Abstract
A therapeutic strategy that targets multiple proinflammatory factors in inflammatory bowel disease (IBD) with minimal systemic side effects would be attractive. Here, we develop a drug-free, biodegradable nanomedicine that acts against IBD by scavenging proinflammatory cell-free DNA (cfDNA) and reactive oxygen species (ROS). Polyethylenimine (PEI) was conjugated to antioxidative diselenide-bridged mesoporous organosilica nanoparticles (MONs) to formulate nanoparticles (MON-PEI) that exhibited high cfDNA binding affinity and ROS-responsive degradation. In ulcerative colitis and Crohn’s disease mouse colitis models, orally administered MON-PEI accumulated preferentially in the inflamed colon and attenuated colonic and peritoneal inflammation by alleviating cfDNA- and ROS-mediated inflammatory responses, allowing a reduced dose frequency and ameliorating colitis even after delayed treatment. This work suggests a new nanomedicine strategy for IBD treatment.
A drug-free nanomedicine ameliorates IBD by scavenging proinflammatory cell-free DNA and ROS.
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic disorder instigated and amplified by complex interactions between genetic, environmental, and immune factors (1–3). During intestinal inflammation, the innate immune system recognizes and responds to danger signals such as pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), and reactive oxygen species (ROS) (4, 5). These danger signals trigger proinflammatory signaling cascades that disturb tissue homeostasis in the intestinal mucosa and drive the progression of colitis (6). Developing effective therapies for IBD is challenging due to the complexity of the inflammatory microenvironment in the intestinal mucosa, due to a subpopulation of patients who are or become unresponsive to a single anti-inflammatory treatment (7). Moreover, frequent, long-term use of small-molecule therapeutics or biologics-based immunosuppressive drugs can result in off-target systemic side effects and serious complications including autoimmunity, opportunistic infections, and organ damage (8). A “drug-free” therapeutic strategy without including any small-molecule therapeutics or biologics-based immunosuppressive drugs involves scavenging of multiple danger signals. This simple design may reduce toxicity and ease eventual translation.
There is growing evidence that abnormal activation of Toll-like receptors (TLRs) on effector immune cells drives the overproduction of inflammatory mediators that promote IBD (9, 10). Cell-free DNA (cfDNA) released by damaged cells and/or gut microbes activates TLR9-mediated proinflammatory signaling in immune cells such as resident macrophages and contributes to the magnitude and duration of inflammation (11, 12). cfDNA is a prognostic biomarker of IBD. In this context, we reasoned that neutralization of cfDNA could modulate the imbalanced immune response during IBD.
Soluble cationic polymers and insoluble cationic nanoparticles have been used as nucleic acid carriers for gene delivery (13). Encouraged by these findings, we develop a variety of nucleic acid–binding polymers (NABPs) that scavenge proinflammatory cfDNA and prevent TLR activation in immune cells (14) and exhibit therapeutic activity against inflammatory diseases such as sepsis (15), psoriasis (16), liver failure (17), cancer metastasis (18), and influenza infection (19). Like these soluble NABPs, insoluble nucleic acid–binding nanoparticles (NABNs) also scavenge cfDNA and reduce TLR9 activation after intracellular internalization into endosomal compartments where TLR9 resides. However, unlike soluble NABPs, NABNs preferentially accumulate in inflamed tissues, allowing greater therapeutic activity and lower systemic toxicity (15, 20). We previously fabricated diselenide-bridged mesoporous organosilica nanoparticles (MONs) (21, 22), a biodegradable and biocompatible silica material with potent anti-oxidative capacity, as silica has been classified as Generally Recognized as Safe (GRAS) list of the U.S. Food and Drug Administration.
With these findings in mind, we hypothesized that a combination strategy that involves scavenging two disparate proinflammatory factors, cfDNA and ROS, would be beneficial for IBD treatment. Here, we develop a cfDNA- and ROS-scavenging nanoparticulate by conjugating DNA binding polyethylenimine (PEI; 25 kDa) to antioxidative diselenide-bridged MON (MON-PEI). We characterize the cfDNA-scavenging, antioxidative, and anti-inflammatory properties of MON-PEI in vitro, and the effects of MON-PEI relative to free PEI and unmodified MON on inflammation and colonic injury in two murine colitis models—dextran sulfate sodium (DSS)–induced acute colitis and 2,4,6-trinitrobenzenesulfonic acid (TNBS)–induced chronic colitis. We optimize the route, dose, and timing of administration to achieve the best therapeutic outcomes in these colitis models. Further, we identify the role of MON-PEI in reducing cfDNA-induced TLR9–MyD88–nuclear factor κB (NF-κB) signaling and proinflammatory macrophage activation to mitigate inflammation and colitis. Preferential accumulation of orally administered MON-PEI in the inflamed colon allowed a lower dose frequency with a better safety profile than mesalazine, a widely used first-line IBD drug. Our study provides a proof of concept for the use of drug-free, cfDNA- and ROS-scavenging nanomedicine to prevent and treat IBD.
RESULTS
Serum cfDNA level and colonic TLR9 expression are elevated in IBD patients
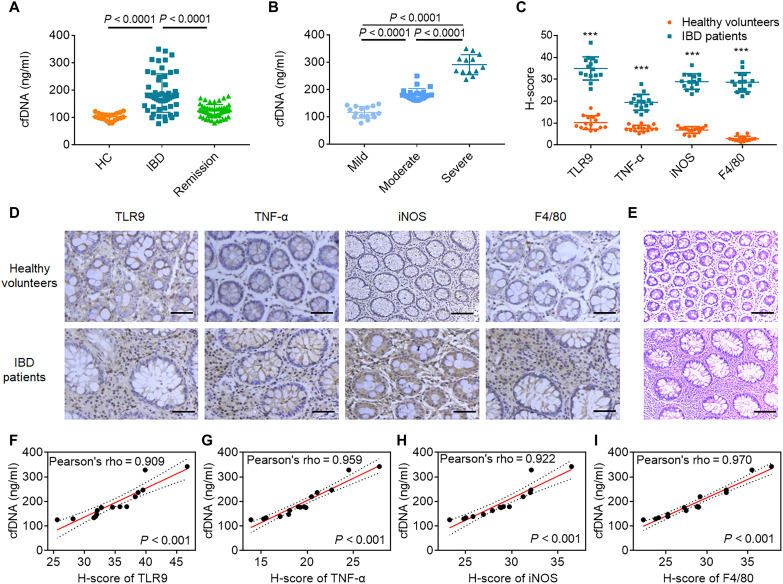
We prospectively recruited and collected serum from 52 IBD patients and 20 healthy volunteers (table S1). Active IBD patients showed significantly higher serum levels of cfDNA than healthy volunteers and IBD patients in remission (Fig. 1A). Patients with acute severe IBD had the highest serum cfDNA levels (Fig. 1B). Gender and age did not correlate with serum cfDNA levels (fig. S1). We performed hematoxylin and eosin (H&E) and immunohistochemical staining for TLR9, tumor necrosis factor–α (TNF-α), inducible nitric oxide synthase (iNOS), and F4/80 in colonic resection specimens (Fig. 1, C to E). We observed a higher frequency of lamina propria TLR9+ cells and higher expression of the proinflammatory markers TNF-α and iNOS and the macrophage marker F4/80 in the colonic immune cells of IBD patients than in those of healthy volunteers. Furthermore, serum cfDNA levels were strongly correlated with expression levels of colonic TLR9, TNF-α, iNOS, and F4/80 (Fig. 1, F to I). Higher serum cfDNA levels were collectively associated with IBD severity and colonic TLR9 expression, pointing to cfDNA-TLR9 signaling as a target for IBD treatment.
Fig. 1. Relationship between cfDNA and colonic inflammation in IBD patients.
(A) Serum levels of cfDNA in healthy volunteers (n = 20) and IBD patients (n = 52) with active IBD or in remission. (B) Serum levels of cfDNA in patients with IBD of different grades were determined using Truelove and Witts criteria (n = 52). In (A) and (B), data are means ± SEM; P values were assessed using Student’s t test with Tukey’s multiple comparison test. (C) Quantification of TLR9, TNF-α, iNOS, and F4/80 protein expression in healthy volunteers and IBD patients. Data are means ± SEM (n = 15; *P < 0.05, **P < 0.01, and ***P < 0.001 by Student’s t test). (D) Images of colon sections stained for TLR9, TNF-α, iNOS, and F4/80. (E) Images of H&E-stained colon sections. In (D) and (E), the scale bars represent 100 μm. (F to I) Linear regression curves illustrating the correlation between serum cfDNA level and (F) TLR9, (G) TNF-α, (H) iNOS, and (I) F4/80 protein expression in colon sections of IBD patients. In (D) to (I), data are means ± SEM (n = 15; *P < 0.05, **P < 0.01, and ***P < 0.001 by Student’s t test).
