Abstract
Phosphoinositides are membrane phospholipids involved in a variety of cellular processes like growth, development, metabolism, and transport. This review focuses on the maintenance of cellular homeostasis of phosphatidylinositol 4,5-bisphosphate (PIP2), and phosphatidylinositol 3,4,5-trisphosphate (PIP3). The critical balance of these PIPs is crucial for regulation of neuronal form and function. The activity of PIP2 and PIP3 can be regulated through kinases, phosphatases, phospholipases and cholesterol microdomains. PIP2 and PIP3 carry out their functions either indirectly through their effectors activating integral signaling pathways, or through direct regulation of membrane channels, transporters, and cytoskeletal proteins. Any perturbations to the balance between PIP2 and PIP3 signaling result in neurodevelopmental and neurodegenerative disorders. This review will discuss the upstream modulators and downstream effectors of the PIP2 and PIP3 signaling, in the context of neuronal health and disease.
Keywords: Phosphoinositides, cholesterol, autism, Alzheimer’s, ion channels, cytoskeleton, AKT, mammalian target of rapamycin
Introduction
Phosphoinositides are membrane phospholipids involved in regulation of key cellular processes that range from cell growth, protein metabolism, membrane remodeling, and trafficking, to cell death [1–5]. Traditionally, phosphoinositides have been characterized as precursors to secondary messengers for cellular pathways [6, 7], however, evidence for direct roles for these phospholipids in membrane dynamics is also piling up [8]. Chemically, the seven distinct species of phosphoinositides are derived from unique phosphorylations of myo-inositol head group of the phosphatidylinositol (PI) at position 3,4 and 5 hydroxyl residues, either singly or in combination [9]. This addition and removal of phosphate groups is selectively regulated by specific phosphoinositide kinases and phosphatases, which are evolutionarily well-conserved [2].
PI 4,5-bisphosphate [PI(4,5)P2, hereon referred to as PIP2], and PI 3,4,5-trisphosphate [PI(3,4,5)P3, hereon referred to as PIP3] are the focus of this review. PIP2 and PIP3 are constituents of plasma membrane and their precisely regulated abundance changes in response to cell intrinsic and/or extrinsic stimuli. The PIP2 is primarily formed as a result of sequential phosphorylations at position 4 and position 5 hydroxyl residues of PI by PI 4-kinase (PI4K), and PI phosphate kinase (PIPK), respectively. PIP3 is generated from PIP2 by addition of a phosphate on position 3 hydroxyl residue by Class I phosphoinositide 3-kinase (PI3K). Conversely, dephosphorylation of PIP3 into PIP2 by phosphatase and tensin homolog deleted on chromosome 10 (PTEN) phosphatase also contributes towards maintenance of a PIP2/PIP3 homeostasis in the cells (Figure 1). In the nervous system, both PIP2 and PIP3 play essential roles in regulating neuronal morphogenesis, electrical activity, ion channels and neurotransmitters mediated signal transduction, synaptic plasticity, and cytoskeletal remodeling [10–13]. Perturbations to the delicate balance between PIP2 and PIP3 levels result in aberrant neurodevelopment and neurodegenerative diseases, like autism spectrum disorders (ASD) and Alzheimer’s disease (AD), among others [14, 15]. This review discusses the upstream modulators that maintain this PIP2/PIP3 balance, and downstream mediators that are influenced by it, in the context of neuronal regulation and disease.
Figure 1.

Biosynthesis of PIP2 and PIP3 at plasma membrane-cytoplasm interface. The equilibrium between synthesis of PIP2 and PIP3 at membrane-cytoplasm interface is maintained through addition of phosphate groups (orange) by kinases (green), and its removal by phosphatases (red) at position 3,4, or 5 of the cytoplasmic inositol head group of phosphoinositides (PI). INPP4: inositol polyphosphate 4-phosphatase; SACM1L: SAC1 like phosphatidylinositide phosphatase; INPP5: inositol polyphosphate 5-phosphatase; TPIP: transmembrane phosphatase with tensin homology (TPTE) and PTEN homologous inositol lipid phosphatase
Modulators of PIP2/PIP3 balance
PIP2 and PIP3 are primarily distributed at the cytoplasmic leaflet of plasma membrane and their localized abundance plays diverse roles in regulation of varied cellular processes. A cell needs to maintain a critical balance of these PIs for its normal function. PIP2 and PIP3 are essential for ligand-associated receptor tyrosine kinase (RTK) and G-protein coupled receptor (GPCR) mediated signaling [16], and their cellular levels and activity can be modulated by PI kinases and PI phosphatases [17], which are described below:
Kinases
Phosphoinositide kinases are diverse group of enzymes that perform the addition of phosphate group on the hydroxyl residues of myo-inositol ring of PIs. These kinases have substrate specificity for PIs, as well as specificity for their target hydroxyl residue on these PIs.
PI4Ks
The formation of PIP2 is catalyzed by two sequential phosphorylations, first of which is catalyzed by PI4Ks. The two classes of PI4Ks (type II and type III) are categorized into two distinct groups of isozymes, each based on their structure, which lends itself to their specific spatiotemporal activities (Figure 2A). Type II PI4Ks are categorized into PI4KIIα (gene names are italicized and put in brackets; PI4K2A) and PI4KIIβ (PI4K2B) isozymes, while Type III PI4Ks are categorized into PI4KIIIα (PI4KA) and PI4KIIIβ (PI4KB) isozymes [18]. Structurally, type II isozymes have a bisected kinase domain, with its cystine-rich (CR) N-terminal half containing a palmitoylation site, which is likely involved in membrane tethering [19, 20]. The type II isozymes are homologous but have differences in their N-terminals. PI4KIIα contains a proline-rich (PR) region at its N-terminal, while PI4KIIβ contains an acidic region (AR) [21] (Figure 2A). PI4KIIα and PI4KIIβ are ubiquitously expressed but PI4KIIα has higher expression in brain tissue when compared to PI4KIIβ and its dysfunction is associated with late onset neurodegenerative disease in mouse models [21, 22]. Both PI4KIIα and PI4KIIβ localize to intracellular membranes and have been reported to be involved in cellular vesicle trafficking [23, 24]. The association of PI4KIIα with synaptic vesicles points to its role in neurotransmission [25].
Figure 2.
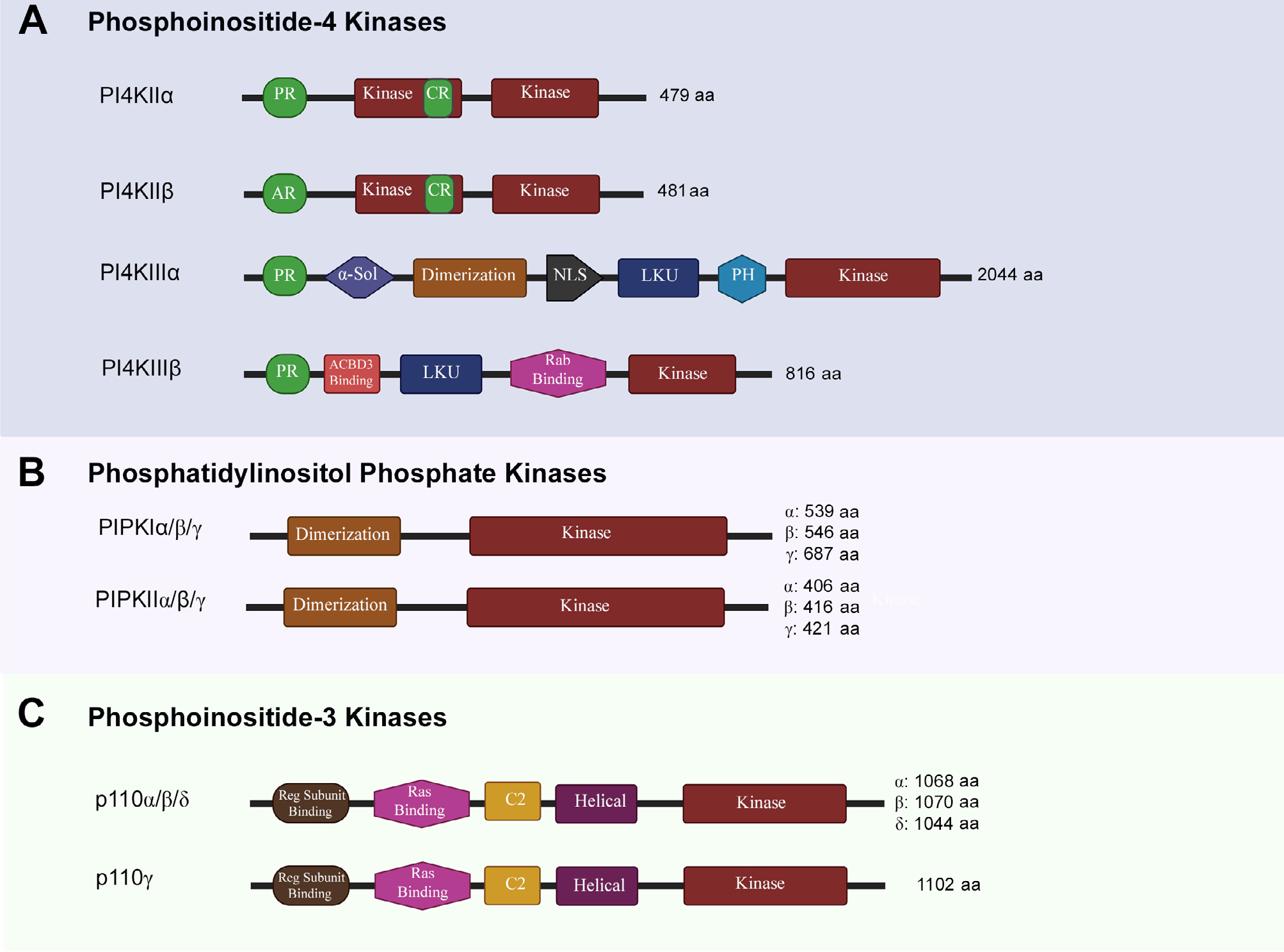
Structural features of phosphoinositide kinases. aa: amino acid; α-Sol: α-solenoid domain; NLS: nuclear localization signal; LKU: lipid kinase unique; PH: pleckstrin homology domain; ACBD3: acyl-CoA-binding protein 3; Reg: regulatory. The schematics for structural domains are not to scale
The Type III isozyme, PI4KIIIα is primarily localized at plasma membrane and is the main source of generation of precursor PIs. Generation of PIP2 from these precursor PIs regulates Ca2+ signaling [26]. Structurally, both type III isozymes contain a N-terminal pleckstrin homology (PH) domain, a LKU domain and a continuous kinase domain on the C-terminal. PI4KIIIα contains a large N-terminal solenoid domain (α-Sol), whose role remains unknown [27]. PI4KIIIα also contains a NLS and a PH domain, implicated in plasma membrane association [28]. PI4KIIIβ contains a Rab-binding region implicated in lipid transport and membrane trafficking [29].
The activity of type III PI4Ks is specified by their association with a myriad of regulatory binding partners. PI4KIIIα directly interacts with tetratricopeptide repeat domain 7 (TTC7)A/B, with FAM126 acting as a scaffold, to form a dimer of heterotrimers [30]. TTC7 interacts with plasma membrane associated EFR3 homolog A (EFR3A) protein to activate PI4KIIIα. On the other hand, PI4KIIIβ interacts with a variety of binding partners of its own, namely Rab11 GTPase, ACBD3, and 14–3-3 regulatory proteins. The Rab11 binding localizes Rab11 to trans-Golgi network (TGN), while the interaction with ACBD3 is reported to be necessary for viral pathogenesis [31]. Protein kinase D mediated S294 phosphorylation stabilizes PI4KIIIβ by allowing the binding of 14–3-3 proteins [29, 32].
PIPKs
PIP2 can be generated by two different biosynthetic pathways. In the canonical route for synthesis of PIP2, a second phosphorylation of PI 4-phosphate (PI4P) at position 5 hydroxyl residue is catalyzed by type I PIPKs (PIPKIs) to generate PIP2 [33]. In the non-canonical route, PI 5-phosphate (PI5P) can be phosphorylated by type II PIPKs (PIPKIIs) at position 4 hydroxyl residue to generate same PIP2 as well [34]. PI5P is relatively low-abundance and this PIPKII mediated phosphorylation is considered to be a mechanism to regulate its levels in the cell. The Type I PIPKs have three distinct isozymes termed as PIPKIα (PIP5K1A), PIPKIβ (PIP5K1B), and PIPKIγ (PIP5K1C) [33]. The mRNA coding for PIPKIγ can be alternatively spliced to give rise to PIPKI γ635, γ661 and γ687 variants [35]. PIPKIIs are PIPKIIα (PIP4K2A), PIPKIIβ (PIP4K2B), and PIPKIIγ (PIP4K2C) isozymes [36]. Structurally, both types of PIPKs share a homologous central lipid kinase domain (PIPKc) and a conserved dimerization domain. The PIPKc has an activation loop at C-terminal that is specific for substrate and subcellular localization. The variability on N- and C-terminal also facilitates isoform-specific functions of PIPKs [37] (Figure 2B).
