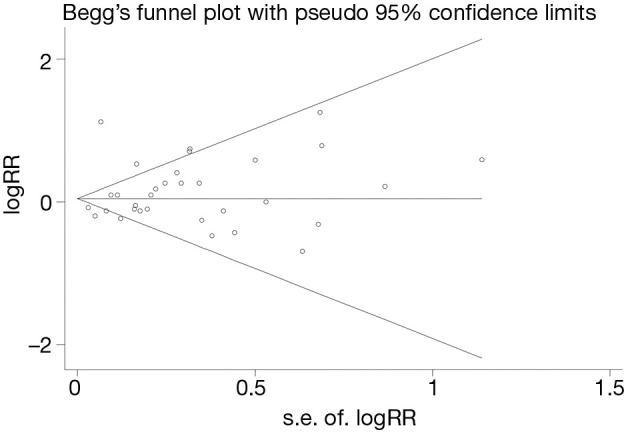
Forest plot of pooled RR with 95% CI for the association between immunosuppression in solid organ transplantation and PCa risk. RR, relative risks; 95%CI, 95% confidence interval; PCa, prostate cancer.
Discussion
The goal of this study is to determine the possible elevated risk of PCa in solid organ transplant recipients. This study, to the best of our knowledge, represents the first meta-analysis estimating the PCa risk in the solid-organ-transplanted population. The pooled RRs indicated that immunosuppression in solid organ transplantation did not increase PCa risk. Independent analysis in different subsets of the transplanted population further validated that individual organ transplantation of the kidney, liver, lung or heart is not associated with elevated PCa risk.
The key finding of this study is that there appears to be no significant association between immune inhibition in solid organ transplantation and PCa risk. The exact mechanism behind this surprising phenomenon is still unknown. In general, solid organ transplant recipients are associated with an increased risk of developing cancers, and it is widely believed that immunosuppression is the key reason. However, this simple concept does not explain why a higher frequency is associated with a few specific cancer types (48). Certainly, the relationship between cancer risk and the degree of immunosuppression might not always go hand in hand, and it could be very cancer-specific (49). Despite immunosuppression being a key factor, other risk factors like oncogenic viral infection may also play an important role (50). In an immunosuppressed population with impaired immunosurveillance, the implicit ability of several viruses to immortalize infected cells by disrupting the cell-cycle control could lead to tumorigenesis; meanwhile, immunosuppressive drugs damaging the immune function controlling the oncogenic viral infections may be involved in the initial effect on carcinogenesis by transforming cells and then evading immune recognition (51). Virus-related cancers consistently associated with an elevated risk in transplant recipients include hepatocellular carcinoma (52), squamous cell carcinoma of the cervix, Kaposi’s sarcoma (53), and Merkel cell carcinoma (54,55). However, to date, there is no conclusive evidence to support that infection is likely to be involved in prostate carcinogenesis (56). Therefore, a possible explanation is that most of the cancers occurring at increased rates in solid organ transplant recipients are infection-related, but PCa is not related to infection (56,57); hence, PCa risk does not increase in solid organ transplanted population.
One previous study indicated that PCa risk differs across race and ethnicity in renal transplanted population, and the black population had an elevated PCa risk following kidney transplantation (58). In our study, we also conducted a subgroup analysis to evaluate the ethnic differences in PCa risk after solid organ transplantation. However, the results indicated that PCa risk did not increase after solid organ transplantation both in Asians and Europeans.
Heterogeneity is considered to be a significant issue in meta-analytical studies. We detected significant between-study heterogeneity in some analyses. Sensitivity analyses showed that no individual study exhibited excessive impact on the pooled RRs, indicating these findings were relatively stable. Also, no evidence of publication bias was detected in Begg’s funnel plots and Begg’s tests, so the results were unbiased.
