Abstract
COVID-19 increases the risk of atrial fibrillation (AF) and thrombotic complications, particularly in severe cases, leading to higher mortality rates. Anticoagulation is the cornerstone to reduce thromboembolic risk in patients with AF. Considering the risk of hepatotoxicity in patients with severe COVID-19 as well as the risk of drug–drug interactions, drug-induced hepatotoxicity and bleeding, the ANIBAL protocol was developed to facilitate the anticoagulation approach at discharge after COVID-19 hospitalization. However, since the publication of the original algorithm, relevant changes have occurred. First, treatment of COVID-19 pneumonia has been modified with the use of dexamethasone or remdesivir during the first week in patients that require oxygen therapy, and of dexamethasone and/or tocilizumab or baricitinib during the second week in patients that necessitate supplementary oxygen or with a high inflammation state, respectively. On the other hand, metabolic syndrome is common in patients with AF as well as metabolic-associated fatty liver disease, and this could negatively impact the prognosis of patients with COVID-19, including high transaminase levels in patients treated with immunomodulators. The EHRA guidelines update also introduce some interesting changes in drug–drug interaction patterns with the reduction of the level of the interaction with dexamethasone, which is of paramount importance in this clinical context. Considering the new information, the protocol, named ANIBAL II, has been updated. In this new protocol, the anticoagulant of choice in patients with AF after COVID-19 hospitalization is provided according to three scenarios: with/without dexamethasone treatment at discharge and normal hepatic function, transaminases ≤2 times the upper limit of normal, or transaminases >2 times the upper limit of normal.
Keywords: atrial fibrillation, COVID-19, dabigatran, direct oral anticoagulants, hepatotoxicity, MAFLD, metabolic syndrome
Introduction
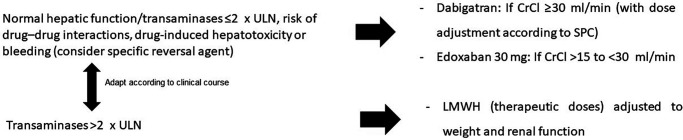
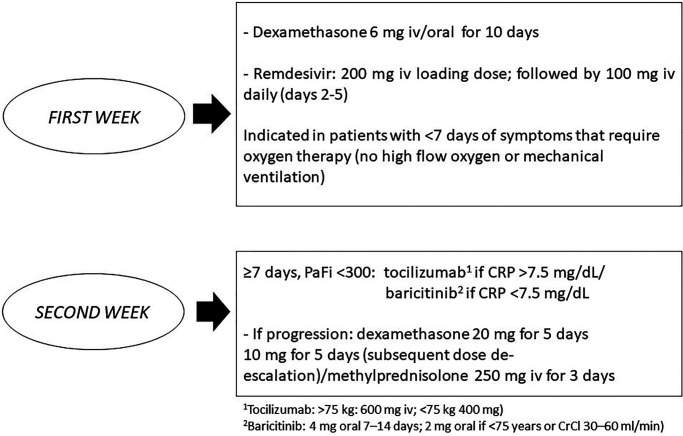
Atrial fibrillation (AF) is common in patients with COVID-19. Thus, a recent meta-analysis of 31 studies found that ~8% of patients with COVID-19 also have AF, and thus have a worse prognosis.1 The ANIBAL (Anticoagulación al alta tras una Neumonía Inducida por Covid-19 y Basada en las Alteraciones Laboratoriales de la función hepática y metabólica – Anticoagulation at COVID-19 pneumonia discharge based on hepatic and metabolic laboratory alterations) protocol recently published (Figure 1) considered dabigatran as the oral anticoagulant of choice in this clinical setting due to the lower risk of hepatotoxicity, drug–drug interactions and the availability of a specific reversal agent.2 However, as the therapeutic approach of COVID-19 pneumonia has substantially changed during this period, the ANIBAL protocol was updated. Whereas certain drugs used during the first months of the COVID-19 pandemic, such as hydroxychloroquine, azithromycin or lopinavir/ritonavir, have become obsolete, current hospital protocols include remdesivir as the antiviral therapeutic of choice during the first week of COVD-19 pneumonia.3,4 In addition, corticosteroids, particularly dexamethasone, have also been considered as the first-line therapy in patients hospitalized with COVID-19 pneumonia,5,6 with a modulation of the risk of drug–drug interactions according to the European Heart Rhythm Association (EHRA) update regarding the use of direct oral anticoagulants (DOACs) in patients with AF.7 The current protocol in our hospital (University Hospital Santa Lucía, Cartagena, Spain) for the treatment of COVID-19 pneumonia (Figure 2) is based on current recommendations according to available evidence.3–6 In addition, the anticoagulation protocol during hospitalization and at discharge according to different clinical circumstances is also presented (Figure 2). During the first week, remdesivir and dexamethasone are indicated in symptomatic patients that require oxygen therapy to improve prognosis. During the second week from the onset of symptoms of COVID-19 pneumonia, tocilizumab or baricitinib, according to C-reactive protein levels, are administered together with dexamethasone in case of disease progression, even at discharge. In this line, the EHRA update has reduced the impact of the interaction between dexamethasone and dabigatran or edoxaban,7 facilitating their use at the end of the hospitalization without the need for a heparin bridge.
