Abstract
Background
The age thresholds for differentiating young and elderly patients are still under debate. This study aimed to evaluate the cut-off age for differentiating patients along with the prognostic value of age for operable gastric cancer (GC).
Methods
Patients diagnosed with resected gastric adenocarcinoma were identified from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database (training cohort and internal validation cohort) and Liaoning Cancer Hospital (external validation cohort). Kaplan-Meier plots were used to compare cancer-specific survival (CSS) across different age groups. Univariate and multivariate analysis was conducted using a Cox regression model. Predictive ability of the nomogram was determined by the Harrell’s concordance index (C-index), calibration curves, and Akaike’s Information Criterion (AIC).
Results
A total of 17,339 patients with GC were included. According to the univariate analysis results, CSS was similar among patients aged 20–69 years old, started to worsen for patients over the age of 70, and was the worst for patients older than 79 years in the training cohort. Thus, we further divided the age groups into 20–69, 70–79, and >79, and multivariate analysis showed that patients above 70 years of age had worse CSS. The nomogram was established based on the results of the multivariate analysis. The C-indexes for the training, internal, and external validation cohorts were 0.7531, 0.7344, and 0.7431, respectively.
Conclusions
This study showed that age had a relative predictive ability for CSS, 70 years should be the cut-off age, and age ≥70 years is an independent prognostic risk factor for GC patients who undergo surgery. These data highlight the importance of individualized treatment to improve the prognosis of patients with GC.
Keywords: Age, gastric cancer (GC), prognosis, nomogram
Introduction
Gastric cancer (GC) is the fifth most common cancer in the world and the third leading cause of cancer-related mortality (1). Although great progress has been made in the treatment of GC, the prognosis is still not optimistic. Apart from typical pathological prognostic factors, the demographic characteristics of patients, especially age, have been proven to affect survival outcomes of multiple cancers, including colorectal (2) and prostate cancer (3). The GC incidence rate increases gradually with age (4). The age of patients at GC onset is generally between 50–70 years old, and 60% of patients with GC are aged over 65 years (5,6), although the incidence of GC among younger people is increasing (7). Old age has been a problem for choosing effective treatment strategies, and clarifying the association between age and long-term survival of GC to improve therapeutic efficacy is critical. Previous findings on the prognosis of young and elderly patients have not formed a consensus. Some studies reported that younger patients had a better prognosis (5,8), whereas others have indicated unfavorable characteristics and poorer prognosis in young patients (9,10). Still other studies found no significant differences in stage-specific survival between the 2 age groups (6,11,12). However, the study population sizes were relatively small or the age thresholds for differentiating young from elderly patients were not fixed in these studies. In contrast, the U.S. National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database comprises larger samples of data on GC than those of other studies, enabling us to evaluate the impact of age at diagnosis on gastric cancer-specific survival (GCSS). Therefore, this study aimed to determine whether differences in cancer-specific survival (CSS) exist between different age groups, to evaluate a cut-off age, and to establish a predictive model for GC patients based on age.
Methods
Patient selection
Data were obtained from the SEER 18 Regs Custom Data (with additional treatment fields), Nov 2017 Sub (1973–2015 varying). From the SEER database, patients diagnosed between 2010 and 2015 were set as the training cohort, and patients diagnosed between 2004 and 2009 were set as the internal validation cohort. Patients from Liaoning Cancer Hospital between 2011 and 2016 formed the external validation cohort. The inclusion criteria were as follows: patients older than 20, pathological confirmation of gastric adenocarcinoma (codes: M-8140/3, M8143-3 to M-8145/3, M-8210/3, M-8211/3, M-8255/3, M-8260/3 to M-8263/3, M-8310/3, M-8323/3, M-8480/3, M-8481/3), patients received surgery, and American Joint Committee on Cancer (AJCC) eighth edition stages I–III. The exclusion criteria were as follows: patients with an unknown number of lymph node (LN) retrieved, an unknown number of positive LNs, an unknown AJCC stage, survival of less than 1 month, unknown survival time or death because of any other cause. The CONSORT diagram is listed in Figures S1,S2.
Figure S1.

CONSORT diagram of the training and internal validation cohorts. AJCC, eighth edition. AJCC, American Joint Committee on Cancer; LN, lymph node.
Figure S2.

CONSORT diagram of the external validation cohort. AJCC, eighth edition. AJCC, American Joint Committee on Cancer; LN, lymph node.