cfDNA- and ROS-scavenging MON-PEI reduce inflammation in vitro
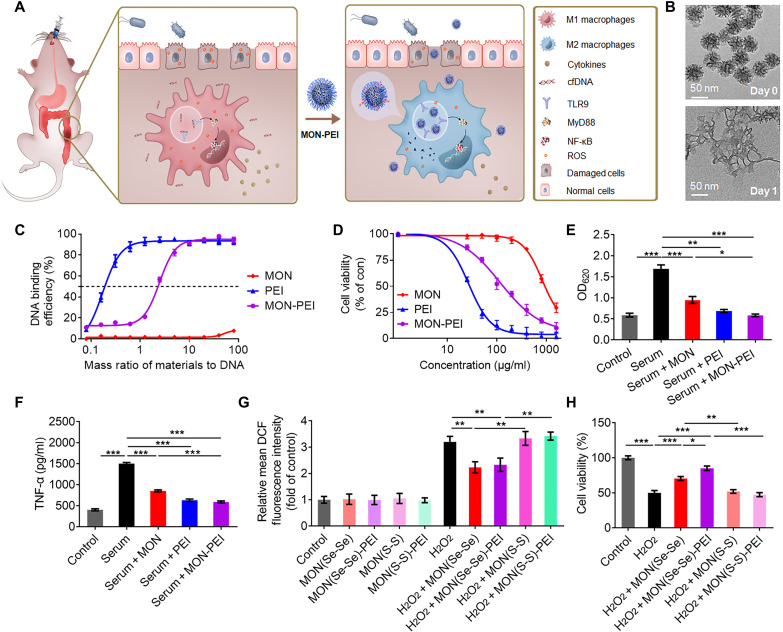
We prepared diselenide-bridged MONs (50 nm diameter, 7 nm pore size), which have previously exhibited redox-responsive matrix degradability and a controlled drug release profile (21, 22). These MONs exhibited ROS-responsive degradation (Fig. 2B and fig. S2), indicating their potential for ROS binding and scavenging. Next, we conjugated PEI [number-average molecular weight (Mn) of 25 kDa] onto the surface of the MON to endow the particles with a positive charge and good stability in simulated gastric fluid (fig. S2 and table S2). Both free PEI and MON-PEI, but not unmodified MON, exhibited a high binding affinity for calf thymus DNA (ct-DNA) (Fig. 2C). MON-PEI had a slightly lower DNA binding affinity than PEI but caused significantly less cytotoxicity than free PEI in colonic epithelial cells (Caco-2) (Fig. 2D) and in human embryonic kidney (HEK)–TLR9 reporter cells and RAW 264.7 macrophages (fig. S3). In HEK-TLR9 reporter cells, both free PEI and MON-PEI significantly inhibited TLR9 activation induced by CpG oligodeoxynucleotides (fig. S3) and by IBD patient serum (Fig. 2E). Free PEI and MON-PEI also reduced TNF-α secretion by danger signal–activated macrophages (Fig. 2F and fig. S3). Unmodified MON also reduced TLR9 activation and proinflammatory cytokine secretion. Since ROS play a crucial role in amplifying TLR-mediated inflammation (5), we compared the ROS-scavenging capability of diselenide-bridged MON with that of disulfide-bridged MON and found that diselenide-bridged MON exhibited significantly greater ROS-scavenging activity in H2O2-challenged Caco-2 cells (Fig. 2G) and lipopolysaccharide (LPS)–challenged RAW 264.7 cells (fig. S3). Diselenide-bridged MON and MON-PEI protected Caco-2 and RAW 264.7 cells from H2O2-mediated cytotoxicity; their disulfide-bridged counterparts did not (Fig. 2H and fig. S3). Notably, diselenide-bridged MON and MON-PEI attenuated LPS-induced TLR4 activation and proinflammatory cytokine secretion (fig. S3), indicating their anti-inflammatory capacity due to inhibition of TLR activation. In addition, MON and MON-PEI could scavenge reactive sulfur species and partly reverse the Na2S4-induced cell death (fig. S3).
Fig. 2. MON-PEI reduces cfDNA- and ROS-induced inflammation in vitro.
(A) Schematic of the design of a biodegradable nanomedicine with cfDNA- and ROS-scavenging activity for IBD therapy. (B) Transmission electron microscopy images of MON before and after a 1-day incubation in simulated body fluid solution containing 100 μM H2O2. (C) DNA binding efficiency of MON, PEI, and MON-PEI at different nanoparticle:DNA mass ratios at 37°C. (D) Viability of Caco-2 cells treated for 24 hours with various concentrations of MON, PEI, and MON-PEI. (E) Activation of HEK-TLR9 reporter cells by IBD patient sera in the absence or presence of MON, PEI, and MON-PEI for 24 hours. The corresponding SEAP activity in supernatants from each group was determined with a QUANTI-Blue assay at OD620. (F) RAW 264.7 macrophages were stimulated with IBD patient sera in the absence or presence of MON, PEI, and MON-PEI for 24 hours. Supernatants were assayed for TNF-α by ELISA. (G) Relative fluorescence intensity of oxidized DCF in Caco-2 cells after incubation with different formulations in the presence or absence of 100 μM H2O2 for 4 hours. (H) The viability of Caco-2 cells was measured after treatment with different formulations in the presence of 100 μM H2O2 for 24 hours. Data are means ± SEM (n = 3 independent experiments; *P < 0.05, **P < 0.01, and ***P < 0.001 by one-way ANOVA with Tukey’s multiple comparison test).
Oral administration of MON-PEI ameliorates acute colitis in mice
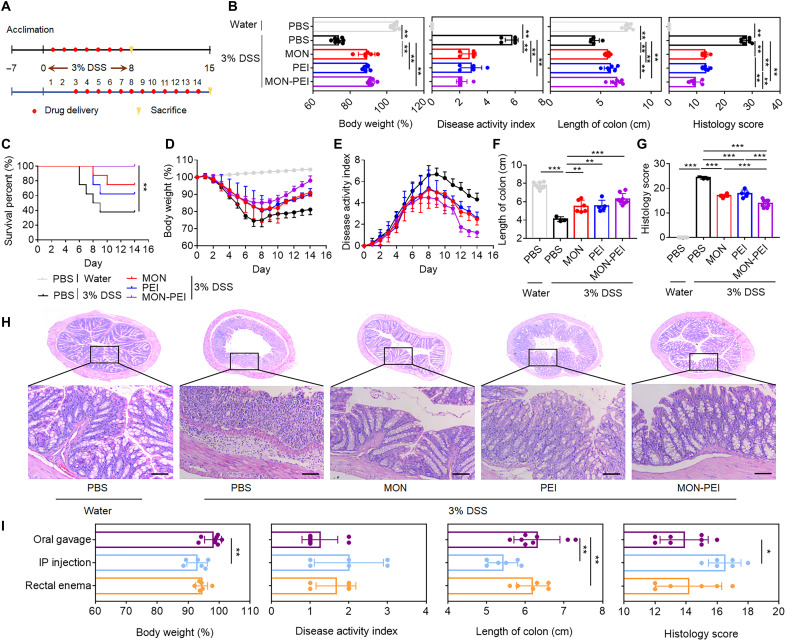
We investigated the effects of MON-PEI on DSS-induced acute colitis in mice. C57BL/6 mice were given 3% DSS in drinking water for 7 days (Fig. 3A). For the preventive setting, treatments were administered daily starting on day 1. Oral administration of MON, PEI, and MON-PEI significantly protected mice against DSS-induced body weight loss, increased disease activity index (DAI), reduced colon length, colonic tissue damage, and inflammation at day 7 versus phosphate-buffered saline (PBS)–only treatment (Fig. 3B and fig. S4). For the therapeutic setting, treatments were administered daily starting on day 3 (Fig. 3A). As in the preventive setting, therapeutic administration of MON, PEI, and MON-PEI increased survival rates (Fig. 3C), reduced body weight loss (Fig. 3D), and reduced DAI values (Fig. 3E). MON, PEI, and MON-PEI also substantially reversed DSS-induced shortening of the colon at day 14 (Fig. 3G and fig. S5) and alleviated colonic damage as indicated by reduced epithelial disruption, goblet cell depletion, and granulocyte infiltration (Fig. 3, G and H, and fig. S5). Although the effects of MON-PEI were similar to those of MON and PEI for prevention, when administered on day 3 to treat acute colitis, MON-PEI showed greater efficacy than MON or PEI, including greater body weight recovery, lower DAI, reduced shortening of the colon, and less colonic damage.
Fig. 3. MON-PEI ameliorates DSS-induced acute colitis in vivo.
(A) Experimental scheme for DSS-induced acute colitis and preventive and therapeutic administration of scavengers (MON, PEI, and MON-PEI). (B) For the preventive setting, body weight, DAI, colon length, and colonic damage scores of mice in each group via oral gavage were determined at day 7. (C to H) For the therapeutic setting, daily (C) survival rate, (D) body weight, and (E) DAI value changes of mice in each group via oral gavage were monitored. (F) Colon length and (G) colonic damage scores of mice of indicated groups were determined at day 14. (H) Representative images of colon sections of indicated groups were stained with H&E. Scale bars, 100 μm. (I) For the therapeutic setting, body weight, DAI, colon length, and colonic damage scores of MON-PEI–treated mice via oral gavage, intraperitoneal (IP) injection, and rectal enema were determined at day 14. Data are means ± SEM (n = 6 to 8 mice per group; *P < 0.05, **P < 0.01, and ***P < 0.001 by one-way ANOVA with Tukey’s multiple comparison test).
Since the administration route may affect the performance of IBD treatments, we compared the therapeutic efficacy of MON-PEI administered via three routes: oral gavage, intraperitoneal injection, and rectal enema. Intraperitoneal injection (fig. S6) and rectal enema (fig. S7) of MON-PEI significantly mitigated acute colitis symptoms in both preventive and therapeutic models, but oral administration showed the best therapeutic performance in terms of survival rate, body weight, DAI, colon length, and colonic tissue damage (Fig. 3I and fig. S8). To assess the toxicity profile of MON-PEI, we monitored the general health conditions of healthy mice until day 28 after receiving 14 daily doses of MON, PEI, and MON-PEI via oral gavage, intraperitoneal injection, or rectal enema. Body weights, serum biochemical parameters, and histopathology of the heart, liver, spleen, lung, kidney, and colon in each group were not significantly different from those of the control group (fig. S9 to S11). Collectively, oral administration of MON-PEI showed the best preventive and therapeutic effects in acute colitis models, without any observed toxicity.
