Abstract
Background
To evaluate the surgical safety and quality of transurethral en bloc resection with monopolar current for non-muscle invasive bladder cancer (NMIBC) based on the tumour, node, metastasis (TNM) classification system, and report the midterm oncological outcome.
Methods
From October 2015 to June 2017, en bloc resection of bladder tumor (ERBT) and transurethral resection of bladder tumor (TURBT) were performed in 96 and 87 patients clinically diagnosed with NMIBC in the prospective case-control trial, respectively. Operative details, intraoperative and postoperative complications regarded as safety outcomes were documented. The quality of ERBT was judged by the histopathological examination of tumor specimens from initial resection and second TURBT, random bladder biopsy and follow-up recurrence rate.
Results
Operative time, obturator nerve reflex, irrigation and catheterization time were similar in the two groups. Bladder perforation was occurred in 2 patients during ERBT and 9 patients during TURBT (2/96 vs. 9/87, P=0.019). Compared with TURBT group, the ratio of detrusor muscle (DM) identified in pathologic T1 tumor specimens was higher (P=0.024), but lower in pathologic Ta tumor specimens in ERBT group (P<0.001). The residual tumor identified in ERBT group was lower than that in TURBT group during second TURBT (2/28 vs. 10/32, P=0.020). The recurrence-free survival rate did not differ significantly between the two groups after 24 months follow-up.
Conclusions
ERBT based on TNM system is a safe and feasible technique to treat patients with NMIBC. Besides, ERBT may reduce the proportion of bladder perforation and residual tumor during initial resection.
Keywords: Bladder cancer, en bloc resection, transurethral resection of bladder tumor (TURBT), monopolar current, TNM system
Introduction
Bladder cancer (BC) is the second common malignant tumor of genitourinary system in the United States of America (USA). It was estimated that the number of newly diagnosed BC patients would rise to about 80,470 in 2019, equally to an average of more than 220 new cases per day (1). About 75% of newly diagnosed BC with the lesions confined to the mucosa [stage Ta and carcinoma in situ (CIS)] or submucosa (stage T1) and the proportion is more higher in younger patients (<40 years), which is named non-muscle invasive bladder cancer (NMIBC) (2). The standard treatment scheme of NMIBC is transurethral resection of bladder tumor (TURBT), and followed with tailored intravesical therapy with chemotherapeutic drugs or Bacillus Calmette-Guerin (BCG) corresponding to the individual risk of tumor recurrence and progression. The recurrence and progression rate ranged from 15% to 61% and from 0.2% to 17% for NMIBC after TURBT at one year, respectively, and the rate ranged from 31% to 78% and from 0.8% to 45% at five years (3). Due to the high recurrence and progression rate of bladder tumor, a rigorous follow-up program of cystoscopy and instillation therapy regularly is needed. Thus, the economic burden of BC is huge. And in 2012, the disease was responsible for 3% of all cancer-related medical expenses in the European Union (EU) (€143 billion) (4).
Although TURBT is used widely and the procedure has amassed enormous expertise over the years, many disadvantages are still accompanied with it. Such as some recognized intraoperative complications: obturator nerve reflex, acute bleeding and bladder perforation, which are occurred during the tumor resection, especially when the neoplastic tissues are located in the lateral wall and around the ureteral orifice (5). Moreover, piecemeal resection of the tumor lead to the tumor cells dissemination and seeding, and thermal injury of the tumor samples make it difficult to accurately evaluate the pathological of tumor stage (6). To alleviate the above-mentioned drawbacks and achieve optimal results, en bloc resection of bladder tumor (ERBT) as a possible alternative to TURBT has obtained more and more interest among urologists (7). ERBT can be performed with either electrical ERBT (e-ERBT) or laser ERBT (l-ERBT), and the results indicate that there is not much of a difference between various energy sources in performing the procedure (8). However, laser technology used in ERBT has some disadvantage, such as pulsed emission mode and disruption of tissue at incision line. And not every hospital has access to laser devices, which are expensive for small hospital. Then using an electrical current instead of laser devices to perform ERBT may be a promising alternative.
According to our clinical practice, here we introduce a novel technology of en bloc resection with monopolar current according to TNM classification system, and evaluate its safety and quality by comparison to TURBT in terms of perioperative features and midterm oncological outcome. According to our best knowledge, this is the first report about ERBT with monopolar current based on TNM system.