Statistical analysis
Patient data including age at diagnosis, gender, race, histological grade, T stage, N stage, TNM stage, radiotherapy, positive number of LNs, total number of retrieved LNs, tumor size, cause-specific death classification, survival time (months), and status were retrieved from both the SEER database and the Liaoning Cancer Hospital dataset. To better establish the impact of age on CSS, age at diagnosis was classified into the following groups: 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, and older than 79 years. Categorical variables were compared by χ2 test. Survival rates were estimated using the Kaplan-Meier method. The nomogram, Harrell’s concordance index (C-index), Akaike’s Information Criterion (AIC), and calibration curves were generated to compare the predictive accuracy for different age groups. Statistical Package for Social Science (SPSS; IBM, Armonk, NY, USA) version 23.0 and R software (version 3.4.4) were used to conduct all statistical analyses. P<0.05 was considered a statistically significant value.
Results
Clinicopathological characteristics
A total of 17,339 patients were eligible for this study, and the clinicopathological characteristics of these patients are summarized in Table 1. Statistical significance (P<0.05) was found for all the variables when the training set and the external validation set were compared. Meanwhile, except for race, the variables of the internal validation set were significantly different from those of the training set. It was apparent that the patients in the external validation set were younger than those in the training set (23.2% vs. 38.3% of the group ≥70 years). Patients in the internal and external validation sets had a greater number of unfavorable features, including larger tumor size, advanced T stage, LN metastasis, and poorer histological grade. However, more patients in the external validation set had ≥15 LNs retrieved and less in the internal validation set than those of training set.
Table 1. Clinicopathological characteristics of study populations.
| Characteristics | Training set (A) (N=7,493), N (%) | Internal validation set (B) (N=8,129), N (%) | P value (A vs. B) | External validation set (C) (N=1,717), N (%) | P value (A vs. C) |
|---|---|---|---|---|---|
| Age (years) | <0.001 | <0.001 | |||
| 20–29 | 47 (0.6) | 29 (0.4) | 6 (0.3) | ||
| 30–39 | 208 (2.8) | 224 (2.8) | 34 (2.0) | ||
| 40–49 | 638 (8.5) | 788 (9.7) | 129 (7.5) | ||
| 50–59 | 1,547 (20.6) | 1,549 (19.1) | 440 (25.6) | ||
| 60–69 | 2,188 (29.2) | 2,150 (26.4) | 709 (41.3) | ||
| 70–79 | 1,975 (26.4) | 2,179 (26.8) | 318 (18.5) | ||
| >79 | 890 (11.9) | 1,210 (14.9) | 81 (4.7) | ||
| Gender | 0.025 | <0.001 | |||
| Male | 4,800 (64.1) | 5,067 (62.3) | 1,252 (72.9) | ||
| Female | 2,693 (35.9) | 3,062 (37.7) | 465 (27.1) | ||
| Race | 0.708 | – | |||
| White | 4,952 (66.1) | 5,380 (66.2) | – | ||
| Black | 928 (12.4) | 1,034 (12.7) | – | ||
| Othera | 1,613 (21.5) | 1,715 (21.1) | – | ||
| T stage | <0.001 | <0.001 | |||
| T1 | 1,863 (24.9) | 1,868 (23.0) | 218 (12.7) | ||
| T2 | 924 (12.3) | 1,000 (12.3) | 307 (17.9) | ||
| T3 | 2,546 (34.0) | 2,845 (35.0) | 34 (2.0) | ||
| T4a | 1,721 (23.0) | 1,818 (22.4) | 970 (56.5) | ||
| T4b | 439 (5.9) | 598 (7.4) | 188 (10.9) | ||
| N stage | <0.001 | <0.001 | |||
| N0 | 3,532 (47.1) | 3,363 (41.4) | 590 (34.4) | ||
| N1 | 1,337 (17.8) | 1,456 (17.9) | 335 (19.5) | ||
| N2 | 1,198 (16.0) | 1,455 (17.9) | 373 (21.7) | ||
| N3a | 999 (13.3) | 1,321 (16.3) | 297 (17.3) | ||
| N3b | 427 (5.7) | 534 (6.6) | 122 (7.1) | ||
| TNM stage | <0.001 | <0.001 | |||
| I | 2,268 (30.3) | 2,290 (28.2) | 370 (21.5) | ||
| II | 2,331 (31.1) | 2,240 (27.6) | 368 (21.4) | ||
| III | 2,894 (38.6) | 3,599 (44.2) | 979 (57.0) | ||
| LN examined | <0.001 | <0.001 | |||
| <15 | 3,069 (41.0) | 4,241 (52.2) | 345 (20.1) | ||
| ≥15 | 4,424 (59.0) | 3,888 (47.8) | 1,372 (79.9) | ||
| Grade | 0.001 | <0.001 | |||
| 1 | 416 (5.8) | 360 (4.6) | 51 (3.0) | ||
| 2 | 1,988 (27.9) | 2,092 (27.0) | 437 (25.5) | ||
| 3 and UD | 4,728 (66.3) | 5,310 (68.4) | 1,229 (71.6) | ||
| Site | 0.005 | <0.001 | |||
| Upper stomach | 2,436 (41.5) | 2,464 (39.8) | 696 (41.9) | ||
| Middle stomach | 720 (12.3) | 697 (11.3) | 253 (15.2) | ||
| Lower stomach | 2,174 (37.0) | 2,483 (40.1) | 711 (42.8) | ||
| Overlapping type | 543 (9.2) | 543 (8.8) | – | ||
| Tumor size (cm) | <0.001 | <0.001 | |||
| <5 | 4,055 (60.9) | 3,963 (56.5) | 652 (49.1) | ||
| ≥5 | 2,603 (39.1) | 3,056 (43.5) | 677 (50.9) |
a, Other includes American Indian/AK Native, Asian/Pacific Islander. LN, lymph node; UD, undifferentiated type.
