Abstract
Infection with Lactobacillus is rare, and only a handful of species have been identified as being clinically significant: Lactobacillus casei, Lactobacillus rhamnosus, and Lactobacillus leichmannii. The literature contains one case report of bacteremia caused by Weissella confusa (basonym: Lactobacillus confusus), but the clinical significance of the infection was unclear. We describe a case of W. confusa bacteremia in a 46-year-old man with a history of abdominal aortic dissection and repair. This procedure was complicated by gut ischemia, which necessitated massive small bowel resection. He subsequently developed short-bowel syndrome, which required him to have total parenteral nutrition. He later developed an Enterococcus faecalis aortic valve endocarditis that required a coronary artery bypass graft and aortic root replacement with homograft and 6 weeks of intravenous ampicillin and gentamicin. Three months prior to his most recent admission, he was diagnosed with Klebsiella pneumoniae bacteremia and candidemia. At the present admission, he had fever (Tmax, 39.5°C) and chills of 2 days' duration and was admitted to the intensive care unit because of hemodynamic instability. Blood cultures grew K. pneumoniae and W. confusa in four of four blood culture bottles (both aerobe and anaerobe bottles). Imaging studies failed to find any foci of infection. A transesophageal echocardiogram revealed no vegetations. A culture of the patient's Hickman catheter tip was negative. The patient was treated with piperacillin-tazobactam and gentamicin. His condition improved, and he was discharged home, where he completed 4 weeks of piperacillin-tazobactam therapy. Lactobacillemia seldom results in mortality; however, it may be a marker of a serious underlying disease. It is usually seen in patients who have a complex medical history or in patients who receive multiple antibiotics. Lactobacillus spp. are generally associated with polymicrobial infections, and when isolated from the blood, they need to be considered possible pathogens. The presence of a vancomycin-resistant, gram-positive coccobacilli on a blood culture should alert clinicians to the possibility of bacteremia caused by W. confusa or other small gram-positive rods.
Lactobacillus sp. bacteremia is rare. This is complicated by the fact that lactobacilli are usually considered contaminants and are not identified to the species level. The true occurrence of Weissella confusa cannot be determined. However, more cases of Lactobacillus sp.-related bacteremia have been reported recently (9). W. confusa (basonym: Lactobacillus confusus) (3) bacteremia has been reported only once in the literature (6), but its clinical significance was not described. The objective of this report is to present a case with a polymicrobial bacteremia including W. confusa and to describe the Gram stain morphology, biochemical characteristics, and clinical manifestations of W. confusa.
(This case report was presented as a poster [number 213] at the 38th annual meeting of the Infectious Diseases Society of America on 7 to 10 September 2000 at the Ernest N. Memorial convention center in New Orleans, La.)
Case report.
A 46-year-old man was admitted to the Cleveland Clinic Foundation (Cleveland, Ohio) with fever, chills, and dehydration.
One year before the present admission, the patient underwent abdominal aortic dissection and repair. The procedure was complicated by gut ischemia, which necessitated massive small bowel resection. Total parenteral nutrition (TPN) was required because of the short-bowel syndrome he developed secondary to the small bowel resection.
Five months later, the patient developed an Enterococcus faecalis aortic valve endocarditis. At that time, he underwent a coronary artery bypass graft and aortic root replacement with homograft and 6 weeks of intravenous ampicillin and gentamicin.
Three months prior to the present admission, the patient was diagnosed with Klebsiella pneumoniae bacteremia, candidemia, and methicillin-resistant Staphylococcus aureus (which had colonized in his endotracheal tube). He was treated with fluconazole for 2 weeks and cefepime, amikacin, ciprofloxacin, and vancomycin for 1.5 weeks.
He then developed TPN-related cholestatic jaundice for which he was admitted 2 months prior to the present admission. After a 1-month stay, he was released. He was clinically stable until 1 month after discharge, when he developed chills, fever (Tmax, 39.5°C), and dehydration.
He presented to our facility's emergency room after 2 days of symptoms. There were no significant findings in the history or physical examination to explain the cause of fever, and he had not recently undergone any invasive procedures. Blood cultures obtained during admission grew K. pneumoniae and a vancomycin-resistant, gram-positive coccobacillus in four out of four blood culture bottles (in both aerobe and anaerobe bottles). The vancomycin-resistant, gram-positive coccobacillus was identified later as W. confusa.
