Abstract
Two patients with rare GCC2-ALK fusion G13:A20 which were found in Chinese population by next generation sequencing (NGS) developed resistant to crizotinib with a prolonged progression-free survival (PFS). Both patients showed unfavorable response to subsequent second or third generation tyrosine kinase inhibitors (TKIs) treatment with shorten PFS. In conclusion, non-small cell lung cancer (NSCLC) patients with rare GCC2-ALK fusion G13:A20 may be optimal candidates for crizotinib as front-line therapy and may have a high possibility to exhibit unsatisfactory response to subsequent second or third generation TKIs target therapy after acquiring resistance to crizotinib.
Keywords: GCC2-ALK fusion, non-small cell lung cancer (NSCLC), target therapy, next generation sequencing (NGS), tyrosine kinase inhibitor (TKI)
Introduction
Treatment strategy for lung cancer has changed significantly over the past decade, from histology to molecular-driven therapy. Anaplastic lymphoma kinase (ALK) rearrangement has been detected in 3% to 7% of non-small cell lung cancer (NSCLC) patients (1). For ALK-positive NSCLC, ALK-related treatment is the most effective treatment, therefore, accurate diagnosis of ALK rearrangement is critical. Besides the standard diagnostic method fluorescence in situ hybridization (FISH) and immunohistochemistry (IHC), most recently, the advent of targeted next generation sequencing (NGS) for ALK rearrangement has become possible and many ALK inhibitors have been profoundly used in treating ALK-positive NSCLC patients.
In our previous study, we found a 29-year-old female lung adenocarcinoma patient with rare GCC2-ALK fusion G13:A20 using NGS. The patient received crizotinib treatment as first-line therapy and reached a progression-free survival (PFS) of 18 months (2). GCC2-ALK fusion increased up to a mutant allele frequency (MAF) of 20% at the end of crizotinib treatment and ALK E1407K was detected upon crizotinib resistance at a MAF of 1% without any known crizotinib resistant mechanism. Similar GCC2-ALK fusion G12:A20 has been reported in one of the 158 ALK-positive patients in a study of 3,000 lung cancer patients from Korea although without clinical follow-up (3). Another GCC2-ALK fusion G19:A20 has been detected by France group with a continuation of response to crizotinib treatment with stabilization of the skeletal metastases but evidence of local extension of brain metastases (4).
In this study, we reported another patient with the same rare GCC2-ALK fusion G13:A20 received crizotinib as front-line therapy and second or third generation tyrosine kinase inhibitors (TKIs) as subsequent target therapy. Treatment history of both patients with rare GCC2-ALK fusion was also presented.
Methods
DNA extraction from tumor tissue, plasma and whole blood, library preparation and quantification, hybridization-based targeted enrichment and sequence were carried out as previously described (5). Basically, circulating cell-free DNA (cfDNA) was extracted using the NucleoSpin Plasma XS kit (Macherey Nagel) following optimized manufacturer’s protocols. Fresh tissue DNA and whole blood DNA were extracted using the DNeasy Blood & Tissue kit (Qiagen) while DNA from FFPE samples was extracted using the QIAamp DNA FFPE Tissue Kit (Qiagen) according to the manufacturer’s protocols. Purified DNA was qualified by Nanodrop 2000 (Thermo Fisher Scientific) and quantified by Qubit 2.0 using the dsDNA HS Assay Kit (Life Technologies) according to the manufacturer’s recommendations. The size distribution of cfDNA was analyzed by the Agilent Technologies 2100 Bioanalyzer using the Agilent High Sensitivity DNA kit (Agilent Technologies) according to the manufacturer’s instructions. For cfDNA samples contaminated with genomic DNA, size selection was performed using Agencourt AMPure XP beads (Beckman Coulter) according to the manufacturer’s recommendations. Sequencing libraries were prepared using the KAPA Hyper Prep kit (KAPA Biosystems) with an optimized manufacturer’s protocol.
