Abstract
Background
The constitutive activation of the mammalian target of rapamycin (mTOR) is involved in the pathogenesis of many cancers. Rapamycin (RAPA), a specific inhibitor of mTOR, has been applied to the clinical treatment of tumors, and its anti-leukemia effect has also been confirmed.
Methods
We detected apoptosis and the NKG2D ligands expression in acute myeloid leukemia (AML) cells using flow cytometry and investigated the cytotoxicity of AML cells that had been co-cultured with natural killer (NK) cells using CFSE staining. We evaluated the signal pathways with a western blot assay.
Results
In this study, we found that RAPA can significantly inhibit the proliferation of AML cells. Further studies showed that the use of RAPA alone reduced the expression of NKG2D ligands on the membranes of HL-60 and THP-1 AML cells. Also, RAPA blocked the upregulation of the NKG2D ligand when AML cells were cultured with the demethylation drug decitabine (DAC). We found that RAPA decreased the expression of the NKG2D ligands by inducing the STAT3 phosphorylation of AML cells.
Conclusions
The discovery of this mechanism might further optimize the clinical use of RAPA for the treatment of AML.
Keywords: Rapamycin (RAPA), NKG2D ligand, acute myeloid leukemia (AML), STAT3, immune escape
Introduction
Acute myeloid leukemia (AML) is a life-threatening, malignant hyperplasia disease of the hematopoietic system, which is characterized by an unregulated proliferation and the survival of hematopoietic stem cells and myeloid progenitor cells (1,2). The overall 5-year survival rate of AML patients younger than 60 years is below 45%, and for those older than 60 years, the rate is less than 10% (3). Therefore, according to the abnormal activation signal pathway in AML cells, targeted anti-leukemic drugs have been developed in recent years, with rapamycin (RAPA) being one of them. In AML, 50% to 80% of all patients show constitutive activation of the PI3K/AKT/mammalian target of rapamycin (mTOR) (1). mTOR is a serine/threonine kinase with a crucial role in the regulation of cell growth and proliferation by translational control that is required for tumor development (4). The inhibition of the activation of the mTOR pathway is beneficial in prolonging the life span of AML patients.
RAPA, which is a specific inhibitor of mTOR, has attracted considerable attention because of its antitumor effect which was discovered in recent years. RAPA has achieved good clinical efficacy in the treatment of solid tumors (5-7). In the treatment of leukemia, the effect of RAPA and its analogs on the progression of leukemic cells has been confirmed (8). A study has shown that RAPA can arrest the cell cycle progression of AML cells in the G1 phase and promote its apoptosis with the collapse of the mitochondrial potential and caspase-3 activation, while the combination with chemotherapeutic drug Ara-C has yielded the most prominent proapoptosis effects (9).
Natural killer (NK) cells are cytotoxic lymphocytes that play a vital role in the antitumor immune response. The killing ability of these NK cells depends on the balance of activation and inhibitory signals mediated by the activated receptors and inhibitory receptors (inhibitory killer immunoglobulin-like receptors) that are expressed on the surface of NK cells, especially the activation signal mediated by activated receptor NKG2D and its ligand. The activation signal mediated by the NKG2D can antagonize the inhibitory signals produced by the binding of the killer inhibitory receptors to major histocompatibility complex (MHC) class I molecules, which leads to the activation of NK cells, thus killing the tumor cells. NKG2D ligands, including MHC class I chain-related proteins A and B (MICA/B) and UL16 binding protein family (ULBPS), are the primary ligands for exerting cytotoxic activity (10). The downregulation or loss of NKG2D ligand expression is frequently found in tumor cells; thus, they cannot be easily identified and killed by NK cells, resulting in the immune escape of the tumor (11).
Reports have shown that RAPA is involved in the regulation of the NK cell activity through the mTOR signal pathway, and has inhibited the growth of NKbright and the secretion of interferon gamma (IFN-γ) (12). However, it is rarely reported how RAPA regulates the expression of the NKG2D ligand. In this study, AML cell lines HL-60 and THP-1 were used as research models. We found that the expression of the NKG2D ligand on the surface of AML cells decreased after using a RAPA therapy, and cytotoxic sensitivity against NK cells also declined. RAPA can antagonize the upregulation of the NKG2D ligand, which was induced by the demethylation drug decitabine (DAC). Our study observed that the downregulation of the NKG2D ligand by RAPA was achieved by inducing STAT3 phosphorylation in AML cells. Also, the phosphorylation of the ERK protein related to proliferation and differentiation also increased.
