Abstract
Background
Endometrial cancer is the fourth most frequent gynecological cancer and the most frequent type of uterine cancer. There is an increase in the incidence and mortality of uterine cancers in the past few decades, and there are no well-established screening programs for endometrial cancer currently. Most endometrial cancers arise through the interplay of familial, genetic, and lifestyle factors. Although a number of genetic factors modify endometrial cancer susceptibility, they are not of standard use in the clinical assessment of prognosis. We conducted a comprehensive systematic literature review to provide an overview of the relationship between genetic factors and risk for endometrial cancer.
Methods
MEDLINE and EMBASE databases were searched for studies between January 2010 to March 2020 reporting the genes associated with endometrial cancer.
Results
Through the selection process, we retrieved 186 studies comprising 329 genes identified using several molecular methodologies in all human chromosomes and in mitochondrial DNA. Endometrial cancer exhibits a molecular complexity and heterogeneity coherent with its clinical and histologic variability. Improved characterization of molecular alterations of each histological type provides relevant information about the prognosis and potential response to new therapies.
Conclusions
The current challenge is the integration of clinicopathologic and molecular factors to improve the diagnosis, prognosis, and treatment of endometrial cancer.
Keywords: Endometrial cancer, genetic, systematic review, genetic marker
Introduction
Endometrial cancer is globally the fourth most frequent gynecological cancer and the most frequent type of uterine cancer, with the highest rates observed in developed countries. There is a lifetime estimated occurrence of endometrial cancer in 3% of women (1). In Brazil, the mortality due to endometrial cancer in 2015 was 1,454, and the expected incidence of the cancer is 6,540 in 2020 (2). In the United States, the incidence of endometrial cancer was 63,230 and the mortality was 11,350 in 2018, with uterine cancer being the fourth most common cancer in women and the fifth leading cause of cancer deaths in the county (1,3).
Endometrial cancer has a heterogeneous pathophysiology, encompassing many histological types, microscopical features, pathogenesis, behaviors, and prognosis. Endometrial cancers have been classified into two groups: type I is mostly low-grade endometrioid tumors developing from glandular cells in the lining of the endometrium, expressing high levels of estrogen receptor α with a favorable overall prognosis. They represent 80–90% of the endometrial cancer incidence and 40% of the cancer mortality. Type II endometrial tumors mainly include serous papillary or clear cell histology, are non-estrogen dependent, and exhibit a more aggressive clinical course with poor prognosis (4-9). The nine different subtypes of endometrial cancer according to a recently updated classification by WHO (10) are mucinous carcinoma, endometrioid carcinoma, serous carcinoma, serous endometrial intraepithelial carcinoma, clear cell carcinoma, neuroendocrine tumor, mixed cell adenocarcinoma, undifferentiated carcinoma, and dedifferentiated carcinoma. However, endometrioid carcinomas and serous carcinoma are together responsible for 85% and 3–10% of the endometrial cancer cases, respectively (11). Endometrioid carcinomas are regarded as the prototypical type I tumor, whereas serous carcinomas are regarded as the prototypical type II tumors (12).
Rates of uterine cancers have increased by 21% during the past decade with increased mortality, similar to the rates in the early 1980s. This increase may be attributed to longer life expectancy, increased rates of obesity, and shifts in female reproductive patterns (13,14). Recently, increased incidence has been reported in low-middle income countries, such as Brazil and South Africa (14).
Currently, there are no well-established screening programs for endometrial cancer, and full hysterectomy is the most widely used and effective treatment for early-stage tumors (15). The risk assessment for endometrial carcinoma is inaccurate and relies on less reproducible histological examinations (16,17). Most patients receive a diagnosis in the early stages (I/II) owing to abnormal vaginal bleeding, observed in 94% of the cases (18). The patients show improved prognosis after surgery alone, with 5- and 10-year survival rates of 95% and 77%, respectively. However, diagnosis in late stages (IV) results in a drastic reduction of survival, with the 5-year survival rate being only 14% (19,20).
Several risk factors such as obesity, physical inactivity, excess exogenous estrogen, and insulin resistance are associated with increased risk of endometrial cancer (21). Women with a family history of endometrial cancer have approximately a 2-fold increase in the risk of developing the disease, with heritability between 27% and 52% (9,22,23). A prospective study of 203,691 twins (monozygotic and same-sex dizygotic) from Nordic countries revealed a cumulative cancer incidence of 32%. In a median 32 years of follow-up, the authors observed a significant overall familial risk for cancer and specific types of cancer, including uterine cancers (27%; 95% CI, 11–43%) (23).
In addition, approximately 3–5% of endometrial cancers occur in women with Lynch syndrome, an autosomal dominant hereditary predisposition to cancer, where patients have an increased risk of multiple cancers, including gastric, colorectal, ovarian, and endometrial cancers, during their lifetime. Lynch syndrome is caused by variations in the MLH1, MLH3, MSH2, MSH6, PMS2, TGFBR2, or EPCAM genes (24). The Society of Gynecologic Oncology since 2014 recommends a deep screening for Lynch syndrome in every woman diagnosed with endometrial cancer (25). Additionally, the much rarer autosomal dominant Cowden syndrome substantially increases the risk of endometrial cancer. Cowden syndrome is a cancer predisposition syndrome caused by pathogenic variants in PTEN, with affected patients having an increased risk of breast, thyroid, renal, colorectal, and endometrial cancers and malignant melanoma (26). Carriers of BRCA1 mutations may also have an increased risk of serous endometrial cancer (20).
