Cutaneous melanoma represents a major worldwide health issue, nearly accounting for 75% of skin cancer deaths (1). Although in the last decade novel promising therapies have been developed, surgical treatment in melanoma early stages still provides the best opportunity for cure. Indeed, despite important successes obtained with targeted therapy and immune checkpoint inhibitors, most patients undergo resistance and responses are not durable (2). In addition, a number of meta-analyses reported significant gender-specific differences in the efficacy of therapeutic approaches, unexpectedly indicating a higher increment of overall survival in men than in women, particularly when treated with immune checkpoint inhibitors (3). Taking in mind all these points, the identification of informative biomarkers, useful either to choose first line therapies, or to predict relapses and clinical outcomes, represent a fundamental point providing a rationale for making the best therapeutic choice for each single patient.
In the multistep process leading to metastases, among many other mechanisms of intracellular communication, exosomes (EXOs), microvesicles ranging from 40 to 100 nm, emerged as a key one as they can incorporate and efficiently transport to distant sites different bioactive molecules, such as lipids, proteins and RNAs (4). Although initially considered as garbage bags utilized by tumor cells for removing disturbing molecules, growing evidences indicated that usually contents of tumor-released EXOs well represents their cell of origin. Indeed, EXOs secreted by tumor cells are able to transfer their properties via horizontal propagation into the acceptor cells in autocrine and paracrine fashions being able to regulate nearly all the main biological processes, including tumor progression (5). The general increased number of EXOs associated with cancer progression, further emphasizes their importance (6). On this basis and in view of their easy accessibility in many body fluids (i.e., blood, urine, saliva, bile, breast milk), EXOs represent a valuable tool, even for repeated analyses, in healthy persons and cancer patients (7).
Actually, the transfer of tumor-derived exosomal microRNAs into recipient cells appears of particular interest, as they are able to induce major epigenetic modifications into the acceptor cells. These small non-coding RNAs (18–25 nucleotides) act by direct binding to specific consensus sequences in the 3'UTR of their target RNAs, thus leading to posttranscriptional regulation. Indeed, although the encapsuled RNAs are usually fragment shorter than 200 bp, these small non coding RNAs can play their regulatory actions in the recipient cells as they are protected from degradation and do not require additional maturation steps, as would be for coding RNAs (8).
Until recently, the EXO cargos were mostly considered casual and not dependent on a specific sorting process (9), but a number of recent results did not totally confirm this idea. Indeed in many cases the exosomal RNA content has been shown to be different from that of the donor cells, as for example shown by the lack of 18S and 28S ribosomal RNAs and even by the presence in the exosomal cargos of miRs apparently not expressed in the releasing cells (10,11). Actually, one possibility justifying the presence of different molecules in cells and corresponding released EXOs is the existence of subsets of EXOs containing different collection of miRs (11). Also, concerning serum EXOs, we should consider that they could be produced not only by tumors, but also released by the tumor microenvironment or by circulating immune cells (7).
Although the mechanisms that regulate the inclusion of specific RNAs into EXOs were not completely clarified, their encapsulation was reported to be regulated by the presence of flanking consensus sequences favoring or not their incorporation. Actually, specific short motifs present in miRNAs determine their sorting into EXOs, being controlled by the sumoylated fraction of the RNA-binding protein hnRNPA2B1, but additional mechanisms for miRNA sorting should exist (12).
