Abstract
Background
Nav1.5, an isoform of voltage-gated sodium channel alpha subunits, has been found to be over-expressed in cancer cells and linked to disease progression. Nav1.5-third extracellular region antibody (E3Ab) specifically targets the E3 of Nav1.5 and can lead to a subtype-specific inhibition of Nav1.5. We were interested in the therapeutic potential of E3Ab in cancer.
Methods
The Nav1.5 expression in Caov-3 cells was assessed by immunocytochemistry. The effect of E3Ab on tumor growth in vivo was evaluated by using a nude mouse xenograft model derived from Caov-3 cells. In vitro, SiHa, MDA-MB-231, and Caov-3 cells were treated with E3Ab and cell migration, invasion, and proliferation were evaluated by transwell assays and EdU. Metal matrix protease-9 (MMP-9) expression was analyzed by Western blot.
Results
We found that Caov-3 cells highly expressed Nav1.5 and its binding with E3Ab was confirmed. In vivo, the growth of Caov-3 xenografts was significantly inhibited by E3Ab or lidocaine compared to control. The treatment with E3Ab and lidocaine significantly reduced the mitotic activity in tumor. E3Ab, as well as lidocaine, could inhibit the migration and invasion ability of cancer cells in vitro.
Conclusions
These findings suggest that E3Ab is a potential new therapeutic antibody for treatment of cancers expressing Nav1.5.
Keywords: Ion channel, invasion, migration, Nav1.5, cancer
Introduction
Generally, metastasis is tightly associated with poor survival outcomes in patients with cancer (1). Thus, there is an urgent need to understand the mechanisms involved in cancer metastasis so as to identify new molecular targets.
Voltage-sensitive ion channels facilitate diffusion of ions down the electrochemical gradient across cell membranes in excitable cells and tissues, which have been implicated in a variety of oncogenic processes, from proliferation to metastatic cascade, and have been proposed as promising early functional biomarkers and prognostic factors in tumors (2). Among these ion channels, voltage-gated sodium channels (VGSCs) were associated with metastasis and poor prognosis in a variety of cancers, such as malignancies of prostate, breast, lung, and colon (3-5). VGSCs are composed of ten pore-forming α subunits (VGSCα) and several modulating β subunits (VGSCβs). Both VGSCαs (Nav1.1 to Nav1.9, and Nax) and VGSCβs (β1 to β4) play a critical role in regulating electrical excitability and tumor metastasis (6). Nav1.5 is one of the VGSCαs that is predominantly expressed in heart, being sensitive to micromolar concentrations of the tetrodotoxin (TTX); Nav1.5 dysfunction could lead to some cardiac arrhythmias’ diseases (7). Emerging evidence suggests that Nav1.5 is also widely expressed in non-excitable cells, including immune cells and cancer cells, and the up-regulation of Nav1.5 expression and activity contribute to tumor metastasis (3). In a previous study, we found the mRNA expression of Nav1.5 were significantly increased in highly metastatic ovarian cancer cell lines compared with those of low-metastatic cells, and increased Nav1.5 expression was associated with high histological grade and metastasis in ovarian cancer (8). These findings indicated metastasis-promoting effects of Nav1.5 in cancer.
Indeed, blockers of VGSCs are very effective in suppressing cancer cell invasion and migration (5). Local anesthetics, such as lidocaine and ropivacaine, have been indicated to be potent inhibitors of VGSCs activity and cancer cell invasion (9). Natural VGSCs blocking agents, such as TTX that blocks single sodium channels in an all-or-none manner on binding, are unequivocally toxic to mammals. TTX treatment could suppress migration and invasion of ovarian cancer cells in vitro (10). However, neither lidocaine nor TTX is isoform-specific, i.e., they inhibit VGSC isoforms unselectively.