Despite the involvement of genetic factors in the risk of endometrial cancer, they are not incorporated in the standard clinical procedures for prognosis assessment. Currently, variables adopted for risk stratification comprise stage, lymph node involvement, depth of myometrial invasion, histology, and grade (27).
The identification of biomarkers and their relationship with the risk factors are necessary for the improvement in risk stratification and early detection of endometrial cancers in view of the rise in disease incidence and mortality. We conducted a comprehensive systematic review of the literature to provide an overview of the associations between genetic factors and risk of endometrial cancer. We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-2334).
Methods
Search strategy
The systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations (28). The search was performed in March 2020. Databases searched were Embase and MEDLINE with time spam restrictions from 2010 to 2020 aiming to studies reporting genes and genetic polymorphisms associated to endometrial cancer. Terms included MeSH terms and words in both title and abstract comprising “Endometrial Neoplasms”, “Endometrial Cancer”, “Polymorphism, Genetic”, “Genetic Profile”, “Genetic Markers” for MEDLINE. Terms for Embase were “endometrium tumor”, “endometrium cancer”, “genetic marker”, “single nucleotide polymorphism” and related terms. No language restrictions were applied.
Eligibility criteria
Studies were selected by three independent investigators in a prepiloted form, with disagreements resolved by a fourth member. The criteria for full-text evaluation were if they were primary studies reporting genes involved in the pathophysiology of the endometrial cancer disregarding the study design or technique used for detection. Study outcome was the detection of alterations in genes related to endometrial cancer reported.
Data extraction and synthesis
Data extracted from each study were gene, chromosome location, year of publication, first author, type of study, country of the study and doi number.
Results
A total of 483 articles were evaluated and 186 (the complete list of references of the summary of evidence is available in Supplementary file 1) were retrieved in the selection process, comprising 329 genes that were studied in all human chromosomes and in mitochondrial DNA by several molecular methodologies (Table 1). The PRISMA flow of included studies is presented in Figure 1. The complete summary of findings is available in the online table (Table S1).
Table 1. Genes studied in endometrial cancer.
| Chromosome | Location | Genes studied |
|---|---|---|
| 1 | 1p | ARID1A; ELAPOR1; C1QTNF12; MTOR; GADD45A; GSTM1; LEPR; MACF1; MTHFR; NOTCH2; NRAS |
| 1q | ADIPOR1; AGT; AKT3; CAPN9; CD247; CFHR4; CRP; DNAH14; FASLG; IL24; MDM4; MUC1; PTGS2; RHEX; ZBTB7B | |
| 2 | 2p | CYP1B1; DNMT3A; EIF2AK3; FABP1; MEIS1; MSH2; MSH6 |
| 2q | BARD1; BCL2L11; CASP8; CXCR4; ERBB4; ERCC3; IL1A; IL1R2; IMP4; MCM6; UGT1A1; UGT1A8 | |
| 3 | 3p | CTDSPL; CTNNB1; OGG1; MLH1; NPRL2; GFBR2; TLR9; VGLL4; XPC |
| 3q | ADIPOQ; AGTR1; FOXL2; IGSF10; PIK3CA; PIK3CB; TNK2 | |
| 4 | 4p | FGFR3; JAKMIP1; MSX1; STX18 |
| 4q | BMP3; KIT; CXCL3; FBXW7; GC; KIT; NFKB1; PDGFRA; SULT1E1; TLR2; UGT2B4 | |