Overall survival (OS) of GC among different age groups
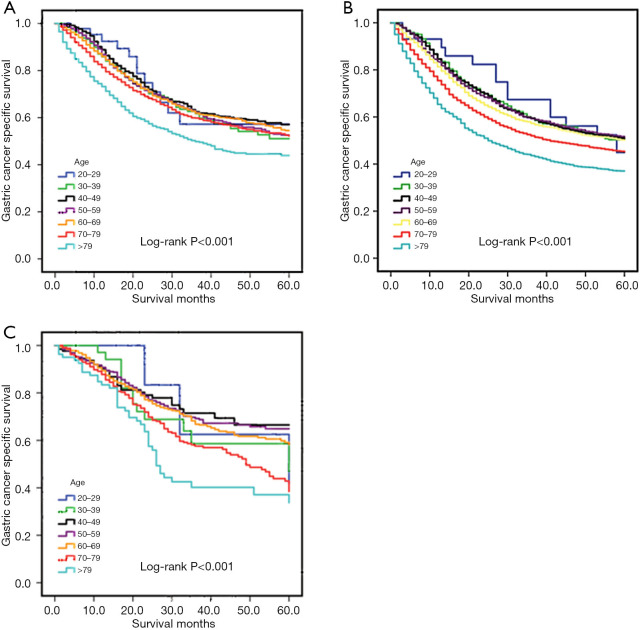
The Kaplan-Meier plot for the training set is shown in Figure 1A. The patients who were older than 79 years of age at the time of diagnosis presented with the worst survival rate with a 5-year CSS rate of 57.2%, 51.1%, 57.0%, 52.7%, 54.6%, 52.2%, and 43.9% for patients ages 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, and >79 years, respectively. We then evaluated the long-term survival of the validation sets (Figure 1B,C). The results were consistent with those of the training set. Mean survival times were 44.2, 41.5, 41.3, 41.2, 39.9, 36.9, and 32.0 months for each age group, respectively, in the internal validation set, and 48.0, 44.2, 46.9, 46.4, 45.3, 40.9, and 34.8 months for each age group, respectively, in the external validation set. There were significant differences found in all analyses (log-rank: P<0.001).
Figure 1.
Kaplan-Meier estimates of OS in the training set (A), internal validation set (B), and external validation set (C). OS, overall survival.
Comparisons of survival using Cox regression model in the training cohort
Univariate analysis results of the training set using Cox regression model are listed in Table S1. In the univariate analysis, using ages 60–69 years as reference, the patients who were younger than 69 years had almost the same prognosis and showed no significant difference (P=0.596, 0.708, 0.307, and 0.895 for the age groups 20–29, 30–39, 40–49, and 50–59 years, respectively). The CSS decreased with age only over 70 years [hazard radio (HR), 1.14; 95% confidence interval (CI), 1.02–1.26; P=0.021] and was the worst in the >79-year age group (HR, 1.63; 95% CI, 1.44–1.85; P<0.001) (Table S1). The age groups were then divided into 20–69, 70–79, and >79 years for multivariate analysis to be performed. Moreover, race, histological grade, T stage, N stage, number of retrieved LNs, site, tumor size, and radiation were also associated with CSS. Each of these variables was included in the multivariate analysis. The multivariate analysis showed that older age (70–79 and >79 years), race (white), histological grade [poorly differentiated and undifferentiated type (UD)], T stage (T2/3/4), N stage (N1/2/3), number of retrieved LNs (<15), site (upper stomach), tumor size (≥5 cm), and the absence of radiation therapy were independent risk factors for prognosis (Table 2).