Methods
Cell culture and reagents
The AML cell line HL-60 (human acute granulocytic leukemia cell line) was obtained from the Cell Resource Center, the Institute of Life Sciences, and the Chinese Academy of Sciences. THP-1 cells were purchased from the American Type Culture Collection. These cells were maintained in the RPMI1640, supplemented with a 10% fetal bovine serum (FBS), 100 U/mL of penicillin, and 100 µg/mL of streptomycin at 37 °C in a humidified atmosphere with a CO2 level of 5%. NK92 cells (human NK cell line) were provided by Prof. Ji from the Xinguangwuhuoshi Hospital, Taipei, and grown in a minimum essential Eagle medium-alpha modification (α-MEM) which included 12.5% FBS, 12.5% horse bovine serum (HBS), 100 U/mL of IL-2, and 100 IU/mL of penicillin and doxorubicin. RPMI1640, α-MEM, and HBS were produced by Gibco Grand Island (New York, NY, USA). RAPA and decitabine (DAC) were purchased from Sigma (St Louis, MO, USA). Annexin-V/PI was purchased from Invitrogen (Carlsbad, CA, USA). STAT3 inhibitor VII was the product of Calbiochem Corporation (Darmstadt, Germany). MICA/B-PE, ULBP1-PE, ULBP2/5/6-APC, ULBP3-PE, ULBP4-APC, and PE and APC-labeled isotype control immunoglobulin G1 (IgG1) were purchased from R&D System Company (Minneapolis, MN, USA). Antibodies against STAT3, p-STAT3 on Ser727 and Tyr705, p42/44 MAPK, p-p42/44 MAPK, and β-actin were purchased from Cell Signaling Technology (Beverly, MA, USA). A Cell Counting Kit-8 (CCK-8) was the product of Shanghai Dojindo Molecular Technologies, Inc. (Shanghai, China). An enhanced chemiluminescence detection kit for the western blot assay was purchased from Santa Cruz Biotechnology, Inc (Dallas, TX, USA).
Measurement of cell proliferation by CCK-8
CCK-8 analyzed the cell proliferation of HL-60 and THP-1. AML cells were collected and washed with a phosphate buffered saline (PBS) once, with an adjusted cell density of 1×105/mL. HL-60 and THP-1 cells were exposed to the indicated concentration (0, 0.01, 0.1, 1.0 µg/mL) of RAPA. AML cells of different treatment methods were seeded in 96-well plates with 100 µL per well and 4 multiple wells in each group for 24, 48, and 96 h. These cells were then incubated with a CCK-8 solution (10 µL per well) for a further 4 h incubation time at 37 °C at the 24, 48, and 72 h time points. The absorbance was determined at 450 nm. The experiments were repeated 3 times, and cell viability was determined with the following formula: cell viability (%) = [(OD450sample − OD450blank)/(ODcontrol − OD450blank)] ×100.
Detection of apoptotic cells by annexin-V/PI double staining
AML cells (HL-60, THP-1) were treated with different concentrations of RAPA (0, 0.01, 0.1, 1.0 µg/mL) for 24, 48, and 72 h. AML cells were collected and washed with PBS once. The cells were suspended in a 100 µL of 1× annexin-V binding buffer at a density of 1×106/mL. The cells were incubated with 5 µL of annexin-V and 1 µL PI for 15 min and then detected using flow cytometry.
Detection of NKG2D ligands expression on NK cells by flow cytometry
Cells were harvested and washed with PBS once and resuspended in a 100 µL PBS. The 5 anti-human NKG2D ligand antibodies (MICA/B, ULBP1, ULBP2, ULBP3, ULBP4) were added and followed by an incubation in the dark for 30 min at 4 °C. The cells were then washed twice with PBS and suspended in 500 µL PBS. The expression of NKG2D ligand on AML cells was detected using flow cytometry, and the values were expressed as the mean fluorescence intensity (MFI). The isotype IgG1 was used as a negative control. The experiment was performed at least 3 times.
Determination of cytotoxicity of NK cells by CFSE
The human NK92 cell line was used as the effector cells and HL-60 cells as the target cells. Collected HL-60 cells were treated with RAPA for 24 h and washed with PBS once. After that, the cells were resuspended with PBS. CFSE was added to the system at a final concentration of 5 µmol/L. The liquid was mixed gently and incubated for 10 min at 37 °C. After removal of the liquid, 5 times the volume of precooled FBS was added, centrifuged, washed twice with PBS, and the cell density was adjusted to 1×105/mL. Then, the effector cells were mixed with target cells at different ratios (5:1, 10:1, 20:1), and incubated at 37 °C for 24 h. After incubation, cells were collected and washed twice with PBS. Next, PI was added and incubated for 15 min at room temperature. NK92 cells against AML cells were assessed by flow cytometry. The experiment was repeated 3 times.
Detection of expressions of STAT3 by western blot assay
HL-60 and THP-1 cells were treated with RAPA for 24 h and combined with DAC for 48 h, and cells were harvested and lysed in radioimmunoprecipitation assay lysis buffer for 30 min on ice. The lysates were cleared by centrifugation at 12,000 rpm for 10 min at 4 °C, and the protein concentration was determined with bicinchoninic acid assays. Then, proteins were separated by a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis membrane and transferred onto polyvinylidene membranes. After inoculation in the blocking buffer (5% nonfat milk), the membranes were probed overnight at 4 °C with the indicated antibodies. All the bands were visualized with horseradish peroxidase-conjugated secondary antibodies and an enhanced chemiluminescence kit.