| 5 | 5p | CLPTM1L; IL7R; PRLR; TERT |
| 5q | APC; CMYA5; CYFIP2; HSD17B4; MSH3; PCDHGB4; PDGFRB; PIK3R1; RAD50; TNFAIP8 | |
| 6 | 6p | CDKN1A; DDR1; DST; HFE; CDKN1A; VEGFA; YIPF3 |
| 6q | ARID1B; ESR1; IGF2R; RNASET2; SYNE1; TBP | |
| 7 | 7p | AHR; C7orf50; EGFR; IL6; NOD1; PMS2; TWIST1 |
| 7q | BRAF; CAPZA2; DPP6; LEP; MET; POT1; SERPINE1; XRCC2 | |
| 8 | 8p | BMP1; MSR1; PPP2R2A |
| 8q | CCAT1; CNBD1; CPQ; LAPTM4B; MYC; NCOA2; PCMTD1; PRKDC; PSCA; PTK2; PTP4A3; SOX17; TERF1 | |
| 9 | 9p | CDKN2A; LINGO2; CD274; PDCD1LG2; TLN1 |
| 9q | DNM1; NIPSNAP3B; TGFBR1; TLR4; TSC1; XPA | |
| 10 | 10p | TRDMT1; NUDT5 |
| 10q | ADAM12; C10orf88; CYP2E1; SLF2; FAZ; FGFR2; HHEX; MBL2; MGMT; PLAU; PRKG1; PTEN; RNLS; CXCL12; SIRT1; TNKS2 | |
| 11 | 11p | CYP2R1; HRAS; IGF2; P2RX3; CAVIN3 |
| 11q | ASRGL1; ATM; BIRC2; DHCR7; GAB2; HMBS; MMP3; MMP7; P2RX3; PGR; SCGB2A1 | |
| 12 | 12p | ADIPOR2; CHD4; KRAS |
| 12q | CDK2; IGF1; MDM2; POLE; SH2B3; UBC; VDR; ZNF605 | |
| 13 | 13q | BRCA2; DCT; ERCC5; IRS2; PCDH17; RB1 |
| 14 | 14q | AKT1; BMP4; CIDEB; DICER1; ESR2; HIF1A; MLH3; SNX6; XRCC3 |
| 15 | 15q | B2M; BLM; CYP19A1; CYP1A1; IGF1R; RAD51; SYNM; TICRR; TJP1; TTC23; VPS13C; ZSCAN29 |
| 16 | 16p | CIITA; CREBBP; ERCC4; HCFC1R1; IL32; PALB2; PDPK1; SULT1A1 |
| 16q | CDH1; CTCF; E2F4; FTO; HSD17B2; MMP2; NOD2; TERF2; WWOX; ZFHX3 | |
| 17 | 17p | SHBG; SMYD4; SREBF1; TP53 |
| 17q | ACE; BIRC5; BRCA1; BRIP1; CDK12; ERBB2; CYGB; HNF1B; MAPT; NF1; NME1; RAD51C; RAD51D; SPOP | |
| 18 | 18q | BCL2; C18orf21; MC4R; MOCOS; NEDD4L; SMAD4 |
| 19 | 19p | MUC16; DNMT1; INSR; KEAP1; NFIC; PIK3R2; RETN; SMARCA4 |
| 19q | AKT2; APOE; BAX; CCNE1; DYRK1B; ERCC1; ERCC2; LIG1; PAK4; NOP53; PLAUR; POLD1; PPP2R1A; TGFB1; URI1; XRCC1 | |
| 20 | 20p | BMP2 |
| 20q | ASXL1; AURKA; BMP7; DNMT3B; EYA2; MMP9; SNAI1; ZNF217 | |
| 21 | 21q | GABPA; TFF3 |
| 22 | 22q | BIK; CBY1; CHEK2; CHEK2; COMT; GSTT1 |
| X | Xp | ATP6AP2; FOXP3 |
| Xq | ARMCX4; DACH2; HPRT1; TAF1; XIST | |
| Mitochondrial | – | MT-CO2; MT-ND1 |
Figure 1.
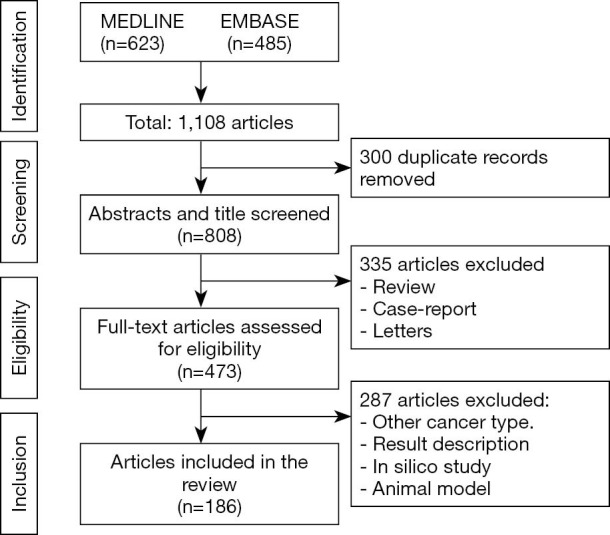
Flow diagram of the included studies.
Evidence retrieved
It has become well known in the past two decades that endometrial cancers present the highest molecular complexity among common tumor types, and its mechanistic heterogeneity is conformable with histologic and clinical variability (29).
Endometrial carcinomas have distinguishing molecular features. The most frequently mutated genes in endometrioid carcinomas are PTEN (>77%), PIK3CA (53%), PIK3R1 (37%), CTNNB1 (36%), ARID1A (35%), K-RAS (24%), CTCF (20%), RPL22 (12%), TP53 (11%), FGFR2 (11%), and ARID5B (11%). The most frequently mutated genes in serous carcinomas are TP53 (80–90%), PIK3CA (41.9%), PPP2R1A (36.6%), FBXW7 (30.2%), CHD4 (16.3%), CSMD3 (11.6%), and COLA11 (11.6%), along with loss of heterozygosity (LOH) on many chromosomes and several molecular changes (STK15, p16, E-cadherin, and C-erbB2) (3,11,30,31). Moreover, microsatellite instability (MSI) is detected in 25–40% of type I carcinomas, although it is rare in type II carcinomas (<5%). TP53 is mutated in more than 90% of type II carcinomas and in 11% of type I carcinomas. From the pathogenetic viewpoint, type I versus type II classification is interesting, despite the challenging application to clinical practice (11).