Methods
From October 2015 to June 2017, ERBT and TURBT were performed in 96 patients with odd registration number and 87 patients with even registration number clinically diagnosed with NMIBC in the prospective case-control trial, respectively. The surgical operation was performed by same urologist who was well trained and experienced with endoscopic practices. Cystoscopy, ultrasonography, intravenous pyelography and computed tomography were performed to choose the appropriate patients routinely. The inclusion criteria were cystoscopically proved primary, not the recurrent BC, with the diameter of the tumor between 1.0 to 3.0 cm. The exclusion criteria were CIS, coupled with upper urinary tract tumor and preoperative examination results suggestive of a high probability of distant metastasis or tumor stage ≥ T3. All patients gave their consent for the procedure and their clinical data were stored for the current scientific purpose. This study protocol was approved by the Ethics Committee of First Affiliated Hospital of Shanxi Medical University.
Surgical procedure
Both ERBT and TURBT were performed using an Olympus 26F continuous flow resectoscope with monopolar current (Olympus, Japan). The energy magnitude of cutting and coagulation was 110W and 70W (Valleylab, USA), respectively, and 5% mannitol solution was utilized as the irrigant during surgical operation. Under combined spinal and epidural anesthesia, all patients were positioned in the lithotomy position. The time for tumor resection, taking out the specimens and dwelling catheterization were included in the operative time. The data of the demographic characteristics, intraoperative complications, postoperative conditions and follow-up information in the two groups were collected. The bladder perforation was judged by the cystoscope during the operation preliminarily. If the surgeon is not sure during the operation, postoperative cystography was performed to confirm bladder perforation.
ERBT based on TNM system
Examine the bladder thoroughly. Recording the tumor location, diameter, and finding the tumor base and adjacent mucosa.
Define the boundary between tumor base and adjacent normal mucosa. The boundary between normal bladder mucosa and tumor base is defined according to the mucosal color and distribution of blood supply vessels under direct vision.
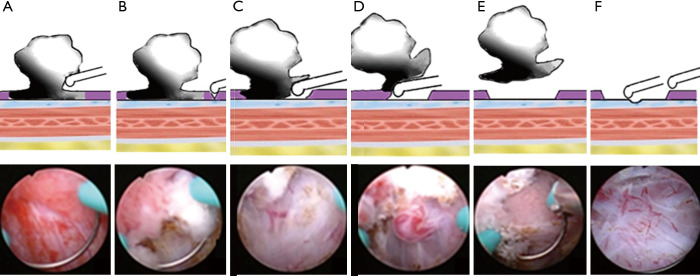
Preliminary judgement of clinical stage of Ta and T1. Pushing the tumor base with monopolar current and observing whether the interlace movement happened among the tumor base and submucosa. Interlace movement occurred in Ta instead of T1 bladder tumor (Figure 1).
Make a small incision in proximal position of the tumor base. Applying gentle pressure on the proximal normal bladder mucosa about 1.0–2.0 cm away from the tumor base and making a small incision reached to the muscular propria layers (Figure 1B).
Remove the tumor in one piece. Identifying the anatomical structure and morphology of normal mucosa, submucosa and muscular propria layers, then blunt shearing of the whole mucosal and submucosal patch around the tumor base and gradually reached to the deepest infiltration of tumor tissue. Coagulating the blood supply arteries of the tumor and removing the tumor in one piece (Figure 1C,D,E).
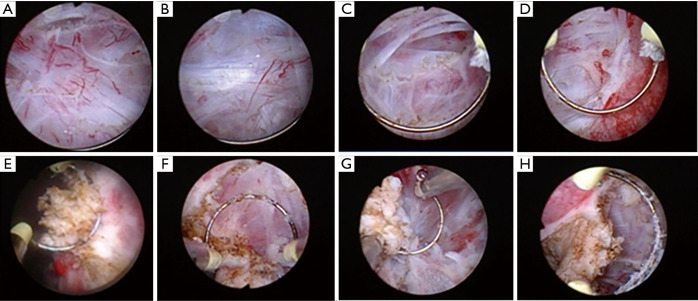
Identify the mucosa, submucosa, muscularis propria surface, muscular propria layers and tumor tissue, and then distinguish the clinical stage of the tumor in real-time during tumor resection according to tumor stage stratification of TNM system. If the tumor is confined to the mucosa layer, then the mucosa and submucosa could be easily separated by mechanical thrust with monopolar current. At this time, the submucosa structure is integrity and the clinical stage of tumor is Ta (Figure 2A). If the tumor is limited to the submucosa, it is not easy to separate the mucosa from submucosa and need to blunt shear the whole mucosal and submucosa. At this time, the muscularis propria surface is covered with a silver-white intact myolemma and the clinical stage of tumor is T1 (Figure 2B). If the tumor invades superficial muscular propria layers, then the detrusor muscle (DM) needs to be cut partially and pink deep muscle tissue can be seen after removing the tumor. At this time, the tumor clinical stage is T2a (Figure 2C). If the whole muscular propria layers need to be removed and extravesical fat is normal, the clinical stage of the tumor is T2b (Figure 2D).