Statistical analysis
Statistical analysis was performed with the GraphPad Prism 5 Software. Data are presented as the mean ± standard deviation (SD) of the mean of 3 experiments. The t-test was used to determine the significance of differences between the two datasets. The value of P<0.05 was considered to have statistical significance.
Results
RAPA inhibited the proliferation of AML cells
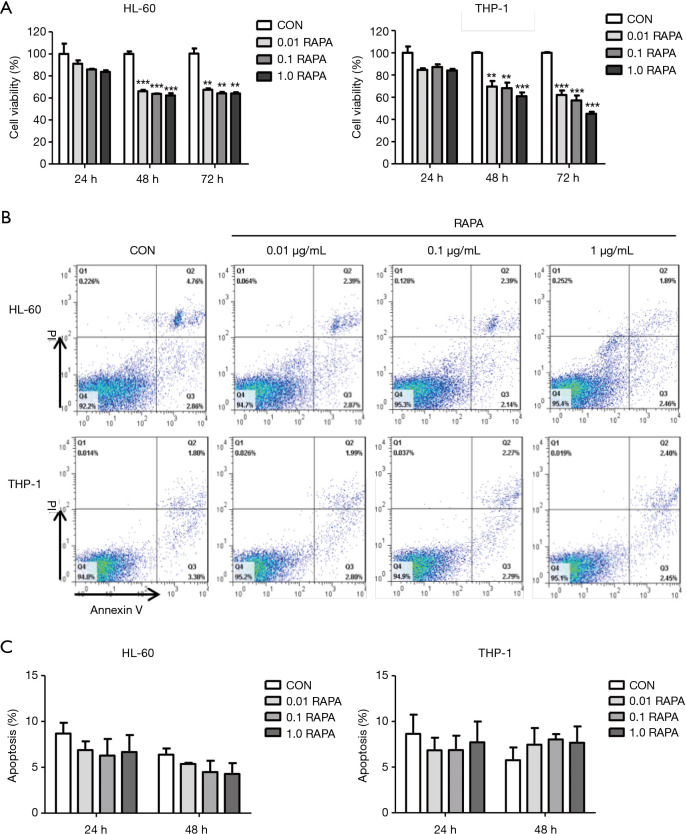
The AML cell lines HL-60 and THP-1 were treated with different concentrations of RAPA for 24, 48, and 72 h. The results of CCK-8 showed that there was no significant inhibition of cell proliferation when AML cells were treated with RAPA for 24 h. The proliferation inhibitory rates of 0.1 µg/mL RAPA on HL-60 and THP-1 were 14.1%±0.5% and 12.7%±2.6%, respectively. With prolongation of the action time of RAPA, the proliferation of HL-60 and THP-1 cells were significantly inhibited after treatment with RAPA for 48 and 72 h. For HL-60 cells treated with 0.1 µg/mL RAPA for 48 and 72 h, the viability significantly declined by 36.3%±0.4% and 35.8%±1.2%, respectively, as compared to the control cells. THP-1 cells were more sensitive to RAPA, and the viability of THP-1 at 48 and 72 h declined by 31.9%±5.0% and 42.9%±4.5% (Figure 1A).
Figure 1.
Anti-leukemia effect of RAPA on AML cells. (A) Proliferation inhibition efficiency of various concentrations of RAPA (0, 0.01, 0.1, 1.0 µg/mL) on AML cells (HL-60 and THP-1) for 24, 48, and 72 h. AML cells were incubated in 96-well plates at a density of 1×104 cells per well for 24, 48, and 72 h, and then CCK-8 solution was added for a further 4 h incubation. Results are reported as mean ± SD of the 3 independent experiments. Statistical significance was reported as **, P<0.01, ***, P<0.001; (B) HL-60 and THP-1 cells treated with different doses of RAPA (0, 0.01, 0.1, 1.0 µg/mL) were analyzed by FACS for the percentage of apoptotic cells for 48 h. Q1, dead cells; Q2, advanced apoptosis cells; Q3, living cells; Q4, early apoptosis cells; (C) cell apoptosis of AML cells (HL-60 and THP-1) following RAPA treatment. Each column and error bar represent the mean ± SD of apoptosis level (n≥2). RAPA, rapamycin; AML, acute myeloid leukemia; SD, standard deviation.
We further tested the apoptosis of the AML cells in different concentrations of RAPA. Results found that RAPA did not induce specific apoptosis in AML cells, even in the concentration of RAPA which was as high as 1.0 µg/mL (Figure 1B,C). Although studies have shown that RAPA induced early apoptosis of HL-60 cells (13), there was no difference in the induction of apoptosis found in our study. The inhibitory effect of RAPA on AML cells is mainly in the inhibition of cell proliferation.