Different homo-dimerization mechanisms have been reported for PIPKIs and PIPKIIs in animal models, giving rise to the idea of different interaction surfaces for their binding partners. The PIPKIs have been reported to be activated by Ras homolog family member A (RhoA)/Rac family small GTPase 1 (Rac1), ADP ribosylation factor 6 (ARF6) GTPases, and Wnt signaling through dishevelled segment polarity protein (DVL), in an isoform-selective manner [38–40]. PIP5KII’s isoforms, on the other hand, can form both homo- and heterodimers which may modulate its activity. The activity of PIPKIIα is modulated by phosphorylation of its activation loop by protein kinase D, while the activity of PIPKIIβ is regulated by p38 mitogen activated protein kinase (MAP kinase) [41–43]. PIPKIIβ is also proposed as a GTP sensor in cells, because of its preference for GTP instead of ATP [44]. Not much is known when it comes to regulation of PIPKIIγ activity, due to it being non-functional when expressed in bacterial cells [45]. Both types of PIPKs play important role in development, actin dynamics, autophagy, and polarity of cells [17, 46]. A type III class of PIPKs (PIPKIII, or PIKfyve) also exists which is involved in phosphorylating PI and PI3P at position 5 hydroxyl residue to produce PI5P and PI(3,5)P2 respectively, but its characterization is beyond the scope of this review.
PI3K
The PI3Ks are divided into three classes based on their structure and substrate specificity. Class I PI3K catalyzes phosphorylation of position 3 hydroxyl residue of PIP2 to generate PIP3. Based on their structure and composition, Class I PI3Ks are further divided into two subclasses i.e., Class IA PI3Ks and Class IB PI3Ks. Class IA PI3Ks are heterodimeric proteins consisting of a catalytic subunit p110α, p110β, or p110δ; encoded by PIK3CA/B/D genes and a regulatory subunit [p85/55/50α, p85β, or p55γ variants; encoded by PIK3R1/2/3 genes respectively) [47, 48]. These regulatory subunits play important role in stabilizing the catalytic subunits, inhibiting their activity, and recognize the phosphorylated YXXM motif on intracellular receptors and adaptors through their Src homology 2 (SH2) domains to allow for plasma membrane localization, adaptor binding and increased kinase activity [49, 50]. Class IB PI3Ks consist of a p110γ (PIK3CG) catalytic subunit, which can associate with either p101 (PIK3R5), or p84 (also known as p87; PIK3R6) regulatory subunits to form a heterodimer that can get activated through interaction with Gβγ subunits of GTP-binding proteins [51]. In fact, both subclasses can be activated through interaction of GTP-Ras at the Ras-binding domain. The C2 and helical domains are thought to take part in membrane tethering [52] (Figure 2C).
The ability to generate PIP3 depends on the tissue specific expression and activation of the specific isoforms by the RTKs and GPCRs. There is considerable overlap in RTKs and GPCRs mediated downstream signaling, leading to synergistic activation of different PI3K isoforms [53]. In the context of neurons, the p110α subunit is mainly activated through RTKs and plays important role in insulin signaling which is important for cell survival, energy metabolism, synaptic development, and plasticity [54–56]. The p110β subunit is primarily associated with GPCRs and is reported to regulate glutamate receptor dependent (mGlu1/5) form of plasticity and protein synthesis in the brain [57, 58]. The P110γ has been implicated in N-methyl-D-aspartate (NMDA)-dependent neuronal plasticity, while P110δ seems to be involved in axonal growth and regeneration [59, 60].
Phosphatases
Phosphoinositide phosphatases are enzymes that catalyze removal of phosphate groups from the position 3,4 or 5 hydroxyl residues of myo-inositol ring of PIs. Just like PI kinases, these phosphatases also have substrate and catalytic site specificity. Here, phosphatases that recognize only PIP2 or PIP3 as their substrates are in focus.
Phosphoinositide 3-phosphatases
Phosphoinositide 3-phosphatases (PI3 phosphatases) primarily dephosphorylate PIP3 on position 3, thereby antagonizing the activity of Class I PI3 kinases and generating PIP2. The activity of these phosphatases is important for maintaining a PIP2/PIP3 balance and even small changes may have dramatic effects on neuronal growth and development. The PI3 phosphatases include PTEN, TPTE, and TPIP. PTEN is a dual-specificity lipid and protein phosphatase. Structurally, PTEN contains a CX5R catalytic motif in its phosphatase domain which is common to all PI3 phosphatases; a lipid-binding C2 domain which mediates membrane binding; two C-terminal PEST (proline, glutamine, serine, threonine) sequences that enhance sensitivity to proteolysis; and a PDZ domain important for stability and binding proteins [61] (Figure 3A). PTEN is distributed throughout the cell but has the highest catalytic activity when associated with membranes [62]. Nuclear-associated PTEN has tumor-suppressor activity [63], while nuclear-excluded PTEN has been associated with dysregulation of neuronal growth [64]. PTEN gene activity is reported to be modulated by its splice variants and post transcriptional modifications [65]. Whether PTEN can access nuclear phospholipids is currently a debated topic in the literature.
Figure 3.
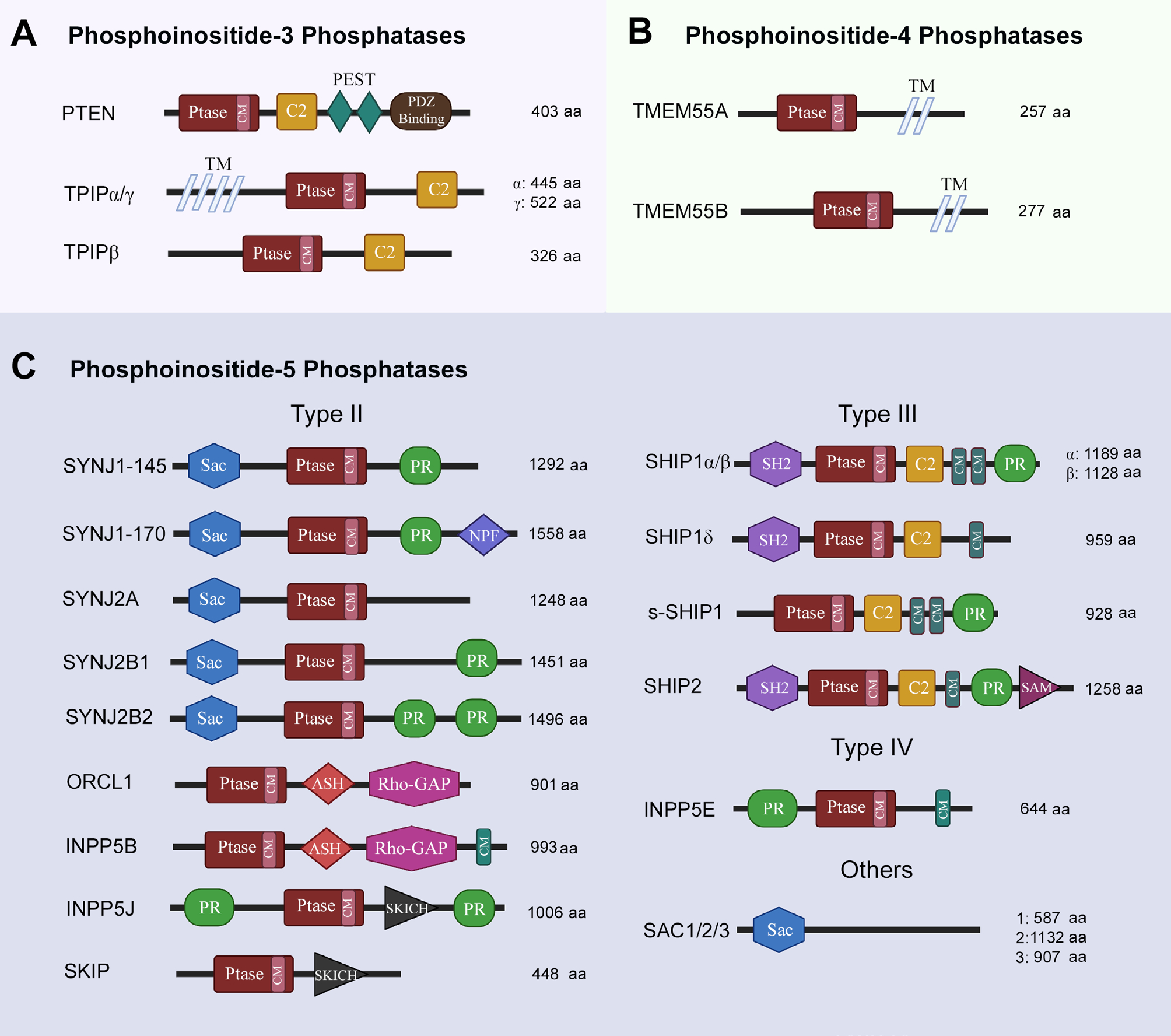
Structural features of phosphoinositide phosphatases. Ptase: phosphatase domain; CM: consensus motif; PEST: proline, glutamine, serine, threonine-rich sequence; TM: transmembrane spanning region; NPF: asparagine, proline, phenylalanine repeats; SKICH: skeletal muscle- and kidney-enriched inositol polyphosphate phosphatase (SKIP) carboxyl hydroxy domain; SH2: Src homology 2 domain; SAM: sterile α motif; SYNJ: synaptojanin; ORCL1: oculocerebrorenal syndrome of Lowe-1; ASH: ASPM-SPD-2-Hydin; Rho-GAP: Rho GTPase activating protein; SHIP: SH2 containing inositol phosphatase; s-SHIP: stem cell specific isoform of SHIP. The schematics for structural domains are not to scale
TPTE (TPTE) is found in plasma membrane but is reported to lack PI phosphatase activity [66]. TPIP (TPTE2) exists as three isoforms (TPIPα, β and γ). TPIPα is endoplasmic reticulum (ER)-localized while TPIPγ is reported to be cytosolic [67]. All isoforms have a phosphatase domain and a C2 domain. TPIPα and TPIPγ are predicted to have transmembrane (TM) segments that display homology to voltage-sensing phosphatases (VSP) [67, 68]. The best characterized VSP is found in marine invertebrate Ciona intestinalis, which contains a voltage sensing domain (VSD). This ci-VSP recognizes both PIP3 and PIP2 as its substrate for dephosphorylation and gets activated in response to membrane depolarization [69].
Phosphoinositide 4-phosphatases
Phosphoinositide 4-phosphatases (PI4 phosphatases) catalyze the removal of phosphorylation from position 4 of myo-inositol has group of PIs. Two types of PI4 phosphatases have been identified in mammals, namely INPP4 and TMEM55. Out of these two, only TMEM55 proteins (TMEM55A, TMEM55B; encoded by TMEM55A/B) will be discussed as they recognize PIP2 as their substrate to dephosphorylate position 4, converting it into PI5P [17]. Both TMEM55A and TMEM55B proteins are expressed ubiquitously; contain a CX5R motif in their phosphatase domain, and are named after two TM segments on their C-terminal [70] (Figure 3B). TMEM55 proteins are not well-characterized but have been reported to be involved in embryonic growth factor receptor (EGFR) degradation, cholesterol homeostasis, DNA damage response and p53-mediated cell death [70–73].
Phosphoinositide 5-phosphatases
Phosphoinositide 5-phosphatases (PI5 phosphatases) are the most abundant lipid phosphatases. There are three classes (II, III, and IV) of INPP5s with an inositol 5-phosphatase (5-Ptase) domain, which contains motifs for phosphoinositide substrate selectivity [74, 75]. The type II enzymes are synaptojanins, ORCL1, INPP5B, INPP5J, and SKIP. Both synaptojanin isozymes (SYNJ1 and SYNJ2), including their splice variants (145, 170, A, B1, B2), share similar structures consisting of a N-terminal Sac domain, a central 5-Ptase domain and a C-terminal PR region. All synaptojanins dephosphorylate PIP2 and PIP3 on position 5 [76, 77]. Both SYNJ1–145 and SYNJ2B are reported to be especially localized in nerve terminals and synapsis [78, 79]. The ORCL1 (ORCL) also removes the 5-phosphate from PIP2 and PIP3; contains 5-Ptase, ASH and Rho-GAP-like domains; but only one of its splice variants (ORCL1a) is expressed in brain [80–82]. INPP5B (INPP5B) is structurally similar to ORCL, but with an additional CAAX motif on C-terminal [83]. INPP5B expression has not been reported in brain. INPP5J (INPP5J), on the other hand, has been detected in brain; contains an additional SKICH domain; and seems to be involved in neurite elongation [84–86]. SKIP (SKIP) itself has a preference for PIP3 as a substrate and is expressed ubiquitously [87].