Imaging studies of the patient's abdomen and chest failed to reveal any foci of infection. A transesophageal echocardiogram did not reveal any evidence of vegetations. The patient's Hickman catheter was removed and the tip was cultured; the results were negative. The patient was admitted to our hospital, and he began treatment with piperacillin-tazobactam and vancomycin. He developed hypotension and dyspnea, which required intubation and admission to the intensive care unit. Vancomycin was discontinued and gentamicin was added when the results of the blood cultures identified the pathogen. The patient's Hickman catheter was replaced, and he was discharged on the 11th hospital day to complete 4 weeks of piperacillin-tazobactam at home.
Microbiology.
Blood cultures that were obtained during admission grew two organisms, K. pneumoniae and a vancomycin-resistant, gram-positive coccobacillus that was identified as W. confusa. The W. confusa grew on blood agar plates (BAPs) that were incubated aerobically and anaerobically as pinpoint colonies showing alpha hemolysis. The organism grew at 25, 35, and 42°C. It was catalase negative and was positive for esculin hydrolysis and arginine deamination. The organism was incubated in Andrades broth (Carr-Scarborough Microbiologicals, Inc., Decatur, Ga.) for 10 days to test the fermentation of 11 carbohydrates. Results of both the physiologic and biochemical tests are shown in Table 1. We identified the organism by comparing our results with those from the literature (5) (Table 1). The only difference was with the acidification of xylose.
TABLE 1.
Biochemical and physiologic characteristics of W. confusa (L. confusus) isolate in a patient with polymicrobial bacteremia
| Test | Result with patient's isolate | Result from the literature |
|---|---|---|
| Hemolysis | ||
| BAP, ambient | Alpha | Alpha |
| BAP, anaerobic | Alpha | Alpha |
| BAP, CO2 | Alpha | Alpha |
| Gram stain | Gram-positive coccobacilli/short rods | Gram-positive coccobacilli/short rods |
| Arrangement | Chain | Chain |
| Catalase | Negative | Negative |
| Bile-esculin | Positive | Positive |
| Growth: | ||
| At 25°C | Positive | Positive |
| At 35°C | Positive | Positive |
| At 42°C | Positive | Positive |
| Pyroglutamyl aminopeptidase | Negative | Negative (positive in some [11]) |
| Esculin hydrolysis | Positive | Positive |
| Motility | Nonmotile | Nonmotile |
| Arginine deamination | Positive | Positive |
| Acetoin production | Negative | Negative |
| H2S production | Negative | Negative |
| Acidification of: | ||
| Glucose | Positive | Positive |
| Xylose | Positive | Negative |
| Mannitol | Negative | Negative |
| Lactose | Negative | Negative |
| Sucrose | Positive | Positive |
| Maltose | Positive | Positive |
| Fructose | Positive | Positive |
| Salicin | Positive | Positive |
| Arabinose | Negative | Negative |
| Rhamnose | Negative | Negative |
| Trehalose | Negative | Negative |
Antimicrobial susceptibility testing was performed on this isolate using the microdilution method and haemophilus test medium with lysed horse blood. The results showed that the isolate was sensitive to penicillin (MIC, 0.5 μg/ml), cefuroxime (MIC, 4 μg/ml), ceftriaxone (MIC, 4 μg/ml), amoxicillin (MIC, 1 μg/ml), erythromycin (MIC ≤ 0.12 μg/ml), clindamycin (MIC ≤ 0.1 μg/ml), and tetracycline (MIC, 4 μg/ml). It was resistant to vancomycin (MIC > 16 μg/ml) and trimethoprim-sulfamethoxazole (MIC > 4 μg/ml).
Discussion.
Lactobacillus spp. are increasingly being recognized as a cause, although a rare one, of bacteremia. Lactobacillus-related bacteremia has been reported in patients with complex medical histories, diabetes, cancer, and recent surgery and gastrointestinal procedures (1). It has also been reported in immunocompromised patients, especially in recipients of liver transplants (7, 12). The use of antibiotics such as vancomycin and the use of selective bowel decontamination have been associated with Lactobacillus bacteremia (12). The infection can be caused by a number of factors, including changes in the normal flora of the throat, gut, and vaginal tract and disruption of mucous integrity by invasive procedures, surgery, and/or antibiotic use.