RAPA downregulates the cytotoxic sensitivity of AML to NK cells by decreasing NKG2DL expression on AML cells
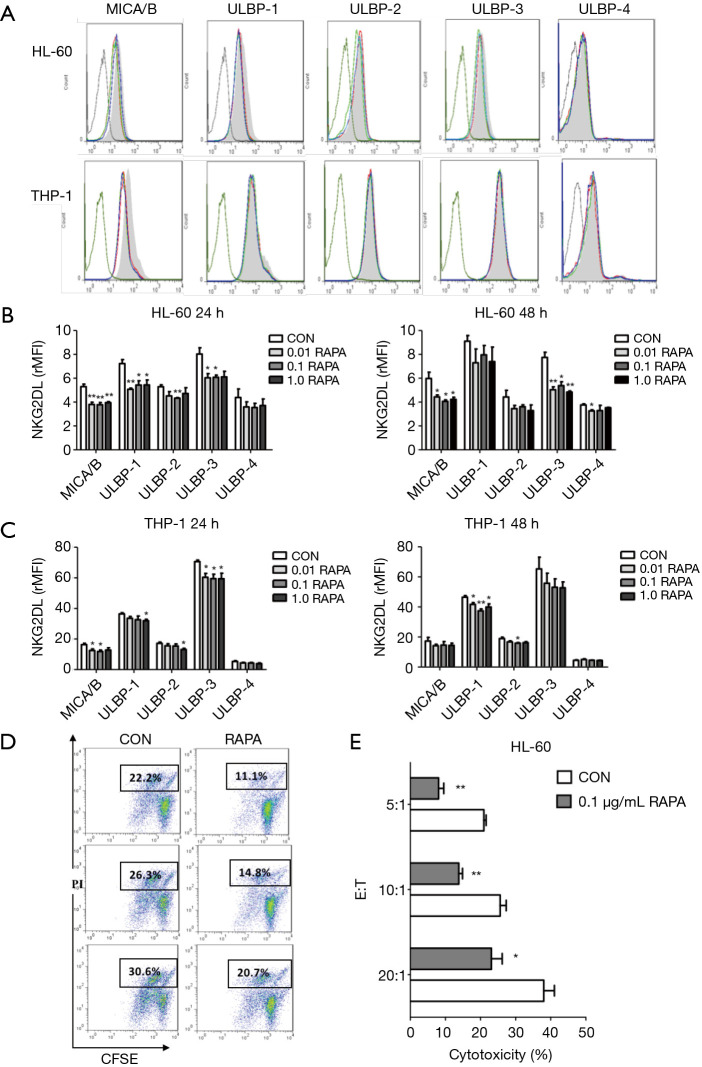
To further investigate whether RAPA was able to regulate the expression of NKG2D ligand on AML cells, the expression of 5 NKG2D ligands (MICA/B and ULBP1-4) on AML cells was detected using flow cytometry. In this study, HL-60 and THP-1 cells were treated with different concentrations of RAPA (0.01, 0.1 and 1.0 µg/mL) for 24 and 48 h. The results showed that HL-60 cells were more sensitive to the inhibitory effect of RAPA. After treatment with RAPA for 24 h, the expression of MICA/B, ULBP-1, ULBP-2, and ULBP-3 were decreased significantly, whereas the expression of ULBP-4 was not affected (Figure 2).
Figure 2.
RAPA downregulates the cytotoxic sensitivity of AML to NK cells by decreasing NKG2DL expression on AML cells. (A) Flow cytometry results of the expression of 5 NKG2D ligands expressed on both AML cells (HL-60 and THP-1) after various concentrations of RAPA (0, 0.01, 0.1, 1.0 µg/mL) treatment for 24 h. Black line, IgG; grey line, Con; red line, 0.01 µg/mL; green line, 0.1 µg/mL; blue line, 1.0 µg/mL; (B,C) the downregulation of rMFI levels of 5 NKG2D ligands (MICA/B, ULBP1-4) in HL-60 cells and THP-1 cells after RAPA treatment; (D) flow cytometry of cytotoxic sensitivity of HL-60 cells to NK cells. After RAPA treatment for 24 h, the cytotoxic sensitivity of HL-60 cells to NK cells significantly declined; (E) RAPA downregulated the cytotoxic sensitivity of HL-60 cells to NK cells at different E:T ratios. HL-60 cells were previously incubated with 0.1 µg/mL RAPA for 24 h and then co-cultured at a different effector-target ratio (E:T ratio). Results are reported as mean ± SD of the 3 independent experiments (B,C,E). *, P<0.05 vs. control group; **, P<0.01 vs. control group. RAPA, rapamycin; AML, acute myeloid leukemia; NK, natural killer; IgG, immunoglobulin G; rMFI, relative mean fluorescence intensity; SD, standard deviation.
We also observed that the expression of MICA/B and ULBP-3 on THP-1 cells was inhibited by a significant amount when cells were treated with RAPA for 24 h. For ULBP-1, the downregulation at 48 h was much higher than that at 24 h (Figure 2C). There were no significant differences among the different concentrations. In conclusion, RAPA could inhibit the expressions of the NKG2D ligands on both AML cells; however, there were differences in the regulation of expression of different ligands.
