Abstract
Inflammatory breast cancer (IBC) is a rare and aggressive form of breast cancer characterized by the presence of many dermal tumor emboli in the papillary and reticular dermis of the skin overlying the breast. IBC patients, compared to other breast cancer patients, have more frequently metastatic axillary lymph nodes. IBC is often high grade, negative for hormone receptors and presents with amplification of the HER2 gene. Invasive IBC is frequently of ductal phenotype, even if a specific histological distinction for these lesions has not been described. The pathogenesis and evolution of IBC are strongly dependent upon tumor microenvironment, characterized by several macrophages/monocytes and lymphocytes. The tumor and microenvironment cells are well molecularly characterized, showing the main contributor of inflammatory pathways in tumor biology of IBC. In addition, several molecular alterations are described in this tumor, such as mutations of ERBB2, KRAS, BRAF, EGFR, PIK3CA, PTEN, AKT1, and AKT3 genes that could suggest a therapeutic stratification of IBC patients with the combination of different biological target therapies.
Keywords: Inflammatory breast cancer (IBC), tumor biology, inflammatory pathways
Introduction
Inflammatory breast cancer (IBC) is a rare and aggressive type of invasive cancer clinically characterized by acute inflammatory changes of the breast. It accounts for about 2.5% of all breast cancers frequently affecting younger age women. It shows a rapid progression, frequent local and distant metastases, and lower overall survival compared with other breast cancers (1). In many cases, IBC is lately diagnosed or frequently misdiagnosed as mastitis, dermatitis or infective process, treated with antibiotics lengthen periods for correct diagnosis (2). The molecular and epidemiological characteristics together with an aggressive course of IBC, support the hypothesis that IBC may be a distinct biological entity characterized by two specific clinical varieties: “primary IBC” identifying the de novo development of IBC in a previously normal breast; “secondary IBC” meaning the development of inflammatory skin changes that mimic primary IBC either in a breast that already had cancer or on the chest wall after a mastectomy for non-IBC (3).
The clinical diagnosis of IBC is based upon the observations of the physical appearances of the affected breast, a careful medical history, physical examination, and pathological findings from a skin biopsy and/or needle or core biopsy. The frequency of involvement of axillary and supraclavicular lymph nodes in different studies has ranged from 60% to 85% (4). IBC can be considered a lethal disease with less than a 5% survival rate beyond 5 years even if survival times have been increased with multimodal therapy. Currently, the treatment of IBC starts with neoadjuvant chemotherapy, and if the tumor is HER2-positive, targeted therapy is given along with the chemotherapy. This is typically followed by surgery and radiation therapy (5).
Microscopic pathological characteristics of IBC
According to the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system of breast cancer, IBC is a rare subtype of locally advanced breast cancer and classified as T4d (6,7). Among the non-inflammatory BC (n-IBC) the best-represented histotypes are invasive ductal carcinoma (IDC) and invasive lobular carcinoma (ILC), which have distinct clinic-pathological and molecular profiles, different prognoses as well as a different response to therapy. Although the clinical features of IBC are very clearly described, its histology is not well defined (8). As a matter of fact, a histological distinction between lobular and ductal subtypes has not been described in IBC. Thus, it is not clear whether it represents a heterogeneous group like n-IBC or a strictly homogeneous entity (9).
IBC is characterized by the presence of many dermal tumor emboli in the papillary and reticular dermis of the skin overlying the breast. The emboli in patients with IBC are generally very numerous and larger compared to other non-IBCs. Embolic cells are usually of high nuclear grade with a ductal phenotype. Most of the spaces in the dermis with tumor emboli do not contain red blood cells and are, therefore, considered to be lymphatic spaces (10).
The morphology of cells in the skin of IBC patients is extremely heterogeneous. Areas within and outside the zone of erythema and edema may appear histologically identical, with lymphatic tumor emboli also present (10).
The presence lymphovascular tumor emboli in the dermis are useful for clinical diagnosis of IBC. However, their absence cannot exclude an IBC.