Table S1. Univariate analysis of CSS in the training set.
| Variables | Training set | |
|---|---|---|
| HR (95% CI) | P value | |
| Age (years) | ||
| 20–29 | 0.86 (0.48–1.52) | 0.596 |
| 30–39 | 1.05 (0.82–1.34) | 0.708 |
| 40–49 | 0.92 (0.78–1.08) | 0.307 |
| 50–59 | 1.01 (0.90–1.13) | 0.895 |
| 60–69 | Reference | – |
| 70–79 | 1.14 (1.02–1.26) | 0.021 |
| >79 | 1.63 (1.44–1.85) | <0.001 |
| Gender | ||
| Male | Reference | – |
| Female | 0.97 (0.90–1.06) | 0.517 |
| Race | ||
| White | Reference | – |
| Black | 1.03 (0.91–1.16) | 0.669 |
| Othera | 0.74 (0.67–0.82) | <0.001 |
| T stage | ||
| T1 | Reference | – |
| T2/T3/T4 | 5.39 (4.63–6.28) | <0.001 |
| N stage | ||
| N0 | Reference | – |
| N1/2/3 | 4.03 (3.66–4.43) | <0.001 |
| LN examined | ||
| <15 | Reference | – |
| ≥15 | 0.92 (0.85–1.00) | 0.050 |
| Grade | ||
| 1/2 | Reference | – |
| 3 and UD | 1.95 (1.77–2.15) | <0.001 |
| Site | ||
| Upper stomach | Reference | – |
| Middle stomach | 0.87 (0.74–1.01) | 0.066 |
| Lower stomach | 0.90 (0.82–1.00) | 0.051 |
| Overlapping type | 1.40 (1.21–1.63) | <0.001 |
| Tumor size (cm) | ||
| <5 | Reference | – |
| ≥5 | 2.08 (1.91–2.26) | <0.001 |
| Radiation | ||
| No | Reference | – |
| Yes | 1.15 (1.06–1.24) | 0.001 |
a, Other includes American Indian/AK Native, Asian/Pacific Islander. CSS, cancer-specific survival; HR, hazard radio; CI, confidence interval; LN, lymph node; UD, undifferentiated type.
Table 2. Multivariate analysis of CSS in the training set.
| Variables | HR (95% CI) | P value |
|---|---|---|
| Age (years) | ||
| 20–69 | Reference | – |
| 70–79 | 1.33 (1.21–1.46) | <0.001 |
| >79 | 1.71 (1.52–1.93) | <0.001 |
| Race | ||
| White | Reference | – |
| Black | 1.04 (0.92–1.17) | 0.107 |
| Othera | 0.82 (0.73–0.91) | <0.001 |
| T stage | ||
| T1 | Reference | – |
| T2/T3/T4 | 3.04 (2.58–3.58) | <0.001 |
| N stage | ||
| N0 | Reference | – |
| N1/2/3 | 3.02 (2.72–3.35) | <0.001 |
| LN examined | ||
| <15 | Reference | – |
| ≥15 | 0.72 (0.67–0.78) | <0.001 |
| Grade | ||
| 1/2 | Reference | – |
| 3 and UD | 1.47 (1.33–1.62) | <0.001 |
| Site | ||
| Upper stomach | Reference | – |
| Middle stomach | 0.76 (0.65–0.89) | 0.001 |
| Lower stomach | 0.79 (0.70–0.88) | <0.001 |
| Overlapping type | 0.94 (0.81–1.10) | 0.453 |
| Tumor size (cm) | ||
| <5 | Reference | – |
| ≥5 | 1.31 (1.26–1.50) | <0.001 |
| Radiation | ||
| No | Reference | – |
| Yes | 0.76 (0.69–0.83) | <0.001 |
a, Other includes American Indian/AK Native, Asian/Pacific Islander. CSS, cancer-specific survival; HR, hazard radio; CI, confidence interval; LN, lymph node; UD, undifferentiated type.
Development and validation of a prediction model
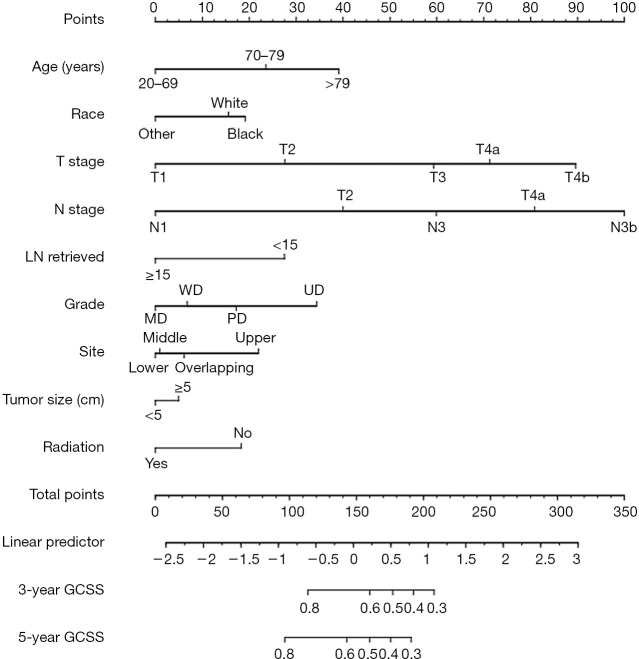
Independent risk factors of the training set were used to generate the nomogram (Figure 2). The calibration curve is shown in Figure 3A, and the C-index was 0.7531. We then validated the results. Relatively good calibrations were also observed between the predictive and actual 5-year CSS for the validation sets (Figure 3B,C). The C-index of the internal and external validation sets was 0.7344 and 0.7431, respectively. Univariate and multivariate analysis of the validation sets indicated that patients aged 70–79 and over 79 years experienced worse prognosis, which also supported the age-based prediction model (Tables 3,S2).