SHIP family enzymes (SHIP1 and SHIP2) are type III PI5 phosphatases whose alternative splicing gives rise to SHIP1α, SHIP1β, SHIP1γ and s-SHIP1 [88]. All SHIP family enzymes contain an SH2 domain, a PR region, and a NPXY motif whose phosphorylation allows for interactions with binding partners containing immunoreceptor tyrosine inhibitory motif (ITIM)/ immunoreceptor tyrosine activating motif (ITAM), phosphotyrosine-binding (PTB) or SH2 domains. SHIP2 also has an additional SAM domain [17, 89]. All SHIP1 isoforms and SHIP2 recognize PIP3 as their substrate, however, only SHIP2 is expressed ubiquitously [90]. Pharbin (INPP5E) is the only type IV PI5 phosphatase, contains a PR region, 5-Ptase domain and a CAAX motif; is expressed in brain; and has the highest affinity (Km = 0.65 μmol/L for PIP3 recognition as a substrate than any other PI5 Phosphatase [91]. Another family of PI5 phosphatases is Sac family of phosphatases (Sac1, Sac2, Sac3), which differ from the rest by the lack of 5-Ptase domain. Sac2 and Sac3 dephosphorylate both PIP2 and PIP3 [92] (Figure 3C). Sac2 and Sac3 are expressed ubiquitously but Sac2 expression is especially high in the brain tissue. Both Sac2 and Sac3 have been implicated in neuronal outgrowth [93, 94].
Phospholipases
Phospholipases are enzymes that hydrolyze phospholipids into its constituent fatty acids. The most relevant class of phospholipases to our discussion is phospholipase C (PLC) class of PI-specific enzymes, which cleave PIP2 to generate diacylglycerol (DAG) and inositol-1,4,5-triphosphate (IP3). DAG and IP3 are important secondary messengers that are involved in protein kinase C (PKC) signaling, intra-neuronal calcium (Ca2+) signaling, and transcription, among other regulatory roles [6]. PI specific PLCs have been classified into six families (β, γ, δ, ε, ζ, and η; Figure 4). Alternative splicing reportedly leads to the generation of about 30 isozymes in mammals [95]. All PI specific isozymes have a homologues core consisting of a N-terminal PH domain, four EF domains, a triose phosphate isomerase (TIM) barrel domain (X, Y), and a C-terminal C2 domain [96, 97]. The subtle structural nuances and specific combinations of these domains can regulate distribution and function of each PLC isozyme. PLC enzymes are activated through either GPCRs (β, δ, and η), or RTKs (γ and ζ), or both (ε) [98]. Of note here is a suggested role for the nuclear PLC-β1 in PIP2 hydrolysis, raising questions about the existence of PI signaling in the nucleus [99]. The isoforms PLC-δ (1,3,4), PLC-β (1,4), PLC-γ (1), PLC-ε and PLC-η (1,2) are most relevant to nervous system and have been implicated in neurodegenerative disorders [100].
Figure 4.

Structural features of phospholipase C family. EF: helix-loop-helix motif; X,Y: TIM barrel domain; SH2: Src homology 2 domain; SH3: Src homology 3 domain. The schematics for structural domains are not to scale
Cholesterol-rich microdomains
The cellular plasma membranes are heterogenous in nature and are organized into microdomains or ordered/disordered liquid phases, based on their lipid and protein constituents. In the last decade or so, it has been reported that composition of these microdomains dictates localization of PIs to specific regions of the plasma membranes [101]. An important regulator of this localization is reported to be cholesterol. Plasma membrane microdomains can be classified, on the basis of cholesterol abundance, into cholesterol-rich, liquid-ordered (Lo) raft domains or cholesterol-poor, liquid-disordered (Ld) non-raft domains. Recent literature suggests that PIP2 is present in both domains, however, it gets hydrolyzed by PLC faster, and is also restored more rapidly in cholesterol-rich (Lo) domain [102]. This compartmentalization of PIP2 signaling seems to be conserved as it has been reported in plants membranes as well [103]. In the context of neurodegenerative diseases, increasing cholesterol levels in the membranes led to PLC-mediated depletion of PIP2 and an increase in AD-associated secretory amyloid β42 in cell lines [104]. PTEN phosphatase binding to PIP2 is also reported to be increased in cholesterol-rich environments [105].
Effectors of PIP2 and PIP3 signaling
Classically, PIP2 and PIP3 were primarily thought of as precursors to secondary messengers that mediate activity of integral cellular signaling pathways through interaction with cytoplasmic proteins. However, PIP2 and PIP3 also play direct roles in mediating the activities of membrane-bound ion channels and transporters. These direct and indirect roles of PIP2 and PIP3 signaling in mediating cellular development and function are described below:
IP3 and DAG signaling
GPCRs or RTKs mediated activation of PLC isoforms leads to hydrolysis of PIP2 into IP3 and DAG [6]. IP3 binds to IP3 receptors (IP3Rs) on the ER to release Ca2+. These IP3Rs are found on nuclear envelope as well. In order for Ca2+ to release from ER into the cytosol, IP3 first needs to bind to all four monomers of an IP3R tetramer, causing a conformational change that allows Ca2+ to pass. This allowance is, however, transient. When Ca2+ levels rise above a certain level, this signaling becomes inhibitory through complex feedback interactions [106–108]. These IP3 induced Ca2+ oscillations are found in multiple cell types. In brains, these oscillations regulate differentiation and proliferation [109]. IP3 levels regulate steering of axonal growth cones, while Ca2+ transients specify if a neuron will be inhibitory or regulatory by way of regulating neurotransmitters release [110]. Low-frequency oscillations lead to release of excitatory neurotransmitters (acetylcholine, glutamate), while higher frequency oscillations lead to expression of inhibitory transmitters (glycine, γ-aminobutyric acid) [111]. These oscillations are important in generating brain rhythms for sleep/wake cycle, memory formation, and synaptic plasticity [112, 113]. Alterations in IP3/Ca2+ signaling are associated with many neurological disorders like AD, ASD, epilepsy, schizophrenia among others, which are discussed in section Disease relevance.
DAG, the second product of PIP2 hydrolysis, is most notably involved in PKC signaling. The PKC family of kinases have two DAG-binding copies of C1 domains, and in the case of conventional PKCs (cPKCs), an additional C2 domain to sense intracellular Ca2+ levels for full activation [114, 115]. The subcellular distribution of cPKCs is cytosolic under basal Ca2+ conditions, and it has now been shown that Ca2+ binding to C2 domain is sufficient and necessary for rapid membrane translocation. The DAG association with C1 domain of cPKC is important for its retention on the membrane [116]. Hence, the downstream signaling of both PIP2 hydrolysis products is intricately connected and has critical roles to play in activation of cPKCs. The activation of cPKCs has been implicated in interfering with inhibitory binding of calmodulin at plasma membrane Ca2+-ATPases (PMCA); regulation of transient receptor potential (TRP), Na+/Ca2+ exchanger (NCX) and sodium proton exchangers (NHE) channels; generation of cyclic adenosine monophosphates (cAMPs); and activation of phospholipase D (PLD) and DAG kinases to coordinate phosphatidic acid (PA) signaling [117–121] (Figure 5). The PA signaling is known to play role in neurite growth associated cytoskeletal and membrane remodeling [122]. Alterations in PA signaling levels are associated with glioblastomas, intellectual disability, and neurodegenerative disorders, which are discussed later.
Figure 5.
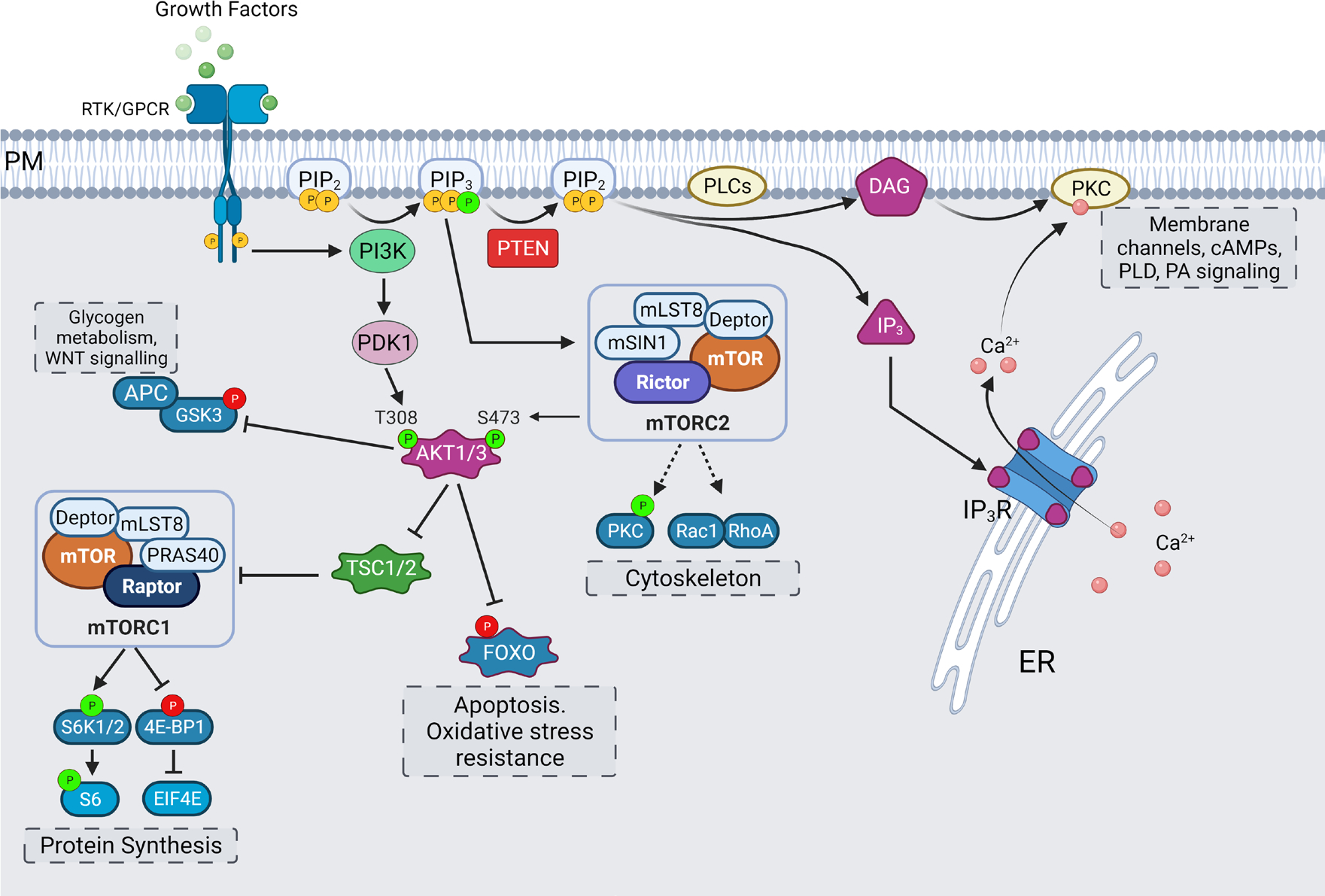
Effectors of PIP2 and PIP3. The hydrolysis of PIP2 by PLC leads to formation of IP3 and DAG which are involved in intracellular calcium release and activation of PKC-mediated signaling. PI3K-mediated synthesis of PIP3 leads to activation of AKT/mammalian target of rapamycin (mTOR) signaling pathway, with mTOR complexes, glycogen synthase kinase 3 (GSK3)β and Forkhead BOX O (FOXO) working as main effectors for regulation of processes of protein synthesis, cytoskeletal organization, and nutrient-sensing and survival. PTEN phosphatase keeps this activation in balance by dephosphorylation of PIP3 into PIP2. Activating phosphorylations are shown in green, while inhibitory phosphorylations are shown in red. 4E-BP1: eukaryotic initiation factor 4E-binding protein 1; EIF4E: eukaryotic translation initiation factor 4E; APC: adenomatous polyposis coli; Deptor: DEP domain-containing mTOR-interacting protein; mLST8: mammalian lethal with sec13 protein 8; mSIN1: mammalian stress activated protein kinase interacting protein; mTORC1: mammalian target of rapamycin complex 1; PDK: phosphoinositide-dependent protein kinase; PM: plasma membrane; PRAS40: proline-rich Akt substrate of 40 kDa; Raptor: regulatory associated protein of mTOR; Rheb: Ras homolog enriched in brain; Rictor: rapamycin-insensitive companion of mTOR; S6: ribosomal protein S6; S6K: ribosomal S6 kinase; TSC: tuberous sclerosis complex
AKT and mTOR pathway
Canonically, the activation of RTKs or GPCRs by extracellular stimuli recruits PI3K to plasma membrane, where it catalyzes the phosphorylation of PIP2, which generates PIP3. This generation of PIP3 leads to activation of AKT (also known as protein kinase B)/mTOR signaling pathway, which is a central regulator for cell growth, metabolism, protein translation, cytoskeletal organization, membrane trafficking and survival [123]. PTEN is a negative regulator of this pathway which keeps the activation of this pathway in check, by dephosphorylating PIP3 into PIP2 [124]. The formation of PIP3 recruits PH domain containing proteins like PDK and AKT to the plasma membrane [125]. The PDK is reported to localize in cholesterol-rich membrane rafts [126]. This close proximity allows PDK to phosphorylate AKT at T308 [127]. Phosphorylation at T308 stabilizes and activates AKT.