The third extracellular region (E3) of VGSCα is diverse between different isoforms. An antibody recognizing E3 of Nav1.7 has been used as a specific inhibitor in human to inhibit the invasive capacities of non-small-cell lung cancer cells (11). In the present study, we used a Nav1.5-third extracellular region antibody (E3Ab) to specifically bind to and inhibit Nav1.5. E3Ab is a peptide specific polyclonal antibody recognizing E3 of Nav1.5, which could inhibit approximately 60% of Na+ current in HEK-293 and Chinese hamster ovary epithelia cells upon an extra-cellular application, without effecting Nav1.4 and Nav1.6 isoforms (12). The aim of this study was to investigate the inhibitory effect of E3Ab on cancer cells in vitro and in vivo.
Methods
Cell culture
Three highly invasive cancer cell lines Caov-3 (ovarian adenocarcinoma), MDA-MB-231 (breast adenocarcinoma), SiHa (cervical squamous cell carcinoma) were obtained from the China Type Culture Collection (CTCC). Cells were cultured at 37 °C with 5% CO2, 95% O2 in DMEM containing 10% fetal bovine serum, 50 IU/mL penicillin, 50 ng/L streptomycin and 0.3 ng/L glutamine.
Antibodies
The E3Ab was a gift of University Laboratory of Physiology, Oxford, London. It was produced in a rabbit immunized against a synthetic 18 amino acid peptide (CVRNFTALNGTNGSVEAD) derived from the coding sequences of D1:S5/6. The antibody against Nav1.5 was purchased from Alomone Labs (Cat. #ASC-005, Jerusalem, Israel). It was produced in a mouse immunized against a synthetic 17/19 amino acid peptide [DRLPKSDSEDGPRALNQLS(C)], corresponding to amino acid residues 493–511 of rat Nav1.5, the intracellular loop between domains I and II.
Immunocytochemistry
Cells were cultured on glass cover-slips for 24 h and then fixed with 4% paraformaldehyde at room temperature for 10 min. Non-specific sites were saturated by incubating with 3% normal goat serum in PBS for 30 min. Nav1.5 was detected by incubating the cells with E3Ab (15 µg/mL) and Nav1.5 antibody (8 µg/mL, Alomone Labs) for 60 min. Cells were then washed and incubated with a goat anti-rabbit secondary antibody conjugated to fluorescein isothiocyanate (FITC; Pierce, USA) for 60 min. Cell nuclei were visualized by DAPI staining (Beyotime Biotechnology, Shanghai, China). The results of immunocytochemistry were evaluated under a fluorescence microscope (Olympus IX71, Japan).
Tumor xenograft mouse model
The animal experiments were approved by the University Committee on Use and Care of Animals (UCUCA) at the hospital and conformed to their relevant regulatory standards. Female BALB/c mice (Silaikejinda, Hunan, China) of 4–6-week-old weighted 17–18 g was used for examining the effect of E3Ab on growth of ovarian cancer. The mice were raised under SPF (specific pathogen-free) conditions and were acclimatized for one week before inoculation. To establish tumor xenografts, Caov-3 cells were re-suspended in PBS at 2×106 cells per 100 µL and then subcutaneously injected into the right flank of the mice. One week after inoculation with confirmation of xenograft formation (i.e., tumor size approached 0.1 cm3), mice were randomly divided into three groups (ten mice per group) and received intratumoral injection of E3Ab (15 µg/mL, 200 µL, weekly) (12), lidocaine (200 µg/mL, 200 µL, weekly), and PBS (200 µL, weekly) for four weeks, respectively. Body weight and tumor volume were recorded weekly (13). Tumor volume was estimated using the formula (π/6) (L × W2), where L is the length of the tumor and W is the width. One week after the last drug administration, the mice were sacrificed and their xenografts were removed, paraffin embedded and sliced. Sections were stained with haematoxylin and eosin (HE) and the histological features of tumors were determined.
Blockade of Nav1.5 in vitro
Cells were treated with 15 µg/mL E3Ab for 48 h in order to achieve an effective inhibition of Nav1.5 activity (12). Cells treated with lidocaine (200 µg/mL; Huayuan Medicines, Fuyang, China) for 48 h served as positive control and untreated cells as negative control.