Lactobacillus spp., in general, are seldom identified to the species level, since the rate of infection is rare, and are usually considered contaminants. The greatest difficulty is not necessarily identifying them but establishing their clinical relevance. The clinical manifestations are mostly similar for the entire genus. Species identification, though, may be important for determining the epidemiology of Lactobacillus-associated infections. W. confusa can be identified and differentiated from other species such as Enterococcus spp., Streptococcus spp., Lactococcus spp., and Leuconostoc spp. by its biochemical and physiologic properties. These include arginine deamination, esculin hydrolysis, growth at 42°C, and acidification of certain carbohydrates (Table 1).
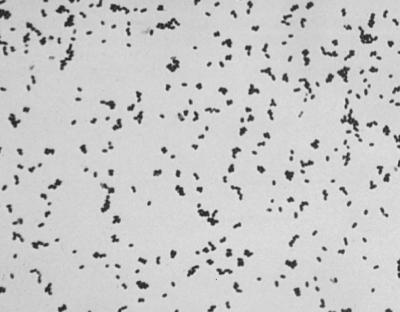
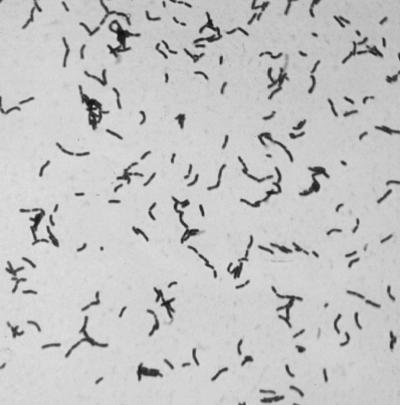
Enterococcus spp. are and W. confusa can be pyroglutamyl aminopeptidase positive and can have similar morphologies on Gram stains from blood agar plates (Fig. 1). Additional biochemical testing and evaluation of Gram stain morphology from a broth culture may be necessary to differentiate the two. The broth Gram stains of W. confusa usually reveal elongated gram-positive bacilli (Fig. 2), whereas Enterococcus spp. usually resemble gram-positive cocci in pairs and chains.
FIG. 1.
W. confusa (L. confusus) Gram stain morphology from BAP (×100).
FIG. 2.
W. confusa (L. confusus) Gram stain morphology from thioglycolate broth (×100).
Of all the Lactobacillus species associated with bacteremia reported in the literature, only a handful have been identified as being clinically significant: Lactobacillus casei, Lactobacillus rhamnosus, and Lactobacillus leichmannii (4, 8, 9). Bacteremia of unclear significance caused by W. confusa has been reported only once in the literature; the infection developed in a pediatric patient (6). W. confusa has also caused a thumb abscess (2) and has been isolated from the peritoneal fluid and abdominal wall of two patients, but with unclear significance (13). Paludan-Muller et al. and Kandler and Weiss reported that W. confusa can be found in garlic mix, banana leaves, sugarcane, carrot juice, raw milk, and sewage as well as in humans (10, 11).
Lactobacillus sp. bacteremias are usually associated with a polymicrobial infection (1, 9), which was the case with our patient. In the past, Lactobacillus has been treated merely as a contaminant. However, according to Antony et al., the isolation of Lactobacillus spp. in the blood may suggest a true infection rather than a skin contamination since these bacteria do not normally reside on the skin (1). Our patient's complex medical history of vascular and gut surgery and multidrug antibiotic therapy could have been a risk factor for the bacteremia. In a study by Husni et al., the researchers observed that many patients with Lactobacillus sp. bacteremia were receiving TPN, but they did not determine if this was a risk factor (9).
Lactobacillus spp. can cause low-grade infections (1) and endocarditis. Given the history of an aortic valve graft replacement in our patient, a transesophageal echocardiogram was performed, but it did not show any evidence of vegetations. Despite its ability to cause endocarditis, Lactobacillus sp. bacteremia has a very low mortality rate (1, 9). In a study by Antony et al., the researchers determined that only 3 deaths out of 53 could probably be explained by the Lactobacillus infection (1). Husni et al. recorded a single death out of 45 patients with bacteremia that the researchers thought was caused by Lactobacillus spp. These authors concluded that although Lactobacillus sp. bacteremia was rarely life threatening, it may serve as a marker of more serious underlying disease (9).
Not all isolates of Lactobacillus spp. are vancomycin resistant. In the study by Husni et al., only 73% of the strains were vancomycin resistant. Few isolates of Weissella spp. have been tested. The antibiotics of choice for treatment include clindamycin, penicillin, erythromycin, aminoglycosides, and imipenem (1).