To investigate further whether the low expression of NKG2D ligand used after treatment with RAPA could reduce the recognition and lysis mediated by the NK cells, we used a well-known model that enabled the NKG2D-NKG2DL interaction to be accurately analyzed. The NK92 cell line was used as a source of effector cells for its high expression level of NKG2D. AML cells were previously incubated with 0.1 µg/mL RAPA for 24 h and then co-cultured at a different effector-target ratio (E:T ratio). The results showed that the percentage of specific lysis in the RAPA treatment group was significantly lower than the control group with a measured E:T ratio (Figure 2D,E). Although the killing efficiency of the two treatment groups increased with the increase of the E:T ratio, the killing efficiency of the RAPA group was significantly lower than that of the control group (Figure 2E). We examined further the effect of RAPA on the expression of NKG2D, an activated receptor on NK92 cells. The results showed that the measured concentration of RAPA did not affect the expression of NKG2D on the surface of NK cells (data not shown) for 24 h. The decrease in the sensitivity of AML cells to NK cells after the treatment of RAPA is due to the decrease of NKG2D ligand expression in AML cells. In summary, we found that RAPA can downregulate the expression of NKG2D ligand on the surface of AML cells, reduce the killing effect of NK cells, and result in immune escape of the tumor.
RAPA inhibits the expression of NKG2D ligand by a STAT3 signaling pathway
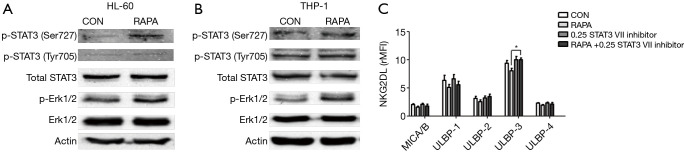
The STAT3 protein was evaluated by western blot analysis to investigate whether STAT3 participates in the regulation of RAPA for the expression of NKG2D ligand in the membrane of AML cells. Western blot analysis revealed that RAPA treatment resulted in strong upregulation of the STAT3 phosphorylation at Ser727 and a moderate increase at Tyr705 in both of the AML cells. We also found that there was an increase in phosphorylation of ERK protein, which can also modulate the expression of NKG2D ligand (Figure 3A,B).
Figure 3.
RAPA inhibited the expression of NKG2D ligand on the STAT3 signaling pathway. (A,B) RAPA upregulated the activity of STAT3 and ERK in HL-60 and THP-1 cells. AML cells were exposed to 0.1 µg/mL RAPA for 24 h. Cell lysates were prepared and subjected to western blot assay for p-STAT3 (Tyr705 and Ser727), total STAT3 protein, p-ERK, and total ERK. The phosphorylated STAT3 and ERK in HL-60 cells (A) and THP-1 cells (B) were increased after the treatment of RAPA; (C) without changing the basic expression of NKG2D ligand, STAT3 specific inhibitor upregulated the expression of ULBP3. HL-60 cells were treated with 0.1 µg/mL RAPA, 0.25 µmol/L STAT3 VII inhibitor or 0.1 µg/mL RAPA +0.25 µmol/L STAT3 VII inhibitor for 24 h. The expression of NKG2D ligands was analyzed using flow cytometry. Results are reported as mean ± SD of the 3 independent experiments. *, P<0.05 vs. RAPA group. RAPA, rapamycin; AML, acute myeloid leukemia; SD, standard deviation.
Next, we investigated whether the inhibition of the expression of STAT3, ERK affected the expression of NKG2D ligands. RAPA and chemical inhibitors were combined on HL-60 cells to detect the change of the expression of NKG2D ligands. Results revealed that inhibition of STAT3 by STAT3 inhibitor VIIincreased cell surface expression of ULBP-3 (Figure 3C), whereas inhibition of ERK by LY3214996 did not affect the expression of NKG2D ligand (data not shown). These findings suggested that RAPA down-regulated the expression of NKG2D ligand on AML cells by upregulation of the phosphorylation of STAT3.
RAPA antagonizes the anti-leukemia effect of DAC by upregulating phosphorylation of STAT3 protein
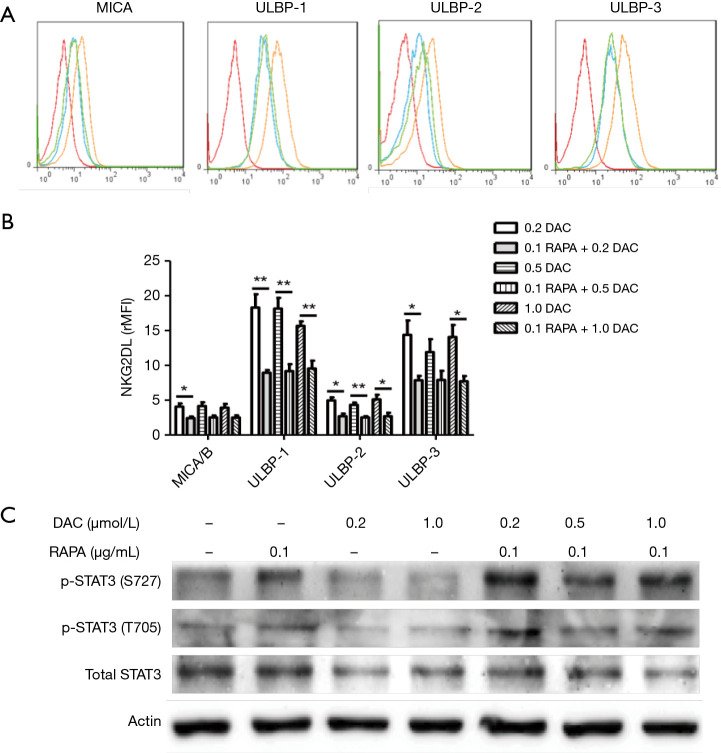
Previous data have shown that it is safe to combine DAC with RAPA in the treatment of AML (14). However, in our study, we found that RAPA can induce low expression of NKG2D ligands on the surface of AML cells. We further found that RAPA could inhibit the upregulation of the NKG2D ligand which is induced by different concentrations of DAC (Figure 4A). RAPA can downregulate the expression of 4 NKG2D ligands (MICA/B, ULBP1-3) expressed on HL-60 cells (Figure 4B). The results of western blot analysis showed that RAPA antagonized the inhibitory effect of DAC on STAT3 and induced the increase in the phosphorylation of Ser727 and Tyr705 (Figure 4C). Consistent with these data, the downregulation of the expression of NKG2D ligand when combining RAPA with DAC in the treatment of AML cells may be one of the reasons for the recurrence of leukemia.