Lymphoplasmacytic infiltration may surround the lymphovascular spaces, even if there is no correlation between the extent of this infiltration and severity and distribution of the cutaneous manifestations of the disease (7).
The invasive IBC is generally of high histologic and nuclear grades, and it diffusely infiltrates the stroma. Consequently, the evaluation of skin margins in surgically excised samples may not be useful because of the possibility of finding emboli in grossly unremarkable skin as well as in the skin beyond the extent of the invasive tumor in the breast (10).
Differential diagnosis with other types of cancer is with high-grade non-Hodgkin lymphoma, acute leukemia, melanoma, angiosarcoma, and metastatic poorly differentiated carcinomas from other organ sites uncertainty can usually be excluded easily by morphological and immunohistochemical findings; however, for others, the diagnosis is more complicated (5). For example, non-inflammatory, locally advanced breast cancer that involves the skin, can have a similar tumor presentation, with ulceration, satellite skin nodules, edema, skin dimpling, or nipple retraction. At same time, infectious mastitis and breast abscess which typically occurs in lactating women, and ductal ectasia, a benign condition of middle age related to the collection of debris in and swelling of the ducts, can have a similar clinical and mammographic appearance to IBC (5). This is a microscopic section. I would cancel or move it to the introduction.
Since there are no specific biomarkers for accurate and early diagnosis of IBC, recent studies have focused attention on plasma membrane proteins (PMPs) that play an important role in the progression of IBC and could be optimal candidates as biomarkers (11). A series of identified PMPs have been validated by immunohistochemical analysis. They show the overexpression of the novel plasminogen receptor with A C-terminal Lysine (PLGRKT) and of the carrier protein, Secretory Carrier Membrane Protein 3 (SCAMP3), in IBC (11).
Molecular characteristics of IBC
Although IBC represents a distinct tumor entity based on clinical presentation, it shows the same molecular heterogeneity observed in non-IBC (12). IBCs are characterized by a combination of hormone receptors and oncogenes, which allows for the classification of them in the Her2 amplified, basal-like, breast cluster and “claudin-low”. This classification of IBC tumors is consistent with previous gene expression studies reporting that IBC tumors are characterized by amplification/over-expression of growth factor receptor HER2 (13) and down-regulation of hormone receptors ER/PR (1).
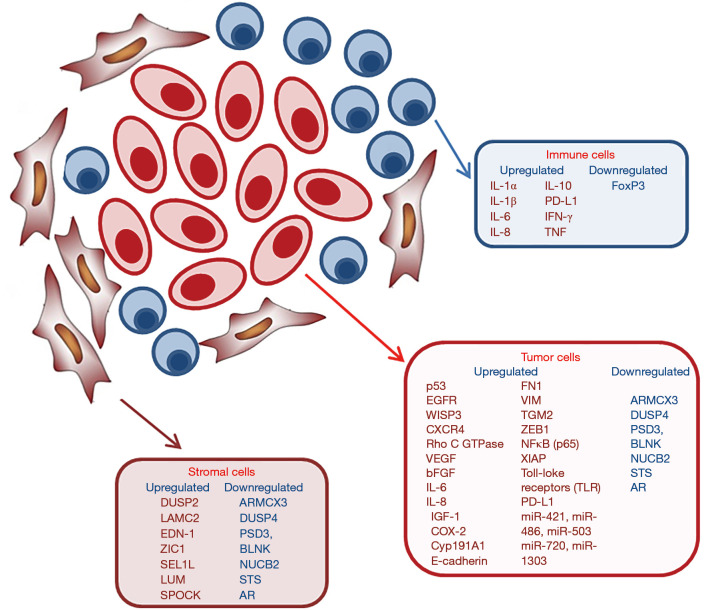
Other characteristics of IBC tumors include the most frequently mutated or amplified genes, such as TP53, MYC, and components of the RAS and phosphoinositide 3-kinase (PI3K) pathways. Mutations of the RAS pathway included those encoded by the ERBB2, KRAS, BRAF, and EGFR genes, while in the PI3K pathway, PIK3CA, PTEN, AKT1, and AKT3 were found to be mutated (14). Moreover, the combined overexpression of EGFR and p53 (15) and of EGFR and CXCR4 (16) are both associated with poor prognosis of IBC patients (Figure 1).