Recognize the residual tumor. After tumor resection, the organizational structure of operation site in bladder is smooth and clear (Figure 1F). According to the clinical experience, the structure of residual tumor in the operation site is obviously different from that of normal mucosa, submucosa and muscular propria layers. The residual tumor is disordered in structure and uneven in texture (Figure 2E,F,G,H). Taking a cold cup biopsy to confirm whether the tumor resection is complete.
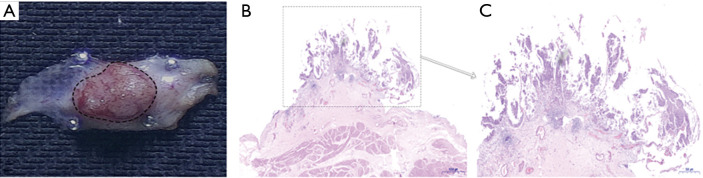
Retrieve the tumor in one piece. Extracting the tumor with an Ellick evacuator through the resectoscope. In the case of larger tumor, the lesion is grasped with laparoscopic forceps and retrieved from the bladder with (and not through) the resectoscope (9). Representative images of the integrated tumor specimen are shown in Figure 3.
Figure 1.
The key steps and techniques of ERBT based on TNM system. (A) Observe the interlace movement between tumor base and submucosa by pushing tumor base with monopolar current. (B) Make a small incision. (C) Blunt shear the whole mucosa and submucosa, and gradually reached to the deepest infiltration of tumor tissue. (D) Coagulate the blood supply arteries of the tumor. (E) Remove the tumor in one piece. (F) Take a cold cup biopsy.
Figure 2.
Distinguish the clinical stage of the tumor and residual tumor during ERBT. Distinguish the clinical stage of the tumor and residual tumor. (A) Ta, submucosa is a normal and translucent tissue. (B) T1, detrusor muscle (DM) is a intact silver-white fibrous tissue. (C) T2a, part of muscular propria layers is destroyed and the deep muscular structure is intact. (D) T2b, no normal muscle tissue was left after tumor resection. (E,F,G,H) The residual tumor that invaded into the muscular propria layer was not completely excised and it is disordered in structure and uneven in texture.
Figure 3.
Representative images of the integrated tumor specimen. (A) The image of fresh integrated tumor specimen. (B) The HE staining image of fresh integrated tumor specimen. (C) The HE staining representative image of tumor area.
TURBT
During TURBT, perform resection in fractions including the exophytic part of the tumor, the underlying bladder tissue with the DM, and the edges of the resection area. The tumor specimens was extracted from the bladder with an Ellick evacuator through the resectoscope.
Second TURBT
According to the European Association of Urology (EAU) Guidelines on non-muscle invasive urothelial carcinoma of the bladder, a second TURBT was performed 2-6 weeks after initial resection among the patients with the characteristic that positive cold cup biopsy, high-grade tumor and/or T1 tumor in the two groups (10). The normal anatomic structure in initial operation site was destroyed, and the TURBT method was chose as a method to perform second TURBT in all patients.
Postoperative management
All patients were indwelled catheter for postoperative bladder irrigation. Patients with no bladder perforation accepted immediate intravesical chemotherapy with 30 mg pirarubicin within 24 hours after surgical operation for the first-time treatment, and administered weekly for 8 weeks and then followed by monthly to 1 year for both groups. The follow-up methods and schedule included ultrasonography and cystoscopy every 3 months in the first year and 6 months thereafter to the second year. The resected specimen was sent for histopathologic examination and evaluated by the same pathologist. The 2009 version of TNM system and the 2004 World Health Organization (WHO) grading system were used for tumor stage and histological grade examination, respectively.