Figure 2.
Nomogram of OS in the training set. OS, overall survival; LN, lymph node; GCSS, gastric cancer-specific survival.
Figure 3.
The calibration curves for predicting OS of patients at 3 and 5 years, in the training set (A), internal validation set (B), and external validation set (C). OS, overall survival; GCSS, gastric cancer-specific survival.
Table 3. Multivariate analysis of CSS in the internal and external validation sets.
| Variables | Internal validation set | External validation set | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) | |||||
| 20–69 | Reference | – | Reference | – | |
| 70–79 | 1.42 (1.32–1.52) | <0.001 | 1.34 (1.10–1.63) | 0.004 | |
| >79 | 1.81 (1.66–1.98) | <0.001 | 1.76 (1.28–2.42) | 0.001 | |
| Gender | |||||
| Male | – | – | – | – | |
| Female | – | – | – | – | |
| Race | |||||
| White | Reference | – | – | – | |
| Black | 1.03 (0.94–1.14) | 0.485 | – | – | |
| Othera | 0.77 (0.71–0.84) | <0.001 | – | – | |
| T stage | |||||
| T1 | Reference | – | Reference | – | |
| T2/T3/T4 | 2.94 (2.62–3.31) | <0.001 | 2.59 (1.59–4.22) | <0.001 | |
| N stage | |||||
| N0 | Reference | – | Reference | – | |
| N1/2/3 | 2.78 (2.56–3.00) | <0.001 | 3.68 (2.83–4.80) | <0.001 | |
| LN examined | |||||
| <15 | – | – | Reference | – | |
| ≥15 | – | – | 0.76 (0.63-0.93) | 0.007 | |
| Grade | |||||
| 1/2 | Reference | – | Reference | – | |
| 3 and UD | 1.43 (1.33–1.54) | <0.001 | 1.25 (1.02–1.53) | 0.028 | |
| Site | |||||
| Upper stomach | Reference | – | Reference | – | |
| Middle stomach | 0.73 (0.64–0.82) | <0.001 | 0.70 (0.52–0.94) | 0.018 | |
| Lower stomach | 0.76 (0.70–0.83) | <0.001 | 0.82 (0.68–0.98) | 0.032 | |
| Overlapping type | 0.98 (0.89–1.08) | 0.422 | – | – | |
| Tumor size (cm) | |||||
| <5 | Reference | – | Reference | – | |
| ≥5 | 1.16 (1.09–1.24) | <0.001 | 1.32 (1.09–1.61) | 0.006 | |
| Radiation | |||||
| No | Reference | – | – | – | |
| Yes | 0.72 (0.67–0.76) | <0.001 | – | – | |
a, Other includes American Indian/AK Native, Asian/Pacific Islander. CSS, cancer-specific survival; HR, hazard radio; CI, confidence interval; LN, lymph node; UD, undifferentiated type.
Table S2. Univariate analysis of CSS in the internal and external validation sets.