For AKT to reach its maximal activity potential a further phosphorylation at S473 is needed [128, 129]. It is thought that first phosphorylation event at T308 primes AKT for second phosphorylation at S473, which in turn, stabilizes the first T308 phosphorylation. This idea is supported by the observations that T308 phosphorylation can occur without prior S473 phosphorylation, but not vice versa [130]. The S473 phosphorylation is brought about by mTOR complex 2 (mTORC2). This complex is assembled by PIP3 binding to the PH-domain containing mSIN1, and thereby relieving its suppression on mTOR kinase activity and localizing it to plasma membrane as well [131]. mTORC2 complex also contains a scaffolding protein Rictor which regulates its assembly. mTORC2, as the name of its scaffolding protein suggests, is insensitive to acute rapamycin treatment and is known to regulate cytoskeletal rearrangements to support formation of new dendritic branches [129, 132].
Targeting Rictor to inhibit mTORC2 activity has been shown to result in inhibition of basal synaptic transmission and dendritic outgrowth in hippocampal neurons [133]. Another candidate for mediating this S473 phosphorylation on AKT is DNA-dependent protein kinase catalytic subunit (DNA-PKcs), but its role has been described mostly in the context of DNA damage in the nucleus [134]. Reports of PIP3 being enriched at endosome and nuclear envelope also support the idea of localized phosphorylation and activation of AKT in intracellular compartments [135, 136]. In addition, three subtypes of AKT (AKT1, AKT2, AKT3) exist, with a high degree of homology in amino acid sequence and corresponding phosphorylation sites. Mostly, only AKT1 and AKT3 expression has been reported in hippocampus, while AKT2 expression is limited to cerebellum [137].
AKT targets many substrates which are at the nodes of different signaling pathways. One such target protein is GSK3 whose inhibitory phosphorylations of its targets are relieved by an inactivating phosphorylation by AKT. GSK3 regulates cell growth and development by regulating glycogen metabolism. It is also involved in Wnt/β-catenin signaling pathway, indicating its role in crosstalk between these two pathways [138, 139]. Another downstream effect of AKT activation is the assembly of mTORC1. mTORC1 is not a direct substrate of AKT. AKT mediated inhibitory phosphorylation on TSC relieves its inhibition of Rheb-GTP formation and leads to subsequent activation of mTORC1. In addition, mTORC1 complex assembly is facilitated by a scaffolding protein Raptor, whose suppression is relieved by AKT-mediated phosphorylation of PRAS40 [140–142] (Figure 5).
Broadly, mTORC1 is involved in processes of enhanced protein synthesis and growth through downstream effectors like S6 kinase; lipid synthesis through sterol responsive element binding protein (SRBEP); cellular stress responses through its negative regulator AMPK; and cell survival through autophagy and ubiquitin proteosome regulation [143–146]. In neuronal context, mTORC1 is activated through stimuli like brain-derived neurotrophic factor (BDNF), reelin, glutamate, gamma-aminobutyric acid (GABA), acetyl choline, and neuropeptides [147–149]. The stimulation of mTOR increases dendritic protein synthesis locally and contributes to synaptic and structural plasticity [150, 151]. mTOR activity is also needed for proper dendritic arborization, axonal branching, neuronal polarization, and autophagy mediated differentiation [152–154].
AKT also targets transcription factors of FOXO family. These transcription factors translocate from cytoplasm to nucleus to regulate processes like apoptosis and oxidative stress resistance. In the nervous system, acute FOXO activity is involved in age-dependent axonal degeneration, spine density and consolidation of memories [155, 156].
Ion transporters and channels
Apart from signaling through its downstream effectors, PIP2 may directly bind to and interact with ion transporters and membrane channels to affect their activity [11]. The phospholipid composition of the intracellular compartments has been hypothesized to regulate the activity of membrane channels in space, until they arrive at their target membrane compartment with their signature phospholipid composition. Another hypothesis is about regulation of channels’ activity in time. Cells may respond dynamically to extracellular stimuli by way of changes in PI signaling mediating cell’s electrical and transport activity [157, 158]. This is an area of active investigation and the exact nature of PI binding to membrane channels has been difficult to determine. However, recent crystallography and mutagenesis studies have identified clusters of conserved amino acid residues on the channels that interact with phosphate group of PIs. These interactions might cause conformational changes to stabilize channel protein in a certain state, as observed with crystal structures of PIP2 bound to potassium channels. The conformational change brought about by binding of PIP2 is thought to lead to channel activation [159, 160].
In the context of potassium channels activity, PIP2 seems to be the principal phospholipid regulating their activity. For voltage-gated potassium channels (Kv), the Kv7 or potassium voltage-gated channel subfamily Q (KCNQ) family is most relevant to neuronal excitability [161]. The PLC mediate depletion of PIP2 reduces their current in a matter of seconds [159]. Direct application of PIP2 experimentally, slows this rundown [160]. Structurally, basic residues on the C-terminal TM segment and calmodulin binding may be required for PIP2 coupling to KCNQ channels [162]. Inwardly rectifying potassium channels (Kir) were the first to be identified as PIP2 dependent [163]. All members of this family bind to PIP2, but do so with different affinities (high affinity: Kir1, 2.1 and 4; low affinity: Kir2.3 and 3). High affinity channels were slowest to run down in response to PIP2 depletion [164]. PIP2 stabilizes the open state of these channels by binding to basic and hydrophobic residues in the N- and distal C-terminal [165].
Calcium activated potassium channels have also been shown to regulated by PIP2 levels in the cell [166, 167]. For voltage-gated calcium channels (CaV), PIP2 is required for opening in response to membrane potential changes. PIP2 is hypothesized to bind to CaV as a segmented ligand bringing two parts together, and is competed for interaction by CaV β subunits whose expression governs the PIP2 sensitivity of these channels [168]. Some of the TRP channels are also PIP2 sensitive, i.e., PIP2 promotes their opening by binding to basic residues on cytoplasmic linker and C-terminal [169, 170]. An example being cold-activated TRPM8 channel is that it does not respond to stimuli in the absence of PIP2 [171]. The P2X receptor, CNG, and TMEM16-ANO1 are examples of some other channels that are also sensitive to PIP2 binding [158].
Actin regulatory proteins
Actin provides an architectural scaffold for the cell, and is an important regulator of cellular shape, trafficking, and migration [172]. This actin network is integral to the morphological remodeling of highly specialized cells like neurons. PIs regulate the activity of actin-binding proteins which are reported to control the initiation of processes like spinogenesis and dendritogenesis.
In general, PIP2 inactivates actin-biding proteins that inhibit actin polymerization, while activating proteins that support polymerization. Gelsolin is an actin severing protein, whose binding with PIP2 frees up the ends of actin filament for polymerization [173]. Interestingly, this binding is enhanced by calcium ions to promote actin polymerization [174]. The actin-related protein 2/3 (Arp2/3) complex which is responsible for branching of actin filaments is also activated through binding of PIP2 with its activation protein, Wiskott-Aldrich syndrome protein (WASP) [175]. PIP2 has also been shown to bind to other actin regulatory proteins like cofilin and profilin. The severing action of cofilin is inhibited by binding with PIP2, while PIP2-profilin interaction inhibits PLCγ-mediated hydrolysis of PIP2 [176, 177].
The neuronal membrane branching/bending into dendrites, axons or spines is observed to protrude from specialized filopodia like structures which are filled with actin. PIP2 is reported to mediate the formation of these filopodia. PIP2 recruits the inverse bin-amphiphysin-rvs (I-BAR) protein missing-in-metastasis (MIM)/MTSS1 and Arp2/3-mediated actin assembly to nucleate the formation of protrusion that are pre-cursors to spinogenesis [178]. Nerve growth factor (NGF) treatment is known to increase the formation of patches that are precursors to the formation of axonal filopodia in a PI3K mediated activity dependent manner [179]. Localized microdomains of PIP3 are also reported to be synchronous with formation of these precursor patches that are formed in response to NGF treatment [180]. Application of brain derived neurotrophic factor was reported to enhance PIP3 localization to dendritic filopodia as well [181].
Disease relevance
The intricate balance between the levels of PIP2 and PIP3 is necessary for proper regulation and maintenance of several critical cellular processes in the nervous system. Any perturbation to this balance, be it through kinases or phosphatases, may lead to deficits in brain development and neuronal regulation, manifesting itself in disease and disorders of the nervous system.
Disorders of neurodevelopment
Mutations in genes encoding for proteins involved in PI synthesis and metabolism have been associated with ASD. Of the kinases involved in PIP2 and PIP3 synthesis, mutations in catalytic and regulatory isoforms of PI3K have been observed to be overrepresented when it comes to disorders affecting brain development [15]. In the context of dysregulation of catalytic subunits of PI3K, mutations in PIK3CA gene have been observed in clinical cases of cortical dysplasia and megalencephaly [182]. Another study described missense mutations in gene coding for p110α in a patient with autism and macrocephaly [183]. There is also strong evidence for overexpression of p110β catalytic subunit in some cases of autism [184]. This overexpression has been shown to be caused by chromosomal duplication.
More studies have linked a loss of Fragile X mental retardation protein (FMRP) in Fragile X syndrome (FXS) to p110β overexpression [185]. Since p110β mRNA binds to FMRP, this loss of FMRP is associated with increase in p110β expression. This increase has been observed in mouse models [185], as well as human patients cell lines [186, 187]. Dysregulation of another p110δ subunit of PI3K has also been observed in autism and schizophrenia [188, 189]. The location for gene coding for p110γ has been identified as a potential autism susceptible locus [190]. As for the PI3K regulatory subunits, mutations in p85β have been described to be associated with autism and megalencephaly [191]. So far, members of other classes of PI3K (class II and III) that are involved in generation of PIs other than PIP2 and PIP3 have not been shown to be strongly associated with the incidence of autism, further highlighting the importance of maintaining PIP2 an PIP3 balance for neuronal health.
In addition to PI3K, PI4K and PIPKs have also been shown to be altered in autism and other related disorders. Deleterious mutations in a regulator of PI4K have been identified in patients with autism [192]. PIPK isoform 3 has been found to be duplicated in patients with developmental delay and autism [193]. Among the phosphatases regulating PIP2 and PIP3 balance, PTEN stands out for its role in developmental delay, autism, and epilepsy [194–196]. In multiple animal model studies, PTEN loss has been shown to cause autism-like phenotypes and behavior [197–199]. PTEN loss associated phenotypes include anxiety, seizures, macrocephaly, and deficits in neuronal migration, growth, electrical activity, and social behavior [197, 199–201].
Trisomy of the locus containing SYNJ1 phosphatase has been shown to result in enlarged endosomes in cell lines developed from patients with Down’s syndrome (DS) [202]. Other PI phosphatases have not been linked with autism but have been associated with neurodegeneration.
Disorders of neurodegeneration
The PIP2/PIP3 balance in the intracellular environment is reported to be perturbed in neurodegenerative disorders like Alzheimer’s and Parkinson’s [203]. SYNJ1, a PI phosphatase regulating synaptic activity, was observed to be increased in autopsy of adult brains of DS and early-onset AD patients [204]. The excess of SYNJ1 was also found to contribute to memory deficits in mouse models of AD. Reducing this excess experimentally was found to accelerate clearance of Amyloid β (Aβ) and associated cognitive decline. In another study, restoring PIP2 levels was sufficient to ameliorate such synaptic dysfunction [205, 206]. Presence of tau protein has also been detected in patients with SYNJ1 mutations [207]. Missense mutation in sac1 domain of SYNJ1 has also been found in patients with early onset PD [208]. These patients experienced tremors and some cortical atrophy as well. Loss-of-function mutations in SYNJ1 have been observed in patients with infantile epileptic encephalopathy [209, 210].