Statistical analysis
Continuous data were analyzed using the mean ± standard deviation and compared by independent t test. Categorical variables were compared using the chi-square test or Fisher exact test. Recurrence-free survival was calculated using the Kaplan-Meier method and were compared using the log-rank test. Statistical analyses were performed using the SPSS statistical software package, version 19.0 (IBM Co., Armonk, NY, USA) for Windows. All tests were two-sided, and statistical significance was defined as P<0.05.
Results
Pathological outcome
The pathologic results after initial resection of the two groups are listed in Table 1. The tumor grade and stage in ERBT group were similar to that in TURBT group. At histopathological examination of tumor specimens from initial resection, 10 cases in ERBT group and 4 cases in TURBT group were proved with DM invasion. 5 tumor specimens with stage Ta and 24 tumor specimens with stage T1 in ERBT group contained DM. In contrast, 44 tumor specimens with stage Ta and 18 tumor specimens with stage T1 in TURBT group contained DM. The random cold cup biopsy was performed after tumor resection, and the pathological evaluation demonstrated no significantly difference between the two groups. Twenty-eight patients (include 1 patient with positive cold cup biopsy) in ERBT group and 32 patients (include 4 patients with positive cold cup biopsy) in TURBT group underwent second TURBT. The proportion of residual tumor identified at the original resection site in TURBT group was higher than that in the ERBT group (10/32 vs. 2/28, P=0.020). Among these patients, 1 patient with stage T1 upstage to T2 in ERBT group, while 2 patients with stage Ta upstage to T1 and 3 patients with stage T1 upstage to T2 in TURBT group. Except for the 11 patients in ERBT group and 7 patients in TURBT group with DM invaded in final pathology diagnosis, the other patients were subdivided into the low-risk, intermediate-risk and high-risk groups according to the EAU Guidelines (Table 2).
Table 1. Demographic and tumor characteristics of ERBT group vs. TURBT group.
| Variable | ERBT (n=96) | TURBT (n=87) | P |
|---|---|---|---|
| Age (year) | 54.63±12.07 | 55.43±11.67 | 0.963 |
| Gender, n (%) | 0.803 | ||
| Male | 70 (72.92%) | 62 (71.26%) | |
| Female | 26 (20.08%) | 25 (28.74%) | |
| Tumor diameter (cm) | 1.79±0.47 | 1.72±0.57 | 0.337 |
| Tumor number | 1.25±0.73 | 1.21±0.69 | 0.763 |
| Tumor location, n (%) | 0.885 | ||
| Lateral wall | 56 (55.00%) | 51 (44.44%) | |
| Posterior wall | 19 (12.50%) | 17 (15.56%) | |
| Anterior wall | 23 (15.00%) | 16 (14.44%) | |
| Dome | 6 (2.50%) | 8 (5.56%) | |
| Trigone/bladder neck | 16 (5.00%) | 14 (8.89%) | |
| Grade (WHO2004), n (%) | 0.954 | ||
| PUNLMP | 17 (13.64%) | 14 (15.12%) | |
| LG | 59 (60.00%) | 55 (53.49%) | |
| HG | 20 (26.36%) | 18 (31.39%) | |
| T stage, n (%) | 0.318 | ||
| pTa | 61 (50.83%) | 57 (52.22%) | |
| pT1 | 25 (40.83%) | 26 (43.33%) | |
| pT2 | 10 (8.34%) | 4 (4.45%) | |
| Detrusor muscle available, n (%) | |||
| pTa | 5/61 (8.20%) | 44/57 (68.09%) | <0.001 |
| pT1 | 24/25 (95.92%) | 18/26 (71.79%) | 0.024 |
| pT2 | 10/10 (100.00%) | 4/4 (100.00%) | – |
| Positive bladder biopsy, n (%) | |||
| pTa | 0/61 (0.00%) | 0/57 (0.00%) | – |
| pT1 | 1/25 (2.04%) | 4/26 (15.38%) | 0.350 |
| pT2 | 2/10 (20.00%) | 3/4 (75.00%) | 0.095 |
WHO, World Health Organization; PUNLMP, papillary urothelial neoplasms of low malignant potential; LG, low grade; HG, high grade.