Cell invasion assays and migration assays
The 8 µm pore membrane of the culture inserts for 24-well plates (Corning Costar, Cambridge, MA, USA) were pre-coated with Matrigel (BD, USA). Cells were re-suspended in serum-free medium, seeded on the upper chambers at a density of 4×104 cells/well, and treated with E3Ab and lidocaine for 48 h. The lower chambers were filled with medium containing 10% FBS as chemo-attractant. After incubation for 24 h at 37 °C, the invasive cells on the lower surface of the filter membrane were fixed with 70% ethanol, stained with HE, and counted in 10 randomly selected high-power fields (×400). The procedure for migration assays was the same as the invasive assays except the inserts were not pre-coated with Matrigel as an extra-cellular mimicking matrix. Data were obtained from three separate experiments and each assay was performed in triplicate.
5-Ethynyl-2'-deoxyuridine (EdU) incorporation assays
Cell proliferation was assessed by EdU assay. Cells were treated with a Cell-Light EdU kit (Ribobio, Guangzhou, China) in triplicate according to the manufacturer’s instructions. The results of EdU incorporation were evaluated under a fluorescence microscopy (Olympus IX71, Japan) and cells were counted in five randomly selected high-power fields (×400). The percentage of EdU-positive cells was used as the parameter reflecting cell proliferation.
Western blotting for metal matrix protease-9 (MMP-9) detection
MMP-9 protein was detected by Western blot analyses using a rabbit monoclonal antibody purchased from Abcam, Cambridge, UK (1:1,000 dilution), as described in our previous report (14) β-actin served as internal control.
Statistical analysis
All data were expressed as mean ± standard deviation. Statistical significance was determined by one-way ANOVA combined with least significant difference (LSD) post hoc multiple comparison using SPSS13.0 statistic software (SPSS, Chicago, IL, USA). All tests were 2-tailed and differences with P<0.05 were considered to be statistically significant.
Results
Nav1.5 expression in Caov-3 cells
To verify the expression and subcellular location of Nav1.5 in Caov-3 cells, immunocytochemistry assays have been performed. E3Ab and Nav1.5 antibody was used in parallel. Nav1.5 protein was detected predominantly in plasma membrane of Caov-3 cells by both antibodies (Figure 1), confirming the high Nav1.5 expression in Caov-3 cells and the specific binding of E3Ab to Nav1.5
Figure 1.
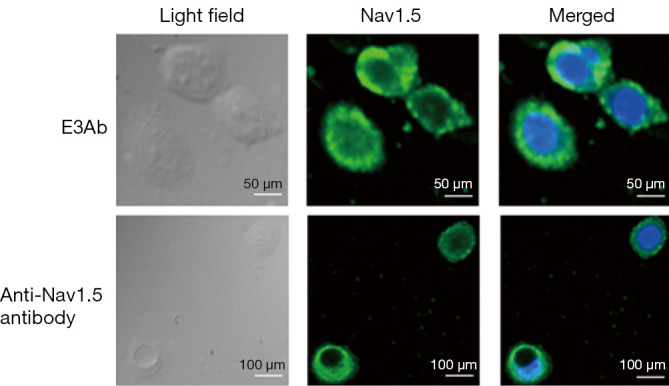
Nav 1.5 expression in Caov-3 cells. Immunocytochemistry assays show positive Nav 1.5 expression (green) in Caov-3 by E3Ab and Nav1.5 antibody. Cell nuclei are visualized by DAPI staining (blue). E3Ab, third extracellular region antibody.