Results
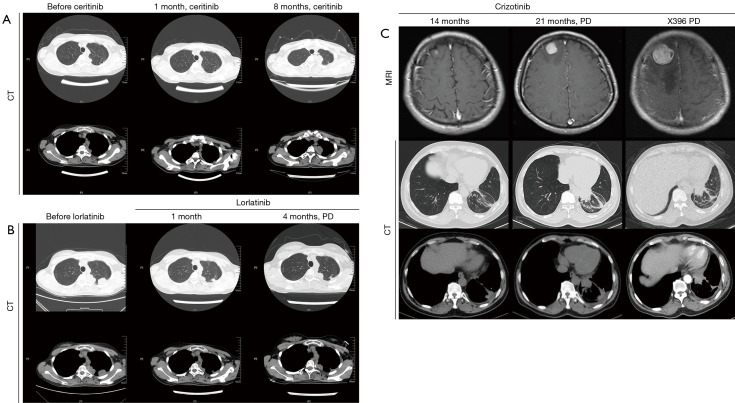
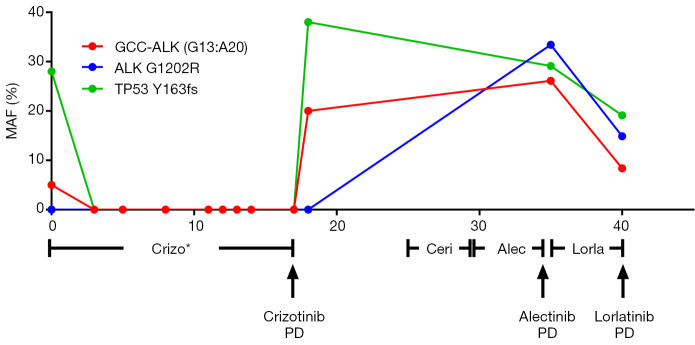
In our previous study, we described a 29-year-old female lung adenocarcinoma patient diagnosed as lung adenocarcinoma with rare GCC2-ALK fusion G13:A20 found in tumor sample using NGS (2). The patient remained on follow-up visits in First Affiliated Hospital of Soochow University after developing resistance to crizotinib. In April 2017, the patient underwent ceritinib treatment and showed response one month later with shrunken tumors. The patient suffered from sickness and severe vomit after four months of ceritinib treatment and switched to alectinib. The patient experienced no discomfort during alectinib treatment but computed tomography (CT) scans showed increased tumor size in left upper lung after three months. Percutaneous lung biopsy was done and tumor samples were sent for NGS detection. CT images from treatment course of patients were shown in Figure 1. Mutation allele frequencies (MAFs) of genes from NGS results were shown in Figure 2. NGS result showed the MAF of GCC2-ALK fusion increased to 26.1% and resistant ALK mutation G1202R was detected at the same time at a MAF of 33.4% (2). Combinational therapy with pemetrexed and carboplatin was used for 2 cycles and CT evaluation was SD. The patient was then administrated with lorlatinib. Chest CT showed decreased tumor size in left upper lung after one month of lorlatinib treatment. The patient achieved progressive disease (PD) four months later. The level of GCC2-ALK decreased to 8.4% and ALK mutation G1202R decreased to 14.9%. However, a new ALK mutation T1151M was developed at a MAF of 15.2%.
Figure 1.
Clinical monitoring of the non-small cell lung cancer patients during TKI treatment. CT images during treatment are shown at different time points to monitor metastatic tumor size in the brain area and chest of two patients with GCC2-ALK fusion. (A) Ceritinib and alectinib treatment; (B) lorlatininb treatment; (C) crizotinib and X396 treatment. TKI, tyrosine kinase inhibitor.
Figure 2.
Target NGS of the tumor tissues or the cfDNA samples from plasma to monitor the genomic changes of the tumor during the treatment course. Liquid biopsies were performed at different time point during TKI treatment after the patient acquired resistance to crizotinib. *, published data. NGS, next generation sequencing; TKI, tyrosine kinase inhibitor; PD, progressive disease.
Same GCC2-ALK fusion was found in a 59-year-old male patient who was referred to Cancer Hospital Chinese Academy of Medical Sciences with blood in phlegm lasting for a week in November 2015. The patient was diagnosed as stage IV lung adenocarcinoma accompanied by left adrenal metastasis. Then the patient received two cycles of pemetrexed plus cisplatin (PC) combined with endostar from December 2015 to January 2016 and achieved partial response (PR). The molecular pathological examination showed no mutations in EGFR exon 18, 19, 20, 21, KRAS exon 2 and amino acid 12 and 13. IHC showed that tumor cells were positive for ALK-Ventana D5F3. FISH test confirmed ALK positive. Due to the severe loss of appetite and constipation caused by previous chemotherapy, in February 2016, the patient switched to crizotinib and showed improvement.
The patient was then referred to the Zhejiang Cancer Hospital. Brain magnetic resonance imaging (MRI) examinations showed abnormal enhanced shadow (7.5 mm) in patient right frontal lobe which suggested brain metastasis combined with previous medical history. Chest CT evaluation was stable and the patient continued crizotinib treatment. Later, the patient experienced a general discomfort in November 2017, characterized by dizziness and tinnitus. Second brain MRI scan showed significantly enhanced shadow (20 mm) in right frontal lobe and chest CT showed occupancy in left inferior lob (37 mm × 29 mm). The patient showed no response to crizotinib at this point and reached a PFS of 21 months. Later, the patient was administrated with brigatinib and the treatment stopped one month later due to disease progression. X396 (ensartinib) was used for another month but the disease continued to progress. NGS showed GCC2-ALK fusion G13:A20 was detected at a MAF of 15.4% in FFPE sample. Then the patient went back to chemotherapy with PC for two cycles and PC combined with bevacizumab for another two cycles from February 2018 to April 2018. Evaluation was done every 2 cycles and the patient achieved PR. Gamma knife was used for single brain metastases during the third and fourth cycles of chemotherapy. Unfortunately, suspicious liver metastasis was revealed in May 2018 by CT scan and the patient received another two cycles of PC chemotherapy. In September 2018, the patient was administrated with ceritinib and showed bad response to the treatment. One month later, the patient switched to lorlatinib, however, the tumor continued to grow. Currently, the patient received palliative chemotherapy of docetaxel for one cycle and remained on follow up in our hospital.