Figure 1.
Molecular markers of IBC tumor and microenvironment cells. IBC, inflammatory breast cancer.
About 80% of IBC is characterized by a loss of WNT1-inducible-signaling pathway protein 3 (WISP3). WISP3 is a cysteine-rich protein able to inhibit invasive and angiogenic potential of IBC cells in vitro and in vivo models (17).
One of the most highly upregulated genes in IBC is the transforming oncogene Rho C GTPase which encodes a member of the ras homology (Rho) GTPase family of proteins involved in cytoskeletal reorganization during invasion as well as regulation of angiogenic growth factors and production of inflammatory cytokines (18). Rho C GTPase is overexpressed in 90% of IBC tumors examined (19). Moreover, RhoC-GTPase is associated with the up-regulation of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), interleukin-6 (IL-6), and interleukin-8 (IL-8), contributing to a distinct type of angiogenic stroma formation in IBC tumors (20).
Other microarray analysis showed a distinct IBC molecular signature that includes genes involved in IGF signaling and loss of IGF-binding genes. In particular alterations in IGF-1 signaling in IBC have important consequences in the regulation of a proinflammatory microenvironment (21).
Another de-regulated gene is cyclooxygenase-2 (COX-2), overexpressed together with its enzymatic product prostaglandin E (PGE) in tumor cells in particular in the metastatic phenotype of IBC. Cox-2 directly regulates the P450 Cyp191A1 aromatase enzyme, thereby regulating estrogen production, and interacts with both HER-2 and EGFR (5).
Several studies showed that IBC is characterized by aberrant expression of E-cadherin, a marker of tumor emboli, which is also present on the surface of circulating tumor cells isolated from IBC patients. Generally, loss of E-cadherin and gain of N-cadherin have been reported to occur during the epithelial-mesenchymal transition (EMT) associated tumor progression and metastasis (22). IBC tumors represent an exception having the anomalous overexpression of E-cadherin but a most aggressive and metastatic phenotype. Several studies suggest E-cadherin molecule is central to the intravasation of IBC tumor emboli and represents an important target for effective treatment of IBC (23). Moreover, Cohen et al. described a correlation between the circulating levels of TNF-α and EMT biomarkers in IBC patients and showed that multiple cytokines originated from the activated T cells stimulate the expressions of fibronectin (FN1), vimentin (VIM), tissue transglutaminase (TGM2) and zinc-finger E-box binding homeobox1 (ZEB1) in different IBC cell lines. This data suggests that EMT may play a significant role in cytokine promotion of IBC cell invasion and metastasis (24).
Many studies have identified the role of anti-apoptotic signaling, in particular, hyperactivation of NF-κB and its target genes, in IBC pathobiology and therapeutic resistance (25). Moreover, a recent study showed that immunohistochemical analysis of IBC tumor samples with tumor emboli revealed high NF-κB (p65) staining and XIAP, a potent anti-apoptotic protein known to interact with NF-κB signaling in enhancing survival of malignant cells (26).
Different DNA microarrays studies comparing IBC and non-IBC tumor samples, showed an over-expression of Toll-like receptors (TLR) in IBC tissues (27). Over-expression of TLR suggests infiltration of IBC by immune cells and the possibility of viral etiology in IBC progression.
Finally, other studies comparing microRNAs (miRNAs) expression profiles in non-IBC, IBC, and normal breast tissues found that IBC patients are characterized by five over-expressed miRNAs comprising miR-421, miR-486, miR-503, miR-720, and miR-1303 (Figure 1) (28).