Table 2. Patient and recurrence rate after 24 months of ERBT group vs. TURBT group.
| Risk stratification | ERBT (n=85) | TURBT (n=80) | |||
|---|---|---|---|---|---|
| Patients | Recurrence (%) | Patients | Recurrence (%) | ||
| Low-risk | 26 | 4 (15.38%) | 30 | 5 (16.67%) | |
| Intermediate-risk | 32 | 6 (18.75%) | 24 | 7 (29.17%) | |
| High-risk | 27 | 8 (29.63%) | 26 | 10 (38.46%) | |
| All | 85 | 18 (21.18%) | 80 | 22 (27.50%) | |
Intraoperative and postoperative complication
The intra- and postoperative outcomes of ERBT group and TURBT group are listed in Table 3. Based on the modified Clavien-Dindo classification system for surgical complications (11), no Grade ≥3 complications occurred in ERBT group, but 2 patients in TURBT group required an endoscopic haemostasis (Grade 3). Obturator nerve reflex happened in the two groups (23.96% and 24.14% in ERBT vs. TURBT group, respectively, P=0.977). And 9 patients in TURBT group encountered bladder perforation, but only 2 patients in ERBT group (9/87 vs. 2/96, P=0.019). The time of operation, postoperative irrigation, catheterization and hospitalization shown no significant difference in the two groups.
Table 3. Intra- and postoperative characteristics of ERBT group vs. TURBT group.
| Variable | ERBT (n=96) | TURBT (n=87) | P |
|---|---|---|---|
| Operative time (min) | 31.09±8.79 | 33.43±8.33 | 0.239 |
| Complications, n (%) | |||
| Grade I | 3 (3.13%) | 4 (4.60%) | 0.710 |
| Grade II | 7 (7.29%) | 8 (9.20%) | 0.789 |
| Grade III | 0 (0.00%) | 2 (2.30%) | 0.225 |
| Obturator nerve reflex, n (%) | 23 (23.96%) | 21 (24.14%) | 0.977 |
| Bladder perforation, n (%) | 2 (2.08%) | 9 (10.34%) | 0.019 |
| Irrigation (h) | 21.09±6.32 | 21.49±6.40 | 0.299 |
| Catheterization (day) | 2.12±0.84 | 2.31±1.14 | 0.416 |
| Postoperative hospital-stay (day) | 3.71±0.97 | 4.02±1.47 | 0.283 |
Follow-up information
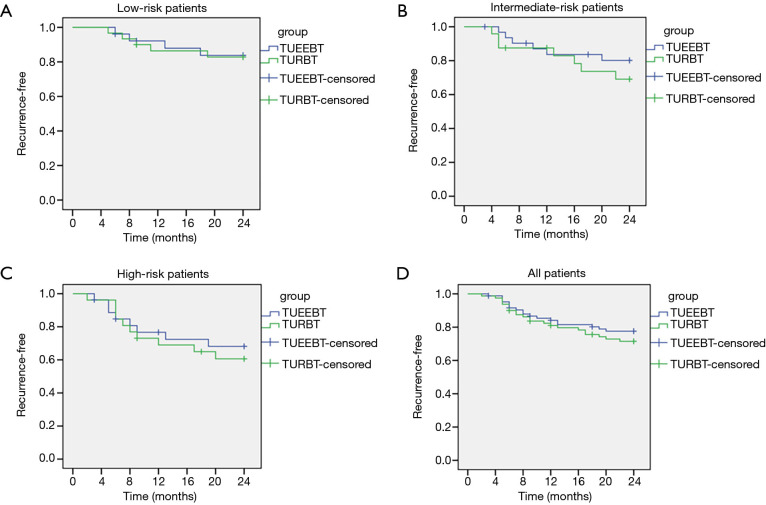
For follow-up analyses, 11 patients in ERBT group and 7 patients in TURBT group with muscle invasive bladder cancer (MIBC) were excluded. The difference of recurrence-free survival between the two groups was calculated using the Kaplan-Meier method, and four curves were obtained: all patients and patients in three subgroups of low-risk, intermediate-risk and high-risk (Figure 4). In the low-risk subgroup, the recurrence-free survival rate of ERBT group after 24 months follow-up was higher compared with TURBT group (84.62% vs. 83.33%), but no significantly difference was observed between the two groups (P=0.0.910) (Figure 4A). In the intermediate-risk and high-risk subgroups, although the recurrence-free survival rate in ERBT group was higher compared to TURBT group, (81.25% vs. 70.83% and 70.37% vs. 61.54%, respectively), the difference was not statistically significant between the two groups (P=0.385 and 0.630) (Figure 4B,C). The curve of all patients shown that the recurrence-free survival rate after 24 months follow-up did not differ between the ERBT and TURBT group (78.82% vs. 72.50%, P=0.398) (Figure 4D).