Rectal enema administration of MON-PEI alleviates TNBS-induced chronic colitis in mice
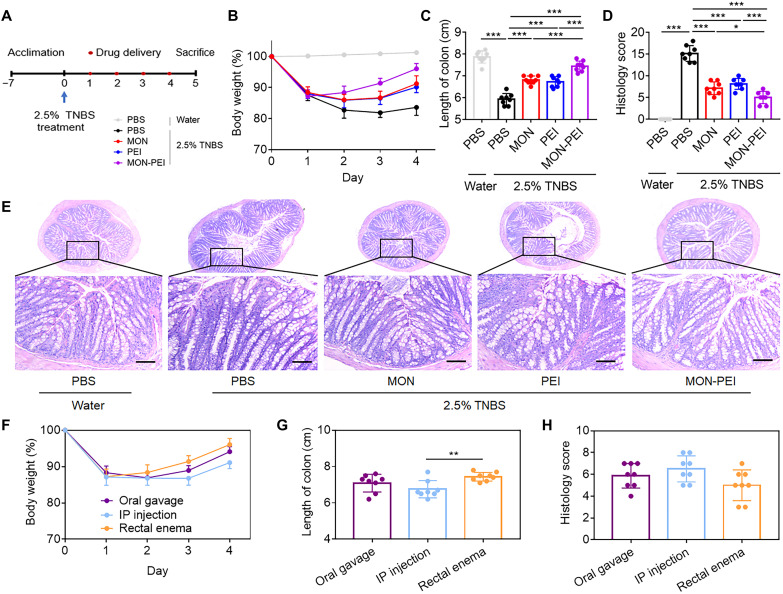
To explore the breadth of potential therapeutic applications of MON-PEI, we evaluated its efficacy against TNBS-induced chronic colitis in mice. C57BL/6 mice were given a 2.5 weight % (wt %) TNBS enema at day 0 (Fig. 4A). Onset of chronic colitis occurred on day 4 as indicated by body weight loss (Fig. 4B), shortening of the colon (Fig. 4C and fig. S12), colonic tissue damage (Fig. 4, D and E), and increased proinflammatory cytokine levels (fig. S13). Enemas of MON, PEI, or MON-PEI to TNBS-challenged mice on three consecutive days (days 1 to 3) demonstrated therapeutic efficacy, with MON-PEI providing more robust protection than PEI or MON against TNBS-induced body weight loss, colon shortening, colonic damage, and inflammation (Fig. 4, B to D). We also investigated the therapeutic efficacy of MON-PEI in this chronic model (day 3 treatment) via oral gavage, intraperitoneal injection, and rectal enema (Fig. 4, F to H, and figs. S14 and S15). Rectal enema of MON-PEI performed marginally better than oral gavage or intraperitoneal injection (fig. S16) in terms of reversal of body weight loss (Fig. 4F), colon shortening (Fig. 4G), and colonic tissue damage (Fig. 4H). Together, these findings indicate the potential of MON-PEI in the chronic colitis model via rectal enema.
Fig. 4. MON-PEI ameliorates TNBS-induced chronic colitis in vivo.
(A) Experimental scheme for TNBS-induced chronic acute colitis and scavenger (MON, PEI, and MON-PEI) administration. (B to D) Daily body weight, (C) colon length, and (D) colonic damage scores of mice in each group via rectal enema were monitored at day 5. (E) Representative images of colon sections of indicated groups via oral gavage were stained with H&E. Scale bars, 100 μm. (F) Body weight, (G) colon length, and (H) colonic damage scores of MON-PEI–treated mice via oral gavage, intraperitoneal injection, and rectal enema were determined at day 5. Data are means ± SEM (n = 6 to 8 mice per group; *P < 0.05, **P < 0.01, and ***P < 0.001 by one-way ANOVA with Tukey’s multiple comparison test).
MON-PEI inhibits macrophage polarization induced by TLR9–MyD88–NF-κB signaling
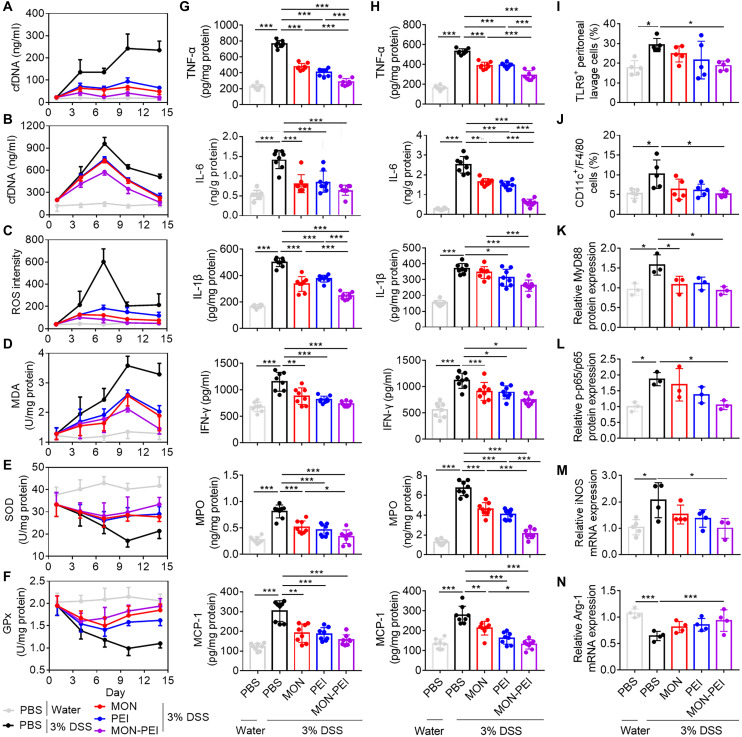
We next sought to decipher the role of MON-PEI in scavenging cfDNA and ROS, and the mechanism of its anti-inflammatory effect in the DSS-induced acute colitis model. cfDNA levels in serum (Fig. 5A) and peritoneum (Fig. 5B) of DSS-challenged mice increased over time. Oral administration of MON-PEI significantly reduced cfDNA levels at each time point. DSS-challenged mice exhibited higher levels of ROS (Fig. 5C) and the oxidative stress marker malondialdehyde (MDA; Fig. 5D) than healthy mice, along with lower activities of the antioxidant enzymes superoxide dismutase (SOD; Fig. 5E) and glutathione peroxidase (GPx; Fig. 5F) in colonic tissue. Treatment with MON-PEI remarkably suppressed the oxidative stress while restoring antioxidant activity in colonic tissue. Peak values of DAI, cfDNA, oxide markers, and cytokines in DSS-challenged mice occurred mainly on day 7; therefore, we selected this time point for studying changes in the immune system. MON-PEI treatment corresponded with a significant reduction at day 7 in colonic (Fig. 5G) and peritoneal (Fig. 5H) levels of TNF-α, interleukin-6 (IL-6), IL-1β, interferon-γ (IFN-γ), myeloperoxidase (MPO), and monocyte chemoattractant protein-1 (MCP-1). Similarly, MON-PEI significantly increased the IL-10 level and decreased the IL-17 level in colonic tissue when compared with the DSS group (fig. S17). In addition, depressed serum selenium levels were found in the model mice, and MON-PEI and MON treatment could significantly restore the selenium levels (fig. S17). Together, MON-PEI scavenged cfDNA, ameliorated oxidative stress, and alleviated inflammation in the acute colitis model.
Fig. 5. MON-PEI inhibits macrophage polarization via TLR9–MyD88–NF-κB signaling.
For the therapeutic DSS-induced acute colitis model, scavengers were administrated daily via oral gavage. Several inflammatory factors were analyzed on days 1, 4, 7, 10, and 14 after DSS challenge. (A) Serum and (B) peritoneal cfDNA levels were analyzed. Colonic (C) ROS level, (D) MDA level, (E) SOD activity, and (F) GPx activity were measured. (G) Colonic and (H) peritoneal TNF-α, IL-6, IL-1β, IFN-γ, MPO, and MCP-1 levels were analyzed on day 7. (I) The number of TLR9+ cells and (J) the percentage of M1-polarized macrophages (CD11c+F4/80+) were assessed in peritoneal lavage fluid by flow cytometry on day 7. (K to N) Peritoneal macrophages were collected on day 7 and lysed in radioimmunoprecipitation assay (RIPA) buffer before analysis of (K) MyD88 and (L) p-p65 protein expression via Western blotting. The data are expressed as fold change relative to the control group and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or p65 protein expression. In parallel, mRNA of indicated macrophages was extracted, converted to cDNA, and analyzed via real-time PCR for (M) iNOS and (N) Arg-1 gene expression. The data are expressed as fold change relative to the PBS-treated normal group and normalized to GAPDH gene expression. Data are means ± SEM (n = 3 to 6 mice per group; *P < 0.05, **P < 0.01, and ***P < 0.001 by one-way ANOVA with Tukey’s multiple comparison test).
