Abstract
Background
There is a paucity of data on outcomes for people with gout and COVID-19. We aimed to assess whether gout is a risk factor for diagnosis of COVID-19 and COVID-19-related death, and to test for sex- and drug-specific differences in risk.
Methods
We used data from the UK Biobank, which included 15 871 people with gout. We used multivariable-adjusted logistic regression in the following analyses using a case-control study design: to test for an association between gout and COVID-19 diagnosis in the entire UK Biobank cohort (n=459 837); to test for an association between gout and COVID-19-related death in people who were known to have died or survived with COVID-19 (n=15 772); to test for an association between gout and COVID-19-related death in the entire UK Biobank cohort (n=459 837); and to assess risk of COVID-19-related death in a subset of patients from the UK Biobank cohort with prescription data, stratified by prescription of urate-lowering therapy and colchicine (n=341 398). Models 1 and 2 were adjusted for age group, sex, ethnicity, Townsend deprivation index, BMI, and smoking status. Model 2 was also adjusted for diagnosis of 16 other diseases that are established comorbidities of gout or established risk factors for COVID-19-related death.
Findings
Gout was associated with diagnosis of COVID-19 (odds ratio [OR] 1·20, 95% CI 1·11–1·29) but not with risk of COVID-19-related death in the cohort of patients diagnosed with COVID-19 (1·20, 0·96–1·51). In the entire cohort, gout was associated with COVID-19-related death (1·29, 1·06–1·56); women with gout had an increased risk of COVID-19-related death (1·98, 1·34–2·94), whereas men with gout did not (1·16, 0·93–1·45). We found no significant differences in the risk of COVID-19-related death according to prescription of urate-lowering therapy or colchicine. When patients with gout were stratified by vaccination status, the risk of diagnosis with COVID-19 was significant in the non-vaccinated group (1·21, 1·11–1·30) but not the vaccinated group (1·09, 0·65–1·85).
Interpretation
Gout is a risk factor for COVID-19-related death in the UK Biobank cohort, with an increased risk in women with gout, which was driven by risk factors independent of the metabolic comorbidities of gout.
Funding
Health Research Council of New Zealand.
Introduction
COVID-19 outcomes for people with inflammatory rheumatic disease are becoming increasingly available. Within a cohort of 3729 patients with rheumatic disease, the COVID-19 Global Rheumatology Alliance identified hypertension associated with cardiovascular disease and chronic lung disease as a comorbidity associated with COVID-19-related death, in addition to the established risk factors of older age and male sex.1 Despite its high prevalence (eg, 2·5% of the UK population2), gout is underrepresented in the COVID-19 Global Rheumatology Alliance cohort,1 where it was included among in other inflammatory arthritis, which comprises only 2·6% of the cohort.
Our previous study3 in the UK Biobank cohort on outcomes for people with gout and COVID-19 comprised 2059 individuals diagnosed with COVID-19 before Aug 25, 2020; our multivariable-adjusted analysis reported no evidence for gout as a risk factor for COVID-19-related death (odds ratio [OR] 1·2, 95% CI 0·8–1·7). The aim of the present study was to repeat the testing for an association between gout and COVID-19 diagnosis and death in a larger number of individuals diagnosed with COVID-19 in the UK Biobank, up to April 6, 2021. This larger cohort also allowed us to test for association in a sex-specific analysis and stratified by the prescription of urate-lowering therapy and colchicine.
Methods
Study design and population
This research was done using the UK Biobank Resource (approval number 12611). The UK Biobank is a large resource of almost 500 000 volunteers aged 49–86 years at recruitment.4 Recruitment began in 2006, with follow-up intended to last for at least 30 years. SARS-CoV-2 test information, International Classification of Diseases Tenth Revision (ICD-10) hospital codes, death records, and general practice prescription information were obtained via the UK Biobank data portal on Sept 13, 2021. This information covered hospital diagnoses between April 18, 1991, and May 7, 2021, SARS-CoV-2 tests between March 16, 2020, and April 6, 2021, and death records between May 10, 2006, and March 23, 2021. Participants who did not have a body-mass index (BMI) measurement, Townsend index score5 (a measure of material deprivation within a population), or smoking status data were excluded.
Research in context.
Evidence before this study
Very little is known about the risk of COVID-19 diagnosis and death from COVID-19 in people with gout. We searched PubMed up to April 1, 2021, using the terms “gout and COVID”, with no language restrictions. 30 publications were retrieved and reviewed for cohort studies that had outcomes involving COVID-19 in people with gout, with one suitable study identified. That study used a UK cohort that included 2059 people diagnosed with COVID-19 and did not report evidence that supported an association between gout and COVID-19-related death.
Added value of this study
Our study analysed a UK cohort that included 16 898 people diagnosed with COVID-19. The findings showed that gout is both a risk factor for diagnosis of COVID-19 and a risk factor for COVID-19-related death, independent of included comorbidities. Women with gout were at a higher risk of death with COVID-19 than were men with gout.
Implications of the available evidence
Our findings show that gout is a risk factor for death from COVID-19, with a greater effect in women than in men. This information could inform clinical decision making in people with gout diagnosed with COVID-19 and inform advice on vaccination decisions for people with gout. Future research should focus on replicating these findings, including a focus on understanding key factors explaining the increased risk of death with COVID-19 in women with gout.
The UK Biobank was undertaken with ethical approval from the North West Multi-centre Research Ethics Committee of the UK. This study was done under this ethical approval; researchers using the UK Biobank do not require separate ethical approval. The study complies with the Declaration of Helsinki and written informed consent was obtained from all participants.