Figure 4.
Kaplan-Meier estimated of tumor recurrence-free survival in ERBT vs. TURBT group. (A) Recurrence-free survival for low-risk patients. (B) Recurrence-free survival for intermediate-risk patients. (C) Recurrence-free survival for high-risk patients. (D) Recurrence-free survival for all patients.
Discussion
TURBT is considered as the gold standard treatment for NMIBC. However, there are still several limitations associated with TURBT: obturator nerve reflex, bladder perforation and acute bleeding, which can be led to significant perioperative or postoperative morbidity. And it can also make the surgery difficult and delay the first-time intravesical instillation therapy after surgery (12). Meanwhile, thermal injury and absence of DM in the tumor specimen make it difficult in accurate pathological interpretation. And the action of piecemeal resection in TURBT contradicts the basic oncological principles, which may result in a higher tumor recurrence and progression rate. To alleviate these predicaments associated with TURBT, several en bloc resection techniques have emerged recently (13-15).
The surgical technique of ERBT has been performed with different energy sources (monopolar or bipolar electrical current, Hybrid-Knife, Tm:YAG, holmium [Ho]:YAG and KTP laser) in the last decade (16). In the biggest European multicenter study of ERBT to date, the largest entire tumor resected was about 5.0 cm (8). In ERBT group, to reduce the potential risk of tumor cell seeding into the urethra while the tumor retrieved from the bladder, the tumor diameter in the current study was 1.79±0.47 cm and the largest tumor was no more than 3.0 cm. Laser energy used in ERBT has some disadvantages, such as pulsed emission mode and disruption of tissue at incision line. The eschar at incision line caused by electric coagulation would put a negative effect on the judgement of tumor base. During ERBT with monopolar current, electric coagulation only used in disconnection of DM fibers around the tumor boundary to separate the tumor from adjacent normal background bladder tissue and blocking the blood supply arteries of the tumor to reduce perioperative blood loss. The architectural detail of tumor specimen is protected and integrity, and with fewer disruption of tissue at incision line compared with TURBT. This approach can get a clear operative field and has the merits of reduction of electric coagulation, little bleeding and smooth incision surface.
Gontero et al. (17) have shown that the presence of DM within the tumor specimens was the most important parameter related to recurrence-free survival in a recent study with 2451 patients of pT1 high-grade bladder tumor. The current evidence of ERBT of NMIBC punctuate the theory that a high rate of DM presence can be obtained regardless of the energy source (7). A systematic review analyzed the clinical data of 8,409 cases with high-grade Ta and T1 bladder tumor. The residual tumor was found in 17–67% of patients with stage Ta and in 20–71% of patients with T1 cancer, and 36–86% of residual tumors were located in the initial resection site. And the tumor progression was happened in 0–8% (Ta to ≥T1) and 0–32% (T1 to ≥T2) of cases at second TURBT (18). Compared to patients who underwent radical cystectomy (RC) with known muscle invasion at initial diagnosis, the prognosis of patients progressed to pT2 was worse (19). In our study, during ERBT, the organizational structure of incision line in bladder is smooth and clear. When the real-time clinical staging judgement of BC is T1, the superficial muscular propria layers will be resected from the smooth operation surface in order to make accurate pathological interpretation. The pathologic results after initial resection indicated that the present of DM within stage T1 tumor specimen was significantly higher in ERBT group, and the proportion of residual tumor at second TURBT was lower compared with TURBT group. Although not significantly different between the two groups, progression was lower in ERBT group at second TURBT (1/28 vs. 5/32, P=0.201).