E3Ab inhibits tumor growth in vivo
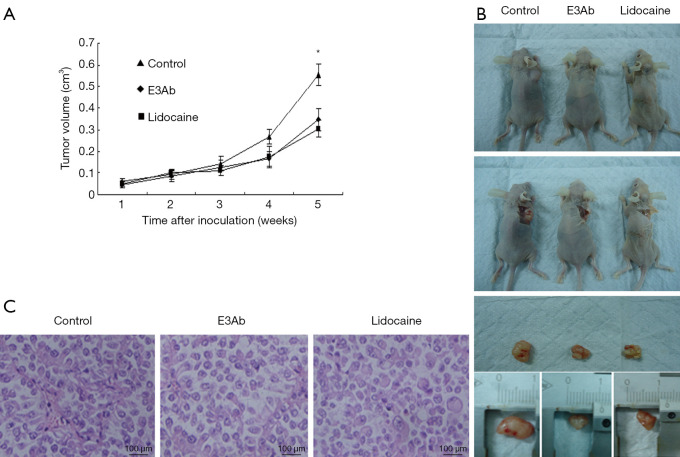
Mouse xenograft models of ovarian cancer were established to evaluate the effect of E3Ab on tumor growth in vivo. The Caov-3 xenografts were treated with PBS, E3Ab, or lidocaine. All mice survived the xenografting and the subsequent drug treatment. Both E3Ab and lidocaine could significantly inhibit the growth of xenografts compared with the control group four weeks after inoculation (Figure 2A). That was further verified by the measurement of autopsy tumor volumes after animals being sacrificed (Figure 2B). The tumor volumes in the E3Ab group and the lidocaine group were decreased to 28.59% and 25.97%, respectively, compared with that in the PBS group (Figure 2A; P<0.05). In addition, xenograft tumors in the control group exhibited higher mitotic activity (6.0±2.4 mitoses per high-power filed) than those treated with E3Ab or lidocaine (3.2±1.2 and 3.0±1.5 mitoses per high-power field, respectively; Figure 2C). The body weight of the mice at the time of sacrifice was not significantly different between groups (PBS group, 22.59±0.88 g; E3Ab group, 21.93±0.84 g; lidocaine group, 22.23±1.37 g; P>0.05).
Figure 2.
E3Ab inhibits ovarian cancer growth in vivo. Caov-3 subcutaneous xenografts mouse models were established and E3Ab, lidocaine, or PBS were intratumoral administrated one week after inoculation (ten mice per group). (A) Tumor growth curves show E3Ab and lidocaine slowed down the growth of xenografts compared with the PBS group; (B) the xenografts in the E3Ab group and the lidocaine group were smaller than those from the PBS group; (C) HE staining of the xenografts. *, P<0.05 vs. control. E3Ab, third extracellular region antibody.
E3Ab inhibits ovarian cancer cell invasion, migration, and proliferation in vitro
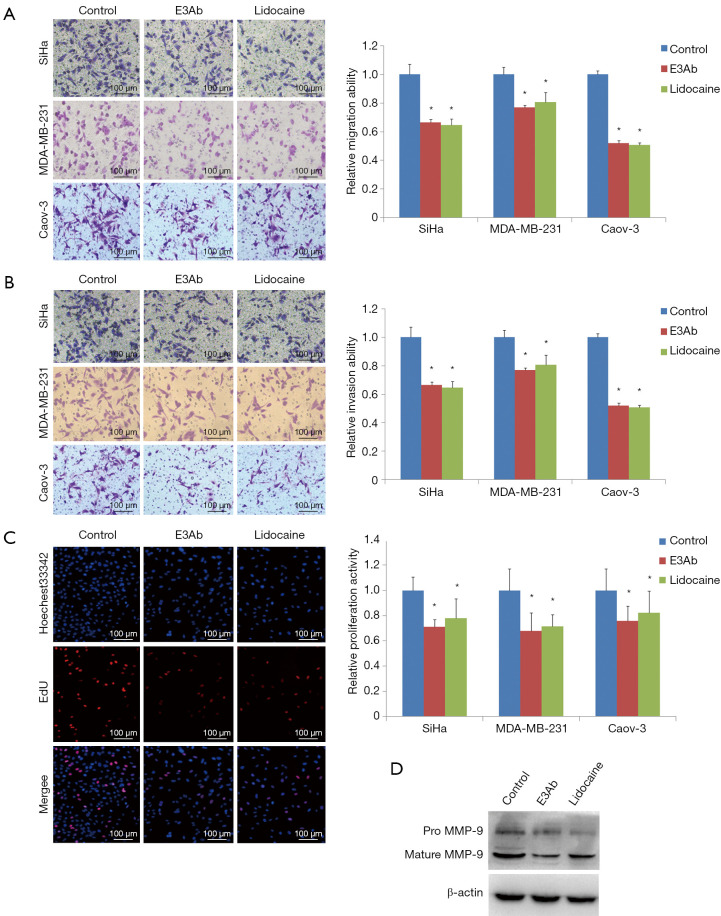
To examine the effect of E3Ab on the malignant biological behavior of ovarian cancer, transwell assays and EdU assays were performed to analyze the alterations in cell migration, invasion, and proliferation caused by administration of E3Ab. Cancer cells were treated with E3Ab or lidocaine for 48 h. Untreated cells served as negative control. The cancer cells pre-treated with E3Ab or lidocaine had a significantly attenuated invasion ability compared with the control group as demonstrated by decreased number of cells that could migrate across the Matrigel filters. The migration ability of SiHa, MDA-MB-231, and Cavo-3 cells that had been treated with E3Ab was decreased by 36.7%, 21.2%, and 49.1% compared with the negative control, respectively (Figure 3A; all P<0.05). Also, the E3Ab treatment leaded to a marked inhibition of cell invasion as compared with the negative control; the SiHa, MDA-MB-231, and Cavo-3 cells that penetrated through the Metrigel were decreased by 34.7%, 23.1%, and 