Cutaneous melanoma is the most aggressive and lethal type of skin cancer with a relatively high prevalence. Recent therapeutic breakthroughs have remarkably improved the survival rate of advanced melanoma patients, however, issues with efficacy as well as safety still persist (1). In order to further understand the mechanisms of melanoma progression and thereby discover novel druggable targets, much effort has been focused on the functions of extracellular vesicles, especially exosomes. Exosomes have long been implicated as drivers of cancer progression (2,3) with recent evidence being strongly suggestive of their protumoral functions (4-9). These observations advocate that targeting exosomes could potentially provide a new therapeutic avenue for treating melanoma.
Exosomes are small, extracellular vesicles which range from 50 to 150 nm in size and are secreted by a variety of benign and malignant cell types (10,11). These double-layered lipid structure vesicles function as messengers facilitating intercellular communication by delivering proteins, lipids, cell surface molecules, RNA and microRNAs (12). The biogenesis of exosomes is regulated through pathways dependent or independent of ESCRT (endosomal sorting complex required for transport). Different mediators/exosome biomarkers are involved in these pathways, such as CD9 in ESCRT-independent and CD63 and Tsg101 in ESCRT-dependent mechanisms (3,13,14). In addition, RAB GTPases have been implicated in exosome formation and secretion; especially RAB27A and RAB27B, which have been demonstrated to be crucial regulators of exosome secretion (15).
MicroRNAs (miRNAs) are a class of non-coding RNAs implicated in the regulation of gene expression. The role of miRNAs in melanoma cell biology, especially in the context of exosomes, has been previously described. While some miRNAs suppress melanoma metastasis, e.g., miR-7-5p (16) or miR-302 (17), a number of miRNAs are prometastatic (18). For example, melanoma exosomes contain miR-155 and miR-210, which in turn modulate metabolism (8) and trigger a proangiogenic switch of cancer-associated fibroblasts (9), and thus may contribute to the creation of a pre-metastatic niche. Felicetti et al. reported that miR-222 is enriched in melanoma exosomes and may be a causative agent of enhanced tumor metastasis (7). Interestingly, Lunavat et al. showed that BRAF-inhibition upregulates miR-211-5p expression in melanoma exosomes, while stable expression of miR-211-5p reduced the sensitivity of melanoma cells to BRAF-inhibitor treatment (19).
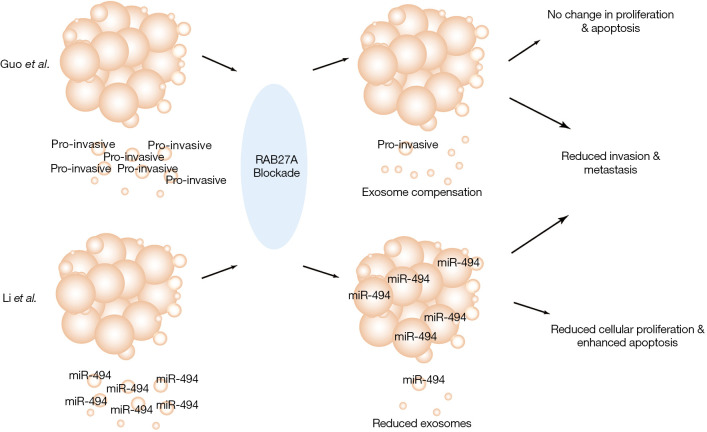
A recent study by Li et al. posits that miR-494 is overexpressed in the serum exosomes of malignant melanoma patients compared to healthy people (20). The authors of this study have employed elegant experimental strategies, such as exosome uptake assay, microarray analysis of serum from melanoma patients and exosomal miRNA expression in cell lines, to reach the conclusion that melanoma cells normally expel miR-494 by releasing it into the extracellular space through exosome secretion. However, an impairment in the release of miR-494 leads to the suppression of melanoma cell proliferation and metastasis (Figure 1). These findings strongly suggest a critical role for miR-494 and exosomes in melanoma progression (20).
Figure 1.
Exosome secretion in melanoma. Schematic representation highlighting core differences in the exosome secretion pathway post-abrogation of RAB27A expression in melanoma cells.