The bladder wall is relatively weak, with thickness measurements ranging from 1mm to 15mm in 25 adult cadavers (20). Therefore, deeper resection may be accompanied by a higher risk of bladder perforation and severe bleeding. Bladder perforation mainly caused by obturator nerve reflex, which potentially can influence the natural history of NMIBC and result in a higher risk for recurrence and progression (21). By using general anesthesia, obturator nerve reflex could be reduced to an extent that it seldom caused bladder perforation during surgery. But general anesthesia may cause a higher tumor recurrence rate comparing with spinal anesthesia (22,23). Unlike fast movement of the monopolar current in TURBT, retrogradely and slowly blunt shearing skill was performed in ERBT, which resulted in easy depth control of resection. Thus, when obturator nerve reflex occurred during surgical operation, the utilization rate of cutting and coagulation will be declined. And for real-time clinical staging judgement of bladder tumor is Ta, the superficial muscular propria layers can be reserved, so the deep of tumor resection is shallower with a less likely for bladder perforation. Comparable to TURBT group, although obturator nerve reflex could not be reduced in ERBT group (23.96% vs. 24.14%, P=0.977). But the ratio of bladder perforation can be reduced to an acceptable level (2.08%), which was consistent with the results of ERBT previously reported by Mori et al. (16).
Certain limitations in this study must be pointed out. First, the number of patients is small and the median follow-up time is limited to 2 years. However, ERBT with monopolar current based on TNM system is a new surgical technique and that the study provided the initial experience. Second, due to it is not provided for treatment of BC in our country, BCG can not be used as the intravesical instillation drug for intermediate and high-risk of NMIBC in the study. Last, the postoperative irrigation time of our study was longer than that reported by most literature (14,15). The primary consideration is that longer irrigation after surgery would remove floating cancer cells and prevent these cells from attaching to the bladder wall (24).
Conclusions
ERBT with monopolar current based on TNM system is a effective, safe and feasible procedure for treating patients with NMIBC. Recurrence rate did not differ between the ERBT and TURBT groups, but ERBT may reduce the proportion of bladder perforation and residual tumor during initial resection. Further validation of these results from large randomized control trials with prolonged follow-up periods are required.
Acknowledgments
The authors are thankful to all the patients and physicians who took part in this study. We also thank Jing Lian for the pathological assistance.
Funding: This research was supported by the National Natural Science Foundation of China (NSFC, Grant No.81172444). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committee of First Affiliated Hospital of Shanxi Medical University (approval ID: 2011033/2011) and conducted according to the principles of the Declaration of Helsinki (as revised in 2013). All patients gave their consent for the procedure and their clinical data were stored for the current scientific purpose.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.48). The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Babjuk M, Burger M, Compérat EM, et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ)-2019 Update. Eur Urol 2019;76:639-57. 10.1016/j.eururo.2019.08.016 [DOI] [PubMed] [Google Scholar]
- 3.Sylvester RJ, van der Meijden APM, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006;49:466-75; discussion 475-77. 10.1016/j.eururo.2005.12.031 [DOI] [PubMed] [Google Scholar]
- 4.Leal J, Luengo-Fernandez R, Sullivan R, et al. Economic Burden of Bladder Cancer Across the European Union. Eur Urol 2016;69:438-47. 10.1016/j.eururo.2015.10.024 [DOI] [PubMed] [Google Scholar]
- 5.Avallone MA, Sack BS, El-Arabi A, et al. Ten-Year Review of Perioperative Complications After Transurethral Resection of Bladder Tumors: Analysis of Monopolar and Plasmakinetic Bipolar Cases. J Endourol 2017;31:767-73. 10.1089/end.2017.0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo CC, Al-Ahmadie HA, Flaig TW, et al. Contribution of bladder cancer pathology assessment in planning clinical trials. Urol Oncol 2018. [Epub ahead of print]. 10.1016/j.urolonc.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 7.Kramer MW, Altieri V, Hurle R, et al. Current Evidence of Transurethral En-bloc Resection of Nonmuscle Invasive Bladder Cancer. Eur Urol Focus 2017;3:567-76. 10.1016/j.euf.2016.12.004 [DOI] [PubMed] [Google Scholar]