We believe our study has several strengths to support the conclusion. This is the first systematic quantitative assessment of the association between immune inhibition in solid organ transplantation and PCa risk. Our study included 33 independent population-based cohort studies with a large sample size of 556,812 recipients that enhanced the power of statistical analysis to lead to a more reliable outcome; this study surpasses the insufficient statistical power of individual studies. Four kinds of regular solid organ transplantation were involved in our included studies, and we further evaluated the association between immunosuppression and PCa risk in four subsets of recipients; the results indicated that none of the four kinds of solid organ replacement were associated with elevated PCa risk, which allows us to draw a comprehensive conclusion. We were able to conduct subgroup analysis to explore the racial differences in PCa risk following solid organ transplantation, and we demonstrated that neither Asians nor Europeans were associated with an elevated risk of PCa after solid organ transplantation.
Nevertheless, this study has some limitations that should be taken into consideration. First, we were not able to conduct a stratified analysis by different age groups to evaluate the impact of immunosuppression in solid organ transplantation on PCa risk because of a lack of data. Second, other confounders like dietary factors (59-62) and smoking (63) may play a role in prostatic carcinogenesis in solid organ recipients which may affect the association between immunosuppression in solid organ transplantation and risk of PCa, but we could not rule out the impact of these factors due to insufficient data. Third, heterogeneity among studies existed in some analyses.
Conclusions
Taken together, immunosuppression in solid organ transplantation is not associated with increased PCa risk. Organ-transplantation-related immunosuppression may not be a risk factor of PCa. Large prospective cohort studies are needed to confirm our findings.
Acknowledgments
Funding: This work was supported by a grant from the China Scholarship Council [Grant number 201508440279 to JM Bao] and a grant from Southern Medical University [Grant number LX2018N001 to JM Bao].
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.06.03). The authors have no conflicts of interest to declare.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. editors. SEER Cancer Statistics Review, 1975-2008, Bethesda, MD, USA: National Cancer Institute; 2011. Based on November 2010 SEER data submission, posted to the SEER web site. Available online: http://seer.cancer.gov/csr/1975-2008/
- 2.Grönberg H. Prostate cancer epidemiology. Lancet 2003;361:859-864. 10.1016/S0140-6736(03)12713-4 [DOI] [PubMed] [Google Scholar]
- 3.Leibovitz A, Baumoehl Y, Segal R. Increased incidence of pathological and clinical prostate cancer with age: age related alterations of local immune surveillance. J Urol 2004;172:435-7. 10.1097/01.ju.0000131908.19114.d3 [DOI] [PubMed] [Google Scholar]
- 4.Burnet FM. Immunological surveillance in neoplasia. Transplant Rev 1971;7:3-25. [DOI] [PubMed] [Google Scholar]
- 5.Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002;3:991-8. 10.1038/ni1102-991 [DOI] [PubMed] [Google Scholar]
- 6.O’Grady JG, Burroughs A, Hardy P, et al. Tacrolimus versus microemulsified ciclosporin in liver transplantation: the TMC randomised controlled trial. Lancet 2002;360:1119-25. 10.1016/S0140-6736(02)11196-2 [DOI] [PubMed] [Google Scholar]
- 7.Cole WH. The increase in immunosuppression and its role in the development of malignant lesions. J Surg Oncol 1985;30:139-44. 10.1002/jso.2930300303 [DOI] [PubMed] [Google Scholar]