Procedures and case-control datasets
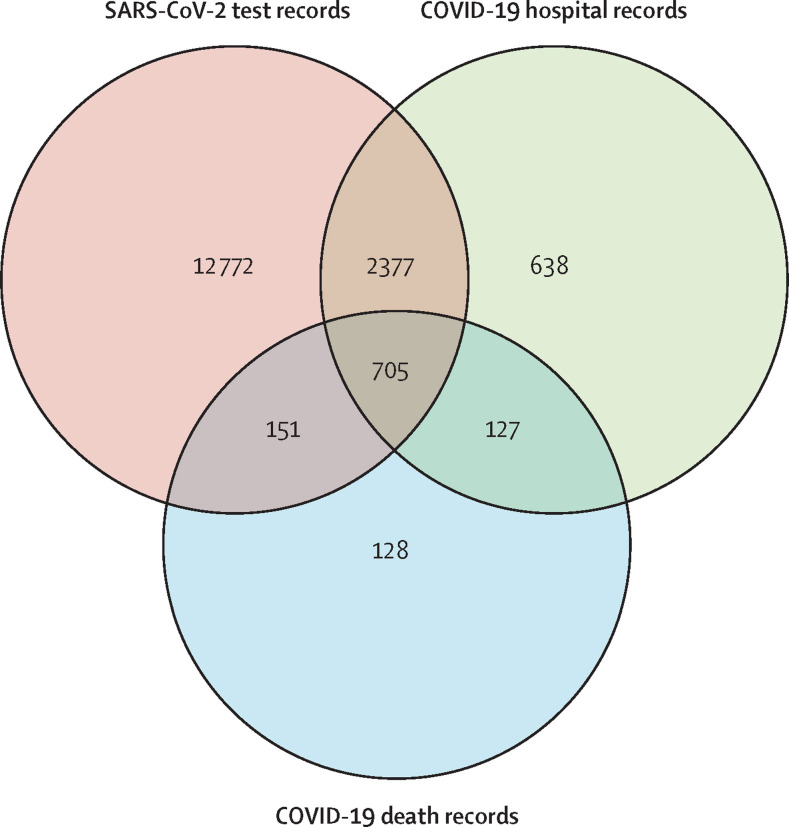
The criteria for COVID-19 diagnosis were defined as participants with a positive SARS-CoV-2 test or ICD-10 code for confirmed COVID-19 (U07.1) or probable COVID-19 (U07.2) in hospital records or death records (figure 1 ). This definition resulted in the identification of 16 898 individuals who were further divided into those that died (n=1111) based on death records, those who were known to survive (n=15 224), and those with uncertain outcome (n=563). Gout was ascertained from the UK Biobank using the following criteria: self-reported gout (at visits 0–2), taking allopurinol or sulfinpyrazone either from self-report or from linked general practice scripts, or hospital-diagnosed gout (ICD-10 code M10;5 15 560 people were ascertained as having gout); this case definition has been validated.6, 7 Additionally, we searched general practice record disease code terms using the term gout, which yielded 76 codes. Of these 76 codes, 11 codes identified 952 individuals who could be classified as having gout, with four terms (acute exacerbation of gout [code 924311000000106], acute exacerbation of gout [code XabbG], history of gout [code 1443], and primary gout [code X40UW]) comprising the majority of patients (n=899). We identified an additional 311 people ascertained to have gout. The total gout cohort consisted of 15 871 patients (840 diagnosed with COVID-19).
Figure 1.
Data sources of individuals diagnosed with COVID-19
Of the 16 898 individuals diagnosed with COVID-19, 16 005 were identified from positive SARS-CoV-2 test results (12 772 identified only by positive SARS-CoV-2 test), 3847 were identified from hospital records (638 identified only from hospital records), and 1111 were identified from death records (128 identified only from death records). 563 individuals diagnosed after Feb 20, 2021 (28 days before the last recorded death), were excluded from the cohort used in analysis B given the unknown outcome of these patients.
We developed four case-control datasets to test for association with outcomes, as follows: dataset A (analysis A) to test for an association between gout and COVID-19 diagnosis in a population-based cohort (16 898 patients with COVID-19 and 442 939 controls); dataset B (analysis B) to test for an association between gout and COVID-19-related death in people diagnosed with COVID-19 (1111 patients with COVID-19 who died and 15 224 people with COVID-19 known to have survived; 563 participants who were diagnosed after Feb 20, 2021 [28 days before the last recorded death], were excluded from the cohort used in analysis B given the unknown outcome in these individuals); dataset C (analysis C) to test for an association between gout and COVID-19-related death in a population-based cohort (1111 people diagnosed with COVID-19 who died and 458 726 other people, including 15 787 people diagnosed with COVID-19 not known to have died); and dataset D (analysis D) to test for an association, in the subset of the UK Biobank with requisite data, between prescription of colchicine and urate-lowering therapy and the risk of COVID-19-related death for people with gout (690 people diagnosed with COVID-19 who died and 340 708 others, including 12 849 people diagnosed with COVID-19 not known to have died).
Self-reported ethnicity was grouped into White British (British, Irish, White, or any other White background), Black British (African, White and Black African, Black or Black British, Caribbean, White and Black Caribbean, or any other Black background), Asian British (Asian or Asian British, Chinese, Indian, Pakistani, Bangladeshi, White and Asian, or any other Asian background), and other (other ethnic group, mixed, any other mixed background, do not know, or prefer not to answer). Age was calculated for 2020 from year of birth. Age groups used in the analysis were younger than 60 years (n=87 045), 60–69 years (n=146 924), 70–74 years (n=107 297), and older than 74 years (n=118 571). We included 16 diseases in model 2 that are known to be a comorbidity of gout8 or a risk factor for COVID-19-related death.9 The ICD-10 hospital codes used to determine additional comorbidity status were as follows: C00–C96 (cancer), D80–D89 (immunodeficiencies), E08–E13 (diabetes), E78 (disorders of lipoprotein metabolism and other lipidaemias), F01–F03 (dementia), I10–I15 (hypertensive diseases), I60–I69 (cerebrovascular diseases), I20–I25 (ischaemic heart diseases), I26–I28 (pulmonary heart disease), I50 (heart failure), J44 (chronic obstructive pulmonary diseases), J45 (asthma), K70.4/K71.1/K72/K91.82 (liver failure), J84 (interstitial lung disease), M19.9 (osteoarthritis), and N18 (chronic kidney disease). Only hospital records before a positive test for SARS-CoV-2 or a diagnosis of COVID-19 were used to determine comorbidities. This strategy was to avoid including outcomes of COVID-19 in the disease associations (eg, liver failure).