Single nucleotide polymorphism (SNP) in INPP5B phosphatase have been shown to be associated with sporadic amyotrophic lateral sclerosis (ALS) [211]. Loss of function mutations in chorein or vacuolar protein sorting-associated protein 13A (VPS13A) have been found in patients with a rare hereditary genetic disorder called chorea-acanthocytosis (ChAc) [212]. ChAc is characterized by progressive movement disorder, seizures, cognitive difficulties, and neurodegeneration-particularly in striatum [213–215]. The mechanism behind disease pathology has remained puzzling for a while. However, recent studies in neuronal cell cultures and animal models have hinted at a role for PI signaling in the development of ChAc. Chorein is reported to be involved in activation of p85 regulatory subunit of PI3K, with subsequent activation of several downstream kinases [215, 216]. Compromised cytoskeleton and cell-survival was observed in patient-derived neuronal cell cultures containing mutations in chorein [217]. Mutations in genes encoding for IP3R, for example inositol 1,4,5-trisphosphate receptor type 1 (ITPR1), have been reported in infantile-onset nonprogressive spinocerebellar ataxia (SCA) [218]. SCA are degenerative disorders related to movement control. The dysfunction of IP3R leads to aberrant calcium signaling in cerebellar neurons which are implicated in SCA pathogenesis [219]. Mutations in FAM126A gene leading to loss of hyccin/FAM126A protein, a scaffolding partner of PIKIIIα, causes disorders of progressive hypomyelination in central and peripheral nervous system [30]. This hypomyelination manifests in form of leukoencephalopathy known as hypomyelination and congenital cataract (HCC), with symptoms of cognitive deficits and neuropathy [220].
Conclusion
Phosphoinositide signaling, especially the levels of PIP2 and PIP3, is critically regulated for maintenance of general neuronal health by varied kinases and phosphatases. The downstream functions for PIP2 and PIP3 are quite diverse, and include roles in neuronal growth, development, connectivity, and activity. Perturbations to the regulators of this balance in PIP2 and PIP3 have been associated with myriad of neurological disorders and diseases. Research in this domain continues and new associations are being discovered regularly. However, the need for effective therapeutic strategies remains.
Acknowledgments
The authors thank members of the Luikart lab for their intellectual assistance to this paper. Figures were created with BioRender and ChemSketch.
Funding
The authors are funded by the National Institute of Health grant R01 MH097949 to BWL and Autism Speaks grant 11857 to KT. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- 5-Ptase
inositol 5-phosphatase
- ACBD3
acyl-CoA-binding protein 3
- AD
Alzheimer’s disease
- ASD
autism spectrum disorder
- CaV
voltage-gated calcium channels
- ChAc
chorea-acanthocytosis
- cPKC
conventional protein kinase C
- DAG
diacylglycerol
- ER
endoplasmic reticulum
- FMRP
Fragile X mental retardation protein
- FOXO
Forkhead BOX O
- GPCR
G-protein coupled receptor
- GSK3
glycogen synthase kinase 3
- INPP5
inositol polyphosphate 5-phosphatase
- IP3
inositol-1,4,5-triphosphate
- IP3R
inositol-1,4,5-triphosphate receptor
- Kir
inwardly rectifying potassium channels
- mTORC1
mammalian target of rapamycin complex 1
- mTORC2
mammalian target of rapamycin complex 2
- ORCL
oculocerebrorenal syndrome of Lowe
- PA
phosphatidic acid
- PD
Parkinson’s disease
- PDK
phosphoinositide-dependent protein kinase
- PH
pleckstrin homology
- PI
phosphoinositides
- PI3K
phosphoinositide 3-kinase
- PI4K
phosphatidylinositol-4 kinases
- PI5 phosphatases
phosphoinositide 5-phosphatases
- PI5P
phosphatidylinositol 5-phosphate
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PIP3
phosphatidylinositol 3,4,5-trisphosphate
- PIPK
phosphatidylinositol phosphate kinase
- PIPKIIs
type II phosphatidylinositol phosphate kinases
- PIPKIs
type I phosphatidylinositol phosphate kinases
- PKC
protein kinase C
- PLC
phospholipase C
- PR
proline-rich
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
- Rictor
rapamycin-insensitive companion of mTOR
- RTK
receptor tyrosine kinase
- SCA
spinocerebellar ataxia
- SH2
Src homology 2
- SHIP
Src homology 2 containing inositol phosphatase
- SKIP
skeletal muscle- and kidney-enriched inositol polyphosphate phosphatase
- SYNJ
synaptojanin
- TM
transmembrane spanning region
- TPIP
transmembrane phosphatase with tensin homology and phosphatase and tensin homolog deleted on chromosome 10 homologous inositol lipid phosphatase
- TPTE
transmembrane phosphatase with tensin homology
- TRP
transient receptor potential
- VSP
voltage-sensing phosphatases
Footnotes
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
References
- 1.Phan TK, Williams SA, Bindra GK, Lay FT, Poon IKH, Hulett MD. Phosphoinositides: multipurpose cellular lipids with emerging roles in cell death. Cell Death Differ. 2019;26:781–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghu P, Joseph A, Krishnan H, Singh P, Saha S. Phosphoinositides: regulators of nervous system function in health and disease. Front Mol Neurosci. 2019;12:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balla T Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol Rev. 2013;93:1019–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–7. [DOI] [PubMed] [Google Scholar]
- 5.De Matteis MA, Godi A. PI-loting membrane traffic. Nat Cell Biol. 2004;6:487–92. [DOI] [PubMed] [Google Scholar]
- 6.Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–21. [DOI] [PubMed] [Google Scholar]
- 7.Kirk CJ, Bone EA, Palmer S, Michell RH. The role of phosphatidylinositol 4,5 bisphosphate breakdown in cell-surface receptor activation. J Recept Res. 1984;4:489–504. [DOI] [PubMed] [Google Scholar]
- 8.Falkenburger BH, Jensen JB, Dickson EJ, Suh BC, Hille B. Phosphoinositides: lipid regulators of membrane proteins. J Physiol. 2010;588:3179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YJ, Jahan N, Bahk YY. Biochemistry and structure of phosphoinositide phosphatases. BMB Rep. 2013;46:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang SX, Duan LH, He SJ, Zhuang GF, Yu X. Phosphatidylinositol 3,4-bisphosphate regulates neurite initiation and dendrite morphogenesis via actin aggregation. Cell Res. 2017;27:253–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys. 2008;37:175–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi SH, Jan LY, Jan YN. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell. 2003;112:63–75. [DOI] [PubMed] [Google Scholar]
- 13.Kirchner MK, Foehring RC, Wang L, Chandaka GK, Callaway JC, Armstrong WE. Phosphatidylinositol 4,5-bisphosphate (PIP2) modulates afterhyperpolarizations in oxytocin neurons of the supraoptic nucleus. J Physiol. 2017;595:4927–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arancio O PIP2: a new key player in Alzheimer’s disease. Cellscience. 2008;5:44–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Gross C Defective phosphoinositide metabolism in autism. J Neurosci Res. 2017;95:1161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlessinger J Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–25. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki T, Takasuga S, Sasaki J, Kofuji S, Eguchi S, Yamazaki M, et al. Mammalian phosphoinositide kinases and phosphatases. Prog Lipid Res. 2009;48:307–43. [DOI] [PubMed] [Google Scholar]
- 18.Balla A, Balla T. Phosphatidylinositol 4-kinases: old enzymes with emerging functions. Trends Cell Biol. 2006;16:351–61. [DOI] [PubMed] [Google Scholar]
- 19.Barylko B, Gerber SH, Binns DD, Grichine N, Khvotchev M, Südhof TC, et al. A novel family of phosphatidylinositol 4-kinases conserved from yeast to humans. J Biol Chem. 2001;276:7705–8. [DOI] [PubMed] [Google Scholar]
- 20.Jung G, Wang J, Wlodarski P, Barylko B, Binns DD, Shu H, et al. Molecular determinants of activation and membrane targeting of phosphoinositol 4-kinase IIβ. Biochem J. 2008;409:501–9. [DOI] [PubMed] [Google Scholar]
- 21.Balla A, Tuymetova G, Barshishat M, Geiszt M, Balla T. Characterization of type II phosphatidylinositol 4-kinase isoforms reveals association of the enzymes with endosomal vesicular compartments. J Biol Chem. 2002;277:20041–50. [DOI] [PubMed] [Google Scholar]
- 22.Minogue S, Anderson JS, Waugh MG, dos Santos M, Corless S, Cramer R, et al. Cloning of a human type II phosphatidylinositol 4-kinase reveals a novel lipid kinase family. J Biol Chem. 2001;276:16635–40. [DOI] [PubMed] [Google Scholar]
- 23.Waugh MG, Minogue S, Anderson JS, Balinger A, Blumenkrantz D, Calnan DP, et al. Localization of a highly active pool of type II phosphatidylinositol 4-kinase in a p97/valosin-containing-protein-rich fraction of the endoplasmic reticulum. Biochem J. 2003;373:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei YJ, Sun HQ, Yamamoto M, Wlodarski P, Kunii K, Martinez M, et al. Type II phosphatidylinositol 4-kinase β is a cytosolic and peripheral membrane protein that is recruited to the plasma membrane and activated by Rac-GTP. J Biol Chem. 2002;277:46586–93. [DOI] [PubMed] [Google Scholar]
- 25.Guo J, Wenk MR, Pellegrini L, Onofri F, Benfenati F, De Camilli P. Phosphatidylinositol 4-kinase type IIα is responsible for the phosphatidylinositol 4-kinase activity associated with synaptic vesicles. Proc Natl Acad Sci U S A. 2003;100:3995–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakanishi S, Catt KJ, Balla T. A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pools of inositolphospholipids. Proc Natl Acad Sci U S A. 1995;92:5317–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu X, Chi RJ, Baskin JM, Lucast L, Burd CG, De Camilli P, et al. Structural insights into assembly and regulation of the plasma membrane phosphatidylinositol 4-kinase complex. Dev Cell. 2014;28:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heilmeyer LM Jr, Vereb G Jr, Vereb G, Kakuk A, Szivák I. Mammalian phosphatidylinositol 4-kinases. IUBMB Life. 2003;55:59–65. [DOI] [PubMed] [Google Scholar]
- 29.de Graaf P, Zwart WT, van Dijken RAJ, Deneka M, Schulz TKF, Geijsen N, et al. Phosphatidylinositol 4-kinaseβ is critical for functional association of rab11 with the Golgi complex. Mol Biol Cell. 2004;15:2038–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baskin JM, Wu X, Christiano R, Oh MS, Schauder CM, Gazzerro E, et al. The leukodystrophy protein FAM126A (hyccin) regulates PtdIns(4)P synthesis at the plasma membrane. Nat Cell Biol. 2016;18:132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasaki J, Ishikawa K, Arita M, Taniguchi K. ACBD3-mediated recruitment of PI4KB to picornavirus RNA replication sites. EMBO J. 2012;31:754–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hausser A, Link G, Hoene M, Russo C, Selchow O, Pfizenmaier K. Phospho-specific binding of 14–3-3 proteins to phosphatidylinositol 4-kinase III β protects from dephosphorylation and stabilizes lipid kinase activity. J Cell Sci. 2006;119:3613–21. [DOI] [PubMed] [Google Scholar]
- 33.Loijens JC, Anderson RA. Type I phosphatidylinositol-4-phosphate 5-kinases are distinct members of this novel lipid kinase family. J Biol Chem. 1996;271:32937–43. [DOI] [PubMed] [Google Scholar]
- 34.Rameh LE, Tolias KF, Duckworth BC, Cantley LC. A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate. Nature. 1997;390:192–6. [DOI] [PubMed] [Google Scholar]
- 35.Giudici ML, Emson PC, Irvine RF. A novel neuronal-specific splice variant of Type I phosphatidylinositol 4-phosphate 5-kinase isoform gamma. Biochem J. 2004;379:489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boronenkov IV, Anderson RA. The sequence of phosphatidylinositol-4-phosphate 5-kinase defines a novel family of lipid kinases. J Biol Chem. 1995;270:2881–4. [DOI] [PubMed] [Google Scholar]
- 37.Rao VD, Misra S, Boronenkov IV, Anderson RA, Hurley JH. Structure of type IIβ phosphatidylinositol phosphate kinase: a protein kinase fold flattened for interfacial phosphorylation. Cell. 1998;94:829–39. [DOI] [PubMed] [Google Scholar]
- 38.Honda A, Nogami M, Yokozeki T, Yamazaki M, Nakamura H, Watanabe H, et al. Phosphatidylinositol 4-phosphate 5-kinase α is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell. 1999;99:521–32. [DOI] [PubMed] [Google Scholar]