- 8.Campistol JM, Cuervas-Mons V, Manito N, et al. New concepts and best practices for management of pre- and post-transplantation cancer. Transplant Rev (Orlando) 2012;26:261-79. 10.1016/j.trre.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 9.Yan L, Chen P, Chen EZ, et al. Risk of bladder cancer in renal transplant recipients: a meta-analysis. Br J Cancer 2014;110:1871-7. 10.1038/bjc.2014.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Q, Yan L, Xu C, et al. Increased incidence of head and neck cancer in liver transplant recipients: a meta-analysis. BMC Cancer 2014;14:776. 10.1186/1471-2407-14-776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karamchandani D, Arias-Amaya R, Donaldson N, et al. Thyroid cancer and renal transplantation: a meta-analysis. Endocr Relat Cancer 2010;17:159-67. 10.1677/ERC-09-0191 [DOI] [PubMed] [Google Scholar]
- 12.Sint Nicolaas J, de Jonge V, Steyerberg EW, et al. Risk of colorectal carcinoma in post-liver transplant patients: a systematic review and meta-analysis. Am J Transplant 2010;10:868-76. 10.1111/j.1600-6143.2010.03049.x [DOI] [PubMed] [Google Scholar]
- 13.Department of Health and Human Services, Health Resources and Services Administration. 2008 Annual Report of the US Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1998-2007. Rockville, MD: US Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; 2008. [Google Scholar]
- 14.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 17.Hedges LV, Olkin I. Statistical Methods for Meta-analysis. London, UK: Academic Press; 1985. [Google Scholar]
- 18.Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech Bull 1999;8:15-7. [Google Scholar]
- 19.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 20.Egger M, Smith GD. Bias in location and selection of studies. BMJ 1998;316:61-6. 10.1136/bmj.316.7124.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adami J, Gäbel H, Lindelöf B, et al. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer 2003;89:1221-7. 10.1038/sj.bjc.6601219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Végso G, Toth M, Hidvegi M, et al. Malignancies after renal transplantation during 33 years at a single center. Pathol Oncol Res 2007;13:63-9. 10.1007/BF02893443 [DOI] [PubMed] [Google Scholar]
- 23.Kellerman L, Neugut A, Burke B, et al. Comparison of the incidence of de novo solid malignancies after heart transplantation to that in the general population. Am J Cardiol 2009;103:562-6. 10.1016/j.amjcard.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 24.Wisgerhof HC, van der Geest LG, de Fijter JW, et al. Incidence of cancer in kidney-transplant recipients: a long-term cohort study in a single center. Cancer Epidemiol 2011;35:105-11. 10.1016/j.canep.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 25.Tessari G, Naldi L, Boschiero L, et al. Incidence of primary and second cancers in renal transplant recipients: a multicenter cohort study. Am J Transplant 2013;13:214-21. 10.1111/j.1600-6143.2012.04294.x [DOI] [PubMed] [Google Scholar]
- 26.Taborelli M, Piselli P, Ettorre GM, et al. Risk of virus and non-virus related malignancies following immunosuppression in a cohort of liver transplant recipients. Italy, 1985-2014. Int J Cancer 2018. [Epub ahead of print]. 10.1002/ijc.31552 [DOI] [PubMed] [Google Scholar]
- 27.Birkeland SA, Storm HH, Lamm LU, et al. Cancer risk after renal transplantation in the Nordic countries, 1964-1986. Int J Cancer 1995;60:183-9. 10.1002/ijc.2910600209 [DOI] [PubMed] [Google Scholar]
- 28.Kasiske BL, Snyder JJ, Gilbertson DT, et al. Cancer after kidney transplantation in the United States. Am J Transplant 2004;4:905-13. 10.1111/j.1600-6143.2004.00450.x [DOI] [PubMed] [Google Scholar]
- 29.Collett D, Mumford L, Banner NR, et al. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant 2010;10:1889-96. 10.1111/j.1600-6143.2010.03181.x [DOI] [PubMed] [Google Scholar]