A recent characterization of 373 endometrial carcinomas using array- and sequencing-based technologies, through an integrated analysis of genomic, transcriptomic, and proteomic data by The Cancer Genome Atlas (TCGA) provided comprehensive information about pathway alterations and molecular mechanisms, describing four different molecular subgroups. Polymerase epsilon (POLE)-mutated subgroup with very high mutation rates (232×106 mutations/Mb) (ultramutated) is associated with good prognosis and account for 7–10% of endometrioid carcinomas. MSI subgroup with frequent hypermethylation of MLH1 promoter and elevated mutation rates (18×106 mutations/Mb) account for 28–30% of endometrioid carcinomas. Low copy number alterations subgroup with low mutation rate (2.9×106 mutations/Mb) represents 39% of endometrioid carcinomas, and high copy number subgroup (serous-like) with low mutation rate (2.3×106 mutations/Mb) but frequent TP53 mutations with worse prognosis represents serous carcinoma (94%). The candidate driver or pathogenic genes varied in all four subgroups, with 190 genes in the POLE subgroup, 21 in the MSI subgroup, 16 in the low copy number subgroup, and 8 in the high copy number subgroup (3,11,12) (Table 2).
Table 2. The most frequently altered genes of endometrial cancer subgroups.
| TCGA subgroup | Mutation profile | Frequency |
|---|---|---|
| POLE ultramutated | PTEN | 94% |
| FBXW7 | 82% | |
| ARID 1A | 76% | |
| PIK3CA | 71% | |
| PIK3R1 | 65% | |
| KRAS | 53% | |
| ARID5B | 47% | |
| Microsatellite instability hypermutated | PTEN | 88% |
| PIK3CA | 54% | |
| KRAS | 35% | |
| PIK3R1 | 40% | |
| ARID 1A | 37% | |
| RPL22 | 33% | |
| Copy number low | PTEN | 77% |
| PIK3CA | 53% | |
| CTNNB1 | 52% | |
| ARID1A | 42% | |
| PIK3R1 | 33% | |
| Copy number high | TP53 | 92% |
| PIK3CA | 47% | |
| PPP2R1A | 22% |
TCGA, The Cancer Genome Atlas.
Endometrioid carcinomas were present in all four subgroups. Endometrioid carcinoma is generally characterized by frequent derangements of the PI3K-PTEN-AKT-mTOR, RAS-MEK-ERK, and canonical WNT-β-catenin pathways. Endometrial cancer presents more mutations than any other tumor type studied thus far in the PI3K/AKT pathway by TCGA. The PI3K-PTEN-AKT-mTOR signal transduction pathway regulates cell growth and survival, synthesis of specific proteins, and metabolism. The RAS-RAF-MEK-ERK pathway plays a central role in regulation of cell proliferation, cell survival, and differentiation, is activated by KRAS mutations in endometrioid carcinoma, and can co-occur with alterations in PTEN, PIK3CA, and/or PIK3R1. The canonical WNT-β-catenin pathway regulates several cellular processes, which are constitutively activated in endometrioid carcinomas through gain-of-function mutations in CTNNB1 (β-catenin) (12). In addition, DNA mismatch repair (MMR) deficiency, a characteristic of 30% endometrioid cancers, is a major pathway enabling genomic instability in human cancers. Non-functional MMR leads to accumulation of insertion/deletion mutations at repetitive DNA stretches, such as microsatellites, and are, therefore, present with the MSI phenotype (32,33). The consequence of epigenetic silencing caused by promoter hypermethylation of MLH1 leads to MSI in sporadic endometrioid carcinomas. This results in the accumulation of somatic mutations at nucleotide repeats throughout the genome by defective MMR. Recently, ATR, CTCF, JAK1, RNF43, and RPL22 were indicated as drivers of the sustained pathogenic frameshift mutations at mononucleotide repeats in MSI-positive endometrioid carcinomas. Other known mechanisms of genomic instability in endometrioid carcinomas are somatic mutations of the exonuclease domain of POLE (12).
All types of serous carcinomas, except for a single tumor, belong to the high copy number subgroup in the TCGA classification. Mutations and/or dysregulation of TP53 or p53 are the most frequent molecular aberrations. Somatic mutations in PPP2R1A, FBXW7, SPOP, CHD4, and TAF1 are also involved in the serous carcinoma pathogenesis. In addition, overexpression or amplification of CCNE1, ERBB2, MYC, synuclein-γ, and p16 is observed (12,34,35).
Endometrial cancer risk may be influenced by common low-penetrance variants, such as single nucleotide variants (SNVs), in genes involved in cell survival, estrogen metabolism, and transcriptional control. A systematic review by Bafligil et al. (36) revealed that SNVs in HNF1B (rs11263761), KLF (rs7981863), EIF2AK (rs937213), CYP19A1 (rs17601876), SOX4 (rs1740828), and MYC (rs4733613, rs35286446, rs139584729) are strongly associated with endometrial cancer.