| Variables | Internal validation set | External validation set | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) | |||||
| 20–29 | 0.98 (0.59–1.63) | 0.930 | 1.18 (0.38–3.69) | 0.778 | |
| 30–39 | 0.95 (0.78–1.16) | 0.612 | 1.20 (0.68–2.10) | 0.531 | |
| 40–49 | 0.95 (0.85–1.07) | 0.389 | 0.87 (0.60–1.27) | 0.468 | |
| 50–59 | 0.95 (0.86–1.04) | 0.240 | 0.90 (0.71–1.12) | 0.338 | |
| 60–69 | Reference | – | Reference | – | |
| 70–79 | 1.18 (1.09–1.28) | <0.001 | 1.45 (1.17–1.80) | 0.001 | |
| >79 | 1.54 (1.40–1.69) | <0.001 | 2.04 (1.47–2.84) | <0.001 | |
| Gender | |||||
| Male | Reference | – | Reference | – | |
| Female | 0.99 (0.93–1.05) | 0.640 | 0.93 (0.77–1.12) | 0.424 | |
| Race | |||||
| White | Reference | – | – | – | |
| Black | 0.96 (0.87–1.05) | 0.324 | – | – | |
| Othera | 0.71 (0.66–0.77) | <0.001 | – | – | |
| T stage | |||||
| T1 | Reference | – | Reference | – | |
| T2/T3/T4 | 4.59 (4.11–5.11) | <0.001 | 5.13 (3.24–8.28) | <0.001 | |
| N stage | |||||
| N0 | Reference | – | Reference | – | |
| N1/2/3 | 3.59 (3.34–3.86) | <0.001 | 4.69 (3.63–6.05) | <0.001 | |
| LN examined | |||||
| <15 | Reference | – | Reference | – | |
| ≥15 | 1.00 (0.94–1.06) | 0.003 | 0.80 (0.65–0.98) | 0.028 | |
| Grade | |||||
| 1/2 | Reference | – | Reference | – | |
| 3 and UD | 1.80 (1.67–1.93) | <0.001 | 1.37 (1.13–1.67) | 0.001 | |
| Site | |||||
| Upper stomach | Reference | – | Reference | – | |
| Middle stomach | 0.74 (0.66–0.84) | <0.001 | 0.58 (0.43–0.77) | <0.001 | |
| Lower stomach | 0.81 (0.75–0.88) | <0.001 | 0.75 (0.62–0.90) | 0.002 | |
| Overlapping type | 1.31 (1.17–1.47) | <0.001 | – | – | |
| Tumor size (cm) | |||||
| <5 | Reference | – | Reference | – | |
| ≥5 | 1.76 (1.65–1.88) | <0.001 | 1.73 (1.42–2.10) | <0.001 | |
| Radiation | |||||
| No | Reference | – | – | – | |
| Yes | 1.10 (1.04–1.17) | 0.002 | – | – | |
a, Other includes American Indian/AK Native, Asian/Pacific Islander. CSS, cancer-specific survival; HR, hazard radio; CI, confidence interval; LN, lymph node; UD, undifferentiated type.
We further compared the predictive efficacy of age serving as a continuous variable and categorical variable (3 groups and 6 groups). As listed in Table 4, when assessed as a continuous type, age had a better predictive accuracy and goodness of fit with the C-index (0.7539 vs. 0.7359) and AIC (7,773.2 vs. 8,695.0) of the training and internal validation sets, respectively. Age in the 3 age groups and the 6 age groups had a similar prognostic performance (C-index: 0.7531 vs. 0.7532; AIC: 7,769.7 vs. 7,777.9 for the training set, and C-index: 0.7344 vs. 0.7350; AIC: 8,714.3 vs. 8,712.9 for the internal validation set). Notably, age divided into 3 groups (C-index: 0.7431; AIC: 1,772.1) had a superior performance compared to 6 groups (C-index: 0.7391; AIC: 1,802.8) and was comparable with continuous type (C-index: 0.7426; AIC: 1,794.1).
Table 4. Predictive accuracy of age in continuous type and categorical type (6 groups and 3 groups).
| Age | Training set | Internal validation set | External validation set | |||||
|---|---|---|---|---|---|---|---|---|
| C-index | AIC | C-index | AIC | C-index | AIC | |||
| Continuous | 0.7539 | 7,773.2 | 0.7359 | 8,695.0 | 0.7426 | 1,794.1 | ||
| Categorical (6 groups) | 0.7532 | 7,777.9 | 0.7350 | 8,712.9 | 0.7391 | 1,802.8 | ||
| Categorical (3 groups) | 0.7531 | 7,769.7 | 0.7344 | 8,714.3 | 0.7431 | 1,772.1 | ||
C-index, Harrell’s concordance index; AIC, Akaike’s Information Criterion.
Discussion
GC is widely recognized as an age-related disease, and despite growing evidence that neoadjuvant therapy or adjuvant therapy is beneficial for locally advanced GC, the prognosis has remained poor. Understanding the related factors of GC progression can lead to better individualized therapy and prognosis prediction for GC. Considering that the relationship between age and OS rates might be complicated, especially in elderly patients, we selected GCSS as the primary study outcome to evaluate the prognostic value of age.
As a key factor, the correlation between age and survival has been widely analyzed in several cancers (3,13,14). In terms of GC, it has been reported that young patients exhibited worse survival due to the high malignancy of tumor characteristics (10). Jiang et al. (15) reported that younger GC patients usually have metastasis to LNs. Seo et al. also indicated that younger patients had more advanced GC, while the overexpression of p53, HER-2, and MSI were found to be significantly decreased in younger age groups (12). Chen et al. (16) indicated that in patients who received surgery, those aged between 56 and 65 years had better CSS and presented with favorable clinicopathological features. By contrast, Song et al. (17) suggested that elderly patients experienced a lower OS rate compared with young patients in operable GC.
However, the cut-off values of age assessed by the above studies were inconsistent. In our study, age groups were divided into 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, and older than 79 years. After adjusting the available data by known GC prognostic factors, CSS changed with age, being lower among patients older than 70 years. The impact of age on CSS was consistent in both the training and validation sets. Thus, our findings indicate that 70 years should be cut-off age and that elderly GC patients have poorer GCSS than younger patients.