General practice prescription data from Jan 1, 2019, to July 25, 2020, available for 341 398 participants, were used to identify people prescribed colchicine and urate-lowering therapy (allopurinol, benzbromarone, febuxostat, probenecid, and sulfinpyrazone). A single prescription was sufficient for inclusion.
General practice-linked data were used to identify participants who had received either the Pfizer-BioNTech BNT162b2 or Oxford-AstraZeneca ChAdOx1 nCoV-19 vaccines (n=218 575), and to retrieve the date of the first dose. The unvaccinated group (n=241 262) comprised participants without a record of vaccination and those diagnosed with COVID-19 before vaccination and within 14 days of vaccination after the first dose.
Statistical analysis
Model 1 was adjusted for age group, sex, ethnicity, Townsend deprivation index, BMI, and smoking status. Model 2 had the same adjustments as model 1 plus adjustment for the 16 other included diseases. Analysis D compared patients with gout with and without medical treatment (colchicine or urate-lowering therapy) with non-gout controls, using model 2. Analysis of sex-specific groups was done using the same modelling. An additional model consisting of model 1 (without adjustment for BMI) plus eight gout-related metabolic comorbidities (hypertension, dyslipidaemia, type 2 diabetes, chronic kidney disease, obesity [BMI >30 kg/m2], coronary heart disease, cerebrovascular disease, and heart failure) was used to investigate the hypothesis that women with gout are at an increased risk of COVID-19-related death due to a metabolic comorbidity burden. Within the cohort diagnosed with COVID-19 (analysis B) we included a quarterly (three-monthly) categorical time variable.
We assumed that p<0·05 indicated nominal evidence for association. ORs and 95% CIs for the risk of the various diseases use the non-disease group as comparison.
This study is reported according to the Strobe statement. All association analyses were done using R version 4.0.2 in RStudio 1.2.5019.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
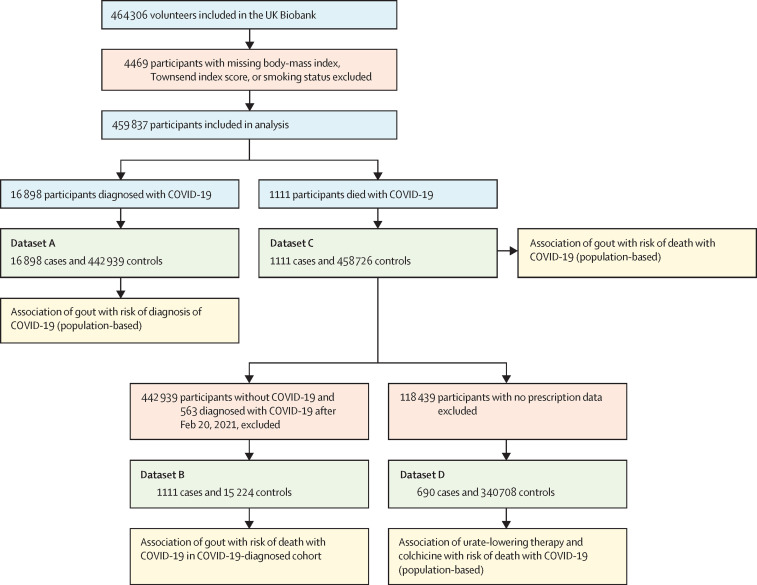
Of 464 306 volunteers included in the UK Biobank, 459 837 participants were included in our population-based cohort (figure 2 ), 15 871 of whom had gout. We recorded the participant characteristics according to diagnosis of COVID-19 and death with COVID-19 (table 1 ). Between March 5, 2020, and March 23, 2021, 136 (0·9%) of 15 871 people with gout died with COVID-19 compared with 1111 (0·2%) of 459 837 in the entire cohort. People who died with COVID-19 had a greater proportion of metabolic-based diseases established as co-morbid with gout,8 for example, chronic kidney disease (212 [19·1%] of 1111 participants who died with COVID-19 vs 14 865 [3·2%] of 459 837 participants in the entire cohort) and diabetes (341 [30·7%] of 1111 participants vs 33 470 [7·3%] of 459 837 participants). We also recorded participant characteristics according to sex (table 2 ). The cohort mainly comprised older people, with 103 006 (50·0%) of 206 147 men and 122 862 (48·4%) of 253 690 women aged 70 years or older. 13 345 (6·5%) of 206 147 men and 2526 (1·0%) of 253 690 women had gout. However, as reported in more detail elsewhere,10 women with gout had a higher proportion of comorbidities than did men, for example, chronic kidney disease (634 [25·1%] of 2526 women vs 1815 [13·6%] of 13 345 men) and diabetes (673 [26·6%] of 2526 women vs 2389 [17·9%] of 13 345 men). In the entire cohort, men had a higher proportion of these conditions (table 2). Notably, in contrast to the higher proportion of men in the COVID-19-diagnosed cohort who died with COVID-19 (712 [64·1%] men vs 399 [35·9%] women; table 1), a greater proportion of women with gout died with COVID-19 than did men with gout (34 [1·3%] of 2526 women vs 102 [0·8%] of 13 345 men; table 2). The proportion of people with gout who were vaccinated against COVID-19 (7475 [47·1%] of 15 871 participants) was similar to the proportion of people without gout who were vaccinated (211 100 [47·5%] of 443 966 of participants).