Our patient was treated with piperacillin-tazobactam since both K. pneumoniae and W. confusa were susceptible to these drugs. Gentamicin was added in the first few days for possible synergy. All subsequent blood cultures were negative for both organisms. The sepsis syndrome was most likely caused by K. pneumoniae.
Conclusion.
W. confusa bacteremia in our patient was associated with polymicrobial infection. We believe that when W. confusa is isolated from the blood, it should be treated as a possible pathogen. In addition, the presence of gram-positive, vancomycin-resistant coccobacilli in blood culture should alert clinicians to the possibility of bacteremia caused by W. confusa or other small gram-positive rods.
REFERENCES
- 1.Antony S, Stratton C W, Dummer J S. Lactobacillus bacteremia: description of the clinical course in adult patients without endocarditis. Clin Infect Dis. 1996;23:773–778. doi: 10.1093/clinids/23.4.773. [DOI] [PubMed] [Google Scholar]
- 2.Bentar C E, Relloso S, Castell F R, Smayevsky J, Bianchini H M. Abscess caused by vancomycin-resistant Lactobacillus confusus. J Clin Microbiol. 1991;29:2063–2064. doi: 10.1128/jcm.29.9.2063-2064.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins S J, Metaxopoulus J, Wallbanks S. Taxonomic studies on some Leuconostoc-like organisms from fermented sausages: description of a new genus Weissella for the Leuconostoc paramesenteroides group of species. J Appl Bacteriol. 1993;75:595–603. doi: 10.1111/j.1365-2672.1993.tb01600.x. [DOI] [PubMed] [Google Scholar]
- 4.Cooper C, Vincent A, Greene J N, Sandin R L, Cobian L. Lactobacillus bacteremia in febrile neutropenic patients in a cancer hospital. Clin Infect Dis. 1998;26:1247–1248. doi: 10.1086/598365. [DOI] [PubMed] [Google Scholar]
- 5.Facklam R, Hollis D, Collins M D. Identification of gram-positive coccal and coccobacillary vancomycin-resistant bacteria. J Clin Microbiol. 1989;27:724–730. doi: 10.1128/jcm.27.4.724-730.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green M, Wadowsky R, Barbadora K. Recovery of vancomycin-resistant gram-positive cocci from children. J Clin Microbiol. 1990;28:484–488. doi: 10.1128/jcm.28.3.484-488.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green M, Barbadora K, Michaels M. Recovery of vancomycin-resistant gram-positive cocci from pediatric liver transplant recipients. J Clin Microbiol. 1991;29:2503–2506. doi: 10.1128/jcm.29.11.2503-2506.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horwitch C A, Furseth H A, Larson A M, Jones T L, Olliffe J F, Spach D H. Lactobacillemia in three patients with AIDS. Clin Infect Dis. 1995;21:1460–1462. doi: 10.1093/clinids/21.6.1460. [DOI] [PubMed] [Google Scholar]
- 9.Husni R, Gordon S M, Washington J A, Longworth D L. Lactobacillus bacteremia and endocarditis: review of 45 cases. Clin Infect Dis. 1997;25:1048–1055. doi: 10.1086/516109. [DOI] [PubMed] [Google Scholar]
- 10.Kandler O, Weiss N. Regular, non-sporing Gram-positive rods. In: Sneath P H A, Mair M S, Sharpe M E, Holt J G, editors. Bergey's manual of systematic bacteriology. 9th ed. Vol. 2. Baltimore, Md: The Williams and Wilkins Co.; 1984. pp. 1208–1260. [Google Scholar]
- 11.Paludan-Muller C, Huss H H, Gram L. Characterization of lactic acid bacteria isolated from a Thai low-salt fermented fish product and the role of garlic as substrate for fermentation. Int J Food Microbiol. 1999;46:219–229. doi: 10.1016/s0168-1605(98)00204-9. [DOI] [PubMed] [Google Scholar]
- 12.Patel R, Cockerill F R, Porayko M K, Osmon D R, Ilstrup D M, Keating M R. Lactobacillemia in liver transplant patients. Clin Infect Dis. 1994;18:207–212. doi: 10.1093/clinids/18.2.207. [DOI] [PubMed] [Google Scholar]
- 13.Riebel W, Washington J. Clinical and microbiologic characteristics of pediococci. J Clin Microbiol. 1990;28:1348–1355. doi: 10.1128/jcm.28.6.1348-1355.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]