Figure 4.
RAPA antagonized the anti-leukemia effect of DAC by upregulating phosphorylation of STAT3 protein. (A) Flow cytometry results of the expression of NKG2D ligand expressed on HL-60 cells after treatment with DAC or cotreatment with DAC and RAPA for 48 h. Red, IgG; blue, Con; orange, 0.2 µmol/L DAC; green, 0.1 µg/mL RAPA +0.2 µmol/L DAC; (B) the downregulation of rMFI levels of 5 NKG2D ligands (MICA/B, ULBP1-3) in HL-60 cells after treated with DAC or DAC combined with RAPA. After HL-60 cells were treated with a different concentration of DAC+0.1 µg/mL RAPA, the expression of NKG2D ligands on HL-60 cells were downregulated. Even at a high concentration of DAC, it cannot further increase the NKG2D ligands expression; (C) RAPA upregulated the phosphorylated STAT3 in DAC-treated HL-60 cells. Results are reported as mean ± SD of the 3 independent experiments. *, P<0.05 vs. DAC group; **, P<0.01 vs. DAC group. RAPA, rapamycin; DAC, demethylation drug decitabine; rMFI, relative mean fluorescence intensity; SD, standard deviation.
Discussion
RAPA, an antineoplastic drug, has seen recent clinical use. A large number of reports have confirmed that RAPA has an anti-leukemia effect (15-17). Our research also confirmed that RAPA inhibited the proliferation of leukemia cells. In this study, 2 AML cell lines were chosen as the study object, in which HL-60 was the MLL-wild-type AML cell line, and THP-1 was the MLL-mutant-type cell line (18). Studies have shown that AML cells with MLL-rearranged subgroup were more sensitive to mTOR inhibitors (18). Therefore, the two cell lines that had different sensitivity to RAPA were selected as research subjects. Our research found that RAPA as an anti-leukemia drug cannot induce early apoptosis in AML cells, but it can inhibit the proliferation of AML cells.
RAPA was isolated from a species of bacteria collected from Rapa Nui, commonly known as Eastern Island, and was first used as a low-toxicity antifungal drug (19). Later, it was discovered that it has an immunosuppressive effect and was used as an immunosuppressive agent for the treatment of organ transplantation rejection (20,21). mTOR, a key regulator of cellular metabolism, has a fundamental role in controlling immune responses and its activity is required for the production of IFN-γ and granzyme B of NK cells (12), whereas it can be inhibited by the specific inhibitor RAPA (22). However, the immune-modulatory effect of RAPA on tumor cells is rarely reported. Our study found that RAPA can downregulate the expression of NKG2D ligands on the membrane of AML cells, whether sensitive or insensitive to mTOR inhibitors, even in a low concentration.
Further research found that RAPA combined with the new antileukemia drug DAC can also inhibit the expression of the NKG2D ligand, and our previous study had shown that the demethylation drug DAC inhibited the proliferation of AML cells, induced its early apoptosis, upregulated the expression of NKG2D ligand, and enhanced its sensitivity to the killing effect on NK cells (23). Although a clinical trial has confirmed that RAPA combined with DAC is safe and effective in the treatment of relapse and refractory leukemia (14), our study found that RAPA can antagonize the effect of DAC on the upregulation of the expression of NKG2D ligands. We also found that the cytotoxicity of NK cells to HL-60 cells was decreased. To investigate the downregulated effect due to the decreased expression of NKG2D ligands or the inhibitory effect of RAPA on NK cells, we stimulated the NK92 cell with RAPA. The data showed that a low concentration of RAPA did not affect the expression of NKG2D on NK92 cells. The expression of NKG2D on NK cell surface can only be affected by long-term treatment of RAPA (12).