Emerging evidence suggests that cfDNA activates TLR9 in immune cells to drive proinflammatory responses (23). We found that the amount of TLR9+ peritoneal and lamina propria cells was remarkably lower in MON-PEI–treated than in untreated DSS-challenged mice (Fig. 5I and fig. S17). Macrophages are the gatekeepers of intestinal immune homeostasis in IBD pathogenesis, and our group and others have previously shown that cfDNA can induce M1 polarization of peritoneal macrophages via the TLR9–MyD88–NF-κB axis (15). Consistent with these findings, we observed a significantly higher portion of proinflammatory CD11c+ F4/80+ monocytes (M1-polarized macrophages) in harvested peritoneal immune cells in untreated versus MON-PEI–treated DSS-challenged mice (Fig. 5J). We isolated peritoneal macrophages to investigate how MON-PEI affects TLR9–MyD88–NF-κB signaling (Fig. 5, K to N, and fig. S17). We found that expression of TLR9 (fig. S17) and MyD88 (Fig. 5K) and the level of NF-κB phosphorylation of p65 (Fig. 5L) were markedly greater in peritoneal macrophages of DSS-challenged mice than in those of healthy mice, and that treatment of DSS-challenged mice with MON-PEI significantly reduced activation of TLR9–MyD88–NF-κB signaling. The M1 polarization markers TNF-α (fig. S17) and iNOS (Fig. 5M) were up-regulated in peritoneal and colonic macrophages of DSS-challenged versus healthy mice but were significantly suppressed in DSS-challenged mice by treatment with MON-PEI. Expression of the M2-polarized marker Arg-1 was also partly restored in DSS-challenged mice by MON-PEI treatment (Fig. 5N). Similarly, MON-PEI treatment remarkably reduced the expression of TLR9, TNF-α, iNOS, and F4/80 in the colonic tissue of DSS-challenged mice (fig. S18). We also found that MON-PEI treatment increased the infiltration of regulatory T (Treg) cells with high expression of FOXP-3 and transforming growth factor–β1 (TGF-β1) in the colonic tissue of DSS-challenged mice (fig. S18). Collectively, MON-PEI exhibited greater anti-inflammatory effects than unmodified MON or free PEI in peritoneal and colonic sites in DSS-challenged mice and reversed M1 polarization of macrophages via the TLR9–MyD88–NF-κB axis during colitis progression.
MON-PEI accumulate in the inflamed colon and suppress colitis after delayed treatment
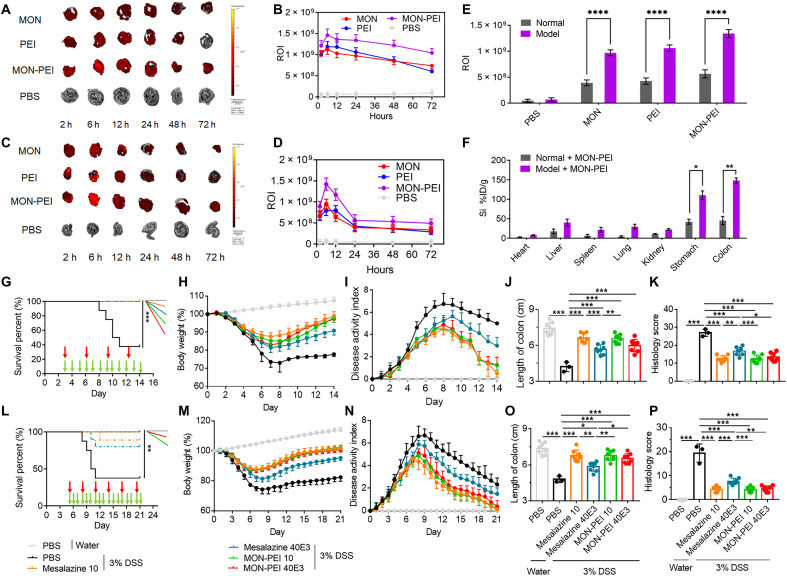
To examine the accumulation of MON-PEI in colonic tissue, mice were either untreated or challenged with 3% DSS for 3 days and then were treated with Cy7-labeled MON, PEI, or MON-PEI via oral administration. All three nanoparticles showed the greatest accumulation in the inflamed colon at 24 hours after oral administration, with colonic retention decreasing after 24 hours (Fig. 6, A to D, and fig. S19). MON-PEI exhibited significantly greater accumulation in the inflamed colon than MON or PEI, and notably, MON-PEI showed greater retention in the inflamed colon of colitis mice than in the normal colon of healthy mice at 24 hours, and more so at 48 and 72 hours—a promising result for long-term administration (Fig. 6E and fig. S19). Similarly, the enhanced accumulation and retention of MON-PEI in the inflamed colon were confirmed by quantitation of silicon in various tissues (Fig. 6F). MON-PEI was located mainly in the colon and stomach, and at lower levels in the liver, spleen, and kidney at 24 hours after oral administration. Together, these results demonstrated preferential accumulation and retention of MON-PEI in the inflamed colon.
Fig. 6. MON-PEI localizes in inflamed colon and shows activity against colitis in a delayed therapeutic setting.
(A) Ex vivo near-infrared fluorescence images of cecum from DSS-challenged mice at 2, 6, 12, 24, 48, and 72 hours after oral delivery of Cy7-labeled MON, PEI, and MON-PEI. (B) Semiquantitative analysis of ex vivo fluorescence images of the cecum in (A). ROI, region of interest. (C) Ex vivo near-infrared fluorescence images of cecum from normal mice at 2, 6, 12, 24, 48, and 72 hours after oral delivery of Cy7-labeled MON, PEI, and MON-PEI. (D) Semiquantitative analysis of ex vivo fluorescence images of the cecum in (C). (E) Semiquantitative analysis of ex vivo fluorescence images of the cecum in (B) and (D) at 24 hours. (F) Quantification analysis of silicon content in the heart, liver, spleen, lung, kidney, stomach, and cecum of control or colitis mice via ICP-OES after oral delivery of MON-PEI for 24 hours. (G to K) DSS-challenged mice were orally administered with different doses of MON-PEI or mesalazine via different administration frequency starting on day 3. (G) Survival rate, (H) body weight, and (I) DAI changes of mice in each group via oral gavage were monitored. (J) Colon length and (K) colonic damage scores of mice of indicated groups were determined on day 14. (L to P) DSS-challenged mice were orally administered with different doses of MON-PEI or mesalazine via different administration frequency starting on day 5. (L) Survival rate, (M) body weight, and (N) DAI changes of mice in each group via oral gavage were monitored. (O) Colon length and (P) colonic damage scores of mice of indicated groups were determined on day 21. Data are means ± SEM (n = 6 to 8 mice per group; *P < 0.05, **P < 0.01, and ***P < 0.001 by one-way ANOVA with Tukey’s multiple comparison test).
To optimize the dose and timing of oral administration of MON-PEI, we first investigated the dose-dependent efficacy of MON-PEI in acute colitis mice (fig. S20). Treatment with MON-PEI at a dose of over 10 mg/kg exhibited therapeutic activity. Encouraged by the long-term retention of MON-PEI in the inflamed colon, we investigated how the frequency of administration affects therapeutic outcomes in colitis mice (figs. S21 to S23). Oral administration of MON-PEI every other day and every 3 days exhibited weaker therapeutic effects than daily treatment at the same dose per administration. Notably, the gap between the effect of administration daily versus every 3 days was reduced as the dose increased, suggesting the possibility of administering a high dose of MON-PEI with a long interval. We next compared the efficacy of MON-PEI with that of mesalazine (5-aminosalicyclic acid), a widely used first-line drug used to treat IBD. Specially, we compared the two formulations at 10 mg/kg daily and 40 mg/kg every 3 days at a similar total dose. Oral administration of MON-PEI at 10 mg/kg daily and at 40 mg/kg every 3 days showed equal therapeutic activity in terms of recovery of survival rate, body weight, and DAI; maintenance of colon length; and reduction of colonic tissue damage (Fig. 6, G to K, and fig. S24). Treatment with mesalazine at 10 mg/kg daily exhibited similar therapeutic performance as treatment with MON-PEI; however, treatment with mesalazine (40 mg/kg) every 3 days showed no significant activity in terms of preventing acute colitis.
Since many IBD patients exhibit a severe form of the disease degree after diagnosis, we examined the efficacy of MON-PEI in a setting of delayed therapy—treatment at day 5 for DSS-challenged mice, at which time point the mice exhibit a high DAI and low survival rate. Animals treated with MON-PEI at day 5 were remarkably protected against DSS-induced death, body weight loss, increased DAI, colon shortening, and colonic damage (Fig. 6, L to P, and fig. S24). Consistent with the previous mesalazine results, only daily administered mesalazine provided potent protection from colitis. Furthermore, daily administration of MON-PEI even at a high dose (40 mg/kg) for 21 days did not cause any obvious systemic toxicity in normal mice (fig. S25). Together, reducing the dose frequency of MON-PEI improved the delayed treatment of severe colitis without systemic toxicity.