Role of tumor microenvironment in IBC
Many experimental pieces of evidence have suggested that the etiology and pathogenesis of IBC may be closely related to inflammation and changes in the tumor microenvironment (29). Tumor environment represents a complicated signal network composed by tumor surrounding blood vessels, inflammatory and immune cells, fibroblasts, and extracellular matrix molecules. Any kind of change affect the metabolism and behavior of tumor cells, and consequently alter the tumor progression, clinical manifestations and outcomes in a direct or indirect manner (30).
In particular, the capacity of migration and invasion of IBC cells dramatically enhanced by the stimulation of inflammatory and immune factors in their tumor microenvironment (Figure 1) (29).
Tumor-associated stromal cells are able to modify the phenotype of cancer cells with the acquisition of a stem cell phenotype, resistant to drug treatment. A recent study analyzed gene expression profiles of tumor stroma and tumor epithelium between IBC and non-IBC tumors showing significant enrichment of tumor stromal genes in IBC cells. Between these, dual specificity phosphatase 2 (DUSP2), and laminin γ2 (LAMC2) are upregulated only in the IBC stroma whereas endothelin-1 (EDN-1) and zinc fingers in cerebellum 1 (ZIC1) are upregulated in both IBC stroma and tumor epithelium. On the contrary, genes downregulated in IBC stroma includes several metastasis suppressor genes such as SEL1L and LUM or lumican, osteonectin (SPOCK). Genes that are downregulated in both stroma and tumor include the candidate tumor suppressors ARMCX3, DUSP4 and PSD3, the B-cell marker and tumor suppressor BLNK, and nucleobindin (NUCB2), steroid sulfatase (STS) and the androgen receptor (AR) (31).
IBC tumor is infiltrated by various types of macrophages/monocytes and lymphocytes (32).
Tumor-associated macrophages (TAMs) produce high levels of inflammatory mediators that promote survival and proliferation of tumor cells and also antagonize the antitumor activity of CD8-positive T cells (33). Several studies have shown that IBC tumors are abundantly infiltrated by TAMs, that also contribute to the metastatic process via releasing mediators such as TNF, IL-6, IL-8, and IL-10 modulating invasion of IBC cells and angiogenesis (32). The expression of these cytokines is significantly higher in tumor-infiltrating monocytes of IBC patients than in those from non-IBC patients (34).
A great significant proportion of tumor-infiltrating lymphocytes are present in IBC tumor. IHC staining identified aggregates of CD8-positive T cells as major subpopulations associated with intratumoral and peritumoral desmoplastic stroma in approximately half of IBC tumors analyzed (35). Moreover, IBC tumors showed low expression of regulatory T cell marker FoxP3, while the immune checkpoint regulator PD-L1 shows an extremely variable expression (35). mRNA expression of PD-L1 in a large case series of IBC tumor samples showed its overexpression in 38% of IBC tumors compared to normal breast tissue. PD-L1 upregulation was associated with a better response to chemotherapy in IBC patients (36,37).
Finally, IBC is also described as a high angiogenic tumor, characterized by increased microvessel density (38). For this reason, molecular targets for lymphangiogenesis and vasculogenesis have demonstrated greater therapeutic potential in IBC than in non-IBC (39).
Main inflammatory pathways in IBC
The abundance of cytokines and chemokines in the IBC tumor microenvironment could be responsible for the high aggressiveness of this tumor and for the increase of angiogenic processes and evasion of immune surveillance (40). However, comparative studies on the expression of many inflammatory cytokines and chemokines, including IFN-γ, TNF, IL-1α, IL-1β, IL-8, and IL-10 between IBC and non-IBC tissue samples did not highlight significant differences (41), suggesting that the molecular mechanisms associated with tumor promotion and progression are more complex.
A series of different molecular pathways have been associated with the regulation of inflammatory processes in IBC. Between these, constitutive activation of the NF-κB pathway, which results in upregulation of antiapoptotic proteins, is frequently observed in ER-negative/HER2-positive tumors (42,43). Moreover, the aberrant activity of two proinflammatory cytokines inducing NF-κB pathway activation, TNF and IL-1β, as described especially in ER-negative breast tumors cells (44). The protumorigenic activity of TNF was also described in the luminal subtypes (45) and elevated TNF levels are associated with increased lymph node metastasis and advanced tumor stage (46).