We included Western and Eastern populations to establish the age-based prediction model. Although clinicopathological characteristics between the training and validation sets were significantly different, the C-index was above 0.70 each time. Further evaluation found that, compared with the initial age grouping, age split into 3 groups had a similar and even better predictive accuracy and goodness of fit, which confirmed the model has good extrapolation and prediction efficiency.
Old age is one of the risk factors that preclude surgical treatment for patients. Contrary to non-elderly patients, elderly patients are more likely to have postoperative complications, and poor compensatory capacity leads to poor tolerance of surgical trauma (18,19). Moreover, the feasibility of inadequate perioperative therapy also increases with age. Previous studies have revealed that chemotherapy and radiotherapy were less likely to be performed on older patients, because they might experience a high incidence and risk of comorbidities, and decreased life expectancy (20,21). This might explain why our results had a poor prognosis for elderly patients.
Other research has demonstrated that elderly patients may be able to gain better prognosis from invasive treatment. Choo et al. showed that although patients aged over 80 years suffered from coronary heart disease, cerebral infarction, renal insufficiency, hypertension, and other diseases, surgical intervention was superior to supportive treatment (22). Pan et al. performed a meta-analysis on laparoscopic gastrectomy (LG) for elderly patients and concluded that short outcomes were acceptable from LG for elderly patients and that old age alone should not be regarded as a contraindication of LG (23).
For adjuvant chemotherapy, the Adjuvant Chemotherapy Trial of TS-1 for Gastric Cancer (ACTS-GC) (24) found that compared with surgery alone, patients aged from 60 to 80 years experienced a better OS and relapse-free survival (RFS) with the postoperative treatment of TS-1. The CLASSIC trial (25) also indicated that patients over 65 years with positive LNs could benefit from surgery in addition to adjuvant chemotherapy.
Numerous studies have also investigated the efficacy and safety of targeted therapies for elderly patients with advanced GC. The Trastuzumab for Gastric Cancer (ToGA) trial (26) revealed that trastuzumab had a beneficial effect on the elderly group (≥60), with no significant increase in the incidence of severe toxicities. The RAINBOW (27) and REGARD (28) trials reported that ramucirumab or ramucirumab plus paclitaxel could serve as an alternative therapy for elderly GC patients with distant metastasis.
However, prospective studies have imposed age limits for eligible populations, so specific evidence of phase III trials regarding treatment for elderly patients is currently unavailable (29,30). Relevant findings were usually derived from subgroup analysis. Further prospective trials are needed to determine guidelines for GC therapy, particularly in elderly patients.
Some limitations existed in our study. First, it was a retrospective study, thus bias might potentially exist. Second, the SEER database did not contain treatment details such as chemotherapy and target therapy. Third, relatively few patients aged 20–29 were involved, which might have affected the results.
Conclusions
In conclusion, this study showed that age had relative predictive ability of GCSS. Furthermore, it found that 70 years should be the cut-off age and that age ≥70 years is an independent prognostic risk factor for GC patients who undergo surgery. These data highlight the importance of individualized treatment for improving the prognosis of patients with GC.
Acknowledgments
Funding: This study was funded by Liaoning provincial special fund for central guiding local science and technology development plan (2018416017).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by an independent ethical committee review board at Liaoning Cancer Hospital & Institute Ethical Committee (No. 20181226). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.02.37). The authors have no conflicts of interest to declare.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Fu J, Ruan H, Zheng H, et al. Impact of old age on resectable colorectal cancer outcomes. PeerJ 2019;7:e6350. 10.7717/peerj.6350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keating MJ, Giscombe L, Tannous T, et al. Age-dependent overall survival benefit of androgen deprivation therapy for metastatic prostate cancer. J Oncol Pharm Pract 2019;25:1927-32. 10.1177/1078155219835597 [DOI] [PubMed] [Google Scholar]
- 4.Jung KW, Won YJ, Kong HJ, et al. Prediction of Cancer Incidence and Mortality in Korea, 2018. Cancer Res Treat 2018;50:317-23. 10.4143/crt.2018.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katai H, Ishikawa T, Akazawa K, et al. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001-2007). Gastric Cancer 2018;21:144-54. 10.1007/s10120-017-0716-7 [DOI] [PubMed] [Google Scholar]
- 6.Takatsu Y, Hiki N, Nunobe S, et al. Clinicopathological features of gastric cancer in young patients. Gastric Cancer 2016;19:472-8. 10.1007/s10120-015-0484-1 [DOI] [PubMed] [Google Scholar]