DISCUSSION
IBDs such as ulcerative colitis and Crohn’s disease involve incurable intestinal inflammation and require lifelong treatment (1). The steadily increasing incidence of IBD worldwide has created an urgent need for more effective therapies. Identifying danger signals that play a crucial role in IBD pathogenesis is essential for developing new therapies. IBD patients often have elevated serum mitochondrial DNA due to its release by damaged cells (11). We found that serum cfDNA was elevated in IBD patients and in DSS-induced colitis mice, and that the level of cfDNA was associated with disease severity and DAI. Lower levels of cfDNA in serum and peritoneum in DSS-induced colitis mice were associated with remission of disease activity and lower colonic inflammation, consistent with the use of serum cfDNA as an IBD biomarker. We previously reported that cationic NABPs and nanoparticles have anti-inflammatory activity (15, 20). Here, we found that cationic MON-PEI bind cfDNA with high affinity and suppress cfDNA-mediated TLR9 activation and proinflammatory macrophage activation. MON-PEI significantly reduced serum and peritoneal cfDNA levels in the colitis model. Although PEI or cationic materials could be selected as gene carriers and cfDNA scavengers due to the high DNA binding capacity (24, 25), gene delivery requires high DNA release, while scavenging applications necessitates low DNA release. We speculated that MON-PEI might bind cfDNA to form a complex, which the macrophages will take to facilitate the degradation without inducing proinflammatory responses (15, 20). Together with the decrease of TLR9+ peritoneal cells in DSS-challenged mice, our results revealed that MON-PEI treatment inhibits the proinflammatory polarized macrophage phenotype in peritoneum and colon via inhibition of TLR9–MyD88–NF-κB signaling.
In addition to damage-associated molecular patterns such as cfDNA, ROS also play a crucial role in mucosal damage and chronic intestinal inflammation in IBD (5, 26). External and internal stimuli induce the formation of ROS, resulting in lipid peroxidation and apoptosis and colonic mucosal damage (27). Neutrophils, monocytes, eosinophils, and macrophages in colon tissues are activated by injury signals in IBD to produce ROS and cytokines, which act as inflammatory mediators to promote a proinflammatory response. Therefore, therapeutic strategies that target oxidative stress signaling and ROS scavenging are promising for IBD prevention and treatment (28). Several nanoparticles with antioxidative properties have recently been explored as potential therapeutics against inflammatory diseases (29–34). A variety of ROS-scavenging and ROS-responsive nanoparticles have been developed for antioxidative IBD therapy and controlled drug release (30–34). Here, we used MON with good biodegradability and with a high surface area for greater PEI conjugation and cfDNA binding. Our group recently developed diselenide-bridged MON with a large pore size and a ROS-degradable matrix for controlled drug release (35, 36) . We hypothesized that these MON may serve not only as a core for PEI functionalization to scavenge cfDNA but also as a ROS scavenger via cleavage of the diselenide bonds and subsequent depletion of ROS. Here, we present data supporting this hypothesis and show that a low dose of MON-PEI significantly reduces intracellular ROS levels and prevents ROS-mediated damage of immune and intestinal cells. We found that diselenide-bridged, rather than disulfide-bridged, MON could inhibit the cfDNA-mediated TLR9 activation and LPS-mediated TLR4 activation in macrophages. It is recognized that ROS can activate IKKs (inhibitor of NF-κB kinases) and/or inhibit phospho-tyrosine and phospho-serine/threonine phosphatases to up-regulate redox-sensitive NF-κB, further exacerbating the TLR9–MyD88–NF-κB axis–mediated inflammation (37). We attributed this phenomenon to the fact that the ROS-scavenging property of the diselenide-bridged MON can reduce activation of ROS-exacerbated TLR-based proinflammatory cascades. Aligning with our in vitro data, these ROS scavengers significantly alleviated oxide stress, reversed antioxidant activity in the inflamed colon, and eventually suppressed the proinflammatory state in colitis mice. On the basis of these findings, diselenide-bridged MON offered a convenient yet powerful platform for regulating other inflammatory diseases of the gastrointestinal (GI) tract.
In light of these findings, we propose that combining PEI and MON to form a dual scavenger of cfDNA and ROS will have considerable benefits for treating colitis. Here, we demonstrated that MON-PEI exhibits better performance than the single scavengers against serum-, CpG-, and LPS-induced TLR activation and inflammation. MON-PEI had better therapeutic outcomes than free PEI or unmodified MON in DSS-induced acute colitis and TNBS-induced chronic colitis mice. MON-PEI exerted greater immunosuppression in inflamed peritoneal and colonic sites than PEI or MON. MON-PEI regulated peritoneal and colonic macrophage polarization in acute colitis mice via two pathways: (i) MON-PEI blocks cfDNA-induced TLR9–MyD88–NF-κB signaling; (ii) MON-PEI reduces the ROS-mediated proinflammatory immune response. We found that free PEI ameliorated oxidative stress in the inflamed colon of colitis mice, possibly because scavenging cfDNA via free PEI might reduce the cfDNA-mediated inflammation-induced oxidative damage. Meanwhile, the reduced cfDNA level in colitis mice treated with diselenide-crosslinked MON may have been due to ROS scavenging that reduced cell damage and subsequent release of cfDNA. Although TLR9 agonist cobitolimod showed a protective induction of IL-10 and down-regulation of IL-17 in DSS-challenged mice (38), CpG, another TLR9 agonist, exacerbated colitis of DSS-treated mice (39). Given that the TLR9 pathway might play complicated roles in developing and protecting IBDs, our current work has demonstrated that MON-PEI could partly reverse cfDNA-based TLR9 activation in the DSS-challenged mice. We attributed the elevated serum Se concentration after treating mesoporous silica nanoparticles (MSNs) to the fact that the Se-Se bond in biodegradable MSNs could be reduced by GSH [glutathione (reduced form)] to form an active selenol group, which could exchange with S element in GSH or cysteine to form Se-containing small compounds (40). These Se-containing small compounds could be metabolized to selenoproteins, including GPx, thioredoxin reductase (TXNRD 1 to 3), and methionine sulfoxide reductase (Msr), which could protect cells from harmful oxidative free radicals and reduce colonic inflammation (41). Moreover, our previous reports have demonstrated that selenium supplement in the DSS-challenged murine model could alleviate colonic inflammation, which is consistent with the therapeutic effects observed for our diselenide-bridged MSNs in this study (42). The increased infiltrations of Treg cells in the colonic tissue of DSS-challenged mice support the unique advantages of MON-PEI against IBD by regulating multiple immune cells. Because of the complexity of colonic inflammation, these anti-inflammatory mechanisms should be further investigated in other neutrophils and T cells, and in the context of the complex relationship between the gut microbiome and colonic inflammation.
Oral administration and rectal enema are two major routes for clinical IBD drug delivery (1). To optimize the administration route of MON-PEI for clinical translation, we systematically investigated how oral gavage, intraperitoneal injection, and rectal enema affect therapeutic outcomes in two colitis models. We selected the DSS-induced acute colitis model accompanied by ROS-mediated colonic damage, a gold standard for representing ulcerative colitis (37). In this model, oral, intraperitoneal, and rectal administration of MON-PEI exhibited considerable anti-colitis and anti-inflammation effects in both preventive and therapeutic models, but oral gavage showed the best therapeutic efficacy of the three administration routes. Orally administered MON-PEI preferentially accumulated throughout the inflamed colon to achieve a better therapeutic outcome. We also used the commonly used TNBS-induced chronic colitis model. This model has more immunological similarities with Crohn’s disease than with ulcerative colitis (43). Although there was no significant difference between some of the groups, the trend in the rectal enema groups was more robust than that in the oral gavage and intraperitoneal injection group. These model-dependent outcomes may be due to a rectal enema achieving more effective delivery of MON-PEI to colon tissue where TNBS-induced damage is localized. When proposing a new therapy, the safety in nanomedicine is paramount (44). We demonstrated that long-term oral, intraperitoneal, and rectal delivery of MON-PEI at a therapeutic dose did not generate noticeable toxicity, indicating a good safety profile. Together, oral delivery and rectal enema of MON-PEI resulted in safe and efficacious treatment of colitis in ulcerative colitis and Crohn’s disease mouse models, respectively.
Nanomedicine is promising for targeted drug delivery to treat IBD due to the unique physicochemical properties of nanoparticulate drug formulations and their preferential accumulation in the inflamed colon via the epithelial enhanced permeability and retention effect (45, 46). Intestinal secretion of mucus also facilitates nanoparticle adhesion at the target site. At the same time, intestinal barrier function is impaired in IBD due to dysfunctional tight junctions, and nanoparticles can pass through damaged inflammatory epithelium to reach the inflamed colon (47). In agreement with previous reports (45, 46), we found that orally administered MON-PEI (50 nm diameter) accumulated more so in inflamed colons than in healthy colons. The long-term retention of MON-PEI in the inflamed colon suggested the possibility of reducing the dose frequency. After demonstrating dose-dependent therapeutic outcomes, we found that oral administration of a high dose of MON-PEI every 3 days showed a similar therapeutic outcome as daily administration. Orally administered MON-PEI showed anti-colitis activity in both preventative and therapeutic (delayed treatment) settings. A higher dose of MON-PEI at a lower frequency (40 mg/kg every 3 days) did not cause any obvious toxicity. Together, these findings demonstrate that MON-PEI have therapeutic potential with a flexible administration that is advantageous for clinical translation. Additional preclinical studies in large animal models are needed to optimize the treatment regimen for MON-PEI before clinical testing.