Discussion
Crizotinib is the first-generation inhibitor targeting ALK. In the PROFILE-1014, PFS for crizotinib to treat ALK-positive NSCLC patients was 10.9 months and majority of the patients develop resistance to crizotinib within one year of the onset of the therapy (6). Here, we reported two patients with rare GCC2-ALK fusion both received crizotinib treatment with prolonged PFS. Crizotinib was used as first-line therapy in the first case and achieved a PFS of 18 months. In the second case, the patient received chemotherapy first and crizotinib later as front-line therapy and achieved a PFS of 21 months. These finding suggested that patients with GCC2-ALK fusion might be optimal candidates for crizotinib as front-line targeted therapy.
To against crizotinib resistance, second and third generation TKIs as subsequent targeted therapy have been applied to these two ALK-positive NSCLC patients but with unfavorable response and shorten PFS. In the first patient, the patient achieved a PFS of 4 months with alectinib which was shorter than the reported 9.6 months median PFS (7). Lorlatinib was administrated due to its activity against G1202R and finally reached a PFS of 4 months which was also shorter than PFS of 11.4 months reported by a phase I study on lorlatinib (8). Furthermore, the second patient showed adverse response to brigatinib, X396, ceritinib and lorlatinib with significant shorten PFS around one month after acquired resistance to crizotinib. While treated at the same dosage, the reported median PFS with brigatinib in a phase II trials of patients with crizotinib-refractory ALK-positive NSCLC were 12.9 months (9). X396, also named ensartinib, was reported with a median PFS of 9.0 months in ALK-positive NSCLC patients with prior crizotinib only (10).
In conclusion, NSCLC patients with rare GCC2-ALK fusion G13:A20 may be optimal candidates for crizotinib as front-line therapy. On the other hand, NSCLC patients with rare GCC2-ALK fusion may also have a high possibility to exhibit unsatisfactory response to second or third generation TKIs as subsequent target therapy with shorten PFS after acquiring resistance to crizotinib.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.08.26). YW Shao, X Wu, R Yu are the shareholders or employees of Geneseeq Technology Inc. K Liu is the employee of Nanjing Geneseeq Technology Inc. The other authors have no conflicts of interest to declare.
References
- 1.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. 10.1200/JCO.2009.22.6993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang J, Wu X, Tong X, et al. GCC2-ALK as a targetable fusion in lung adenocarcinoma and its enduring clinical responses to ALK inhibitors. Lung Cancer 2018;115:5-11. 10.1016/j.lungcan.2017.10.011 [DOI] [PubMed] [Google Scholar]
- 3.Noh KW, Lee MS, Lee SE, et al. Molecular breakdown: a comprehensive view of anaplastic lymphoma kinase (ALK)-rearranged non-small cell lung cancer. J Pathol 2017;243:307-19. 10.1002/path.4950 [DOI] [PubMed] [Google Scholar]
- 4.Vendrell JA, Taviaux S, Beganton B, et al. Detection of known and novel ALK fusion transcripts in lung cancer patients using next-generation sequencing approaches. Sci Rep 2017;7:12510. 10.1038/s41598-017-12679-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shu Y, Wu X, Tong X, et al. Circulating Tumor DNA Mutation Profiling by Targeted Next Generation Sequencing Provides Guidance for Personalized Treatments in Multiple Cancer Types. Sci Rep 2017;7:583. 10.1038/s41598-017-00520-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. 10.1056/NEJMoa1408440 [DOI] [PubMed] [Google Scholar]
- 7.Novello S, Mazieres J, Oh IJ, et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann Oncol 2018;29:1409-16. 10.1093/annonc/mdy121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorlatinib Is Active in Drug-Resistant NSCLC . Cancer Discov 2016;6:OF1. [DOI] [PubMed] [Google Scholar]
- 9.Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol 2017;35:2490-8. 10.1200/JCO.2016.71.5904 [DOI] [PubMed] [Google Scholar]
- 10.Horn L, Infante JR, Reckamp KL, et al. Ensartinib (X-396) in ALK-Positive Non-Small Cell Lung Cancer: Results from a First-in-Human Phase I/II, Multicenter Study. Clin Cancer Res 2018;24:2771-9. 10.1158/1078-0432.CCR-17-2398 [DOI] [PMC free article] [PubMed] [Google Scholar]