- 30.Vajdic CM, Mcdonald SP, Mccredie MR, et al. Cancer incidence before and after kidney transplantation. JAMA 2006;296:2823-31. 10.1001/jama.296.23.2823 [DOI] [PubMed] [Google Scholar]
- 31.Villeneuve PJ, Schaubel DE, Fenton SS, et al. Cancer incidence among Canadian kidney transplant recipients. Am J Transplant 2007;7:941-8. 10.1111/j.1600-6143.2007.01736.x [DOI] [PubMed] [Google Scholar]
- 32.Webster AC, Craig JC, Simpson JM, et al. Identifying high risk groups and quantifying absolute risk of cancer after kidney transplantation: a cohort study of 15,183 recipients. Am J Transplant 2007;7:2140-51. 10.1111/j.1600-6143.2007.01908.x [DOI] [PubMed] [Google Scholar]
- 33.Aberg F, Pukkala E, Hockerstedt K, et al. Risk of malignant neoplasms after liver transplantation: a population-based study. Liver Transpl 2008;14:1428-36. 10.1002/lt.21475 [DOI] [PubMed] [Google Scholar]
- 34.Jiang Y, Villeneuve PJ, Fenton SS, et al. Liver transplantation and subsequent risk of cancer: findings from a Canadian cohort study. Liver Transpl 2008;14:1588-97. 10.1002/lt.21554 [DOI] [PubMed] [Google Scholar]
- 35.Jiang Y, Villeneuve PJ, Wielgosz A, et al. The incidence of cancer in a population-based cohort of Canadian heart transplant recipients. Am J Transplant 2010;10:637-45. 10.1111/j.1600-6143.2009.02973.x [DOI] [PubMed] [Google Scholar]
- 36.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 2011;306:1891-901. 10.1001/jama.2011.1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheung CY, Lam MF, Chu KH, et al. Malignancies after kidney transplantation: Hong Kong Renal Registry. Am J Transplant 2012;12:3039-46. 10.1111/j.1600-6143.2012.04209.x [DOI] [PubMed] [Google Scholar]
- 38.Li WH, Chen YJ, Tseng WC, et al. Malignancies after renal transplantation in Taiwan: a nationwide population-based study. Nephrol Dial Transplant 2012;27:833-9. 10.1093/ndt/gfr277 [DOI] [PubMed] [Google Scholar]
- 39.Sampaio MS, Cho YW, Qazi Y, et al. Posttransplant malignancies in solid organ adult recipients: an analysis of the U.S. National Transplant Database. Transplantation 2012;94:990-8. 10.1097/TP.0b013e318270bc7b [DOI] [PubMed] [Google Scholar]
- 40.Kaneko J, Sugawara Y, Tamura S, et al. De novo malignancies after adult-to-adult livingdonor liver transplantation with a malignancy surveillance program: comparison with a Japanese population-based study. Transplantation 2013;95:1142-7. 10.1097/TP.0b013e318288ca83 [DOI] [PubMed] [Google Scholar]
- 41.Piselli P, Serraino D, Segoloni GP, et al. Risk of de novo cancers after transplantation: results from a cohort of 7217 kidneytransplant recipients, Italy 1997-2009. Eur J Cancer 2013;49:336-44. 10.1016/j.ejca.2012.09.013 [DOI] [PubMed] [Google Scholar]
- 42.Krynitz B, Edgren G, Lindelof B, et al. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008-a Swedish population-based study. Int J Cancer 2013;132:1429-38. 10.1002/ijc.27765 [DOI] [PubMed] [Google Scholar]
- 43.Na R, Grulich AE, Meagher NS, et al. Comparison of de novo cancer incidence in Australian liver, heart and lung transplant recipients. Am J Transplant 2013;13:174-83. 10.1111/j.1600-6143.2012.04302.x [DOI] [PubMed] [Google Scholar]
- 44.Maisonneuve P, Marshall BC, Knapp EA, et al. Cancer risk in cystic fibrosis: a 20-year nationwide study from the United States. J Natl Cancer Inst 2013;105:122-9. 10.1093/jnci/djs481 [DOI] [PubMed] [Google Scholar]
- 45.Secnikova Z, Gopfertova D, Hoskovac L, et al. Significantly higher incidence of skin cancer than other malignancies in patients after heart transplantation. A retrospective cohort study in the Czech Republic. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2015;159:648-51. 10.5507/bp.2015.011 [DOI] [PubMed] [Google Scholar]