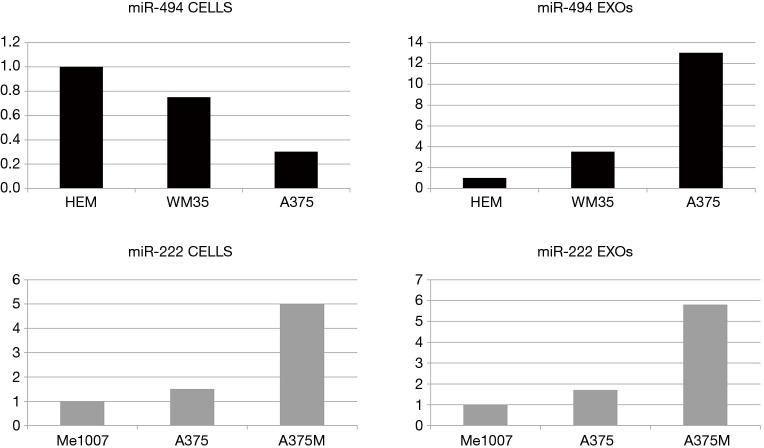
Although tumor-derived EXOs are usually considered pro-tumorigenic, their potential capability to play antitumorigenic roles was recently described in melanoma (13) and in Ewing’s (EWS) sarcoma (14). In melanoma cells and cell lines miR-221 and miR-222 were reported to play an oncogenic role inducing cell growth and blocking differentiation (15). Quantitative reverse transcription polymerase chain reaction (RT-PCR) analysis revealed that EXOs released by metastatic melanoma cells, containing higher levels of miR-222 in comparison with normal melanocytes and primary melanomas, were able to increase malignancy of early stage melanoma used as acceptor cells. In this case, cells and corresponding EXOs showed exactly the same expression pattern of this microRNA (Figure 1). Trying to abrogate the effects produced by miR-222-containing EXOs, microvesicles were recovered from advanced melanoma cells after inhibition of these couple of microRNAs. Indeed, after fusion with metastatic melanoma cells, EXOs devoid of miR-222 were able to reduce the cell cycle rate and the formation of vascular-like structures by modulating the PI3K/AKT, cyclin D1 key axis and inducing the expression of their direct target the cell cycle inhibitor p27kip1 (13).
Figure 1.
Representative results showing miR-494 and miR-222 expression levels during melanoma progression in cell lines and corresponding released exosomes. It is interesting to note that, while the exosomal expression of miR-222 directly reflects that of the parental cells, miR-494 shows an inverse correlation. EXOs, exosomes; HEM, human primary melanocyte.
Likewise, in EWS sarcoma cell lines, the uptake by parental EWS cells of EXOs released from cells silenced for CD99, a key protein in the pathogenesis of EWS, was able to convey the same signals induced by the stable abrogation of CD99, thus leading to cell differentiation associated with the induction of miR-34a (14).
The work of Li and coauthors, recently published on the Journal of Cellular Physiology, proposed an additional regulatory mechanism, in some way going back to the idea of EXO vesicles utilized by tumor cells to expel unnecessary molecules (16). Starting from microarray-based expression profiles of microRNAs in melanocytes, melanoma cell lines and their corresponding secreted EXOs, miR-494 was selected for further analysis in view of its intriguing differential expression. Expression and function of miR-494 were already evaluated in cancers of different origins and its exosomal inclusion described. Interestingly, although mainly reported as a tumor suppressor (17,18), it was described to promote the progression of endometrial cancer (19) and nasopharyngeal carcinoma (20) and identified as one of the key miRs involved in preparing the host niche thus favoring metastatization of adenocarcinoma cells (21). Also, a direct correlation between circulating and tumor levels of miR-494 was described in hepatocellular carcinoma (22).
In melanoma cell lines miR-494 expression was lower compared to normal melanocytes, a result suggestive of tumor suppressor properties. Indeed, along with tumor progression, Li found miR-494 downregulation in melanoma cells paralleled by its exosomal increased levels (16) (Figure 1). Specifically, a comparative analysis of three differently staged melanoma cell lines showed a gradual downregulation of miR-494 associated with progression, whereas increasing amounts were detected in the corresponding EXOs. Accordingly, the expression levels of miR-494, evaluated in serum EXOs indicated a significantly higher miR-494 expression in EXOs derived from melanoma patients than in those obtained from normal controls. Finally, the authors confirmed their results reducing melanoma cell viability, migration and invasion in vitro and tumor growth and metastatization in a xenograft in vivo model either by directly abrogating miR-494 or through Rab-27 silencing. Actually, the Rab27 family, besides a number of oncogenic functions (23), is a positive regulator of EXO secretion (24) and Rab27 silencing, interfering with the EXO release, should favor the antitumorigenic properties associated with the intracellular accumulation of miR-494. Nonetheless, as the regulatory role of Rab27 clearly goes through many different players, its abrogation might be expected to underlie several functions besides EXO release.