Figure 2.
Study flowchart
Table 1.
Characteristics of cohort
| Diagnosed with COVID-19 (n=16 898) | COVID-19-related death (n=1111) | Entire cohort (n=459 837) | ||
|---|---|---|---|---|
| Age, years | ||||
| <60 | 5273 (31·2%) | 33 (3·0%) | 87 045 (18·9%) | |
| 60–69 | 5437 (32·2%) | 168 (15·1%) | 146 924 (32·0%) | |
| 70–74 | 2713 (16·1%) | 220 (19·8%) | 107 297 (23·3%) | |
| >74 | 3475 (20·6%) | 690 (62·1%) | 118 571 (25·8%) | |
| Sex | ||||
| Men | 8040 (47·6%) | 712 (64·1%) | 206 147 (44·8%) | |
| Women | 8858 (52·4%) | 399 (35·9%) | 253 690 (55·2%) | |
| Body-mass index, mg/m2 | 28·34 (5·15) | 30·16 (5·98) | 27·37 (4·74) | |
| Townsend deprivation index | −0·69 (3·27) | −0·14 (3·49) | −1·35 (3·06) | |
| Ethnicity | ||||
| White British | 15 173 (89·8%) | 1012 (91·1%) | 433 636 (94·3%) | |
| Black British | 795 (4·7%) | 42 (3·8%) | 11 199 (2·4%) | |
| Asian British | 571 (3·4%) | 38 (3·4%) | 8318 (1·8%) | |
| Other ethnicity | 359 (2·1%) | 19 (1·7%) | 6684 (1·5%) | |
| Smoking status | ||||
| Never smoker | 8887 (52·6%) | 420 (37·8%) | 256 457 (55·8%) | |
| Current smoker | 6055 (35·8%) | 527 (47·4%) | 157 488 (34·2%) | |
| Ex-smoker | 1956 (11·6%) | 164 (14·8%) | 45 892 (10·0%) | |
| Medical history | ||||
| Gout | 840 (5·0%) | 136 (12·2%) | 15 871 (3·5%) | |
| Asthma | 1985 (11·7%) | 169 (15·2%) | 40 857 (8·9%) | |
| Cancer | 2487 (14·7%) | 314 (28·3%) | 69 911 (15·2%) | |
| Cerebrovascular diseases | 1031 (6·1%) | 221 (19·9%) | 15 465 (3·4%) | |
| Chronic kidney disease | 898 (5·3%) | 212 (19·1%) | 14 865 (3·2%) | |
| Chronic obstructive pulmonary disease | 919 (5·4%) | 200 (18·0%) | 15 131 (3·3%) | |
| Dementia | 493 (2·9%) | 151 (13·6%) | 2892 (0·6%) | |
| Diabetes | 1958 (11·6%) | 341 (30·7%) | 33 470 (7·3%) | |
| Heart failure | 766 (4·5%) | 187 (16·8%) | 10 980 (2·4%) | |
| Hypertensive diseases | 5486 (32·5%) | 705 (63·5%) | 127 648 (27·8%) | |
| Immunodeficiencies | 95 (0·6%) | 18 (1·6%) | 1840 (0·4%) | |
| Interstitial lung disease | 140 (0·8%) | 49 (4·4%) | 2013 (0·4%) | |
| Ischaemic heart disease | 2141 (12·7%) | 331 (29·8%) | 46 630 (10·1%) | |
| Lipoprotein disorders | 2990 (17·7%) | 419 (37·7%) | 63 913 (13·9%) | |
| Liver failure | 37 (0·2%) | 17 (1·5%) | 502 (0·1%) | |
| Osteoarthritis | 3522 (20·8%) | 375 (33·8%) | 81 048 (17·6%) | |
| Pulmonary heart disease | 353 (2·1%) | 77 (6·9%) | 7106 (1·5%) | |
| Medical treatment | ||||
| Colchicine* | 204/13 539 (1·5%) | 15/690 (2·2%) | 3942/341 398 (1·2%) | |
| Urate-lowering therapy* | 549/13 539 (4·1%) | 73/690 (10·6%) | 10 590/341 398 (3·1%) | |
Data are n (%), mean (SD), or n/N (%).
n=341 398 subset.
Table 2.