48.1%, respectively (Figure 3B, all P<0.05). The migration- and invasion-suppressing effect of E3Ab in cancer cells was similar to that induced by lidocaine. Moreover, EdU assays revealed that the cell proliferation activity was reduced by 29.1%, 32.1%, and 24.4% in SiHa, MDA-MB-231, and Cavo-3 cells, respectively (Figure 3C, all P<0.05). In addition, the MMP-9 protein expression was reduced by E3Ab and lidocaine as revealed by Western blot assays (Figure 3D).
Figure 3.
E3Ab inhibits cancer cell migration and invasion in vitro. Tumor cells were treated with E3Ab, lidocaine, or PBS. The transwell migrating cells (A) and the invading cells (B) were significantly decreased in the E3Ab group and the lidocaine group compared with the PBS group; (C) EdU incorporation assays. E3Ab and lidocaine inhibited cell proliferation; (D) Western blot. MMP-9 protein expression in Caov-3 cells was reduced by E3Ab and lidocaine. *, P<0.05 vs. control. E3Ab, third extracellular region antibody; EdU, 5-Ethynyl-2'-deoxyuridine; MMP-9, metal matrix protease-9.
Discussion
Therapeutic antibodies specifically binding to tumor surface antigens have improved the treatment for a wide range of cancers (15). In the present study, we found that E3Ab specifically bound to Nav1.5 in ovarian cancer cells and inhibited tumor growth and invasion, indicating the therapeutic potential of E3Ab in Nav1.5-positive ovarian cancer.
Increasing evidence showed that Nav1.5 was over-expressed in cancers and was implicated in tumor metastasis cascade (3-5). For example, Nav1.5 was found to be up-regulated in breast cancer tissues compared with matched non-cancer breast tissue as well as in a highly metastatic cell line MDA-MB-231 compared with a poorly metastatic cell line MCF-7 (16). In an orthotopic breast cancer model, functionally active Nav1.5 contributed to tumor growth, local invasion, and metastasis to liver, lungs and spleen (17). Similarly, Nav1.5 expression was significantly increased in human colon cancer specimens compared with normal colon tissues. Moreover, ropivacaine, lidocaine, TTX, and a siRNA specifically targeting Nav1.5 could inhibit Nav1.5 channel function and invasion of metastatic colon cancer SW620 cells, and the Nav1.5 channel activator veratridine promoted SW620 cell invasion (9,18). In astrocytomas, increased Nav1.5 expression was positively correlated with the degree of malignancy and Nav1.5 knock-down significantly suppressed tumor cell proliferation, migration and invasion (19). In our previous study, high Nav1.5 was associated with metastatic property in ovarian cancer (8). However, the function of Nav1.5 in cancer remains largely unexplored. In the present study, we found specific blockage of Nav1.5 could inhibit cancer growth and invasive capacity, suggesting a tumor-promoting role and therapeutic implication of Nav1.5 in ovarian cancer.