Recently, Benati et al. (37) investigated the relative length of the telomere in cell-free DNA (cfDNA) of patients with endometrioid endometrial cancer and healthy controls. They showed that the relative telomere length in cfDNA was significantly lower in cancer patients than in healthy controls, with a diagnostic accuracy of 0.87 (95% CI: 0.79–0.95, P<0.0001) and 80.0% (95% CI: 64.35–90.95%) sensitivity and 80.65% (95% CI: 62.53–92.55%) specificity for endometrial cancer. The authors also highlighted that despite the reduced relative length of telomere in cfDNA is not specific for endometrial cancer, it might be useful as an early diagnostic tool for endometrial cancer in high-risk patients, such as those with endometrial hyperplasia, particularly in young patients that may benefit from a fertility-sparing approach.
Providing strategies for translation of genomic profile to clinical practice is garnering interest, especially the use of methods often available in the pathology toolbox (11,38,39). Stello et al. (40) aimed to confirm the prognostic potential of the TCGA subgroups from two large randomized trial populations comprising 834 early-stage endometrioid endometrial carcinomas (PORTEC-1 and -2) with long-term follow-up. They confirmed the effect of the four molecular subgroups on prognosis initially proposed by the TCGA. The substitution of markers for molecular analysis methods applicable clinically was used and proved feasible in >96% of patients with endometrioid endometrial carcinoma. Integration of well-established clinicopathological factors with prognostic molecular alterations resulted in a robust risk assessment. Approximately 15% patients with a markedly unfavorable prognosis and 50% patients with a favorable prognosis could be identified. Another way for clinical translation may be the development of polygenic risk scores for endometrial cancer. Therefore, a major research priority is the expansion of endometrial cancer genomic profile to non-European populations enabling comparable polygenic risk scores of many ethnic groups (9).
Casarin et al. (2020) (41) investigated the potential role of glandular cells as a prognostic factor in cervical cytology in the preoperative identification and assessment of endometrial cancer. They observed no differences in the 5-year disease-free survival and overall survival between the normal cytology and glandular cell groups. However, cervical stromal invasion and presence of glandular cells increased the risk of local recurrence in endometrial cancer.
In addition to the genetic factors, the available data showed that the endocannabinoid system plays an important role in the development and progression of gynecological malignancies. The activation of cannabinoid receptor type 1 (CB1R), a G-protein coupled receptor present in the central nervous system and peripheral tissues such as the ovaries and uterus, can regulate cell proliferation, differentiation, and death. Moreover, cannabinoid receptor activation can inhibit cancer cell invasion through indirect downregulation of metalloproteinase (MMP2) expression and activity. Endocannabinoids also play a role in the inhibition of neoangiogenesis by decreasing the production of proangiogenic factors and/or by directly modulating endothelial cells, decreasing the expression of vascular endothelial growth factor (VEGF) and VEGF receptor (VEGF-R). The cannabinoid receptor type 2 (CB2R) is expressed the most in endometrial cancer biopsies and has a potential role in the control of cancer cell growth through the regulation of mitochondrial function and cell apoptosis (42,43).
Despite the availability of guidelines for assessment, genetic testing, and genetic counseling of patients diagnosed with endometrial cancer, these services are not offered equitably or consistently (44-47). Genetic counseling is a process to support individuals and families affected by or are at risk of genetic or hereditary diseases. Trained healthcare personnel provide counseling, and the assessment is based on a combination of the personal medical and family history. The aim of the counseling is to help individuals understand and adapt to the clinical, psychosocial, economical, and ethical issues raised during the diagnostic process, with a focus on disease information, inheritance, genetic testing, management options, career, and family plan, as well as management of affected or at-risk relatives (47-50).
Hinchcliff et al. (44) summarized the literature on genetic counseling and genetic testing of patients affected by gynecologic cancers and observed that a small proportion of studies provided assessment on guideline compliance or tumor screening completion rate [immunohistochemistry (IHC) and/or MSI], genetic counseling, or genetic testing among patients diagnosed with endometrial cancer in the United States. In addition, the authors draw attention to the variability of tumor screening among hospitals and also among healthcare providers ordering the testing, interpretation of findings, and patient triage to genetics services.
Although endometrial cancer is mostly diagnosed in women over the age of 60 years, approximately 5% of all women diagnosed are of reproductive age (51), and the impairment of reproductive function, especially ovarian insufficiency, is one of the long-term consequences of cancer therapy. The American Society of Clinical Oncology (ASCO) and the American Society for Reproductive Medicine (ASRM) strongly recommend clinicians to inform patients about the potential loss of fertility and possibility of fertility preservation before chemotherapy and radiotherapy (52). Moreover, quality of life represents a relevant concern in patient care; together with potential secondary infertility, the side effects of cancer diagnosis and treatment can affect the physical, mental, and social wellbeing of patients leading to depression and anxiety (53).