Based on all these results the authors propose the idea that melanoma cells at advanced stage utilize EXO vesicles to force out miR-494 and that the intracellular blockage of this microRNA might represent a novel therapeutic approach.
The definitely interesting study published by Li and colleagues faces an important, although still in its early phase, field. Indeed, EXO studies, and even more the option of using them as therapeutic delivery tools (16), will require additional in-depth research. A number of technical issues are still open questions, going from the identification of effective normalizators for exosomal microRNA quantitation to a real scalability into the human models. MiR-494 appears as a particularly complex issue in view of its apparent dual function, oncogenic or tumor suppressive, possibly dependent on tissue-specificities. Many published papers demonstrated miR-494 (specifically miR-494-3p) oncogenic function through various downstream effectors (for example by activating the well-known PI3K/AKT signaling pathway), in turn proposing its abrogation as a therapeutic approach (18). On the other side, many studies, including this one published by Li et al, reported the antitumoral effects associated with the overexpression of miR-494 (16-18).
So, although in the last decades exosomal studies reached relevant results, still a great deal of additional efforts will be required for further dissecting EXO endogenous mechanisms and in turn for their therapeutic manipulation (25). Indeed, data as those reported for miR-494, indicate the existence of complex additional mechanisms that should be further analyzed for effectively and safely transfer the huge therapeutic potential of EXOs to clinical uses.
Acknowledgments
Funding: This work was partly supported by the Italian Association for Cancer Research (AIRC IG18815).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Long Chen, MD (Department of PET-CT center at the Yunnan Tumor Hospital/the Third Affiliated Hospital of Kunming Medical University, Kunming, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.04.08). The authors have no conflicts of interest to declare.
References
- 1.Schadendorf D, van Akkooi ACJ, Berking C, et al. Melanoma. Lancet 2018;392:971-84. 10.1016/S0140-6736(18)31559-9 [DOI] [PubMed] [Google Scholar]
- 2.Ascierto PA, Flaherty K, Goff S. Emerging Strategies in Systemic Therapy for the Treatment of Melanoma. Am Soc Clin Oncol Educ Book 2018;38:751-8. 10.1200/EDBK_199047 [DOI] [PubMed] [Google Scholar]
- 3.Conforti F, Pala L, Bagnardi V, et al. Cancer immunotherapy efficacy and patients' sex: a systematic review and meta-analysis. Lancet Oncol 2018;19:737-46. 10.1016/S1470-2045(18)30261-4 [DOI] [PubMed] [Google Scholar]
- 4.Xu R, Rai A, Chen M, et al. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat Rev Clin Oncol 2018;15:617-38. 10.1038/s41571-018-0036-9 [DOI] [PubMed] [Google Scholar]
- 5.Saleem SN, Abdel-Mageed AB. Tumor-derived exosomes in oncogenic reprogramming and cancer progression. Cell Mol Life Sci 2015;72:1-10. 10.1007/s00018-014-1710-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker A, Thakur BK, Weiss JM, et al. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016;30:836-48. 10.1016/j.ccell.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman EB, Shang S, de Miera EV, et al. Serum microRNAs as biomarkers for recurrence in melanoma. J Transl Med 2012;10:155. 10.1186/1479-5876-10-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anfossi S, Fu X, Nagvekar R, et al. MicroRNAs, Regulatory Messengers Inside and Outside Cancer Cells. Adv Exp Med Biol 2018;1056:87-108. 10.1007/978-3-319-74470-4_6 [DOI] [PubMed] [Google Scholar]