- 7.Sung H, Siegel RL, Rosenberg PS, et al. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health 2019;4:e137-47. 10.1016/S2468-2667(18)30267-6 [DOI] [PubMed] [Google Scholar]
- 8.Liu S, Feng F, Xu G, et al. Clinicopathological features and prognosis of gastric cancer in young patients. BMC Cancer 2016;16:478. 10.1186/s12885-016-2489-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith BR, Stabile BE. Extreme aggressiveness and lethality of gastric adenocarcinoma in the very young. Arch Surg 2009;144:506-10. 10.1001/archsurg.2009.77 [DOI] [PubMed] [Google Scholar]
- 10.Guan WL, Yuan LP, Yan XL, et al. More attention should be paid to adult gastric cancer patients younger than 35 years old: extremely poor prognosis was found. J Cancer 2019;10:472-8. 10.7150/jca.27517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rona KA, Schwameis K, Zehetner J, et al. Gastric cancer in the young: An advanced disease with poor prognostic features. J Surg Oncol 2017;115:371-5. 10.1002/jso.24533 [DOI] [PubMed] [Google Scholar]
- 12.Seo JY, Jin EH, Jo HJ, et al. Clinicopathologic and molecular features associated with patient age in gastric cancer. World J Gastroenterol 2015;21:6905-13. 10.3748/wjg.v21.i22.6905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mauri G, Sartore-Bianchi A, Russo AG, et al. Early-onset colorectal cancer in young individuals. Mol Oncol 2019;13:109-31. 10.1002/1878-0261.12417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu YR, Jiang YZ, Yu KD, et al. Different patterns in the prognostic value of age for breast cancer-specific mortality depending on hormone receptor status: a SEER population-based analysis. Ann Surg Oncol 2015;22:1102-10. 10.1245/s10434-014-4108-5 [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y, Huang W, Xie J, et al. Young age increases risk for lymph node positivity in gastric cancer: a Chinese multi-institutional database and US SEER database study. J Cancer 2020;11:678-85. 10.7150/jca.37531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Chen J, Xu Y, et al. Impact of age on the prognosis of operable gastric cancer patients: an analysis based on SEER database. Medicine (Baltimore) 2016;95:e3944. 10.1097/MD.0000000000003944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song P, Wu L, Jiang B, et al. Age-specific effects on the prognosis after surgery for gastric cancer: a SEER population-based analysis. Oncotarget 2016;7:48614-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nunobe S, Oda I, Ishikawa T, et al. Surgical outcomes of elderly patients with Stage I gastric cancer from the nationwide registry of the Japanese Gastric Cancer Association. Gastric Cancer 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 19.Zhou CJ, Chen FF, Zhuang CL, et al. Feasibility of radical gastrectomy for elderly patients with gastric cancer. Eur J Surg Oncol 2016;42:303-11. 10.1016/j.ejso.2015.11.013 [DOI] [PubMed] [Google Scholar]
- 20.Nelen SD, Verhoeven RHA, Lemmens VEPP, et al. Increasing survival gap between young and elderly gastric cancer patients. Gastric Cancer 2017;20:919-28. 10.1007/s10120-017-0708-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang JS, Choi Y, Shin J, et al. Patterns of care for radiotherapy in the neoadjuvant and adjuvant treatment of gastric cancer: a twelve-year nationwide cohort study in Korea. Cancer Res Treat 2018;50:118-28. 10.4143/crt.2016.575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choo JW, Ju Y, Lim H, et al. Is it beneficial to perform surgical resection in elderly patients more than 80 years old with advanced gastric cancer? Scand J Gastroenterol 2017;52:1057-64. 10.1080/00365521.2017.1339828 [DOI] [PubMed] [Google Scholar]
- 23.Pan Y, Chen K, Yu WH, et al. Laparoscopic gastrectomy for elderly patients with gastric cancer: a systematic review with meta-analysis. Medicine (Baltimore) 2018;97:e0007. 10.1097/MD.0000000000010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007;357:1810-20. 10.1056/NEJMoa072252 [DOI] [PubMed] [Google Scholar]
- 25.Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21. 10.1016/S0140-6736(11)61873-4 [DOI] [PubMed] [Google Scholar]
- 26.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. 10.1016/S0140-6736(10)61121-X [DOI] [PubMed] [Google Scholar]
- 27.Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. 10.1016/S1470-2045(14)70420-6 [DOI] [PubMed] [Google Scholar]
- 28.Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. 10.1016/S0140-6736(13)61719-5 [DOI] [PubMed] [Google Scholar]
- 29.Dunn C, Wilson A, Sitas F. Older cancer patients in cancer clinical trials are underrepresented. Systematic literature review of almost 5000 meta- and pooled analyses of phase III randomized trials of survival from breast, prostate and lung cancer. Cancer Epidemiol 2017;51:113-7. 10.1016/j.canep.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 30.Frérot M, Jooste V, Binquet C, et al. Factors influencing inclusion in digestive cancer clinical trials: a population-based study. Dig Liver Dis 2015;47:891-6. 10.1016/j.dld.2015.05.017 [DOI] [PubMed] [Google Scholar]