Because of the diversity and complexity of the genetic counseling process, in-depth approaches on patient counseling by a team should be considered. An ideal counseling team should consist of clinicians including oncologist, surgeon, obstetrician gynecologist, psychologist and/or psychiatrist, medical geneticist, molecular diagnostic specialist, genetics nurses, and ancillary staff (42-44).
Conclusions
In summary, a combination of genomic characterization along with pathological diagnosis, genetic counseling and psychological support are pivotal in improving the prognostic assessment and clinical management of patients with endometrial cancer.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Endometrial Cancer”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-2334
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-2334). The series “Endometrial Cancer” was commissioned by the editorial office without any funding or sponsorship. Dr. ASL served as the unpaid Guest Editor of the series, and serves as an unpaid editorial board member of Translational Cancer Research from Dec 2019 to Nov 2021. The authors have no other conflicts of interest to declare.
References
- 1.Alves M, Carreira I, Liberato P, et al. Identification of a 0.4 Kb deletion region in 10q26 associated with endometrial carcinoma. Oncol Rep 2010;23:519-22. [PubMed] [Google Scholar]
- 2.Ashton KA, Proietto A, Otton G, et al. Polymorphisms in genes of the steroid hormone biosynthesis and metabolism pathways and endometrial cancer risk. Cancer Epidemiol 2010;34:328-37. 10.1016/j.canep.2010.03.005 [DOI] [PubMed] [Google Scholar]
- 3.Ashton KA, Proietto A, Otton G, et al. Toll-like receptor (TLR) and nucleosome-binding oligomerization domain (NOD) gene polymorphisms and endometrial cancer risk. BMC cancer 2010;10:382. 10.1186/1471-2407-10-382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilbao C, Lara PC, Ramirez R, et al. Microsatellite instability predicts clinical outcome in radiation-treated endometrioid endometrial cancer. Int J Radiat Oncol Biol Phys 2010;76:9-13. 10.1016/j.ijrobp.2009.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cayan F, Tok E, Aras-Ates N, et al. Insulin receptor substrate-2 gene polymorphism: is it associated with endometrial cancer? Gynecol Endocrinol 2010;26:378-82. 10.3109/09513591003632241 [DOI] [PubMed] [Google Scholar]
- 6.Dobrzycka B, Terlikowski SJ, Mazurek A, et al. Circulating free DNA, p53 antibody and mutations of KRAS gene in endometrial cancer. Int J Cancer 2010;127:612-21. 10.1002/ijc.25077 [DOI] [PubMed] [Google Scholar]
- 7.Gaudet MM, Yang HP, Bosquet JG, et al. No association between FTO or HHEX and endometrial cancer risk. Cancer Epidemiol Biomarkers Prev 2010;19:2106-9. 10.1158/1055-9965.EPI-10-0515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghasemi N, Karimi-Zarchi M, Mortazavi-Zadeh MR, et al. Evaluation of the frequency of TP53 gene codon 72 polymorphisms in Iranian patients with endometrial cancer. Cancer Genet Cytogenet 2010;196:167-70. 10.1016/j.cancergencyto.2009.09.013 [DOI] [PubMed] [Google Scholar]
- 9.Janiec-Jankowska A, Konopka B, Goluda C, et al. TP53 mutations in endometrial cancers: relation to PTEN gene defects. Int J Gynecol Cancer 2010;20:196-202. 10.1111/IGC.0b013e3181c83675 [DOI] [PubMed] [Google Scholar]
- 10.Lee E, Hsu C, Haiman CA, et al. Genetic variation in the progesterone receptor gene and risk of endometrial cancer: a haplotype-based approach. Carcinogenesis 2010;31:1392-9. 10.1093/carcin/bgq113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Low YL, Li Y, Humphreys K, et al. Multi-variant pathway association analysis reveals the importance of genetic determinants of estrogen metabolism in breast and endometrial cancer susceptibility. PLoS Genet 2010;6:e1001012. 10.1371/journal.pgen.1001012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murayama-Hosokawa S, Oda K, Nakagawa S, et al. Genome-wide single-nucleotide polymorphism arrays in endometrial carcinomas associate extensive chromosomal instability with poor prognosis and unveil frequent chromosomal imbalances involved in the PI3-kinase pathway. Oncogene 2010;29:1897-908. 10.1038/onc.2009.474 [DOI] [PubMed] [Google Scholar]
- 13.Prescott J, McGrath M, Lee IM, et al. Telomere length and genetic analyses in population-based studies of endometrial cancer risk. Cancer 2010;116:4275-82. 10.1002/cncr.25328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sliwinski T, Sitarek P, Stetkiewicz T, et al. Polymorphism of the ERalpha and CYP1B1 genes in endometrial cancer in a Polish subpopulation. J Obstet Gynaecol Res 2010;36:311-7. 10.1111/j.1447-0756.2009.01143.x [DOI] [PubMed] [Google Scholar]