Many NF-κB target genes are upregulated in ER-negative IBC compared with ER-negative non-IBC tissues (25). Moreover, the inflammatory cytokines IL-6 and IL-8, the best characterized NF-κB target genes, are produced and secreted at high levels within IBC (3).
IL-1β requires inflammasome activation and processing of pro-IL-1β by Caspase-1 or 8 (47). The inflammasome is a multiprotein oligomer responsible for the activation of inflammatory responses. In IBC tumors, the accumulation of XIAP, an essential component of inflammasome signaling, is strongly related to the upregulation of IL-1β in IBC cells (48).
JAK/STAT signaling pathway is also deregulated in IBC tumors, in which the overexpression of IL-6 and the activated isoforms pJAK2 and pSTAT3 are observed (49). Several studies on IBC cell lines and tumor samples have confirmed the up-production and secretion of IL-6 in IBC. In addition, IL-6 also promotes self-renewal of the stem cell characteristics of the IBC cell lines SUM-149 and SUM-190 (50). Serum IL-6 levels are also significantly higher in IBC patients compared to non-IBC patients (51).
Finally, cyclooxygenase (COX) enzymes, in particular of COX-2, induced in response to proinflammatory stimuli, is overexpressed in IBC compared to non-IBC tumors, and this is also reflected by more abundant prostaglandin E2 in primary and metastatic IBC tumors (52). Furthermore, in SUM-149 cell line the suppression of prostaglandin E2 binding to its cognate receptors inhibited the aggressive proliferation and invasion of IBC cells (53).
Conclusions
IBC represents a distinct tumor entity with extremely heterogeneous cell morphology. Although no specific biomarkers for accurate and early diagnosis of IBC have been identified, this tumor has been very well characterized from a molecular point of view (Figure 1). Several microarray analysis showed a distinct IBC molecular signature including alteration of TP53 and MYC genes, RAS pathway and phosphoinositide 3-kinase (PI3K) pathways, suggesting the possibility of more personalized therapies that specifically target oncoproteins encoded by mutated or amplified genes.
Moreover, the contribution of inflammatory tumor microenvironment to disease evolution is fundamental; being IBC tumors strongly infiltrated by various types of macrophages/monocytes and lymphocytes. The abundance of secreted cytokines and chemokines in the tumor microenvironment play an essential role in IBC development and progression, suggesting the chance to perform proinflammatory cytokine blockade and immunotherapy in IBC therapy.
Acknowledgments
Funding: This study was supported by the Italian Ministry of Health.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editors (Emanuela Esposito and Michelino De Laurentiis) for the focused issue “Rare Tumors of the Breast” published in Translational Cancer Research. This article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.03.24). The focused issue “Rare Tumors of the Breast” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
References
- 1.Dawood S, Lei X, Dent R, et al. Survival of women with inflammatory breast cancer: a large population-based study. Ann Oncol 2014;25:1143-51. 10.1093/annonc/mdu121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isichei WM, Misauno MA. Inflammatory breast cancer misdiagnosed as mastitis. J Surg 2014;2:35-7. [Google Scholar]
- 3.Mohamed MM, Al-Raawi D, Sabet SF, et al. Inflammatory breast cancer: New factors contribute to disease etiology: A review. J Adv Res 2014;5:525-36. 10.1016/j.jare.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wecsler JS, Tereffe W, Pedersen RC, et al. Lymph node status in inflammatory breast cancer. Breast Cancer Res Treat 2015;151:113-20. 10.1007/s10549-015-3367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robertson FM, Bondy M, Yang W, et al. Inflammatory breast cancer: the disease, the biology, the treatment. CA Cancer J Clin 2010;60:351-75. 10.3322/caac.20082 [DOI] [PubMed] [Google Scholar]