- 9.Kharaziha P, Ceder S, Li Q, et al. Tumor cell-derived exosomes: a message in a bottle. Biochim Biophys Acta 2012;1826:103-11. [DOI] [PubMed] [Google Scholar]
- 10.Lunavat TR, Cheng L, Kim DK, et al. Small RNA deep sequencing discriminates subsets of extracellular vesicles released by melanoma cells--Evidence of unique microRNA cargos. RNA Biol 2015;12:810-23. 10.1080/15476286.2015.1056975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willms E, Cabañas C, Mäger I, et al. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front Immunol 2018;9:738. 10.3389/fimmu.2018.00738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun 2013;4:2980. 10.1038/ncomms3980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felicetti F, De Feo A, Coscia C, et al. Exosome-mediated transfer of miR-222 is sufficient to increase tumor malignancy in melanoma. J Transl Med 2016;14:56. 10.1186/s12967-016-0811-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ventura S, Aryee DN, Felicetti F, et al. CD99 regulates neural differentiation of Ewing sarcoma cells through miR-34a-Notch-mediated control of NF-κB signaling. Oncogene 2016;35:3944-54. 10.1038/onc.2015.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felicetti F, Errico MC, Bottero L, et al. The promyelocytic leukemia zinc finger-microRNA-221/-222 pathway controls melanoma progression through multiple oncogenic mechanisms. Cancer Res 2008;68:2745-54. 10.1158/0008-5472.CAN-07-2538 [DOI] [PubMed] [Google Scholar]
- 16.Li J, Chen J, Wang S, et al. Blockage of transferred exosome-shuttled miR-494 inhibits melanoma growth and metastasis. J Cell Physiol 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 17.Peng QP, Du DB, Ming Q, et al. MicroRNA 494 increases chemosensitivity to doxorubicin in gastric cancer cells by targeting phosphodiesterases 4D. Cell Mol Biol (Noisy-le-grand) 2018;64:62-6. 10.14715/cmb/2017.64.15.10 [DOI] [PubMed] [Google Scholar]
- 18.Xu S, Li D, Li T, et al. miR-494 Sensitizes Gastric Cancer Cells to TRAIL Treatment Through Downregulation of Survivin. Cell Physiol Biochem 2018;51:2212-23. 10.1159/000495867 [DOI] [PubMed] [Google Scholar]
- 19.Zhu L, Wang X, Wang T, et al. miR 494-3p promotes the progression of endometrial cancer by regulating the PTEN/PI3K/AKT pathway. Mol Med Rep 2019;19:581-8. [DOI] [PubMed] [Google Scholar]
- 20.He H, Liao X, Yang Q, et al. MicroRNA-494-3p Promotes Cell Growth, Migration, and Invasion of Nasopharyngeal Carcinoma by Targeting Sox7. Technol Cancer Res Treat 2018;17:1533033818809993. 10.1177/1533033818809993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rana S, Malinowska K, Zöller M. Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia 2013;15:281-95. 10.1593/neo.122010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fornari F, Ferracin M, Trerè D, et al. Circulating microRNAs, miR-939, miR-595, miR-519d and miR-494, Identify Cirrhotic Patients with HCC. PLoS One 2015;10:e0141448. 10.1371/journal.pone.0141448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bobrie A, Krumeich S, Reyal F, et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res 2012;72:4920-30. 10.1158/0008-5472.CAN-12-0925 [DOI] [PubMed] [Google Scholar]
- 24.Ostrowski M, Carmo NB, Krumeich S, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 2010;12:19-30. sup pp 1-13. [DOI] [PubMed] [Google Scholar]
- 25.Milane L, Singh A, Mattheolabakis G, et al. Exosome mediated communication within the tumor microenvironment. J Control Release 2015;219:278-94. 10.1016/j.jconrel.2015.06.029 [DOI] [PubMed] [Google Scholar]