Stratification of cohort by sex
| All women (n=253 690) | All men (n=206 147) | Women with gout (n=2526) | Men with gout (n=13 345) | ||
|---|---|---|---|---|---|
| Diagnosed with COVID-19 | 8858 (3·5%) | 8040 (3·9%) | 166 (6·6%) | 674 (5·1%) | |
| COVID-19-related death | 399 (0·2%) | 712 (0·3%) | 34 (1·3%) | 102 (0·8%) | |
| Age, years | |||||
| <60 | 47 559 (18·7%) | 39 486 (19·2%) | 131 (5·2%) | 1306 (9·8%) | |
| 60–69 years | 83 269 (32·8%) | 63 655 (30·9%) | 529 (20·9%) | 3534 (26·5%) | |
| 70–74 years | 59 851 (23·6%) | 47 446 (23·0%) | 665 (26·3%) | 3522 (26·4%) | |
| >74 years | 63 011 (24·8%) | 55 560 (27·0%) | 1201 (47·5%) | 4983 (37·3%) | |
| Body-mass index, mg/m2 | 27·03 (5·14) | 27·79 (4·17) | 31·97 (6·37) | 30·06 (4·54) | |
| Townsend deprivation index | −1·37 (3·02) | −1·33 (3·11) | −0·72 (3·27) | −1·19 (3·14) | |
| Ethnicity | |||||
| White British | 239 456 (94·4%) | 194 180 (94·2%) | 2286 (90·5%) | 12 647 (94·8%) | |
| Black British | 5653 (2·2%) | 5546 (2·7%) | 99 (3·9%) | 361 (2·7%) | |
| Asian British | 4889 (1·9%) | 3429 (1·7%) | 73 (2·9%) | 183 (1·4%) | |
| Other ethnicity | 3692 (1·5%) | 2992 (1·5%) | 68 (2·7%) | 154 (1·2%) | |
| Smoking status | |||||
| Never smoker | 152 649 (60·2%) | 103 808 (50·4%) | 1313 (52·0%) | 5582 (41·8%) | |
| Current smoker | 79 367 (31·3%) | 78 121 (37·9%) | 1007 (39·9%) | 6554 (49·1%) | |
| Ex-smoker | 21 674 (8·5%) | 24 218 (11·7%) | 206 (8·2%) | 1209 (9·1%) | |
| Medical history | |||||
| Asthma | 24 955 (9·8%) | 15 902 (7·7%) | 522 (20·7%) | 1409 (10·6%) | |
| Cancer | 36 642 (14·4%) | 33 269 (16·1%) | 588 (23·3%) | 2790 (20·9%) | |
| Cerebrovascular diseases | 6734 (2·7%) | 8731 (4·2%) | 222 (8·8%) | 998 (7·5%) | |
| Chronic kidney disease | 7525 (3·0%) | 7340 (3·6%) | 634 (25·1%) | 1815 (13·6%) | |
| Chronic obstructive pulmonary disease | 7353 (2·9%) | 7778 (3·8%) | 267 (10·6%) | 896 (6·7%) | |
| Dementia | 1427 (0·6%) | 1465 (0·7%) | 49 (1·9%) | 153 (1·1%) | |
| Diabetes | 14 056 (5·5%) | 19 414 (9·4%) | 673 (26·6%) | 2389 (17·9%) | |
| Heart failure | 4018 (1·6%) | 6962 (3·4%) | 316 (12·5%) | 1353 (10·1%) | |
| Hypertensive diseases | 61 929 (24·4%) | 65 719 (31·9%) | 1788 (70·8%) | 7754 (58·1%) | |
| Immunodeficiencies | 983 (0·4%) | 857 (0·4%) | 38 (1·5%) | 99 (0·7%) | |
| Interstitial lung disease | 930 (0·4%) | 1083 (0·5%) | 47 (1·9%) | 145 (1·1%) | |
| Ischaemic heart disease | 16 660 (6·6%) | 29 970 (14·5%) | 614 (24·3%) | 3500 (26·2%) | |
| Lipoprotein disorders | 27 541 (10·9%) | 36 372 (17·6%) | 837 (33·1%) | 4210 (31·5%) | |
| Liver failure | 188 (0·1%) | 314 (0·2%) | 10 (0·4%) | 48 (0·4%) | |
| Osteoarthritis | 48 027 (18·9%) | 33 021 (16·0%) | 1167 (46·2%) | 3745 (28·1%) | |
| Pulmonary heart disease | 3431 (1·4%) | 3675 (1·8%) | 148 (5·9%) | 475 (3·6%) | |
| Medical treatment | |||||
| Colchicine* | 834/190 664 (0·4%) | 3108/150 734 (2·1%) | 334/2250 (14·8%) | 1734/11 789 (14·7%) | |
| Urate-lowering therapy* | 1479/190 664 (0·8%) | 9111/150 734 (6·0%) | 1479/2250 (65·7%) | 9111/11 789 (77·3%) | |
Data are n (%), mean (SD), or n/N (%).
n=341 398 subset.
Gout was associated with diagnosis of COVID-19 both in the unadjusted analysis (OR 1·49, 95% CI 1·39–1·60) and in model 1 (1·41, 1·31–1·51; table 3 ; appendix p 5). Gout remained associated with diagnosis of COVID-19 as more variables were added to the model (model 2; 1·20, 1·11–1·29) Women with gout were more likely to be diagnosed with COVID-19 than were men with gout in model 2 (1·44, 1·22–1·70 for women vs 1·12, 1·03–1·22 for men). In model 2, when stratified by vaccination status, the risk of diagnosis with COVID-19 was significant in the non-vaccinated group (1·21, 1·11–1·30) but not the vaccinated group (1·09, 0·65–1·85; appendix p 1). Data for the association between included covariables and diagnosis of COVID-19 are presented in the appendix (p 2).
Table 3.
Association of gout with diagnosis and outcomes of COVID-19
|
Diagnosis of COVID-19 |
COVID-19-related death in COVID-19 cohort |
COVID-19-related death in entire cohort |
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Unadjusted | ||||||
| Combined | 1·49 (1·39–1·60) | <0·0001 | 2·97 (2·45–3·62) | <0·0001 | 3·93 (3·28–4·70) | <0·0001 |
| Men | 1·34 (1·24–1·45) | <0·0001 | 1·99 (1·58–2·50) | <0·0001 | 2·43 (1·97–3·00) | <0·0001 |
| Women | 1·96 (1·67–2·30) | <0·0001 | 5·74 (3·86–8·53) | <0·0001 | 9·37 (6·58–13·35) | <0·0001 |
| Model 1* | ||||||
| Combined | 1·41 (1·31–1·51) | <0·0001 | 1·44 (1·16–1·78) | 0·00091 | 1·76 (1·46–2·12) | <0·0001 |
| Men | 1·27 (1·17–1·38) | <0·0001 | 1·25 (0·98–1·60) | 0·073 | 1·47 (1·19–1·83) | 0·00044 |
| Women | 1·91 (1·62–2·24) | <0·0001 | 2·34 (1·51–3·62) | 0·00013 | 3·49 (2·41–5·04) | <0·0001 |
| Model 2† | ||||||
| Combined | 1·20 (1·11–1·29) | <0·0001 | 1·20 (0·96–1·51) | 0·11 | 1·29 (1·06–1·56) | 0·013 |
| Men | 1·12 (1·03–1·22) | 0·0066 | 1·11 (0·85–1·44) | 0·44 | 1·16 (0·93–1·45) | 0·20 |
| Women | 1·44 (1·22–1·70) | <0·0001 | 1·65 (1·04–2·64) | 0·035 | 1·98 (1·34–2·94) | 0·00062 |
OR=odds ratio.