The authors have thoroughly evaluated the alterations in melanoma cell biology after blockade of exosomal miR-494 secretion by utilizing cell proliferation assay, wound healing and trans-well assay in vitro as well as tumor xenograft experiments in vivo. They have demonstrated that by blocking RAB27A-mediated exosome secretion, miR-494 failed to be released and thereby accumulated intracellularly. This intracellular accumulation of miRNA-494 resulted in enhanced melanoma cell apoptosis and reduced metastasis (20). They performed both RAB27A shRNA knockdown as well as overexpression of miR-494 in melanoma cell lines to demonstrate that the accumulation or overexpression of miR-494 in melanoma cells is able to facilitate cell apoptosis and inhibit cell proliferation. Additionally, silencing RAB27A and/or overexpressing miR-494 in melanoma cells inhibited invasion and metastasis in vivo. These experiments suggest that retaining miR-494 within melanoma cells could dramatically inhibit melanoma proliferation, migration, invasion, and metastasis. Moreover, all these defects caused by the intracellular containment of miR-494 could be rescued by anti-miR-494 treatment. Overall, the study by Li et al. convincingly indicates a potential therapeutic application in targeting exosomes and miR-494 for the treatment of melanoma (20).
Interestingly, our group has previously reported that RAB27A-mediated exosomes can promote melanoma invasion and metastasis (5). By abrogating RAB27A expression in melanoma cells, we observed a significant reduction in tumor invasion and metastasis both in vitro and in vivo. Through investigating the conditioned medium employing immuno-EM, NanoSight analysis, and Western blotting analysis, we discovered that there were at least two distinct populations of exosomes being secreted by melanoma cells. RAB27A knockdown caused reduction in the secretion of only one exosome population which was responsible for promoting melanoma cell invasion (we described it as a pro-invasive population). This population of exosomes had a CD9hiCD63loTsg101lo profile and a classical exosome structure (21), however, the exosomal cargos responsible for the phenotype require further investigation (5).
Both studies from Li et al. (20) as well as our group (5) have demonstrated that melanoma cell invasion and metastasis can be reduced significantly through disrupting RAB27A-mediated exosome secretion, which supports the role of RAB27A as a melanoma driver gene (22). Notably, Li et al. have also indicated that melanoma exosomes can not only deliver oncogenic signals but also remove tumor suppressing molecules (such like miR-494) from malignant cells (20). Further investigations on the ultimate fate and destination of miR-494 within the melanoma secreted exosomes will be of interest.
Apart from the similarities between Li et al. (20) and our study (5), there are also some significant differences. A few studies have now shown that exosome secretion is reduced after RAB27A knockdown (4,15). Li et al. have also demonstrated a reduction in total exosome secretion by silencing RAB27A. In contrast, in our study (5), RAB27A knockdown or knockout did not reduce the total number of secreted exosomes but only the pro-invasive population (Figure 1). The exosomes released by melanoma cells with RAB27A silenced were not simply reduced but compensated by another population with a CD9loCD63hiTsg101hi-profile, single layer structure and smaller size (<50 nm). The biomarker differences between the two populations of exosomes indicate that they potentially might have undergone different exosome biogenesis pathways. Therefore, we hypothesized that the reduction of total exosome secretion and exosome biomarker expression could only occur before the exosomal compensation (5).
Additionally, in contrast to our study (5), Li et al. (20) have reported a significant reduction in melanoma growth after RAB27A knockdown. Interestingly, we did not find a consistent reduction in the melanoma tumor growth post RAB27A knockdown/knockout. One way these results could be reconciled is by considering the possibility that the melanoma cell apoptosis and proliferation defect caused by RAB27A knockdown is rescued after exosomal compensation. During the course of our study, we indeed found a proliferation defect and cell apoptosis post-RAB27A knockdown as well. However, both of these were transient phenomena and only the defects in cell invasion and metastasis were found to be persistent over the duration of our study.
Targeting or modulating exosomes has opened up a new arena of therapeutic intervention in the treatment of melanoma. The results reported by Li et al. have furthered the implications of investigating exosomes. If the expression of miR-494 in serum exosomes is validated in a larger cohort of patients in the future, it has the potential to become a prognostic marker for melanoma patients. Moreover, if further studies could clearly uncover the functions and mechanisms underlying different populations of melanoma exosomes as well as their cargos, like miR-494, it is also possible that advanced melanoma patients could be treated with therapeutic strategies targeting miR-494 or RAB27A to reduce metastasis and improve progression-free and possibly overall survival.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Long Chen (Department of PET-CT center at the Yunnan Tumor Hospital, The Third Affiliated Hospital of Kunming Medical University, Department of Biochemistry and Molecular Biology of Kunming Medical University, Kunming, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.04.25). The authors have no conflicts of interest to declare.