- 15.Terry K, McGrath M, Lee IM, et al. Genetic variation in CYP11A1 and StAR in relation to endometrial cancer risk. Gynecol Oncol 2010;117:255-9. 10.1016/j.ygyno.2010.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang PH, Yi YC, Tsai HT, et al. Significant association of genetic polymorphism of human nonmetastatic clone 23 type 1 gene with an increased risk of endometrial cancer. Gynecol Oncol 2010;119:70-5. 10.1016/j.ygyno.2010.06.013 [DOI] [PubMed] [Google Scholar]
- 17.Yang HP, Gonzalez Bosquet J, Li Q, et al. Common genetic variation in the sex hormone metabolic pathway and endometrial cancer risk: pathway-based evaluation of candidate genes. Carcinogenesis 2010;31:827-33. 10.1093/carcin/bgp328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi YC, Chou PT, Chen LY, et al. Matrix metalloproteinase-7 (MMP-7) polymorphism is a risk factor for endometrial cancer susceptibility. Clin Chem Lab Med 2010;48:337-44. 10.1515/CCLM.2010.082 [DOI] [PubMed] [Google Scholar]
- 19.Cheung LW, Hennessy BT, Li J, et al. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer Discov 2011;1:170-85. 10.1158/2159-8290.CD-11-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doherty JA, Weiss NS, Fish S, et al. Polymorphisms in nucleotide excision repair genes and endometrial cancer risk. Cancer Epidemiol Biomarkers Prev 2011;20:1873-82. 10.1158/1055-9965.EPI-11-0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hlavna M, Kohut L, Lipkova J, et al. Relationship of resistin levels with endometrial cancer risk. Neoplasma 2011;58:124-8. 10.4149/neo_2011_02_124 [DOI] [PubMed] [Google Scholar]
- 22.Karageorgi S, McGrath M, Lee IM, et al. Polymorphisms in genes hydroxysteroid-dehydrogenase-17b type 2 and type 4 and endometrial cancer risk. Gynecol Oncol 2011;121:54-8. 10.1016/j.ygyno.2010.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karageorgi S, Prescott J, Wong JY, et al. GSTM1 and GSTT1 copy number variation in population-based studies of endometrial cancer risk. Cancer Epidemiol Biomarkers Prev 2011;20:1447-52. 10.1158/1055-9965.EPI-11-0190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konopka B, Janiec-Jankowska A, Kwiatkowska E, et al. PIK3CA mutations and amplification in endometrioid endometrial carcinomas: relation to other genetic defects and clinicopathologic status of the tumors. Hum Pathol 2011;42:1710-9. 10.1016/j.humpath.2010.01.030 [DOI] [PubMed] [Google Scholar]
- 25.Krupa R, Sobczuk A, Poplawski T, et al. DNA damage and repair in endometrial cancer in correlation with the hOGG1 and RAD51 genes polymorphism. Mol Biol Rep 2011;38:1163-70. 10.1007/s11033-010-0214-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacey JV, Jr, Yang H, Gaudet MM, et al. Endometrial cancer and genetic variation in PTEN, PIK3CA, AKT1, MLH1, and MSH2 within a population-based case-control study. Gynecol Oncol 2011;120:167-73. 10.1016/j.ygyno.2010.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li G, Xiang YB, Courtney R, et al. Association of a single nucleotide polymorphism at 6q25.1,rs2046210, with endometrial cancer risk among Chinese women. Chin J Cancer 2011;30:138-43. 10.5732/cjc.010.10516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lurie G, Gaudet MM, Spurdle AB, et al. The obesity-associated polymorphisms FTO rs9939609 and MC4R rs17782313 and endometrial cancer risk in non-Hispanic white women. PloS One 2011;6:e16756. 10.1371/journal.pone.0016756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker JC, Goodfellow PJ. Traditional Approaches to Molecular Genetic Analysis. In: Ellenson LH. Molecular Genetics of Endometrial Carcinoma (Advances in Experimental Medicine and Biology). 1st edition. New York: Springer, 2017:99-118. [DOI] [PubMed] [Google Scholar]
- 30.Yeramian A, Moreno-Bueno G, Dolcet X, et al. Endometrial carcinoma: molecular alterations involved in tumor development and progression. Oncogene 2013;32:403-13. 10.1038/onc.2012.76 [DOI] [PubMed] [Google Scholar]
- 31.Matias-Guiu X, Prat J. Molecular pathology of endometrial carcinoma. Histopathology 2013;62:111-23. 10.1111/his.12053 [DOI] [PubMed] [Google Scholar]