Model 1 was adjusted for age group, sex, ethnicity, Townsend deprivation index, BMI, and smoking status.
Model 2 was adjusted for age group, sex, ethnicity, Townsend deprivation index, BMI, and smoking status, plus 16 other diseases.
In unadjusted analyses, we found an association between gout and the risk of COVID-19-related death within the cohort diagnosed with COVID-19 (OR 2·97, 95% CI 2·45–3·62; table 3; appendix p 5). An association was also evident in model 1 (1·44, 1·16–1·78), however the fully adjusted model (model 2) showed no association between gout and the risk of COVID-19-related death (1·20, 0·96–1·51). To account for improvements in outcome and SARS-CoV-2 evolution over time11, 12, 13, 14 we also included a quarterly (3-monthly) categorical time variable in model 2. The categorical reference group contained diagnoses between Jan 1, 2020, and March 31, 2020 (312 positive cases, including 101 deaths). After the inclusion of the categorical time variable, the association of gout and death in the cohort diagnosed with COVID-19 was significant (OR 1·41, 95% CI 1·12–1·77; p=0·0037). Data for the association between included covariables and the risk of COVID-19-related death in the cohort diagnosed with COVID-19 are in the appendix (p 3).
Gout was associated with the risk of COVID-19-related death in the population-based unadjusted analysis (OR 3·93. 95% CI 3·28–4·70; table 3; appendix p 5). An association between gout and COVID-19-related death was also evident in model 1 (1·76, 1·46–2·12). In model 2, which was additionally adjusted for other diseases, gout remained associated with the risk of COVID-19-related death (1·29, 1·06–1·56). In the non-vaccinated group, the risk of COVID-19-related death was similar to the entire cohort (1·30, 1·07–1·58; appendix p 1). This analysis was not done in the vaccinated group as there was only one COVID-19-related death. Data for the association between the included covariables and the risk of COVID-19-related death in the entire cohort are in the appendix (p 4).
Women with gout had an increased risk of COVID-19-related death in the unadjusted analysis of death in the entire cohort (OR 9·37, 95% CI 6·58–13·35), with a 95% CI that did not overlap the data for men (2·43, 1·97–3·00; table 3; appendix p 5). In model 1, the OR for women was 3·49 (95% CI 2·41–5·04) and for men was 1·47 (1·19–1·83), with non-overlapping 95% CIs (table 3). Addition of the other diseases in model 2 resulted in an OR of 1·98 (1·34–2·94) for women and a non-significant OR of 1·16 (0·93–1·45) for men, with overlapping 95% CIs.
Women with gout have an elevated risk of metabolic comorbidities (hypertension, dyslipidaemia, type 2 diabetes, chronic kidney disease, obesity, coronary heart disease, cerebrovascular disease, and heart failure);15 therefore, to investigate the hypothesis that women with gout are at an increased risk of COVID-19-related death due to a burden of metabolic comorbidity, we added to model 1 only the eight aforementioned metabolic comorbidities, with obesity defined as BMI >30 kg/m2 replacing BMI in the model. The OR for women was 2·10 (95% CI 1·43–3·08; p=0·00016) and for men was 1·13 (0·90–1·41; p=0·28), with 95% CIs that did not overlap.
Finally, we stratified the UK Biobank gout cohort for prescription of colchicine and urate-lowering therapy and tested for an association with COVID-19-related death using model 2 (using the same non-gout comparison cohort). For people with gout with and without colchicine prescription, the ORs were 1·06 (95% CI 0·60–1·89; p=0·84) and 1·38 (1·08–1·76; p=0·0093), and for people with gout with and without urate-lowering therapy the ORs were 1·39 (1·07–1·80; p=0·014) and 1·19 (0·79–1·81; p=0·41). All 95% CIs were overlapping.
Discussion
Gout is a prevalent arthropathy (present in 3·9% of the US population16 and 14% of people of Pacific ethnicity17) yet has received little attention with respect to COVID-19 outcomes.18 In this study, we provide a contribution to the gout-COVID-19 literature. We previously found no evidence for an association between gout and COVID-19 related death in a cohort of 2059 people diagnosed with COVID-19.3 However, for the first time to our knowledge, we report a statistically significant association between gout and COVID-19-related death in a multivariable-adjusted population-based study using a larger cohort of people with COVID-19 than did our previous study. Furthermore, we found that the impact of COVID-19 was greater in women with gout than in men with gout.