- 8.Kramer MW, Rassweiler JJ, Klein J, et al. En bloc resection of urothelium carcinoma of the bladder (EBRUC): a European multicenter study to compare safety, efficacy, and outcome of laser and electrical en bloc transurethral resection of bladder tumor. World J Urol 2015;33:1937-43. 10.1007/s00345-015-1568-6 [DOI] [PubMed] [Google Scholar]
- 9.Naselli A, Introini C, Germinale F, et al. En bloc transurethral resection of bladder lesions: a trick to retrieve specimens up to 4.5 cm. BJU Int 2012;109:960-63. 10.1111/j.1464-410X.2012.10982.x [DOI] [PubMed] [Google Scholar]
- 10.Babjuk M, Burger M, Zigeuner R, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol 2013;64:639-53. 10.1016/j.eururo.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 11.De NC, Franco G, Cindolo L, et al. Transuretral resection of the bladder (TURB): Analysis of complications using a modified Clavien system in an Italian real life cohort. Eur J Surg Oncol 2014;40:90-5. 10.1016/j.ejso.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 12.Bosschieter J, Nieuwenhuijzen JA, van Ginkel T, et al. Value of an Immediate Intravesical Instillation of Mitomycin C in Patients with Non-muscle-invasive Bladder Cancer: A Prospective Multicentre Randomised Study in 2243 patients. Eur Urol 2018;73:226-32. 10.1016/j.eururo.2017.06.038 [DOI] [PubMed] [Google Scholar]
- 13.Huang H, Wang T, Ahmed MG, et al. Retrograde en bloc resection for non-muscle invasive bladder tumor can reduce the risk of seeding cancer cells into the peripheral circulation. World J Surg Oncol 2020;18:33. 10.1186/s12957-020-1808-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Wang L, Mao S, et al. Transurethral en bloc resection with bipolar button electrode for non-muscle invasive bladder cancer. Int Urol Nephrol 2018;50:619-23. 10.1007/s11255-018-1830-0 [DOI] [PubMed] [Google Scholar]
- 15.Li K, Xu Y, Tan M, et al. A retrospective comparison of thulium laser en bloc resection of bladder tumor and plasmakinetic transurethral resection of bladder tumor in primary non-muscle invasive bladder cancer. Lasers Med Sci 2019;34:85-92. 10.1007/s10103-018-2604-8 [DOI] [PubMed] [Google Scholar]
- 16.Mori K, D'Andrea D, Enikeev DV, et al. En bloc resection for nonmuscle invasive bladder cancer: review of the recent literature. Curr Opin Urol 2020;30:41-47. 10.1097/MOU.0000000000000697 [DOI] [PubMed] [Google Scholar]
- 17.Gontero P, Sylvester R, Pisano F, et al. Prognostic factors and risk groups in T1G3 non- muscle-invasive bladder cancer patients initially treated with Bacillus Calmette-Guérin: results of a retrospective multicenter study of 2451 patients. Eur Urol 2015;67:74-82. 10.1016/j.eururo.2014.06.040 [DOI] [PubMed] [Google Scholar]
- 18.Cumberbatch MGK, Foerster B, Catto JWF, et al. Repeat Transurethral Resection in Non- muscle-invasive Bladder Cancer: A Systematic Review. Eur Urol 2018;73:925-33. 10.1016/j.eururo.2018.02.014 [DOI] [PubMed] [Google Scholar]
- 19.Hassan O, Murati AB, Lombardo KA, et al. Clinical significance of urothelial carcinoma ambiguous for muscularis propria invasion on initial transurethral resection of bladder tumor. World J Urol 2020;38:389-95. 10.1007/s00345-019-02782-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asfour V, Gibbs K, DaSilva A ophia, et al. Validation study of ultrasound bladder wall thickness measurements. Int Urogynecol J 2019;30:1575-80. 10.1007/s00192-018-3802-4 [DOI] [PubMed] [Google Scholar]
- 21.Comploj E, Dechet CB, Mian M, et al. Perforation during TUR of bladder tumours influences the natural history of superficial bladder cancer. World J Urol 2014;32:1219-23. 10.1007/s00345-013-1197-x [DOI] [PubMed] [Google Scholar]
- 22.Kweon TD, Lee KY. Spinal anesthesia is associated with lower recurrence rates after resection of non-muscle invasive bladder cancer. Transl Androl Urol 2018;7:283-86. 10.21037/tau.2018.03.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koumpan Y, Jaeger M, Mizubuti GB, et al. Spinal Anesthesia is Associated with Lower Recurrence Rates after Resection of Nonmuscle Invasive Bladder Cancer. J Urol 2018;199:940-6. 10.1016/j.juro.2017.11.064 [DOI] [PubMed] [Google Scholar]
- 24.Onishi T, Sugino Y, Shibahara T, et al. Randomized controlled study of the efficacy and safety of continuous saline bladder irrigation after transurethral resection for the treatment of non-muscle-invasive bladder cancer. BJU Int 2017;119:276-82. 10.1111/bju.13599 [DOI] [PubMed] [Google Scholar]