- 32.Bohaumilitzky L, von Knebel Doeberitz M, Kloor M, et al. Implications of Hereditary Origin on the Immune Phenotype of Mismatch Repair-Deficient Cancers: Systematic Literature Review. J Clin Med 2020;9:E1741. 10.3390/jcm9061741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kloor M, von Knebel Doeberitz M. The Immune Biology of Microsatellite-Unstable Cancer. Trends Cancer 2016;2:121-33. 10.1016/j.trecan.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 34.Wild PJ, Ikenberg K, Fuchs TJ, et al. p53 suppresses type II endometrial carcinomas in mice and governs endometrial tumour aggressiveness in humans. EMBO Mol Med 2012;4:808-24. 10.1002/emmm.201101063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winder AD, Maniar KP, Wei JJ, et al. Synuclein-gamma in uterine serous carcinoma impacts survival: an NRG Oncology/Gynecologic Oncology Group study. Cancer 2017;123:1144-55. 10.1002/cncr.30477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bafligil C, Thompson DJ, Lophatananon A, et al. Association between genetic polymorphisms and endometrial cancer risk: a systematic review. J Med Genet 2020;57:591-600. 10.1136/jmedgenet-2019-106529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benati M, Montagnana M, Danese E, et al. Aberrant Telomere Length in Circulating Cell-Free DNA as Possible Blood Biomarker with High Diagnostic Performance in Endometrial Cancer. Pathol Oncol Res 2020;26:2281-9. 10.1007/s12253-020-00819-x [DOI] [PubMed] [Google Scholar]
- 38.Talhouk A, McConechy MK, Leung S, et al. A clinically applicable molecular-based classification for endometrial cancers, Br J Cancer 2015;113:299-310. 10.1038/bjc.2015.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stelloo E, Bosse T, Nout RA, et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod Pathol 2015;28:836-44. 10.1038/modpathol.2015.43 [DOI] [PubMed] [Google Scholar]
- 40.Stelloo E, Nout RA, Osse EM, et al. Improved Risk Assessment by Integrating Molecular and Clinicopathological Factors in Early-stage Endometrial Cancer-Combined Analysis of the PORTEC Cohorts. Clin Cancer Res 2016;22:4215-24. 10.1158/1078-0432.CCR-15-2878 [DOI] [PubMed] [Google Scholar]
- 41.Casarin J, Bogani G, Serati M, et al. Presence of Glandular Cells at the Preoperative Cervical Cytology and Local Recurrence in Endometrial Cancer. Int J Gynecol Pathol 2020;39:522-8. 10.1097/PGP.0000000000000642 [DOI] [PubMed] [Google Scholar]
- 42.Guida M, Ligresti A, De Filippis D, et al. The levels of the endocannabinoid receptor CB2 and its ligand 2-arachidonoylglycerol are elevated in endometrial carcinoma. Endocrinology 2010;151:921-8. 10.1210/en.2009-0883 [DOI] [PubMed] [Google Scholar]
- 43.Grauso F, De Franciscis P, Schiattarella A, et al. A review on the role of the endocannabinoid system in the gynecological malignancy. Ital J Gynaecol Obstet 2019;31:35-41. [Google Scholar]
- 44.Hinchcliff EM, Bednar EM, Lu KH, et al. Disparities in gynecologic cancer genetics evaluation. Gynecol Oncol 2019;153:184-91. 10.1016/j.ygyno.2019.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laganà AS, La Rosa VL, Rapisarda AM, et al. Comment on: "Needs and priorities of women with endometrial and cervical cancer". J Psychosom Obstet Gynaecol 2017;38:85-6. 10.1080/0167482X.2016.1244186 [DOI] [PubMed] [Google Scholar]
- 46.Vitale SG, Rossetti D, Tropea A, et al. Fertility sparing surgery for stage IA type I and G2 endometrial cancer in reproductive-aged patients: evidence-based approach and future perspectives. Updates Surg 2017;69:29-34. 10.1007/s13304-017-0419-y [DOI] [PubMed] [Google Scholar]
- 47.Vitale SG, Capriglione S, Zito G, et al. Management of endometrial, ovarian and cervical cancer in the elderly: current approach to a challenging condition. Arch Gynecol Obstet 2019;299:299-315. 10.1007/s00404-018-5006-z [DOI] [PubMed] [Google Scholar]
- 48.Lolkema MP, Gadellaa-van Hooijdonk CG, Bredenoord AL, et al. Ethical, legal, and counseling challenges surrounding the return of genetic results in oncology. J Clin Oncol 2013;31:1842-8. 10.1200/JCO.2012.45.2789 [DOI] [PubMed] [Google Scholar]
- 49.Heald B, Rybicki L, Clements D, et al. Assessment of clinical workload for general and specialty genetic counsellors at an academic medical center: a tool for evaluating genetic counselling practices. NPJ Genom Med 2016;1:16010. 10.1038/npjgenmed.2016.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim JY, Byeon JS. Genetic Counseling and Surveillance Focused on Lynch Syndrome. J Anus Rectum Colon 2019;3:60-8. 10.23922/jarc.2019-002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 52.Oktay K, Harvey BE, Partridge AH, et al. Fertility Preservation in Patients with Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol 2018;36:1994-2001. 10.1200/JCO.2018.78.1914 [DOI] [PubMed] [Google Scholar]
- 53.La Rosa VL, Garzon S, Gullo G, et al. Fertility preservation in women affected by gynaecological cancer: the importance of an integrated gynaecological and psychological approach. Ecancermedicalscience 2020;14:1035. 10.3332/ecancer.2020.1035 [DOI] [PMC free article] [PubMed] [Google Scholar]