Most of the risk of COVID-19-related death was accounted for by eight metabolic comorbidities in men (obesity, hypertension, dyslipidaemia, type 2 diabetes, chronic kidney disease, coronary heart disease, cardiovascular disease, and heart failure), but not women, despite the higher risk of metabolic comorbidity in women with gout.15 This finding could indicate the presence of additional risk factors for COVID-19-related death in women. What these risk factors could be was not revealed by our analysis but could be related to factors underlying the known higher risk of metabolic comorbidity in women with gout. When we investigated the influence of colchicine, we found no difference in risk of COVID-19-related death in the gout cohort. The COLCORONA randomised clinical trial provided evidence that colchicine reduces the risk of death or hospital admission in patients with PCR-diagnosed COVID-19.19 However, the RECOVERY randomised trial did not provide evidence for improved outcomes conferred by colchicine for people admitted to hospital with COVID-19.20
Our estimates of the risk of COVID-19-related death could be used in clinician-patient discussions regarding patient decisions to be vaccinated against SARS-CoV-2. There is a paucity of data on vaccine hesitancy in people with gout, although a recent international survey reported only 8·2% of patients with rheumatic disease were hesitant or unwilling to be vaccinated against COVID-19.21 The possibility of vaccine hesitancy in people with gout is concerning in the context of our data, although we note that the proportion of people with gout who had been vaccinated was similar to the non-gout population (47·1% vs 47·5%). Although we are unable to generalise our findings to non-UK populations, in the absence of evidence to the contrary, the safest approach is to assume that the risks for COVID-19-related death in other population groups are at least equivalent to those in our study. For example, the prevalence of gout in the Māori population of Aotearoa New Zealand is 8% and 14% in the Pacific population, compared with to 4% in the non-Māori non-Pacific population.17 These figures justify a targeted strategy for vaccination of Māori and Pacific people with gout in all health-care settings.
Our study has some specific limitations. First, our data are derived from the predominantly middle-aged White British ethnic group of the UK and are not necessarily generalisable to other ethnic groups or other White European ethnic groups. Second, there is the possibility of additional unidentified COVID-19-related deaths in the group diagnosed with COVID-19 in analysis B. Third, COVID-19 outcomes will have been affected over the period of this study (March, 2020, to April, 2021) as clinical treatments and SARS-CoV-2 lineages14 evolved. We were able to account for this only in analysis B by inclusion of a time variable. Fourth, before August, 2020, Public Health England ascribed deaths to COVID-19 if the individual had ever had a positive SARS-CoV-2 test, meaning it was possible that some deaths ascribed to COVID-19 were not related to COVID-19.22 However, Public Health England estimated that before Aug 3, 2020, 96% of deaths from COVID-19 in England were reported according to the revised newly adopted definitions,22 the first being death reported within 28 days of the first positive SARS-CoV-2 test, and the second being death either within 60 days of the first positive SARS-CoV-2 test or if COVID-19 was mentioned on the death certificate if the person died more than 60 days after the first positive SARS-CoV-2 test. The flawed reporting would affect 4% of the 456 COVID-19 UK Biobank deaths in England before August, 2020, which we estimate to be 18 people in our analysis, representing 1·6% (18 of 1111) analysed COVID-19-related deaths, and to have had little effect on our findings. Furthermore, we did not account for non-COVID-19-related risk of death in gout in the 60-day reporting period after diagnosis of COVID-19 by UK public health authorities as any effect was likely to be small. To illustrate, a 10-year study reported a hazard ratio for all-cause death in patients with gout of 1·17 (95% CI 1·14–1·21).10 For a population of 1000 people over a 120-month period, this would equate to 2·8 people per 2-month period, or 0·28%. Given that 136 patients with gout died with COVID-19, any effect on our estimate of the risk of death with COVID-19 that is confounded by other causes of death will be minimal. Fifth, the potential effect of severity (disease activity) in gout could not be assessed (eg, the presence or absence of tophus or flare frequency). Sixth, in the context of reports of a U-shaped relationship between serum urate levels and COVID-19 outcomes,23, 24, 25 we were unable to investigate any effect of serum urate levels on COVID-19 related death, as these data are unavailable in the UK Biobank. Seventh, the medicine prescribing data were single script from general practitioners only; there was no way of ascertaining adherence or whether participants were taking the medication during the COVID-19 pandemic, although we attempted to account for this by only using prescription information from 2019 and 2020. Eighth, we were unable to account for the effect of individual behaviour modification on outcomes. For example, people with rheumatic disease in the Netherlands have been reported to be twice as likely to adhere to strict isolation measures than healthy controls26 and, in the UK, people with risk factors for poor outcomes from COVID-19 show greater risk-mitigating behaviour.27 Finally, we included comorbidities for gout as variables in model 2—there is no consensus as to whether this should be done in epidemiological studies of COVID-19 outcomes in people with rheumatic diseases.28 Therefore, we also included models with fewer adjustors (unadjusted and adjusted by BMI and demographic factors). Several other general limitations (index event [collider] bias in analysis B, inability to consider severity of infection, incomplete testing earlier in the pandemic, and a higher case-fatality ratio in the UK Biobank) have been discussed by us in more detail previously.3
In conclusion, we found an increased risk of COVID-19-related death in people with gout, with a greater burden in women than men. Understanding the drivers of this increased risk in women with gout warrants further investigation in larger datasets.
Data sharing
All data used in this study were accessed from the publicly available UK Biobank Resource under application number 12611. These data cannot be shared with other investigators.
Declaration of interests
PCR reports personal fees from Abbvie, Atom Biosciences, Eli Lilly, Gilead, Janssen, Novartis, UCB Pharma, Roche, and Pfizer, meeting attendance support from BMS, Pfizer, and UCB Pharma, and grant funding from Janssen, Novartis, Pfizer, and UCB Pharma, all outside the submitted work. ND reports personal fees from AbbVie, Horizon, Janssen, Dyve Biosciences, Cello Health, PK Med, JW Pharmaceuticals, Selecta, Arthrosi, and AstraZeneca, grants from Amgen and AstraZeneca, and non-financial support from AbbVie, all outside the submitted work. LKS reports personal fees from the New Zealand PHARMAC Therapeutic Advisory Committee. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We sincerely thank all participants. We thank Riku Takei for figure 2.
Contributors
RKT and TRM substantially contributed to study conception and design, to acquisition and analysis of data, and interpretation of results. AG, LKS, PCR, and ND substantially contributed to study design and interpretation of results. All authors contributed to drafting the article and critical revisions, and all authors approved the final version. RKT and TRM directly accessed and verified the underlying data reported in the manuscript.