- 39.Chong LD, Traynor-Kaplan A, Bokoch GM, Schwartz MA. The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell. 1994;79:507–13. [DOI] [PubMed] [Google Scholar]
- 40.Hu J, Yuan Q, Kang X, Qin Y, Li L, Ha Y, et al. Resolution of structure of PIP5K1A reveals molecular mechanism for its regulation by dimerization and dishevelled. Nat Commun. 2015;6:8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clarke JH, Irvine RF. Evolutionarily conserved structural changes in phosphatidylinositol 5-phosphate 4-kinase (PI5P4K) isoforms are responsible for differences in enzyme activity and localization. Biochem J. 2013;454:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hinchliffe KA, Irvine RF. Regulation of type II PIP kinase by PKD phosphorylation. Cell Signal. 2006;18:1906–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones DR, Bultsma Y, Keune WJ, Halstead JR, Elouarrat D, Mohammed S, et al. Nuclear PtdIns5P as a transducer of stress signaling: an in vivo role for PIP4Kbeta. Mol Cell. 2006;23:685–95. [DOI] [PubMed] [Google Scholar]
- 44.Sumita K, Lo YH, Takeuchi K, Senda M, Kofuji S, Ikeda Y, et al. The lipid kinase PI5P4Kβ is an intracellular GTP sensor for metabolism and tumorigenesis. Mol Cell. 2016;61:187–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clarke JH, Richardson JP, Hinchliffe KA, Irvine RF. Type II PtdInsP kinases: location, regulation and function. Biochem Soc Symp. 2007;74:149–59. [DOI] [PubMed] [Google Scholar]
- 46.Thapa N, Tan X, Choi S, Lambert PF, Rapraeger AC, Anderson RA. The hidden conundrum of phosphoinositide signaling in cancer. Trends Cancer. 2016;2:378–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Domin J, Waterfield MD. Using structure to define the function of phosphoinositide 3-kinase family members. FEBS Lett. 1997;410:91–5. [DOI] [PubMed] [Google Scholar]
- 48.Hawkins PT, Anderson KE, Davidson K, Stephens LR. Signalling through Class I PI3Ks in mammalian cells. Biochem Soc Trans. 2006;34:647–62. [DOI] [PubMed] [Google Scholar]
- 49.Backer JM, Myers MG Jr, Shoelson SE, Chin DJ, Sun XJ, Miralpeix M, et al. Phosphatidylinositol 3’-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992;11:3469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vadas O, Burke JE, Zhang X, Berndt A, Williams RL. Structural basis for activation and inhibition of class I phosphoinositide 3-kinases. Sci Signal. 2011;4:re2. [DOI] [PubMed] [Google Scholar]
- 51.Stephens LR, Eguinoa A, Erdjument-Bromage H, Lui M, Cooke F, Coadwell J, et al. The Gβγ sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell. 1997;89:105–14. [DOI] [PubMed] [Google Scholar]
- 52.Walker EH, Perisic O, Ried C, Stephens L, Williams RL. Structural insights into phosphoinositide 3-kinase catalysis and signalling. Nature. 1999;402:313–20. [DOI] [PubMed] [Google Scholar]
- 53.Burke JE, Williams RL. Synergy in activating class I PI3Ks. Trends Biochem Sci. 2015;40:88–100. [DOI] [PubMed] [Google Scholar]
- 54.Zhao WQ, Alkon DL. Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol. 2001;177:125–34. [DOI] [PubMed] [Google Scholar]
- 55.Lee CC, Huang CC, Hsu KS. Insulin promotes dendritic spine and synapse formation by the PI3K/Akt/mTOR and Rac1 signaling pathways. Neuropharmacology. 2011;61:867–79. [DOI] [PubMed] [Google Scholar]
- 56.Sopasakis VR, Liu P, Suzuki R, Kondo T, Winnay J, Tran TT, et al. Specific roles of the p110α isoform of phosphatidylinsositol 3-kinase in hepatic insulin signaling and metabolic regulation. Cell Metab. 2010;11:220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gross C, Bassell GJ. Neuron-specific regulation of class I PI3K catalytic subunits and their dysfunction in brain disorders. Front Mol Neurosci. 2014;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guillermet-Guibert J, Bjorklof K, Salpekar A, Gonella C, Ramadani F, Bilancio A, et al. The p110β isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110γ. Proc Natl Acad Sci U S A. 2008;105:8292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim JI, Lee HR, Sim SE, Baek J, Yu NK, Choi JH, et al. PI3Kγ is required for NMDA receptor-dependent long-term depression and behavioral flexibility. Nat Neurosci. 2011;14:1447–54. [DOI] [PubMed] [Google Scholar]
- 60.Eickholt BJ, Ahmed AI, Davies M, Papakonstanti EA, Pearce W, Starkey ML, et al. Control of axonal growth and regeneration of sensory neurons by the p110δ PI 3-kinase. PLoS One. 2007;2:e869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, et al. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999;99:323–34. [DOI] [PubMed] [Google Scholar]
- 62.Das S, Dixon JE, Cho W. Membrane-binding and activation mechanism of PTEN. Proc Natl Acad Sci U S A. 2003;100:7491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trotman LC, Wang X, Alimonti A, Chen Z, Teruya-Feldstein J, Yang H, et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128:141–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fricano-Kugler CJ, Getz SA, Williams MR, Zurawel AA, DeSpenza T Jr, Frazel PW, et al. Nuclear excluded autism-associated phosphatase and tensin homolog mutations dysregulate neuronal growth. Biol Psychiatry. 2018;84:265–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fragoso R, Barata JT. Kinases, tails and more: regulation of PTEN function by phosphorylation. Methods. 2015;77–78:75–81. [DOI] [PubMed] [Google Scholar]
- 66.Hsu F, Mao Y. The structure of phosphoinositide phosphatases: insights into substrate specificity and catalysis. Biochim Biophys Acta. 2015;1851:698–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walker SM, Downes CP, Leslie NR. TPIP: a novel phosphoinositide 3-phosphatase. Biochem J. 2001;360:277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–43. [DOI] [PubMed] [Google Scholar]
- 69.Okamura Y, Murata Y, Iwasaki H. Voltage-sensing phosphatase: actions and potentials. J Physiol. 2009;587:513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ungewickell A, Hugge C, Kisseleva M, Chang SC, Zou J, Feng Y, et al. The identification and characterization of two phosphatidylinositol-4,5-bisphosphate 4-phosphatases. Proc Natl Acad Sci U S A. 2005;102:18854–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gozani O, Karuman P, Jones DR, Ivanov D, Cha J, Lugovskoy AA, et al. The PHD finger of the chromatin-associated protein ING2 functions as a nuclear phosphoinositide receptor. Cell. 2003;114:99–111. [DOI] [PubMed] [Google Scholar]
- 72.Zou J, Marjanovic J, Kisseleva MV, Wilson M, Majerus PW. Type I phosphatidylinositol-4,5-bisphosphate 4-phosphatase regulates stress-induced apoptosis. Proc Natl Acad Sci U S A. 2007;104:16834–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Medina MW, Bauzon F, Naidoo D, Theusch E, Stevens K, Schilde J, et al. Transmembrane protein 55B is a novel regulator of cellular cholesterol metabolism. Arterioscler Thromb Vasc Biol. 2014;34:1917–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Majerus PW, Kisseleva MV, Norris FA. The role of phosphatases in inositol signaling reactions. J Biol Chem. 1999;274:10669–72. [DOI] [PubMed] [Google Scholar]
- 75.Tsujishita Y, Guo S, Stolz LE, York JD, Hurley JH. Specificity determinants in phosphoinositide dephosphorylation: crystal structure of an archetypal inositol polyphosphate 5-phosphatase. Cell. 2001;105:379–89. [DOI] [PubMed] [Google Scholar]
- 76.Ramjaun AR, McPherson PS. Tissue-specific alternative splicing generates two synaptojanin isoforms with differential membrane binding properties. J Biol Chem. 1996;271:24856–61. [DOI] [PubMed] [Google Scholar]
- 77.Nemoto Y, Arribas M, Haffner C, DeCamilli P. Synaptojanin 2, a novel synaptojanin isoform with a distinct targeting domain and expression pattern. J Biol Chem. 1997;272:30817–21. [DOI] [PubMed] [Google Scholar]
- 78.McPherson PS, Garcia EP, Slepnev VI, David C, Zhang X, Grabs D, et al. A presynaptic inositol-5-phosphatase. Nature. 1996;379:353–7. [DOI] [PubMed] [Google Scholar]
- 79.Haffner C, Takei K, Chen H, Ringstad N, Hudson A, Butler MH, et al. Synaptojanin 1: localization on coated endocytic intermediates in nerve terminals and interaction of its 170 kDa isoform with Eps15. FEBS Lett. 1997;419:175–80. [DOI] [PubMed] [Google Scholar]
- 80.Ponting CP. A novel domain suggests a ciliary function for ASPM, a brain size determining gene. Bioinformatics. 2006;22:1031–5. [DOI] [PubMed] [Google Scholar]
- 81.Johnson JM, Castle J, Garrett-Engele P, Kan Z, Loerch PM, Armour CD, et al. Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science. 2003;302:2141–4. [DOI] [PubMed] [Google Scholar]
- 82.Peck J, Douglas G 4th, Wu CH, Burbelo PD. Human RhoGAP domain-containing proteins: structure, function and evolutionary relationships. FEBS Lett. 2002;528:27–34. [DOI] [PubMed] [Google Scholar]
- 83.Jefferson AB, Majerus PW. Properties of type II inositol polyphosphate 5-phosphatase. J Biol Chem. 1995;270:9370–7. [DOI] [PubMed] [Google Scholar]
- 84.Ooms LM, Fedele CG, Astle MV, Ivetac I, Cheung V, Pearson RB, et al. The inositol polyphosphate 5-phosphatase, PIPP, is a novel regulator of phosphoinositide 3-kinase-dependent neurite elongation. Mol Biol Cell. 2006;17:607–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mochizuki Y, Takenawa T. Novel inositol polyphosphate 5-phosphatase localizes at membrane ruffles. J Biol Chem. 1999;274:36790–5. [DOI] [PubMed] [Google Scholar]
- 86.Gurung R, Tan A, Ooms LM, McGrath MJ, Huysmans RD, Munday AD, et al. Identification of a novel domain in two mammalian inositol-polyphosphate 5-phosphatases that mediates membrane ruffle localization. The inositol 5-phosphatase skip localizes to the endoplasmic reticulum and translocates to membrane ruffles following epidermal growth factor stimulation. J Biol Chem. 2003;278:11376–85. [DOI] [PubMed] [Google Scholar]
- 87.Ijuin T, Mochizuki Y, Fukami K, Funaki M, Asano T, Takenawa T. Identification and characterization of a novel inositol polyphosphate 5-phosphatase. J Biol Chem. 2000;275:10870–5. [DOI] [PubMed] [Google Scholar]
- 88.Tu Z, Ninos JM, Ma Z, Wang JW, Lemos MP, Desponts C, et al. Embryonic and hematopoietic stem cells express a novel SH2-containing inositol 5’-phosphatase isoform that partners with the Grb2 adapter protein. Blood. 2001;98:2028–38. [DOI] [PubMed] [Google Scholar]
- 89.Lucas DM, Rohrschneider LR. A novel spliced form of SH2-containing inositol phosphatase is expressed during myeloid development. Blood. 1999;93:1922–33. [PubMed] [Google Scholar]
- 90.Liu Q, Shalaby F, Jones J, Bouchard D, Dumont DJ. The SH2-containing inositol polyphosphate 5-phosphatase, ship, is expressed during hematopoiesis and spermatogenesis. Blood. 1998;91:2753–9. [PubMed] [Google Scholar]
- 91.Kisseleva MV, Wilson MP, Majerus PW. The isolation and characterization of a cDNA encoding phospholipid-specific inositol polyphosphate 5-phosphatase. J Biol Chem. 2000;275:20110–6. [DOI] [PubMed] [Google Scholar]
- 92.Minagawa T, Ijuin T, Mochizuki Y, Takenawa T. Identification and characterization of a sac domain-containing phosphoinositide 5-phosphatase. J Biol Chem. 2001;276:22011–5. [DOI] [PubMed] [Google Scholar]
- 93.Sbrissa D, Ikonomov OC, Fu Z, Ijuin T, Gruenberg J, Takenawa T, et al. Core protein machinery for mammalian phosphatidylinositol 3,5-bisphosphate synthesis and turnover that regulates the progression of endosomal transport. Novel Sac phosphatase joins the ArPIKfyve-PIKfyve complex. J Biol Chem. 2007;282:23878–91. [DOI] [PubMed] [Google Scholar]
- 94.Yuan Y, Gao X, Guo N, Zhang H, Xie Z, Jin M, et al. rSac3, a novel Sac domain phosphoinositide phosphatase, promotes neurite outgrowth in PC12 cells. Cell Res. 2007;17:919–32. [DOI] [PubMed] [Google Scholar]
- 95.Gresset A, Sondek J, Harden TK. The phospholipase C isozymes and their regulation. Subcell Biochem. 2012;58:61–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Katan M, Williams RL. Phosphoinositide-specific phospholipase C: structural basis for catalysis and regulatory interactions. Semin Cell Dev Biol. 1997;8:287–96. [DOI] [PubMed] [Google Scholar]
- 97.Yang YR, Follo MY, Cocco L, Suh PG. The physiological roles of primary phospholipase C. Adv Biol Regul. 2013;53:232–41. [DOI] [PubMed] [Google Scholar]