- 6.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4. [DOI] [PubMed] [Google Scholar]
- 7.Vermeulen PB, van Golen KL, Dirix LY. Angiogenesis, lymphangiogenesis, growth pattern, and tumor emboli in inflammatory breast cancer: a review of the current knowledge. Cancer 2010;116:2748-54 10.1002/cncr.25169 [DOI] [PubMed] [Google Scholar]
- 8.van Uden DJ, van Laarhoven HW, Westenberg AH, et al. Inflammatory breast cancer: an overview. Crit Rev Oncol Hematol 2015;93:116-26 10.1016/j.critrevonc.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 9.Woodward WA. Inflammatory breast cancer: unique biological and therapeutic considerations. Lancet Oncol 2015;16:e568-76. 10.1016/S1470-2045(15)00146-1 [DOI] [PubMed] [Google Scholar]
- 10.Dawood S. Biology and management of inflammatory breast cancer. Expert Rev Anticancer Ther 2010;10:209-20. 10.1586/era.09.90 [DOI] [PubMed] [Google Scholar]
- 11.Suárez-Arroyo IJ, Feliz-Mosquea YR, Pérez-Laspiur J, et al. The proteome signature of the inflammatory breast cancer plasma membrane identifies novel molecular markers of disease. Am J Cancer Res 2016;6:1720-40. [PMC free article] [PubMed] [Google Scholar]
- 12.Bertucci F, Finetti P, Rougemont J, et al. Gene expression profiling identifies molecular subtypes of inflammatory breast cancer. Cancer Res 2005;65:2170-8. 10.1158/0008-5472.CAN-04-4115 [DOI] [PubMed] [Google Scholar]
- 13.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25-32. 10.1016/S1470-2045(11)70336-9 [DOI] [PubMed] [Google Scholar]
- 14.Ross JS, Ali SM, Wang K, et al. Comprehensive genomic profiling of inflammatory breast cancer cases reveals a high frequency of clinically relevant genomic alterations. Breast Cancer Res Treat 2015;154:155-62. 10.1007/s10549-015-3592-z [DOI] [PubMed] [Google Scholar]
- 15.Wang XZ, Liu Q, Sun JJ, et al. Correlation between p53 and epidermal growth factor receptor expression in breast cancer classification. Genet Mol Res 2015;14:4282-90. 10.4238/2015.April.28.10 [DOI] [PubMed] [Google Scholar]
- 16.Wurth R, Tarn K, Jernigan D, et al. A Preclinical Model of Inflammatory Breast Cancer to Study the Involvement of CXCR4 and ACKR3 in the Metastatic Process. Transl Oncol 2015;8:358-67. 10.1016/j.tranon.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleer CG, Zhang Y, Pan Q, et al. WISP3 is a novel tumor suppressor gene of inflammatory breast cancer. Oncogene 2002; 21:3172-3180. 10.1038/sj.onc.1205462 [DOI] [PubMed] [Google Scholar]
- 18.Haga RB, Ridley AJ. Rho GTPases: Regulation and roles in cancer cell biology. Small GTPases 2016;7:207-21. 10.1080/21541248.2016.1232583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Golen KL, Wu ZF, Qiao XT, et al. RhoC GTPase Overexpression Modulates Induction of Angiogenic Factors in Breast Cells. Neoplasia 2000;2:418-25. 10.1038/sj.neo.7900115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu M, Wu ZF, Kumar-Sinha C, et al. RhoC induces differential expression of genes involved in invasion and metastasis in MCF10A breast cells. Breast Cancer Res Treat 2004;84:3-12. 10.1023/B:BREA.0000018426.76893.21 [DOI] [PubMed] [Google Scholar]