Supplementary Material
References
- 1.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80:930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Ann Rheum Dis. 2015;74:661–667. doi: 10.1136/annrheumdis-2013-204463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topless RK, Phipps-Green A, Leask M, et al. Gout, rheumatoid arthritis, and the risk of death related to coronavirus disease 2019: an analysis of the UK Biobank. ACR Open Rheumatol. 2021;3:333–340. doi: 10.1002/acr2.11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Townsend P, Phillimore P, Beattie A. Routledge; Abingdon-on-Thames: 1988. Health and deprivation: inequality and the North. [Google Scholar]
- 6.Cadzow M, Merriman TR, Dalbeth N. Performance of gout definitions for genetic epidemiological studies: analysis of UK Biobank. Arthritis Res Ther. 2017;19:181. doi: 10.1186/s13075-017-1390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalbeth N, Schumacher HR, Fransen J, et al. Survey definitions of gout for epidemiologic studies: comparison with crystal identification as the gold standard. Arthritis Care Res (Hoboken) 2016;68:1894–1898. doi: 10.1002/acr.22896. [DOI] [PubMed] [Google Scholar]
- 8.Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007;57:109–115. doi: 10.1002/art.22466. [DOI] [PubMed] [Google Scholar]
- 9.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vargas-Santos AB, Neogi T, da Rocha Castelar-Pinheiro G, Kapetanovic MC, Turkiewicz A. Turkiewicz. Cause-specific mortality in gout: novel findings of elevated risk of non-cardiovascular-related deaths. Arthritis Rheumatol. 2019;71:1935–1942. doi: 10.1002/art.41008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jorge A, D'Silva KM, Cohen A, et al. Temporal trends in severe COVID-19 outcomes in patients with rheumatic disease: a cohort study. Lancet Rheumatol. 2021;3:e131–e137. doi: 10.1016/S2665-9913(20)30422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serling-Boyd N, D'Silva KM, Hsu TY, et al. Coronavirus disease 2019 outcomes among patients with rheumatic diseases 6 months into the pandemic. Ann Rheum Dis. 2021;80:660–666. doi: 10.1136/annrheumdis-2020-219279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gianfrancesco MA, Robinson PC. Changing COVID-19 outcomes in patients with rheumatic disease-are we really getting better at this? Lancet Rheumatol. 2021;3:e88–e90. doi: 10.1016/S2665-9913(21)00008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies NG, Jarvis CI, Edmunds WJ, Jewell NP, Diaz-Ordaz K, Keogh RH. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. 2021;593:270–274. doi: 10.1038/s41586-021-03426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sumpter NA, Cadzow M, So A, Reynolds RJ, Merriman TR. Analysis of common gout comorbidities in the UK Biobank cohort reveals sex-specific effects and genetic differentiation. Arthritis Rheumatol. 2020;72:S10. [Google Scholar]
- 16.Chen-Xu M, Yokose C, Rai SK, Pillinger MH, Choi HK. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: the national health and nutrition examination survey, 2007–2016. Arthritis Rheumatol. 2019;71:991–999. doi: 10.1002/art.40807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalbeth N, Dowell T, Gerard C, et al. Gout in Aotearoa New Zealand: the equity crisis continues in plain sight. N Z Med J. 2018;131:8–12. [PubMed] [Google Scholar]
- 18.Dalbeth N, Robinson PC. Patients with gout: an under-recognised group at high risk of COVID-19. Lancet Rheumatol. 2021;3:e317–e318. doi: 10.1016/S2665-9913(21)00073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tardif J-C, Bouabdallaoui N, L'Allier PL, et al. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir Med. 2021;9:924–932. doi: 10.1016/S2213-2600(21)00222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.RECOVERY Collaborative Group Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet Resp Med. 2021;9:1419–1426. doi: 10.1016/S2213-2600(21)00435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Putman M, Kennedy K, Sirotich E, et al. COVID-19 vaccine perceptions and uptake in people with rheumatic disease: results from the COVID-19 Global Rheumatology Alliance Vaccine Survey. Lancet Rheumatol. 2022 doi: 10.1016/S2665-9913(22)00001-7. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffin S. Covid-19: England comes into line with rest of UK on recording deaths. BMJ. 2020;370 doi: 10.1136/bmj.m3220. [DOI] [PubMed] [Google Scholar]
- 23.Chen B, Lu C, Gu H-Q, et al. Serum uric acid concentrations and risk of adverse outcomes in patients with COVID-19. Front Endocrinol (Lausanne) 2021;12 doi: 10.3389/fendo.2021.633767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu F, Guo Y, Lin J, et al. Association of serum uric acid levels with COVID-19 severity. BMC Endocr Disord. 2021;21:97. doi: 10.1186/s12902-021-00745-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng T, Liu X, Wei Y, et al. Laboratory predictors of COVID-19 mortality: a retrospective analysis from Tongji Hospital in Wuhan. Mediators Inflamm. 2021;2021 doi: 10.1155/2021/6687412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hooijberg F, Boekel L, Vogelzang EH, et al. Patients with rheumatic diseases adhere to COVID-19 isolation measures more strictly than the general population. Lancet Rheumatol. 2020;2:e583–e585. doi: 10.1016/S2665-9913(20)30286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahil SK, Yates M, Langan SM, et al. Risk-mitigating behaviours in people with inflammatory skin and joint disease during the COVID-19 pandemic differ by treatment type: a cross-sectional patient survey. Br J Dermatol. 2021;185:80–90. doi: 10.1111/bjd.19755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curtis JR, Johnson SR, Anthony DD, et al. American College of rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 2. Arthritis Rheumatol. 2021;73:e30–e45. doi: 10.1002/art.41877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this study were accessed from the publicly available UK Biobank Resource under application number 12611. These data cannot be shared with other investigators.