We found that blocking Nav1.5 by E3Ab inhibited migration and invasion properties of cancer cells, accompanied by decreased MMP-9 protein levels. However, the mechanisms through which Nav1.5 promotes tumor metastasis in ovarian cancer remains less investigated. House et al. identified Nav1.5 as a key regulator of a gene transcriptional network that controls colon cancer invasion, involving genes regulating Wnt signaling, cell migration, ectoderm development, response to biotic stimulus, steroid metabolic process, and cell cycle control (18). Nav1.5 and Na(+)/H(+) exchanger type 1 (NHE-1) were functionally coupled and expressed at the sites of matrix remodeling in invadopodia of breast cancer cells. The Na+ current carried by Nav1.5 increased the NHE-1 dependent H+ efflux and enhanced tumor invasion by promoting extra-cellular matrix degradation (20,21). In addition, siRNA knockdown of Nav1.5 could reduce invasion of endocrine-resistant breast cancer cells, in part through reducing ERK1/2 phosphorylation and total MMP activity (22). Similarly, in a previous study, we found that blockade of Nav1.5 channel activity in breast cancer MDA-MB-231 cells resulted in a significant decrease in MMP-9 expression and cell invasion property (23). These findings implicate Nav1.5 in multiple mechanisms of tumor invasion and metastasis.
In the present study, we used a subcutaneous xenograft model to assess the anti-tumor effects of E3Ab and lidocaine. Subcutaneous xenograft model is the most widely used in vivo model for drug screening, because it is simple to operate, high performing, and easy to monitor. However, there are some limitations of this model when compared to an orthotopic tumor model. In an orthotopic ovarian cancer model, tumor cells are inoculated into the animal ovaries, mimicking the natural organ environment. In addition, the subcutaneous ovarian cancer xenograft models are insufficient to show spontaneous metastases and systemic side effects of treatments.
In conclusion, E3Ab can exert inhibitory effects on tumor growth and invasion through blocking Nav1.5, indicating the therapeutic potential of E3Ab for tumor. In addition, we are aiming to knock-down Nav1.5 and explore its effects in further studies, which may help clarify the mechanisms underlying the anti-tumor effects of E3Ab.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No.81572572) and the Research Funds from Science & Technology Department of Hubei Province (2014CFB996).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study followed the national or institutional guidelines for the care and use of animals. The protocol of animal treatment has been approved by the ethic committee of Tongji Medical College, Huazhong University of Science and Technology (S398).
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.12.23). The authors have no conflicts of interest to declare.
References
- 1.Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin 2018;68:284-96. 10.3322/caac.21456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiske JL, Fomin VP, Brown ML, et al. Voltage-sensitive ion channels and cancer. Cancer Metastasis Rev 2006;25:493-500. 10.1007/s10555-006-9017-z [DOI] [PubMed] [Google Scholar]
- 3.Roger S, Potier M, Vandier C, et al. Voltage-gated sodium channels: new targets in cancer therapy? Curr Pharm Des 2006;12:3681-95. 10.2174/138161206778522047 [DOI] [PubMed] [Google Scholar]
- 4.Brackenbury WJ. Voltage-gated sodium channels and metastatic disease. Channels (Austin) 2012;6:352-61. 10.4161/chan.21910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Djamgoz MB, Onkal R. Persistent current blockers of voltage-gated sodium channels: a clinical opportunity for controlling metastatic disease. Recent Pat Anticancer Drug Discov 2013;8:66-84. 10.2174/1574892811308010066 [DOI] [PubMed] [Google Scholar]