- 46.Heo J, Noh OK, Oh YT, et al. Cancer risk after renal transplantation in South Korea: a nationwide population-based study. BMC Nephrol 2018;19:311. 10.1186/s12882-018-1110-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jäämaa-Holmberg S, Salmela B, Lemström K, et al. Cancer incidence and mortality after heart transplantation-A population-based national cohort study. Acta Oncol 2019;58:859-63. 10.1080/0284186X.2019.1580385 [DOI] [PubMed] [Google Scholar]
- 48.Zeier M, Hartschuh W, Wiesel M, et al. Malignancy after renal transplantation. Am J Kidney Dis 2002;39:E5. 10.1053/ajkd.2002.29926 [DOI] [PubMed] [Google Scholar]
- 49.Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007;370:59-67. 10.1016/S0140-6736(07)61050-2 [DOI] [PubMed] [Google Scholar]
- 50.Piselli P, Busnach G, Fratino L, et al. Immunosuppression and Cancer Study Group . De novo malignancies after organ transplantation: focus on viral infections. Curr Mol Med 2013;13:1217-27. 10.2174/15665240113139990041 [DOI] [PubMed] [Google Scholar]
- 51.Grulich AE, Vajdic CM. The epidemiology of cancers in human immunodeficiency virus infection and after organ transplantation. Semin Oncol 2015;42:247-57. 10.1053/j.seminoncol.2014.12.029 [DOI] [PubMed] [Google Scholar]
- 52.Hoffmann CJ, Subramanian AK, Cameron AM, et al. Incidence and risk factors for hepatocellular carcinoma after solid organ transplantation. Transplantation 2008;86:784-90. 10.1097/TP.0b013e3181837761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Busnach G, Piselli P, Arbustini E, et al. Immunosuppression and Cancer Study Group . Immunosuppression and cancer: A comparison of risks in recipients of organ transplants and in HIV-positive individuals. Transplant Proc 2006;38:3533-5. 10.1016/j.transproceed.2006.10.144 [DOI] [PubMed] [Google Scholar]
- 54.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med 2003;348:1681-91. 10.1056/NEJMra022137 [DOI] [PubMed] [Google Scholar]
- 55.Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008;319:1096-100. 10.1126/science.1152586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hrbacek J, Urban M, Hamsikova E, et al. Thirty years of research on infection and prostate cancer: no conclusive evidence for a link. A systematic review. Urol Oncol 2013;31:951-65. 10.1016/j.urolonc.2012.01.013 [DOI] [PubMed] [Google Scholar]
- 57.Guba M, Graeb C, Jauch KW, et al. Pro- and anti-cancer effects of immunosuppressive agents used in organ transplantation. Transplantation 2004;77:1777-82. 10.1097/01.TP.0000120181.89206.54 [DOI] [PubMed] [Google Scholar]
- 58.Hall EC, Segev DL, Engels EA. Racial/ethnic differences in cancer risk after kidney transplantation. Am J Transplant 2013;13:714-20. 10.1111/ajt.12066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Venkateswaran V, Klotz LH. Diet and prostate cancer: mechanisms of action and implications for chemoprevention. Nat Rev Urol 2010;7:442-53. 10.1038/nrurol.2010.102 [DOI] [PubMed] [Google Scholar]
- 60.Cohen JH, Kristal AR, Stanford JL. Fruit and vegetable intakes and prostate cancer risk. J Natl Cancer Inst 2000;92:61-8. 10.1093/jnci/92.1.61 [DOI] [PubMed] [Google Scholar]
- 61.Kirsh VA, Peters U, Mayne ST, et al. Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Prospective study of fruit and vegetable intake and risk of prostate cancer. J Natl Cancer Inst 2007;99:1200-9. 10.1093/jnci/djm065 [DOI] [PubMed] [Google Scholar]
- 62.Kolonel LN, Hankin JH, Whittemore AS, et al. Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. Cancer Epidemiol Biomarkers Prev 2000;9:795-804. [PubMed] [Google Scholar]
- 63.Huncharek M, Haddock KS, Reid R, et al. Smoking as a risk factor for prostate cancer: a meta-analysis of 24 prospective cohort studies. Am J Public Health 2010;100:693-701. 10.2105/AJPH.2008.150508 [DOI] [PMC free article] [PubMed] [Google Scholar]