- 98.Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martelli AM, Gilmour RS, Bertagnolo V, Neri LM, Manzoli L, Cocco L. Nuclear localization and signalling activity of phosphoinositidase Cβ in Swiss 3T3 cells. Nature. 1992;358:242–5. [DOI] [PubMed] [Google Scholar]
- 100.Cocco L, Follo MY, Manzoli L, Suh PG. Phosphoinositide-specific phospholipase C in health and disease. J Lipid Res. 2015;56:1853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Johnson CM, Chichili GR, Rodgers W. Compartmentalization of phosphatidylinositol 4,5-bisphosphate signaling evidenced using targeted phosphatases. J Biol Chem. 2008;283:29920–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Myeong J, Park CG, Suh BC, Hille B. Compartmentalization of phosphatidylinositol 4,5-bisphosphate metabolism into plasma membrane liquid-ordered/raft domains. Proc Natl Acad Sci U S A. 2021;118:e2025343118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Furt F, König S, Bessoule JJ, Sargueil F, Zallot R, Stanislas T, et al. Polyphosphoinositides are enriched in plant membrane rafts and form microdomains in the plasma membrane. Plant Physiol. 2010;152:2173–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chun YS, Oh HG, Park MK, Kim TW, Chung S. Increasing membrane cholesterol level increases the amyloidogenic peptide by enhancing the expression of phospholipase C. J Neurodegener Dis. 2013;2013:407903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jiang Z, Redfern RE, Isler Y, Ross AH, Gericke A. Cholesterol stabilizes fluid phosphoinositide domains. Chem Phys Lipids. 2014;182:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Parys JB, De Smedt H. Inositol 1,4,5-trisphosphate and its receptors. Adv Exp Med Biol. 2012;740:255–79. [DOI] [PubMed] [Google Scholar]
- 107.Prole DL, Taylor CW. Inositol 1,4,5-trisphosphate receptors and their protein partners as signalling hubs. J Physiol. 2016;594:2849–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Berridge MJ. Calcium oscillations. J Biol Chem. 1990;265:9583–6. [PubMed] [Google Scholar]
- 109.Kang DS, Yang YR, Lee C, Kim S, Ryu SH, Suh PG. Roles of phosphoinositide-specific phospholipase Cγ1 in brain development. Adv Biol Regul. 2016;60:167–73. [DOI] [PubMed] [Google Scholar]
- 110.Tojima T, Hines JH, Henley JR, Kamiguchi H. Second messengers and membrane trafficking direct and organize growth cone steering. Nat Rev Neurosci. 2011;12:191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Berridge MJ. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev. 2016;96:1261–96. [DOI] [PubMed] [Google Scholar]
- 112.Berridge MJ. Calcium regulation of neural rhythms, memory and Alzheimer’s disease. J Physiol. 2014;592:281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Inoue T, Kato K, Kohda K, Mikoshiba K. Type 1 inositol 1,4,5-trisphosphate receptor is required for induction of long-term depression in cerebellar Purkinje neurons. J Neurosci. 1998;18:5366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Quest AF. Regulation of protein kinase C: a tale of lipids and proteins. Enzyme Protein. 1996;49:231–61. [DOI] [PubMed] [Google Scholar]
- 115.Newton AC. Protein kinase C. Seeing two domains. Curr Biol. 1995;5:973–6. [DOI] [PubMed] [Google Scholar]
- 116.Reither G, Schaefer M, Lipp P. PKCα: a versatile key for decoding the cellular calcium toolkit. J Cell Biol. 2006;174:521–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang KK, Wright LC, Machan CL, Allen BG, Conigrave AD, Roufogalis BD. Protein kinase C phosphorylates the carboxyl terminus of the plasma membrane Ca2+-ATPase from human erythrocytes. J Biol Chem. 1991;266:9078–85. [PubMed] [Google Scholar]
- 118.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schulze DH, Muqhal M, Lederer WJ, Ruknudin AM. Sodium/calcium exchanger (NCX1) macromolecular complex. J Biol Chem. 2003;278:28849–55. [DOI] [PubMed] [Google Scholar]
- 120.Fujii M, Yi KS, Kim MJ, Ha SH, Ryu SH, Suh PG, et al. Phosphorylation of phospholipase C-δ1 regulates its enzymatic activity. J Cell Biochem. 2009;108:638–50. [DOI] [PubMed] [Google Scholar]
- 121.Halls ML, Cooper DM. Regulation by Ca2+-signaling pathways of adenylyl cyclases. Cold Spring Harb Perspect Biol. 2011;3:a004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tanguy E, Wang Q, Moine H, Vitale N. Phosphatidic acid: from pleiotropic functions to neuronal pathology. Front Cell Neurosci. 2019;13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–8. [DOI] [PubMed] [Google Scholar]
- 125.Risso G, Blaustein M, Pozzi B, Mammi P, Srebrow A. Akt/PKB: one kinase, many modifications. Biochem J. 2015;468:203–14. [DOI] [PubMed] [Google Scholar]
- 126.Gao X, Lowry PR, Zhou X, Depry C, Wei Z, Wong GW, et al. PI3K/Akt signaling requires spatial compartmentalization in plasma membrane microdomains. Proc Natl Acad Sci U S A. 2011;108:14509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim D, Chung J. Akt: versatile mediator of cell survival and beyond. J Biochem Mol Biol. 2002;35:106–15. [DOI] [PubMed] [Google Scholar]
- 128.Okuno S, Kitani T, Matsuzaki H, Konishi H, Kikkawa U, Fujisawa H. Studies on the phosphorylation of protein kinase B by Ca2+/calmodulin-dependent protein kinases. J Biochem. 2000;127:965–70. [DOI] [PubMed] [Google Scholar]
- 129.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. [DOI] [PubMed] [Google Scholar]
- 130.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCα, but not S6K1. Dev Cell. 2006;11:859–71. [DOI] [PubMed] [Google Scholar]
- 131.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–37. [DOI] [PubMed] [Google Scholar]
- 132.Liu P, Gan W, Chin YR, Ogura K, Guo J, Zhang J, et al. PtdIns(3,4,5)P3-dependent activation of the mTORC2 kinase complex. Cancer Discov. 2015;5:1194–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Urbanska M, Gozdz A, Swiech LJ, Jaworski J. Mammalian target of rapamycin complex 1 (mTORC1) and 2 (mTORC2) control the dendritic arbor morphology of hippocampal neurons. J Biol Chem. 2012;287:30240–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bozulic L, Surucu B, Hynx D, Hemmings BA. PKBα/Akt1 acts downstream of DNA-PK in the DNA double-strand break response and promotes survival. Mol Cell. 2008;30:203–13. [DOI] [PubMed] [Google Scholar]
- 135.Jethwa N, Chung GHC, Lete MG, Alonso A, Byrne RD, Calleja V, et al. Endomembrane PtdIns(3,4,5)P3 activates the PI3K-Akt pathway. J Cell Sci. 2015;128:3456–65. [DOI] [PubMed] [Google Scholar]
- 136.Irvine RF. Nuclear inositide signalling -- expansion, structures and clarification. Biochim Biophys Acta. 2006;1761:505–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Levenga J, Wong H, Milstead RA, Keller BN, LaPlante LE, Hoeffer CA. AKT isoforms have distinct hippocampal expression and roles in synaptic plasticity. Elife. 2017;6:e30640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Golpich M, Amini E, Hemmati F, Ibrahim NM, Rahmani B, Mohamed Z, et al. Glycogen synthase kinase-3 beta (GSK-3β) signaling: implications for Parkinson’s disease. Pharmacol Res. 2015;97:16–26. [DOI] [PubMed] [Google Scholar]
- 139.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–76. [DOI] [PubMed] [Google Scholar]
- 140.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol. 2020;21:183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Menon S, Dibble CC, Talbott G, Hoxhaj G, Valvezan AJ, Takahashi H, et al. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell. 2014;156:771–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Alessi DR, Caudwell FB, Andjelkovic M, Hemmings BA, Cohen P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 1996;399:333–8. [DOI] [PubMed] [Google Scholar]
- 144.Yaguchi M, Ikeya S, Kozaki A. The activation mechanism of plant S6 kinase (S6K), a substrate of TOR kinase, is different from that of mammalian S6K. FEBS Lett. 2020;594:776–87. [DOI] [PubMed] [Google Scholar]
- 145.Jhanwar-Uniyal M, Wainwright JV, Mohan AL, Tobias ME, Murali R, Gandhi CD, et al. Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship. Adv Biol Regul. 2019;72:51–62. [DOI] [PubMed] [Google Scholar]
- 146.Yu X, Long YC, Shen HM. Differential regulatory functions of three classes of phosphatidylinositol and phosphoinositide 3-kinases in autophagy. Autophagy. 2015;11:1711–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Silva A, Naia L, Dominguez A, Ribeiro M, Rodrigues J, Vieira OV, et al. Overexpression of BDNF and full-length TrkB receptor ameliorate striatal neural survival in Huntington’s disease. Neurodegener Dis. 2015;15:207–18. [DOI] [PubMed] [Google Scholar]
- 148.Jossin Y, Goffinet AM. Reelin signals through phosphatidylinositol 3-kinase and Akt to control cortical development and through mTor to regulate dendritic growth. Mol Cell Biol. 2007;27:7113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schicknick H, Schott BH, Budinger E, Smalla KH, Riedel A, Seidenbecher CI, et al. Dopaminergic modulation of auditory cortex-dependent memory consolidation through mTOR. Cereb Cortex. 2008;18:2646–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jaworski J, Sheng M. The growing role of mTOR in neuronal development and plasticity. Mol Neurobiol. 2006;34:205–19. [DOI] [PubMed] [Google Scholar]
- 151.Gong R, Park CS, Abbassi NR, Tang SJ. Roles of glutamate receptors and the mammalian target of rapamycin (mTOR) signaling pathway in activity-dependent dendritic protein synthesis in hippocampal neurons. J Biol Chem. 2006;281:18802–15. [DOI] [PubMed] [Google Scholar]
- 152.Noda T, Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem. 1998;273:3963–6. [DOI] [PubMed] [Google Scholar]
- 153.Piper M, Lee AC, van Horck FP, McNeilly H, Lu TB, Harris WA, et al. Differential requirement of F-actin and microtubule cytoskeleton in cue-induced local protein synthesis in axonal growth cones. Neural Dev. 2015;10:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Morita T, Sobue K. Specification of neuronal polarity regulated by local translation of CRMP2 and Tau via the mTOR-p70S6K pathway. J Biol Chem. 2009;284:27734–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Santo EE, Paik J. FOXO in neural cells and diseases of the nervous system. Curr Top Dev Biol. 2018;127:105–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bockaert J, Marin P. mTOR in brain physiology and pathologies. Physiol Rev. 2015;95:1157–87. [DOI] [PubMed] [Google Scholar]
- 157.Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE. 2001;2001:re19. [DOI] [PubMed] [Google Scholar]
- 158.Hille B, Dickson EJ, Kruse M, Vivas O, Suh BC. Phosphoinositides regulate ion channels. Biochim Biophys Acta. 2015;1851:844–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Suh BC, Inoue T, Meyer T, Hille B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. 2006;314:1454–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Suh BC, Hille B. Regulation of KCNQ channels by manipulation of phosphoinositides. J Physiol. 2007;582:911–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang H, Craciun LC, Mirshahi T, Rohács T, Lopes CMB, Jin T, et al. PIP2 activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 2003;37:963–75. [DOI] [PubMed] [Google Scholar]
- 162.Kwon Y, Hofmann T, Montell C. Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Mol Cell. 2007;25:491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hilgemann DW, Ball R. Regulation of cardiac Na+,Ca2+ exchange and KATP potassium channels by PIP2. Science. 1996;273:956–9. [DOI] [PubMed] [Google Scholar]
- 164.Du X, Zhang H, Lopes C, Mirshahi T, Rohacs T, Logothetis DE. Characteristic interactions with phosphatidylinositol 4,5-bisphosphate determine regulation of Kir channels by diverse modulators. J Biol Chem. 2004;279:37271–81. [DOI] [PubMed] [Google Scholar]
- 165.Haider S, Tarasov AI, Craig TJ, Sansom MSP, Ashcroft FM. Identification of the PIP2-binding site on Kir6.2 by molecular modelling and functional analysis. EMBO J. 2007;26:3749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vaithianathan T, Bukiya A, Liu J, Liu P, Asuncion-Chin M, Fan Z, et al. Direct regulation of BK channels by phosphatidylinositol 4,5-bisphosphate as a novel signaling pathway. J Gen Physiol. 2008;132:13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lu M, Hebert SC, Giebisch G. Hydrolyzable ATP and PIP2 modulate the small-conductance K+ channel in apical membranes of rat cortical-collecting duct (CCD). J Gen Physiol. 2002;120:603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Suh BC, Leal K, Hille B. Modulation of high-voltage activated Ca2+ channels by membrane phosphatidylinositol 4,5-bisphosphate. Neuron. 2010;67:224–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rohacs T, Nilius B. Regulation of transient receptor potential (TRP) channels by phosphoinositides. Pflugers Arch. 2007;455:157–68. [DOI] [PubMed] [Google Scholar]