- 21.van Golen KL. Inflammatory breast cancer: Relationship between growth factor signaling and motility in aggressive cancers. Breast Cancer Res 2003;5:174-9. 10.1186/bcr598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014;15:178-96. 10.1038/nrm3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colpaert CG, Vermeulen PB, Benoy I, et al. Inflammatory breast cancer shows angiogenesis with high endothelial proliferation rate and strong E-cadherin expression. Br J Cancer 2003;88:718-25. 10.1038/sj.bjc.6600807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen EN, Gao H, Anfossi S, et al. Inflammation Mediated Metastasis: Immune Induced Epithelial-To-Mesenchymal Transition in Inflammatory Breast Cancer Cells. PLoS One 2015;10:e0132710. 10.1371/journal.pone.0132710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lerebours F, Vacher S, Andrieu C, et al. NF-kappa B genes have a major role in inflammatory breast cancer. BMC Cancer 2008;8:41. 10.1186/1471-2407-8-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arora J, Sauer SJ, Tarpley M, et al. Inflammatory breast cancer tumor emboli express high levels of anti-apoptotic proteins: use of a quantitative high content and high-throughput 3D IBC spheroid assay to identify targeting strategies. Oncotarget 2017;8:25848-63. 10.18632/oncotarget.15667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamauchi H, Woodward WA, Valero V, et al. Inflammatory breast cancer: what we know and what we need to learn. Oncologist 2012;17:891-9. 10.1634/theoncologist.2012-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lerebours F, Cizeron-Clairac G, Susini A, et al. miRNA expression profiling of inflammatory breast cancer identifies a 5-miRNA signature predictive of breast tumor aggressiveness. Int J Cancer 2013;133:1614-23. 10.1002/ijc.28171 [DOI] [PubMed] [Google Scholar]
- 29.Lim B, Woodward WA, Wang X, et al. Inflammatory breast cancer biology: the tumour microenvironment is key. Nat Rev Cancer 2018;18:485-99. 10.1038/s41568-018-0010-y [DOI] [PubMed] [Google Scholar]
- 30.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013;19:1423-37. 10.1038/nm.3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boersma BJ, Reimers M, Yi M, et al. A stromal gene signature associated with inflammatory breast cancer. Int J Cancer 2008;122:1324-32. 10.1002/ijc.23237 [DOI] [PubMed] [Google Scholar]
- 32.Morrow RJ, Etemadi N, Yeo B, et al. Challenging a Misnomer? The Role of Inflammatory Pathways in Inflammatory Breast Cancer. Mediators Inflamm 2017;2017:4754827. [DOI] [PMC free article] [PubMed]
- 33.Condeelis J, Pollard J.W. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006;124:263-6. 10.1016/j.cell.2006.01.007 [DOI] [PubMed] [Google Scholar]
- 34.Mohamed MM, El-Ghonaimy EA, Nouh MA, et al. Cytokines secreted by macrophages isolated from tumor microenvironment of inflammatory breast cancer patients possess chemotactic properties. Int J Biochem Cell Biol 2014;46:138-47. 10.1016/j.biocel.2013.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamm CA, Moran D, Rao K, et al. Genomic and immunological tumor profiling identifies targetable pathways and extensive CD8+/PDL1+ immune infiltration in inflammatory breast cancer tumors. Mol Cancer Ther 2016;15:1746-56. 10.1158/1535-7163.MCT-15-0353 [DOI] [PubMed] [Google Scholar]
- 36.Bertucci F, Finetti P, Birnbaum D, et al. The PD1/PDL1 axis, a promising therapeutic target in aggressive breast cancers. Oncoimmunology 2015;5:e1085148. 10.1080/2162402X.2015.1085148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertucci F, Finetti P, Colpaert C, et al. PDL1 expression in inflammatory breast cancer is frequent and predicts for the pathological response to chemotherapy. Oncotarget 2015;6:13506-19. 10.18632/oncotarget.3642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarthy NJ, Yang X, Linnoila IR, et al. Microvessel density, expression of estrogen receptor alpha, MIB-1, p53, and c-erbB-2 in inflammatory breast cancer. Clin Cancer Res 2002;8:3857-62. [PubMed] [Google Scholar]