Moreover, RAPA had little effect on the rest of the activated receptors that expressed on the surface of NK cells, such as NKP30 and NKP46 (12). The NKG2D ligands on the surface of tumor cells are the key to the activation of NK cells. The downregulation of NKG2D ligands leading to the tumor cannot be easily identified, and NK cells cannot be activated, and thus NK cells fail to recognize and kill tumor cells (11). Therefore, the downregulation of NKG2D ligands expression is the reason for the decrease of cytotoxicity of NK cells to HL-60 cells. Consequently, we speculated that although RAPA can inhibit the proliferation of AML cells, its downregulation of the expression of NKG2D ligands on the surface of AML cells may lead to the emergence of tumor escape and become one of the reasons for leukemia relapse.
Revealing the mechanism of RAPA that can downregulate the expression of the NKG2D ligand could provide a better therapeutic target for AML clinical treatments. It has been reported that the NKG2D ligand MICA was the downstream target gene of STAT3, and STAT3 can specifically bind to the promoter region of MICA and regulate the transcription and expression of the MICA (24). Western blot assay showed that phosphorylated STAT3 was increased in these two AML cells after the treatment of different concentrations of RAPA. The downregulation of the expression of ULBP3 induced by RAPA can be recovered after the treatment of specific STAT3 phosphorylation inhibitor. Also, the phosphorylation of STAT3 protein was increased after RAPA was combined with DAC treatment in AML cells, and our previous study has confirmed that DAC upregulated the expression of NKG2D ligand by inhibiting phosphorylated STAT3 (23).
RAPA is a specific inhibitor of mTOR (25) whose series of effects in the body are almost related to mTOR. Research has proven that mTOR can upregulate the phosphorylation of STAT3 at Ser727 and Tyr705 and induce the activation of STAT3 (26). The further use of mTOR-specific inhibitors of RAPA can downregulate the phosphorylation level of STAT3 in some disease models (27), which is inconsistent with our current results which point to RAPA upregulating the activation of STAT3. It is indicated that RAPA does not downregulate NKG2D ligand through the mTOR pathway. Studies have shown that the phosphorylation level of STAT3 is upregulated in cardiac cells treated with RAPA, which is a central component of cardioprotection (28). We speculated that the activated STAT3 might also be one of the factors contributing to RAPA’s inability to induce the apoptosis of AML cells. However, the reason for these contradictory data of RAPA is not apparent. RAPA may have different ways to trigger the STAT3 signaling pathway in different organs. In this work, we found that the activation of STAT3 induced the phosphorylated ERK.
The results of further detection of Western blot analysis showed that the phosphorylation level of ERK in AML cells that were treated by RAPA increased. However, LY3214996, and ERK-specific inhibitor, did not alter the downregulation of RAPA on the expression of NKG2D ligand, which indicates that the ERK signaling pathway did not participate in the regulation of RAPA on NKG2D ligand. In the current study, we found that RAPA regulated the expression of partial NKG2D ligands by activating the STAT3 signaling pathway, and decreased the cytotoxicity of AML cells to NK cells. The discovery of this mechanism may further improve the clinical use of RAPA for the treatment of AML.
Acknowledgments
Funding: This work was supported by grants from the Youth Talent S&T Project of the Changzhou Commission of Health (QN201821).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.03.01). The authors have no conflicts of interest to declare.
References
- 1.Saultz JN, Garzon R. Acute Myeloid Leukemia: A Concise Review. J Clin Med 2016. doi: . 10.3390/jcm5030033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boehm A, Mayerhofer M, Herndlhofer S, et al. Evaluation of in vivo antineoplastic effects of rapamycin in patients with chemotherapy-refractory AML. Eur J Intern Med 2009;20:775-8. 10.1016/j.ejim.2009.09.007 [DOI] [PubMed] [Google Scholar]
- 3.Ryningen A, Reikvam H, Nepstad I, et al. Inhibition of Mammalian target of rapamycin in human acute myeloid leukemia cells has diverse effects that depend on the environmental in vitro stress. Bone Marrow Res 2012;2012:329061. 10.1155/2012/329061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carnevale J, Ross L, Puissant A, et al. SYK regulates mTOR signaling in AML. Leukemia 2013;27:2118-28. 10.1038/leu.2013.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morran DC, Wu J, Jamieson NB, et al. Targeting mTOR dependency in pancreatic cancer. Gut 2014;63:1481-9. 10.1136/gutjnl-2013-306202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen EE, Wu K, Hartford C, et al. Phase I studies of sirolimus alone or in combination with pharmacokinetic modulators in advanced cancer patients. Clin Cancer Res 2012;18:4785-93. 10.1158/1078-0432.CCR-12-0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng LH, Zheng XF. Toward rapamycin analog (rapalog)-based precision cancer therapy. Acta Pharmacol Sin 2015;36:1163-9. 10.1038/aps.2015.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aleffi S, Navari N, Delogu W, et al. Mammalian target of rapamycin mediates the angiogenic effects of leptin in human hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol 2011;301:G210-9. 10.1152/ajpgi.00047.2010 [DOI] [PubMed] [Google Scholar]