- 170.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–8. [DOI] [PubMed] [Google Scholar]
- 171.Liu B, Qin F. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:1674–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mandal K Review of PIP2 in cellular signaling, functions and diseases. Int J Mol Sci. 2020;21:8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Janmey PA, Stossel TP. Modulation of gelsolin function by phosphatidylinositol 4,5-bisphosphate. Nature. 1987;325:362–4. [DOI] [PubMed] [Google Scholar]
- 174.Tuominen EK, Holopainen JM, Chen J, Prestwich GD, Bachiller PR, Kinnunen PK, et al. Fluorescent phosphoinositide derivatives reveal specific binding of gelsolin and other actin regulatory proteins to mixed lipid bilayers. Eur J Biochem. 1999;263:85–92. [DOI] [PubMed] [Google Scholar]
- 175.Bucki R, Wang YH, Yang C, Kandy SK, Fatunmbi O, Bradley R, et al. Lateral distribution of phosphatidylinositol 4,5-bisphosphate in membranes regulates formin- and ARP2/3-mediated actin nucleation. J Biol Chem. 2019;294:4704–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Goldschmidt-Clermont PJ, Machesky LM, Baldassare JJ, Pollard TD. The actin-binding protein profilin binds to PIP2 and inhibits its hydrolysis by phospholipase C. Science. 1990;247:1575–8. [DOI] [PubMed] [Google Scholar]
- 177.Zhao H, Hakala M, Lappalainen P. ADF/cofilin binds phosphoinositides in a multivalent manner to act as a PIP2-density sensor. Biophys J. 2010;98:2327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Saarikangas J, Kourdougli N, Senju Y, Chazal G, Segerstråle M, Minkeviciene R, et al. MIM-induced membrane bending promotes dendritic spine initiation. Dev Cell. 2015;33:644–59. [DOI] [PubMed] [Google Scholar]
- 179.Spillane M, Ketschek A, Donnelly CJ, Pacheco A, Twiss JL, Gallo G. Nerve growth factor-induced formation of axonal filopodia and collateral branches involves the intra-axonal synthesis of regulators of the actin-nucleating Arp2/3 complex. J Neurosci. 2012;32:17671–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ketschek A, Gallo G. Nerve growth factor induces axonal filopodia through localized microdomains of phosphoinositide 3-kinase activity that drive the formation of cytoskeletal precursors to filopodia. J Neurosci. 2010;30:12185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Luikart BW, Zhang W, Wayman GA, Kwon CH, Westbrook GL, Parada LF. Neurotrophin-dependent dendritic filopodial motility: a convergence on PI3K signaling. J Neurosci. 2008;28:7006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jansen LA, Mirzaa GM, Ishak GE, O’Roak BJ, Hiatt JB, Roden WH, et al. PI3K/AKT pathway mutations cause a spectrum of brain malformations from megalencephaly to focal cortical dysplasia. Brain. 2015;138:1613–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Turner TN, Hormozdiari F, Duyzend MH, McClymont SA, Hook PW, Iossifov I, et al. Genome sequencing of autism-affected families reveals disruption of putative noncoding regulatory DNA. Am J Hum Genet. 2016;98:58–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pinto D, Delaby E, Merico D, Barbosa M, Merikangas A, Klei L, et al. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am J Hum Genet. 2014;94:677–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gross C, Raj N, Molinaro G, Allen AG, Whyte AJ, Gibson JR, et al. Selective role of the catalytic PI3K subunit p110β in impaired higher order cognition in fragile X syndrome. Cell Rep. 2015;11:681–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kumari D, Bhattacharya A, Nadel J, Moulton K, Zeak NM, Glicksman A, et al. Identification of fragile X syndrome specific molecular markers in human fibroblasts: a useful model to test the efficacy of therapeutic drugs. Hum Mutat. 2014;35:1485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gross C, Bassell GJ. Excess protein synthesis in FXS patient lymphoblastoid cells can be rescued with a p110β-selective inhibitor. Mol Med. 2012;18:336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Poopal AC, Schroeder LM, Horn PS, Bassell GJ, Gross C. Increased expression of the PI3K catalytic subunit p110δ underlies elevated S6 phosphorylation and protein synthesis in an individual with autism from a multiplex family. Mol Autism. 2016;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Law AJ, Wang Y, Sei Y, O’Donnell P, Piantadosi P, Papaleo F, et al. Neuregulin 1-ErbB4-PI3K signaling in schizophrenia and phosphoinositide 3-kinase-p110δ inhibition as a potential therapeutic strategy. Proc Natl Acad Sci U S A. 2012;109:12165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cukier HN, Skaar DA, Rayner-Evans MY, Konidari I, Whitehead PL, Jaworski JM, et al. Identification of chromosome 7 inversion breakpoints in an autistic family narrows candidate region for autism susceptibility. Autism Res. 2009;2:258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rivière JB, Mirzaa GM, O’Roak BJ, Beddaoui M, Alcantara D, Conway RL, et al. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat Genet. 2012;44:934–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gupta AR, Pirruccello M, Cheng F, Kang HJ, Fernandez TV, Baskin JM, et al. Rare deleterious mutations of the gene EFR3A in autism spectrum disorders. Mol Autism. 2014;5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Usui D, Shimada S, Shimojima K, Sugawara M, Kawasaki H, Shigematu H, et al. Interstitial duplication of 2q32.1-q33.3 in a patient with epilepsy, developmental delay, and autistic behavior. Am J Med Genet A. 2013;161A:1078–84. [DOI] [PubMed] [Google Scholar]
- 194.Satterstrom FK, Kosmicki JA, Wang J, Breen MS, De Rubeis S, An JY, et al. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell. 2020;180:568–84.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yehia L, Keel E, Eng C. The clinical spectrum of PTEN mutations. Annu Rev Med. 2020;71:103–16. [DOI] [PubMed] [Google Scholar]
- 196.Hansen-Kiss E, Beinkampen S, Adler B, Frazier T, Prior T, Erdman S, et al. A retrospective chart review of the features of PTEN hamartoma tumour syndrome in children. J Med Genet. 2017;54:471–8. [DOI] [PubMed] [Google Scholar]
- 197.Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, et al. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Williams MR, DeSpenza T Jr, Li M, Gulledge AT, Luikart BW. Hyperactivity of newborn Pten knock-out neurons results from increased excitatory synaptic drive. J Neurosci. 2015;35:943–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Skelton PD, Frazel PW, Lee D, Suh H, Luikart BW. Pten loss results in inappropriate excitatory connectivity. Mol Psychiatry. 2019;24:1627–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Getz SA, DeSpenza T Jr, Li M, Luikart BW. Rapamycin prevents, but does not reverse, aberrant migration in Pten knockout neurons. Neurobiol Dis. 2016;93:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Luikart BW, Schnell E, Washburn EK, Bensen AL, Tovar KR, Westbrook GL. Pten knockdown in vivo increases excitatory drive onto dentate granule cells. J Neurosci. 2011;31:4345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Cossec JC, Lavaur J, Berman DE, Rivals I, Hoischen A, Stora S, et al. Trisomy for synaptojanin1 in Down syndrome is functionally linked to the enlargement of early endosomes. Hum Mol Genet. 2012;21:3156–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Volpatti JR, Al-Maawali A, Smith L, Al-Hashim A, Brill JA, Dowling JJ. The expanding spectrum of neurological disorders of phosphoinositide metabolism. Dis Model Mech. 2019;12:dmm038174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Miranda AM, Herman M, Cheng R, Nahmani E, Barrett G, Micevska E, et al. Excess synaptojanin 1 contributes to place cell dysfunction and memory deficits in the aging hippocampus in three types of Alzheimer’s disease. Cell Rep. 2018;23:2967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Voronov SV, Frere SG, Giovedi S, Pollina EA, Borel C, Zhang H, et al. Synaptojanin 1-linked phosphoinositide dyshomeostasis and cognitive deficits in mouse models of Down’s syndrome. Proc Natl Acad Sci U S A. 2008;105:9415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhu L, Zhong M, Zhao J, Rhee H, Caesar I, Knight EM, et al. Reduction of synaptojanin 1 accelerates Aβ clearance and attenuates cognitive deterioration in an Alzheimer mouse model. J Biol Chem. 2013;288:32050–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Dyment DA, Smith AC, Humphreys P, Schwartzentruber J, Beaulieu CL, FORGE Canada Consortium, et al. Homozygous nonsense mutation in SYNJ1 associated with intractable epilepsy and tau pathology. Neurobiol Aging. 2015;36:1222.e1–5. [DOI] [PubMed] [Google Scholar]
- 208.Krebs CE, Karkheiran S, Powell JC, Cao M, Makarov V, Darvish H, et al. The Sac1 domain of SYNJ1 identified mutated in a family with early-onset progressive Parkinsonism with generalized seizures. Hum Mutat. 2013;34:1200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Al Zaabi N, Al Menhali N, Al-Jasmi F. SYNJ1 gene associated with neonatal onset of neurodegenerative disorder and intractable seizure. Mol Genet Genomic Med. 2018;6:109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hardies K, Cai Y, Jardel C, Jansen AC, Cao M, May P, et al. Loss of SYNJ1 dual phosphatase activity leads to early onset refractory seizures and progressive neurological decline. Brain. 2016;139:2420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Xie T, Deng L, Mei P, Zhou Y, Wang B, Zhang J, et al. Genome-wide association study combining pathway analysis for typical sporadic amyotrophic lateral sclerosis in Chinese Han populations. Neurobiol Aging. 2014;35:1778.e9–23. [DOI] [PubMed] [Google Scholar]
- 212.Henkel K, Danek A, Grafman J, Butman J, Kassubek J. Head of the caudate nucleus is most vulnerable in chorea-acanthocytosis: a voxel-based morphometry study. Mov Disord. 2006;21:1728–31. [DOI] [PubMed] [Google Scholar]
- 213.Tomemori Y, Ichiba M, Kusumoto A, Mizuno E, Sato D, Muroya S, et al. A gene-targeted mouse model for chorea-acanthocytosis. J Neurochem. 2005;92:759–66. [DOI] [PubMed] [Google Scholar]
- 214.Luo FM, Deng MX, Yu R, Liu L, Fan LL. Case report: chorea-acanthocytosis presents as epilepsy in a consanguineous family with a nonsense mutation of in VPS13A. Front Neurosci. 2021;15:604715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lang F, Pelzl L, Schols L, Hermann A, Foller M, Schaffer TE, et al. Neurons, erythrocytes and beyond -the diverse functions of chorein. Neurosignals. 2017;25:117–26. [DOI] [PubMed] [Google Scholar]
- 216.Park JS, Halegoua S, Kishida S, Neiman AM. A conserved function in phosphatidylinositol metabolism for mammalian Vps13 family proteins. PLoS One. 2015;10:e0124836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Föller M, Hermann A, Gu S, Alesutan I, Qadri SM, Borst O, et al. Chorein-sensitive polymerization of cortical actin and suicidal cell death in chorea-acanthocytosis. FASEB J. 2012;26:1526–34. [DOI] [PubMed] [Google Scholar]
- 218.Shadrina MI, Shulskaya MV, Klyushnikov SA, Nikopensius T, Nelis M, Kivistik PA, et al. ITPR1 gene p.Val1553Met mutation in Russian family with mild spinocerebellar ataxia. Cerebellum Ataxias. 2016;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ando H, Hirose M, Mikoshiba K. Aberrant IP3 receptor activities revealed by comprehensive analysis of pathological mutations causing spinocerebellar ataxia 29. Proc Natl Acad Sci U S A. 2018;115:12259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Miyamoto Y, Torii T, Eguchi T, Nakamura K, Tanoue A, Yamauchi J. Hypomyelinating leukodystrophy-associated missense mutant of FAM126A/hyccin/DRCTNNB1A aggregates in the endoplasmic reticulum. J Clin Neurosci. 2014;21:1033–9. [DOI] [PubMed] [Google Scholar]