There is currently no curative therapy for IBD, and lifelong medication is typically the only option for IBD management (48). Multifunctional nanoparticulate drug delivery systems for IBD treatment that target the inflamed colon, allow stimuli-responsive drug release, and exhibit therapeutic efficacy have attracted substantial attention (30–34, 46, 49–52). Compared to our previous reports in sepsis, we deciphered that cfDNA played an important role in the IBD and demonstrated the possibility of cfDNA scavenging strategy for effective IBD treatment. We instead disulfide-bridged MSNs to diselenide-bridged MSNs to render our nanoplatform ROS scavenger ability, leading to the development of cfDNA- and ROS-scavenging approach in IBD management. Drug-free nanoparticulate scavengers such as MON-PEI have several advantages versus small-molecule drugs that are used to treat IBD: (i) Drug-free nanoscavengers can achieve excellent therapeutic efficacy by scavenging proinflammatory cfDNA and ROS without the off-target side effects and serious complications that are typical of small-molecule anti-inflammatory drugs. (ii) Nanoparticulate scavengers are suitable for both oral and rectal administration, as demonstrated here for MON-PEI in ulcerative colitis and Crohn’s disease models. MON-PEI exhibited greater efficacy in treating DSS-induced acute colitis than the widely used IBD drug mesalazine. (iii) Preferential accumulation and retention of nanoparticulate scavengers in the inflamed colon allows a reduced dose frequency while ameliorating severe colitis. (iv) Nanoparticulate scavengers such as the MON used here can be prepared from a U.S. Food and Drug Administration–approved silica material, allowing higher doses that retain a good safety profile, as shown here for MON-PEI. (v) ROS-scavenging nanoparticles such as the MON used here can allow ROS-responsive drug release. It would be tempting to further investigate whether using these MON-PEI nanoscavengers to deliver clinically approved anti-IBD drugs would achieve a synergistic effect to induce and maintain long-term remission of IBD.
In summary, we have developed a nanoparticulate scavenger for anti-inflammatory therapy of IBD via dual scavenging of cfDNA and ROS. Through the targeted accumulation in the inflamed colon, the nanoscavenger showed therapeutic activity in both ulcerative colitis and Crohn’s disease models with flexible administration route and allowing a reduced dosing frequency. Oral administration of MON-PEI attenuated colonic and peritoneal inflammation and prevented tissue damage by blocking cfDNA-induced TLR9–MyD88–NF-κB signaling while reducing ROS-mediated proinflammatory responses. Together, our findings suggest a new cfDNA- and ROS-scavenging approach to treating IBD and encourage the development of similar nanoparticulate scavengers for treating other inflammatory diseases of GI tract.
MATERIALS AND METHODS
Patient samples
Plasma samples from 52 IBD patients and 20 healthy volunteers were collected from the First Affiliated Hospital of Xi’an Jiaotong University. Histochemical staining samples were taken from 15 IBD patients and 15 healthy volunteers. Collection of samples was approved by the Ethics Committee at the First Affiliated Hospital of Xi’an Jiaotong University.
Synthesis of diselenide-bridged MON-PEI
Diselenide-bridged MONs were prepared on the basis of our protocol reported previously (21). To synthesize MON-PEI, 1.0 g of MON was dispersed in 250 ml of toluene with ultrasonic. After adding 1.5 ml of (3-glycidyloxypropyl)trimethoxysilane, the mixture was refluxed at 80°C for 24 hours to obtain epoxysilane-functionalized MON. After purification, 500 mg of epoxysilane-functionalized MON was dispersed in 250 ml of PEI 25K solution (1 mg/ml) at room temperature with stirring for 24 hours. Diselenide-bridged MON-PEI were collected, washed, and dried for further use. Disulfide-bridged MON-PEI were fabricated on the basis of our protocol reported previously (14).
DNA binding assay
The binding ability of MON, PEI, and MON-PEI with ct-DNA was evaluated as described previously (14). Briefly, ct-DNA solution (25 μl, 10 μg/ml), PicoGreen (25 μl), and MilliQ water (50 μl) were mixed in 96-well plate. Then, the mixture was shaken for 30 min in the dark to form complex. After that, different concentrations of MON, PEI, or MON-PEI solutions (100 μl) were added. After incubation at 37°C for 1 hour, the fluorescence intensity of complex at wavelength of 520 nm was measured with a multi-mode microplate reader (SpectraMax iD3, Molecular Devices) with excitation at wavelength of 490 nm.
In vitro TLR activation assay
HEK-Blue reporter cells (TLR4 and TLR9) were purchased from InvivoGen (San Diego, CA) and cultured in Dulbecco’s minimal essential medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin at 37°C in a humidified atmosphere with 5% CO2.
To study the inhibition of TLR9 activation by MON, PEI, or MON-PEI in HEK-Blue reporter cells, CpG 2006 (1 μg/ml) and human sera (5 μl) were incubated with HEK-Blue TLR9 at a density of 8 × 104 cells per well in a 96-well plate for 30 min. Then, MON, PEI, or MON-PEI (10 μg/ml) were added, respectively. After incubation for 24 hours, the supernatants were collected and the QUANTI-Blue (InvivoGen, San Diego, CA) assay was performed according to the manual instructions. Embryonic alkaline phosphatase (SEAP) activity was quantified by the optical density at wavelength of 620 nm (OD620) with a multi-mode microplate reader (SpectraMax iD3, Molecular Devices).
To study the inhibition of TLR4 activation by MON, PEI, or MON-PEI in HEK-Blue reporter cells, LPS (10 ng/ml) was incubated with HEK-Blue TLR4 reporter cells at a density of 2.5 × 104 cells per well in a 96-well plate for 30 min. Then, MON, PEI, or MON-PEI (10 μg/ml) were added. The following steps were described as above.
Anti-inflammatory assay in vitro
RAW 264.7 macrophages were cultured in DMEM with 10% FBS, 1 mM sodium pyruvate, and 1% penicillin-streptomycin at 37°C in a humidified atmosphere with 5% CO2. RAW 264.7 cells were plated in 96-well plates at a density of 2 × 104 cells per well and incubated with CpG 1826 (1 μg/ml), LPS (1 μg/ml), and 5 μl of human sera for 30 min. Then, MON, PEI, and MON-PEI (10 μg/ml) were added by final volume adjusting to 200 μl. The supernatants were collected after incubation for 24 hours, and the TNF-α level was measured with an enzyme-linked immunosorbent assay (ELISA) kit according to the manual instruction.
Cytotoxicity assay in vitro
Human colon carcinoma cell lines (Caco-2), RAW 264.7, and HEK-Blue TLR9 cells were cultured in DMEM with 10% FBS, 1 mM sodium pyruvate, and 1% penicillin-streptomycin at 37°C in a humidified atmosphere with 5% CO2. Cells (104 per well) were plated in 96-well plates. After 12 hours, MON, PEI, or MON-PEI with different concentrations were added, and plates were incubated for 24 hours. Cell viability was measured with a sulforhodamine B (SRB) assay. To determine the protective effect of nanoscavengers from oxide damage, RAW 264.7 and Caco-2 cells were grown in 96-well culture plates at 104 cells per well and were cultured. After 12 hours, H2O2 (100 μM) with or without different concentrations of MON, PEI, or MON-PEI was added to each well, and plates were incubated for 24 hours. Cell viability was measured by SRB assay.
In vitro ROS assay
RAW 264.7 and Caco-2 cells were grown in 96-well culture plates at 1 × 104 cells per well and were cultured. RAW 264.7 cells were treated with LPS (1 μg/ml), and Caco-2 cells were treated with 100 μM H2O2. After 4 hours, the cells were stained with 2′-7′ dichlorofluorescin diacetate (DCFH-DA) for 15 min and washed three times with PBS. Intracellular ROS generation was analyzed with a microplate reader at wavelength of 490-nm excitation and 520-nm emission.
DSS-induced acute colitis model and treatment
Acute ulcerative colitis was induced by addition of 3% (w/v) DSS (36 to 50 kDa; MP Biomedicals) to the mouse drinking water for 7 days. For the prevention setting, mice treated with DSS were administered with MON, PEI, or MON-PEI (10 mg/kg) via oral gavage, intraperitoneal injection, or rectal enema. For all diseased mice, daily administration was performed during 7 days of DSS treatment, while normal mice were administered with PBS. Body weight, fecal bleeding, and visible stool consistency changes of mice were observed daily. On days 1, 3, 5, and 7, the mice were sacrificed and serum was collected for analysis of TNF-α, IL-6, IL-1β, and IL-4 levels. Colon tissue was collected for determining colon length and histopathological examination on day 7. For the therapeutic setting, PBS or MON, PEI, or MON-PEI at 10 mg/kg were administered starting on the third day up to day 14 via oral gavage, intraperitoneal injection, or rectal enema. Body weight, fecal bleeding, and visible stool consistency changes of mice were monitored. The survival rate in different groups was recorded for 14 days. The mice were sacrificed, and colon tissue was collected for determining colon length, histopathological examination, and protein content by immunohistochemical staining on day 14. Serum and peritoneal fluids were collected for detection of cfDNA, and colon was collected for analysis of ROS, MDA, SOD, and GPx on days 1, 4, 7, 10, and 14. Colon tissue and peritoneal fluids were collected for analysis of TNF-α, IL-6, IL-1β, IFN-γ, MPO, and MCP-1 levels on day 7. Peritoneal fluids were collected for determining the number of TLR9+ cells and isolated macrophages for detecting protein content by Western blot on day 7.
TNBS-induced colitis model and treatment
After the 6-hour starvation period, the mice were provided a 200-μl saline enema by using a feeding tube to clear the colorectal distal of approximately 3 to 4 cm. After 10 min, the mice under isoflurane anesthesia were administrated with 65 μl of TNBS (2.5% TNBS in 50% ethanol) using a Wiretrol to minimize backflow (day 0). These protocols revealed an acceptable reduction in body weight within 24 hours after TNBS administration (day 1). The mice that did not show minimum 7% weight reduction after 24 hours of TNBS administration were excluded from the study, while the remaining were arranged in different groups to ensure the average weight loss.