- 9.Janus A, Linke A, Cebula B, et al. Rapamycin, the mTOR kinase inhibitor, sensitizes acute myeloid leukemia cells, HL-60 cells, to the cytotoxic effect of arabinozide cytarabine. Anticancer Drugs 2009;20:693-701. 10.1097/CAD.0b013e32832e89b4 [DOI] [PubMed] [Google Scholar]
- 10.Cho H, Chung JY, Kim S, et al. MICA/B and ULBP1 NKG2D ligands are independent predictors of good prognosis in cervical cancer. BMC Cancer 2014;14:957. 10.1186/1471-2407-14-957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol 2003;3:781-90. 10.1038/nri1199 [DOI] [PubMed] [Google Scholar]
- 12.Eissens DN, Van Der Meer A, Van Cranenbroek B, et al. Rapamycin and MPA, but not CsA, impair human NK cell cytotoxicity due to differential effects on NK cell phenotype. Am J Transplant 2010;10:1981-90. 10.1111/j.1600-6143.2010.03242.x [DOI] [PubMed] [Google Scholar]
- 13.Zhang YH, Lin QF, Wang XL, et al. Rapamycin induces human acute promyelocytic leukemia cell HL-60 autophagic apoptosis. Eur Rev Med Pharmacol Sci 2017;21:5506-14. [DOI] [PubMed] [Google Scholar]
- 14.Liesveld JL, O'Dwyer K, Walker A, et al. A phase I study of decitabine and rapamycin in relapsed/refractory AML. Leuk Res 2013;37:1622-7. 10.1016/j.leukres.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 15.Maude SL, Tasian SK, Vincent T, et al. Targeting JAK1/2 and mTOR in murine xenograft models of Ph-like acute lymphoblastic leukemia. Blood 2012;120:3510-8. 10.1182/blood-2012-03-415448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giles FJ, Albitar M. Mammalian target of rapamycin as a therapeutic target in leukemia. Curr Mol Med 2005;5:653-61. 10.2174/156652405774641034 [DOI] [PubMed] [Google Scholar]
- 17.Li J, Xue L, Hao H, et al. Rapamycin combined with celecoxib enhanced antitumor effects of mono treatment on chronic myelogenous leukemia cells through downregulating mTOR pathway. Tumour Biol 2014;35:6467-74. 10.1007/s13277-014-1820-5 [DOI] [PubMed] [Google Scholar]
- 18.Sandhofer N, Metzeler KH, Rothenberg M, et al. Dual PI3K/mTOR inhibition shows antileukemic activity in MLL-rearranged acute myeloid leukemia. Leukemia 2015;29:828-38. 10.1038/leu.2014.305 [DOI] [PubMed] [Google Scholar]
- 19.Morris RE. Rapamycins: antifungal, antitumor, antiproliferative, and immunosuppressive macrolides. Transplantation Reviews 1992;6:39-87. 10.1016/S0955-470X(10)80014-X [DOI] [Google Scholar]
- 20.Janes MR, Fruman DA. Immune regulation by rapamycin: moving beyond T cells. Sci Signal 2009;2:pe25. 10.1126/scisignal.267pe25 [DOI] [PubMed] [Google Scholar]
- 21.Ferrer IR, Araki K, Ford ML. Paradoxical aspects of rapamycin immunobiology in transplantation. Am J Transplant 2011;11:654-9. 10.1111/j.1600-6143.2011.03473.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcais A, Cherfils-Vicini J, Viant C, et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat Immunol 2014;15:749-57. 10.1038/ni.2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu Z, Lu X, Jiang L, et al. STAT3 signaling pathway is involved in decitabine induced biological phenotype regulation of acute myeloid leukemia cells. Am J Transl Res 2015;7:1896-907. [PMC free article] [PubMed] [Google Scholar]
- 24.Bedel R, Thiery-Vuillemin A, Grandclement C, et al. Novel role for STAT3 in transcriptional regulation of NK immune cell targeting receptor MICA on cancer cells. Cancer Res 2011;71:1615-26. 10.1158/0008-5472.CAN-09-4540 [DOI] [PubMed] [Google Scholar]
- 25.Brown EJ, Albers MW, Shin TB, et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature 1994;369:756-8. 10.1038/369756a0 [DOI] [PubMed] [Google Scholar]
- 26.Bezzerri V, Vella A, Calcaterra E, et al. New insights into the Shwachman-Diamond Syndrome-related haematological disorder: hyper-activation of mTOR and STAT3 in leukocytes. Sci Rep 2016;6:33165. 10.1038/srep33165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang B, Xiao Z, Chen B, et al. Nogo-66 promotes the differentiation of neural progenitors into astroglial lineage cells through mTOR-STAT3 pathway. PLoS One 2008;3:e1856. 10.1371/journal.pone.0001856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das A, Salloum FN, Durrant D, et al. Rapamycin protects against myocardial ischemia-reperfusion injury through JAK2-STAT3 signaling pathway. J Mol Cell Cardiol 2012;53:858-69. 10.1016/j.yjmcc.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]