References
- 1.Luke JJ, Flaherty KT, Ribas A, et al. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol 2017;14:463-82. 10.1038/nrclinonc.2017.43 [DOI] [PubMed] [Google Scholar]
- 2.Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev 2013;32:623-42. 10.1007/s10555-013-9441-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zöller M. Exosomes in Cancer Disease. Methods Mol Biol 2016;1381:111-49. 10.1007/978-1-4939-3204-7_7 [DOI] [PubMed] [Google Scholar]
- 4.Peinado H, Aleckovic M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 2012;18:883-91. 10.1038/nm.2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo D, Lui GY, Lai SL, et al. RAB27A promotes melanoma cell invasion and metastasis via regulation of pro-invasive exosomes. Int J Cancer 2019;144:3070-85. 10.1002/ijc.32064 [DOI] [PubMed] [Google Scholar]
- 6.Chen G, Huang AC, Zhang W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018;560:382. 10.1038/s41586-018-0392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felicetti F, De Feo A, Coscia C, et al. Exosome-mediated transfer of miR-222 is sufficient to increase tumor malignancy in melanoma. J Transl Med 2016;14:56. 10.1186/s12967-016-0811-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shu SL, Yang Y, Allen CL, et al. Metabolic reprogramming of stromal fibroblasts by melanoma exosome microRNA favours a pre-metastatic microenvironment. Sci Rep 2018;8:12905. 10.1038/s41598-018-31323-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou X, Yan T, Huang C, et al. Melanoma cell-secreted exosomal miR-155-5p induce proangiogenic switch of cancer-associated fibroblasts via SOCS1/JAK2/STAT3 signaling pathway. J Exp Clin Cancer Res 2018;37:242. 10.1186/s13046-018-0911-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathieu M, Martin-Jaular L, Lavieu G, et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 2019;21:9-17. 10.1038/s41556-018-0250-9 [DOI] [PubMed] [Google Scholar]
- 11.Somasundaram R, Herlyn M. Melanoma exosomes: messengers of metastasis. Nat Med 2012;18:853-4. 10.1038/nm.2775 [DOI] [PubMed] [Google Scholar]
- 12.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002;2:569-79. 10.1038/nri855 [DOI] [PubMed] [Google Scholar]
- 13.Chairoungdua A, Smith DL, Pochard P, et al. Exosome release of β-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol 2010;190:1079-91. 10.1083/jcb.201002049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buschow SI, Nolte-‘t Hoen EN, Van Niel G, et al. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic 2009;10:1528-42. 10.1111/j.1600-0854.2009.00963.x [DOI] [PubMed] [Google Scholar]
- 15.Ostrowski M, Carmo NB, Krumeich S, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 2010;12:19-30; sup pp 1-13. [DOI] [PubMed] [Google Scholar]
- 16.Giles KM, Brown RAM, Ganda C, et al. microRNA-7-5p inhibits melanoma cell proliferation and metastasis by suppressing RelA/NF-kappa B. Oncotarget 2016;7:31663-80. 10.18632/oncotarget.9421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maadi H, Moshtaghian A, Taha MF, et al. Multimodal tumor suppression by miR-302 cluster in melanoma and colon cancer. Int J Biochem Cell Biol 2016;81:121-32. 10.1016/j.biocel.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 18.Gajos-Michniewicz A, Czyz M. Role of miRNAs in Melanoma Metastasis. Cancers (Basel) 2019;11. doi: . 10.3390/cancers11030326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lunavat TR, Cheng L, Einarsdottir BO, et al. BRAF(V600) inhibition alters the microRNA cargo in the vesicular secretome of malignant melanoma cells. Proc Natl Acad Sci U S A 2017;114:E5930-9. 10.1073/pnas.1705206114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Chen J, Wang S, et al. Blockage of transferred exosome-shuttled miR-494 inhibits melanoma growth and metastasis. J Cell Physiol 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21.Jeppesen DK, Fenix AM, Franklin JL, et al. Reassessment of Exosome Composition. Cell 2019;177:428-45.e18. 10.1016/j.cell.2019.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akavia UD, Litvin O, Kim J, et al. An integrated approach to uncover drivers of cancer. Cell 2010;143:1005-17. 10.1016/j.cell.2010.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]