The mice treated with TNBS were administrated with PBS MON, PEI, or MON-PEI at 10 mg/kg from day 1 to day 4 via intraperitoneal injection, oral gavage, or rectal enema. Body weight changes were monitored daily. The mice were sacrificed, and colon tissue was collected for determining colon length and histopathological examination on day 5. Serum was collected for analysis of TNF-α, IL-6, IL-1β, and IL-4 levels on day 5.
Disease activity index
The DAI was scored using the following criteria: stool consistency (hard: 0, soft: 2, and diarrhea: 4), fecal occult blood using Hemoccult Sensa (Beckman Coulter) (negative: 0, positive: 2, and macroscopic: 4), and weight loss (less than 1%: 0, 1 to 5%: 1, 5 to 10%: 2, 10 to 20%: 3, and more than 20%: 4).
Analysis of cytokines
The length of the colon was determined by the measurement of the distance between the most distal part of the cecum and rectum. To determine the cytokine contents of colon tissue, the colon segments were homogenized in 50 mM phosphate buffer (pH 6.0) in the ratio of 1:10 (w/v). The resulting samples were then subjected to centrifugation at 10,000g for 10 min at 4°C. Colonic and peritoneal TNF-α, IL-1β, IL-6, IFN-γ, MPO, IL-10, IL-17, and MCP-1 levels were determined by using an ELISA assay kit. The colonic levels of redox factors were determined by MDA assay kit, ROS assay kit, SOD assay kit, and GPx assay kit.
Histology
The distal section of colonic tissue about 0.5 cm was stored in 4% buffered saline before paraffin embedding for the histological evaluation. Colonic tissues were fixed by incubation with 4% (v/v) paraformaldehyde and 70% (v/v) alcohol and then embedded in paraffin. There was an independent observer present on H&E slides of paraffin colon sections to perform the histological examination. The histological scores were assessed in a blinded manner for inflammation severity (none: 0, slight: 1, moderate: 2, and severe: 3), polymorphonuclear neutrophil (PMN) infiltration/high power field (HPF) (less than 5: 0, 5 to 20: 1, 21 to 60: 2, 61 to 100: 3, and more than 100: 4), injury depth (none: 0, mucosa: 1, submucosa and mucosa: 2, and transmural: 3), crypt damage (none: 0, basal 1/3: 1, basal 2/3: 2, only surface epithelium intact: 3, and total crypt lost: 4), and adjustment to the tissue involvement multiplied by the percentage factor (0 to 25%: ×1, 26 to 50%: ×2, 51 to 75%: ×3, and 76 to 100%: ×4) (53).
Extraction and quantification of cfDNA
Extraction of cfDNA from serum in patients or peritoneal fluid in mice was carried out via the QIAamp DNA Blood Mini Kit (Qiagen, Germany). The Quant-iT PicoGreen dsDNA Assay Kit (Thermo Fisher Scientific, Waltham, MA) was used for determining the concentration of cfDNA.
In vivo fluorescence imaging and biodistribution
To evaluate the retention and toxicity of MON-PEI in the colon, Cy7-labeled MON, PEI, and MON-PEI were orally administered to mice in the normal and DSS groups after treatment with 3% DSS water or normal water for 4 days. After 2, 6, 12, 24, 48, and 72 hours of oral delivery, mice were sacrificed; the heart, liver, spleen, lung, kidney, and colon were collected; and fluorescence intensities were analyzed with the NIR Imaging System (IVIS Spectrum, Caliper Life Sciences) with a Cy7 filter channel.
To quantify MON-PEI biodistribution, the liver, heart, spleen, kidney, lung, stomach, and colon were disrupted with concentrated nitric acid. The amount of silicon in samples was quantified using inductively coupled plasma atomic emission spectroscopy (ICP-OES).
Statistical analysis
Statistical analyses were performed using Prism 7 software (GraphPad Software, La Jolla, CA), and values were expressed as means ± SEM. The statistical differences between groups were assessed by Student’s t test for two-group comparison or one-way analysis of variance (ANOVA) for multiple comparison. Comparison of Kaplan-Meier survival curves was performed with the log-rank Mantel-Cox test. Values of P < 0.05 were considered to be statistically significant.
Acknowledgments
We thank X. Zheng, Z. Tu, Y. Zhang, X. Meng, X. Xie, and K. Waqar for expertise and technical assistance.
Funding: This project was supported by the National Natural Science Foundation of China (nos. 81870380 and 82072049); the Shaanxi Province Science Foundation (2020ZDLSF01-03 and 2020KWZ-020); Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University, China (no. XJTU1AF-CRF-2020-004); and the Fundamental Research Funds for the Central Universities.
Author contributions: C.S., J.D., F.S., C.Y., Q.Q., T.S., L.W., H.H., M.S., F.C., and Y.Z. contributed to the collection of experimental data. C.S., J.D., F.S., C.Y., L.R., F.L., M.L., L.M., and D.L. analyzed the data. C.S., J.D., F.S., C.Y., D.S., K.W.L., and J.S. contributed to writing and revising the manuscript. D.S., K.W.L., and J.S. supervised the research.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Materials and Methods
Figs. S1 to S25
Tables S1 and S2
REFERENCES AND NOTES
- 1.Loftus E. V. Jr., Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 126, 1504–1517 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Khor B., Gardet A., Xavier R. J., Genetics and pathogenesis of inflammatory bowel disease. Nature 474, 307–317 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caruso R., Lo B. C., Núñez G., Host-microbiota interactions in inflammatory bowel disease. Nat. Rev. Immunol. 20, 411–426 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Nanini H. F., Bernardazzi C., Castro F., de Souza H. S. P., Damage-associated molecular patterns in inflammatory bowel disease: From biomarkers to therapeutic targets. World J. Gastroenterol. 24, 4622–4634 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu H., Li Y. R., Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: Updated experimental and clinical evidence. Exp. Biol. Med. 237, 474–480 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Cader M. Z., Kaser A., Recent advances in inflammatory bowel disease: Mucosal immune cells in intestinal inflammation. Gut 62, 1653–1664 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Taylor K. M., Irving P. M., Optimization of conventional therapy in patients with IBD. Nat. Rev. Gastroenterol. Hepatol. 8, 646–656 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Stallmach A., Hagel S., Bruns T., Adverse effects of biologics used for treating IBD. Best Pract. Res. Clin. Gastroenterol. 24, 167–182 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Huang Y., Chen Z., Inflammatory bowel disease related innate immunity and adaptive immunity. Am. J. Transl. Res. 8, 2490–2497 (2016). [PMC free article] [PubMed] [Google Scholar]
- 10.Lu Y., Li X., Liu S., Zhang Y., Zhang D., Toll-like receptors and inflammatory bowel disease. Front. Immunol. 9, 72 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyapati R. K., Dorward D. A., Tamborska A., Kalla R., Ventham N. T., Doherty M. K., Whitfield P. D., Gray M., Loane J., Rossi A. G., Satsangi J., Ho G. T., Mitochondrial DNA is a pro-inflammatory damage-associated molecular pattern released during active IBD. Inflamm. Bowel Dis. 24, 2113–2122 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latz E., Schoenemeyer A., Visintin A., Fitzgerald K. A., Monks B. G., Knetter C. F., Lien E., Nilsen N. J., Espevik T., Golenbock D. T., TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 5, 190–198 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Kilpinen H., Waszak S. M., Gschwind A. R., Raghav S. K., Witwicki R. M., Orioli A., Migliavacca E., Wiederkehr M., Gutierrez-Arcelus M., Panousis N. I., Yurovsky A., Lappalainen T., Romano-Palumbo L., Planchon A., Bielser D., Bryois J., Padioleau I., Udin G., Thurnheer S., Hacker D., Core L. J., Lis J. T., Hernandez N., Reymond A., Deplancke B., Dermitzakis E. T., Coordinated effects of sequence variation on DNA binding, chromatin structure, and transcription. Science 342, 744–747 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pisetsky D. S., Lee J., Leong K. W., Sullenger B. A., Nucleic acid-binding polymers as anti-inflammatory agents: Reducing the danger of nuclear attack. Expert Rev. Clin. Immunol. 8, 1–3 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Dawulieti J., Sun M., Zhao Y., Shao D., Yan H., Lao Y. H., Hu H., Cui L., Lv X., Liu F., Chi C. W., Zhang Y., Li M., Zhang M., Tian H., Chen X., Leong K. W., Chen L., Treatment of severe sepsis with nanoparticulate cell-free DNA scavengers. Sci. Adv. 6, eaay7148 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang H., Yan Y., Wu J., Ge X., Wei L., Liu L., Chen Y., Topical nanoparticles interfering with the DNA-LL37 complex to alleviate psoriatic inflammation in mice and monkeys. Sci. Adv. 6, eabb5274 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J., Sohn J. W., Zhang Y., Leong K. W., Pisetsky D., Sullenger B. A., Nucleic acid-binding polymers as anti-inflammatory agents. Proc. Natl. Acad. Sci. U.S.A. 108, 14055–14060 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naqvi I., Gunaratne R., McDade J. E., Moreno A., Rempel R. E., Rouse D. C., Herrera S. G., Pisetsky D. S., Lee J., White R. R., Sullenger B. A., Polymer-mediated inhibition of pro-invasive nucleic acid DAMPs and microvesicles limits pancreatic cancer metastasis. Mol. Ther. 26, 1020–1031 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holl E. K., Shumansky K. L., Pitoc G., Ramsburg E., Sullenger B. A., Nucleic acid scavenging polymers inhibit extracellular DNA-mediated innate immune activation without inhibiting anti-viral responses. PLOS ONE 8, e69413 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang H., Peng B., Dong C., Liu L., Mao J., Wei S., Wang X., Xu H., Shen J., Mao H. Q., Gao X., Leong K. W., Chen Y., Cationic nanoparticle as an inhibitor of cell-free DNA-induced inflammation. Nat. Commun. 