- 39.Bertucci F, Fekih M, Autret A, et al. Bevacizumab plus neoadjuvant chemotherapy in patients with HER2-negative inflammatory breast cancer (BEVERLY-1): a multicentre, single-arm, phase 2 study. Lancet Oncol 2016;17:600-11. 10.1016/S1470-2045(16)00011-5 [DOI] [PubMed] [Google Scholar]
- 40.Cohen E, Lee B, Gao H, et al. Soluble Factors and Circulating Tumor Cells in Inflammatory Breast Cancer. Abstracts: Thirty-Second Annual CTRC-AACR San Antonio Breast Cancer Symposium-- Dec 10-13, 2009; San Antonio, TX. [Google Scholar]
- 41.Van Laere S, Van der Auwera I, Van den Eynden G, et al. Distinct molecular phenotype of inflammatory breast cancer compared to non-inflammatory breast cancer using affymetrix-based genome-wide gene-expression analysis. Br J Cancer 2007;97:1165-74. 10.1038/sj.bjc.6603967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shostak K, Chariot A. NF-kappaB, stem cells and breast cancer: the links get stronger. Breast Cancer Res 2011;13:214. 10.1186/bcr2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xia Y, Shen S, Verma IM. NF-kappaB, an active player in human cancers. Cancer Immunol Res 2014;2:823-30. 10.1158/2326-6066.CIR-14-0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pileczki V, Braicu C, Gherman CD, et al. TNF-α gene knockout in triple negative breast cancer cell line induces apoptosis. Int J Mol Sci 2012;14:411-20. 10.3390/ijms14010411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubio MF, Werbajh S, Cafferata EG, et al. TNF-alpha enhances estrogen-induced cell proliferation of estrogen-dependent breast tumor cells through a complex containing nuclear factor-kappa B. Oncogene 2006;25:1367-77. 10.1038/sj.onc.1209176 [DOI] [PubMed] [Google Scholar]
- 46.Esquivel-Velázquez M, Ostoa-Saloma P, Palacios-Arreola MI, et al. The Role of Cytokines in Breast Cancer Development and Progression. J Interferon Cytokine Res 2015;35:1-16. 10.1089/jir.2014.0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watanabe H, Gaide O, Petrilli V, et al. Activation of the IL-1beta-processing inflammasome is involved in contact hypersensitivity. J Invest Dermatol 2007;127:1956-63. 10.1038/sj.jid.5700819 [DOI] [PubMed] [Google Scholar]
- 48.Aird KM, Ghanayem RB, Peplinski S, et al. X-linked inhibitor of apoptosis protein inhibits apoptosis in inflammatory breast cancer cells with acquired resistance to an ErbB1/2 tyrosine kinase inhibitor. Mol Cancer Ther 2010;9:1432-42. 10.1158/1535-7163.MCT-10-0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jhaveri K, Teplinsky E, Silvera D, et al. Hyperactivated mTOR and JAK2/STAT3 pathways: molecular drivers and potential therapeutic targets of inflammatory and invasive ductal breast cancers after neoadjuvant chemotherapy. Clin Breast Cancer 2016;16:113-22.e1. 10.1016/j.clbc.2015.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Debeb BG, Cohen EN, Boley K, et al. Pre-Clinical studies of Notch Signaling Inhibitor RO4929097 in Inflammatory Breast Cancer Cells. Breast Cancer Res Treat 2012;134:495-510. 10.1007/s10549-012-2075-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drygin D, Ho CB, Omori M, et al. Protein kinase CK2 modulates IL-6 expression in inflammatory breast cancer. Biochem Biophys Res Commun 2011;415:163-7. 10.1016/j.bbrc.2011.10.046 [DOI] [PubMed] [Google Scholar]
- 52.Huang A, Cao S, Tang L. The tumor microenvironment and inflammatory breast cancer. J Cancer 2017;8:1884-91. 10.7150/jca.17595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh B, Irving LR, Tai K, et al. Overexpression of COX-2 in celecoxib-resistant breast cancer cell lines. J Surg Res 2010;163:235-43. 10.1016/j.jss.2010.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]