- 6.Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron 2000;26:13-25. 10.1016/S0896-6273(00)81133-2 [DOI] [PubMed] [Google Scholar]
- 7.Sottas V, Abriel H. Negative-dominance phenomenon with genetic variants of the cardiac sodium channel Nav1.5. Biochim Biophys Acta 2016;1863:1791-8. 10.1016/j.bbamcr.2016.02.013 [DOI] [PubMed] [Google Scholar]
- 8.Gao R, Shen Y, Cai J, et al. Expression of voltage-gated sodium channel alpha subunit in human ovarian cancer. Oncol Rep 2010;23:1293-9. [DOI] [PubMed] [Google Scholar]
- 9.Baptista-Hon DT, Robertson FM, Robertson GB, et al. Potent inhibition by ropivacaine of metastatic colon cancer SW620 cell invasion and NaV1.5 channel function. Br J Anaesth 2014;113 Suppl 1:i39-48. 10.1093/bja/aeu104 [DOI] [PubMed] [Google Scholar]
- 10.Moczydlowski EG. The molecular mystique of tetrodotoxin. Toxicon 2013;63:165-83. 10.1016/j.toxicon.2012.11.026 [DOI] [PubMed] [Google Scholar]
- 11.Roger S, Rollin J, Barascu A, et al. Voltage-gated sodium channels potentiate the invasive capacities of human non-small-cell lung cancer cell lines. Int J Biochem Cell Biol 2007;39:774-86. 10.1016/j.biocel.2006.12.007 [DOI] [PubMed] [Google Scholar]
- 12.Xu SZ, Zeng F, Lei M, et al. Generation of functional ion-channel tools by E3 targeting. Nat Biotechnol 2005;23:1289-93. 10.1038/nbt1148 [DOI] [PubMed] [Google Scholar]
- 13.Mitra AK, Davis DA, Tomar S, et al. In vivo tumor growth of high-grade serous ovarian cancer cell lines. Gynecol Oncol 2015;138:372-7. 10.1016/j.ygyno.2015.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi X, Guo J, Guo J, et al. EZH2-mediated epigenetic silencing of TIMP2 promotes ovarian cancer migration and invasion. Sci Rep 2017;7:3568. 10.1038/s41598-017-03362-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panowski S, Bhakta S, Raab H, et al. Site-specific antibody drug conjugates for cancer therapy. MAbs 2014;6:34-45. 10.4161/mabs.27022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraser SP, Diss JK, Chioni AM, et al. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin Cancer Res 2005;11:5381-9. 10.1158/1078-0432.CCR-05-0327 [DOI] [PubMed] [Google Scholar]
- 17.Nelson M, Yang M, Millican-Slater R, et al. Nav1.5 regulates breast tumor growth and metastatic dissemination in vivo. Oncotarget 2015;6:32914-29. 10.18632/oncotarget.5441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.House CD, Vaske CJ, Schwartz AM, et al. Voltage-gated Na+ channel SCN5A is a key regulator of a gene transcriptional network that controls colon cancer invasion. Cancer Res 2010;70:6957-67. 10.1158/0008-5472.CAN-10-1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xing D, Wang J, Ou S, et al. Expression of neonatal Nav1.5 in human brain astrocytoma and its effect on proliferation, invasion and apoptosis of astrocytoma cells. Oncol Rep 2014;31:2692-700. 10.3892/or.2014.3143 [DOI] [PubMed] [Google Scholar]
- 20.Brisson L, Gillet L, Calaghan S, et al. Na(V)1.5 enhances breast cancer cell invasiveness by increasing NHE1-dependent H(+) efflux in caveolae. Oncogene 2011;30:2070-6. 10.1038/onc.2010.574 [DOI] [PubMed] [Google Scholar]
- 21.Brisson L, Driffort V, Benoist L, et al. NaV1.5 Na+ channels allosterically regulate the NHE-1 exchanger and promote the activity of breast cancer cell invadopodia. J Cell Sci 2013;126:4835-42. 10.1242/jcs.123901 [DOI] [PubMed] [Google Scholar]
- 22.Mohammed FH, Khajah MA, Yang M, et al. Blockade of voltage-gated sodium channels inhibits invasion of endocrine-resistant breast cancer cells. Int J Oncol 2016;48:73-83. 10.3892/ijo.2015.3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao R, Wang J, Shen Y, et al. Functional expression of voltage-gated sodium channels Nav1.5 in human breast cancer cell line MDA-MB-231. J Huazhong Univ Sci Technolog Med Sci 2009;29:64-7. 10.1007/s11596-009-0113-5 [DOI] [PubMed] [Google Scholar]