9, 4291 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shao D., Li M., Wang Z., Zheng X., Lao Y. H., Chang Z., Zhang F., Lu M., Yue J., Hu H., Yan H., Chen L., Dong W. F., Leong K. W., Bioinspired diselenide-bridged mesoporous silica nanoparticles for dual-responsive protein delivery. Adv. Mater. 1801198 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Shao D., Zhang F., Chen F., Zheng X., Hu H., Yang C., Tu Z., Wang Z., Chang Z., Lu J., Li T., Zhang Y., Chen L., Leong K. W., Dong W. F., Biomimetic diselenide-bridged mesoporous organosilica nanoparticles as an X-ray-responsive biodegradable carrier for chemo-immunotherapy. Adv. Mater. 32, e2004385 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Marsman G., Zeerleder S., Luken B. M., Extracellular histones, cell-free DNA, or nucleosomes: Differences in immunostimulation. Cell Death Dis. 7, e2518 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wegmann F., Gartlan K. H., Harandi A. M., Brinckmann S. A., Coccia M., Hillson W. R., Kok W. L., Cole S., Ho L. P., Lambe T., Puthia M., Svanborg C., Scherer E. M., Krashias G., Williams A., Blattman J. N., Greenberg P. D., Flavell R. A., Moghaddam A. E., Sheppard N. C., Sattentau Q. J., Polyethyleneimine is a potent mucosal adjuvant for viral glycoprotein antigens. Nat. Biotechnol. 30, 883–888 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li A. W., Sobral M. C., Badrinath S., Choi Y., Graveline A., Stafford A. G., Weaver J. C., Dellacherie M. O., Shih T. Y., Ali O. A., Kim J., Wucherpfennig K. W., Mooney D. J., A facile approach to enhance antigen response for personalized cancer vaccination. Nat. Mater. 17, 528–534 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dudzińska E., Gryzinska M., Ognik K., Gil-Kulik P., Kocki J., Oxidative stress and effect of treatment on the oxidation product decomposition processes in IBD. Oxid. Med. Cell. Longev. 2018, 7918261 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian T., Wang Z., Zhang J., Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid. Med. Cell. Longev. 2017, 4535194 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bourgonje A. R., Feelisch M., Faber K. N., Pasch A., Dijkstra G., van Goor H., Oxidative stress and redox-modulating therapeutics in inflammatory bowel disease. Trends Mol. Med. 26, 1034–1046 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Alaarg A., Pérez-Medina C., Metselaar J. M., Nahrendorf M., Fayad Z. A., Storm G., Mulder W. J. M., Applying nanomedicine in maladaptive inflammation and angiogenesis. Adv. Drug Deliv. Rev. 119, 143–158 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee Y., Sugihara K., Gillilland M. G. III, Jon S., Kamada N., Moon J. J., Hyaluronic acid-bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat. Mater. 19, 118–126 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee B. C., Lee J. Y., Kim J., Yoo J. M., Kang I., Kim J. J., Shin N., Kim D. J., Choi S. W., Kim D., Hong B. H., Kang K. S., Graphene quantum dots as anti-inflammatory therapy for colitis. Sci. Adv. 6, eaaz2630 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li S., Xie A., Li H., Zou X., Zhang Q., A self-assembled, ROS-responsive Janus-prodrug for targeted therapy of inflammatory bowel disease. J. Control. Release 316, 66–78 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Liu Y., Cheng Y., Zhang H., Zhou M., Yu Y., Lin S., Jiang B., Zhao X., Miao L., Wei C. W., Liu Q., Lin Y. W., Du Y., Butch C. J., Wei H., Integrated cascade nanozyme catalyzes in vivo ROS scavenging for anti-inflammatory therapy. Sci. Adv. 6, eabb2695 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li C., Zhao Y., Cheng J., Guo J., Zhang Q., Zhang X., Ren J., Wang F., Huang J., Hu H., Wang R., Zhang J., Proresolving A., A proresolving peptide nanotherapy for site-specific treatment of inflammatory bowel disease by regulating proinflammatory microenvironment and gut microbiota. Adv. Sci. 6, 1900610 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang F., Chen F., Yang C., Wang L., Hu H., Li X., Zheng X., Wang Z., Chang Z., Li T., Li L., Ge M., Du J., Sun W., Dong W. F., Shao D., Coordination and redox dual-responsive mesoporous organosilica nanoparticles amplify immunogenic cell death for cancer chemoimmunotherapy. Small 17, e2100006 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Hu H., Yang C., Zhang F., Li M., Tu Z., Mu L., Dawulieti J., Lao Y. H., Xiao Z., Yan H., Sun W., Shao D., Leong K. W., A versatile and robust platform for the scalable manufacture of biomimetic nanovaccines. Adv. Sci. 8, 2002020 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chassaing B., Aitken J. D., Malleshappa M., Vijay-Kumar M., Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 104, 15.25.1–15.25.14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmitt H., Ulmschneider J., Billmeier U., Vieth M., Scarozza P., Sonnewald S., Reid S., Atreya I., Rath T., Zundler S., Langheinrich M., Schüttler J., Hartmann A., Winkler T., Admyre C., Knittel T., Dieterich Johansson C., Zargari A., Neurath M. F., Atreya R., The TLR9 agonist cobitolimod induces IL10-producing wound healing macrophages and regulatory T cells in ulcerative colitis. J. Crohns Colitis 14, 508–524 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Obermeier F., Dunger N., Deml L., Herfarth H., Schölmerich J., Falk W., CpG motifs of bacterial DNA exacerbate colitis of dextran sulfate sodium-treated mice. Eur. J. Immunol. 32, 2084–2092 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Han W., Zhang S., Qian J., Zhang J., Wang X., Xie Z., Xu B., Han Y., Tian W., Redox-responsive fluorescent nanoparticles based on diselenide-containing AIEgens for cell imaging and selective cancer therapy. Chem. Asian J. 14, 1745–1753 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Kaushal N., Kudva A. K., Patterson A. D., Chiaro C., Kennett M. J., Desai D., Amin S., Carlson B. A., Cantorna M. T., Prabhu K. S., Crucial role of macrophage selenoproteins in experimental colitis. J. Immunol. 193, 3683–3692 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi C., Yue F., Shi F., Qin Q., Wang L., Wang G., Mu L., Liu D., Li Y., Yu T., She J., Selenium-containing amino acids protect dextran sulfate sodium-induced colitis via ameliorating oxidative stress and intestinal inflammation. J. Inflamm. Res. 14, 85–95 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alex P., Zachos N. C., Nguyen T., Gonzales L., Chen T. E., Conklin L. S., Centola M., Li X., Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm. Bowel Dis. 15, 341–352 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oberdörster G., Safety assessment for nanotechnology and nanomedicine: Concepts of nanotoxicology. J. Intern. Med. 267, 89–105 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Zhang S., Langer R., Traverso G., Nanoparticulate drug delivery systems targeting inflammation for treatment of inflammatory bowel disease. Nano Today 16, 82–96 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nunes R., Neves J. D., Sarmento B., Nanoparticles for the regulation of intestinal inflammation: Opportunities and challenges. Nanomedicine 14, 2631–2644 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y., Thanou M., Vllasaliu D., Exploiting disease-induced changes for targeted oral delivery of biologics and nanomedicines in inflammatory bowel disease. Eur. J. Pharm. Biopharm. 155, 128–138 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Chudy-Onwugaje K. O., Christian K. E., Farraye F. A., Cross R. K., A state-of-the-art review of new and emerging therapies for the treatment of IBD. Inflamm. Bowel Dis. 25, 820–830 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun T., Kwong C. H. T., Gao C., Wei J., Yue L., Zhang J., Ye R. D., Wang R., Amelioration of ulcerative colitis via inflammatory regulation by macrophage-biomimetic nanomedicine. Theranostics 10, 10106–10119 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dou G., Tian R., Liu X., Yuan P., Ye Q., Liu J., Liu S., Zhou J., Deng Z., Chen X., Liu S., Jin Y., Chimeric apoptotic bodies functionalized with natural membrane and modular delivery system for inflammation modulation. Sci. Adv. 6, eaba2987 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zu M., Ma Y., Cannup B., Xie D., Jung Y., Zhang J., Yang C., Gao F., Merlin D., Xiao B., Oral delivery of natural active small molecules by polymeric nanoparticles for the treatment of inflammatory bowel diseases. Adv. Drug Deliv. Rev. 113887 (2021). [DOI] [PubMed] [Google Scholar]
- 52.Shi H., Zhao X., Gao J., Liu Z., Liu Z., Wang K., Jiang J., Acid-resistant ROS-responsive hyperbranched polythioether micelles for ulcerative colitis therapy. Chin. Chem. Lett. 31, 3102–3106 (2020). [Google Scholar]
- 53.Herrera Estrada L., Wu H., Ling K., Zhang G., Sumagin R., Parkos C. A., Jones R. M., Champion J. A., Neish A. S., Bioengineering bacterially derived immunomodulants: A therapeutic approach to inflammatory bowel disease. ACS Nano 11, 9650–9662 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials and Methods
Figs. S1 to S25
Tables S1 and S2