Figure 1.
Algorithm of management of patients with atrial fibrillation at discharge after COVID-19 pneumonia. The ANIBAL I protocol.
CrCl, creatinine clearance; LMWH, low-molecular-weight heparin; ULN, upper limit of normal. Adapted from ref.2
Figure 2.
Therapeutic approach of patients hospitalized with COVID-19 pneumonia at the University Hospital Santa Lucía, Cartagena, Spain.
CrCl, creatinine clearance; CRP, C-reactive protein; PaFi, inspired oxygen fraction. Reproduced from ref.2 with permission.
Metabolic syndrome is a cluster of cardiometabolic risk factors, including abdominal obesity, hypertension, hyperglycaemia, hypertriglyceridaemia and low serum HDL cholesterol, in which insulin resistance, as well as prothrombotic and pro-inflammatory states, play a key role in the aetiopathogenesis.8,9 Metabolic syndrome is a very common condition. In fact, approximately one out of four adult individuals in the United States exhibit this condition, and this proportion is expected to increase in the following years due to the increasing elderly population, sedentarism and unbalanced dietary patterns.10 Metabolic syndrome is associated with a marked risk of developing cardiovascular disease and type 2 diabetes.11 Similarly, metabolic syndrome also increases the risk of AF up to three-fold, being more frequent as the components of metabolic syndrome increase, particularly in young and middle-age patients.12,13 In addition, metabolic syndrome worsens AF burden and outcomes, thus increasing the risk of AF recurrence after single-catheter ablation.14
Fatty liver associated with metabolic dysfunction, termed metabolic-associated fatty liver disease (MAFLD), is very common in clinical practice, affecting approximately one-quarter of the population worldwide. Remarkably, COVID-19 can negatively affect liver function, particularly in patients with pre-existing liver diseases, including MAFLD. Conversely, MAFLD patients have a higher risk of progression to severe COVID-19. Thus, amongst patients with COVID-19 pneumonia, patients with MAFLD could have a higher risk of disease progression, higher likelihood of abnormal liver function during hospitalization and a longer viral shedding time compared to patients without MAFLD. With regard to the pathogenesis, both conditions share inflammatory pathways and the presence of fibrosis in MAFLD patients independently increases the severity of COVID-19. The pattern of liver injury is mostly hepatocellular rather than cholestatic.15–21
Non-alcoholic fatty liver disease has been associated with arterial hypertension, arterial stiffness, aortic valvular sclerosis, atherosclerosis, coronary artery disease and AF. In fact, non-obese, non-alcoholic fatty liver disease induces early cardiac electrical and structural changes that could promote the development of AF.22,23 As a result, the presence of MAFLD in patients with AF may be particularly problematic in individuals with COVID-19. On the other hand, immunomodulators, such as tocilizumab and baricitinib, may increase transaminase levels that could aggravate the previous liver state of these patients.24,25
Considering the current therapeutic approach of COVID-19 pneumonia and the frequent concomitance of fatty liver disease in patients with metabolic syndrome and AF, we have updated the ANIBAL protocol (ANIBAL II protocol) with the aim to facilitate the anticoagulation approach at discharge amongst patients with AF after hospitalization for COVID-19 pneumonia.
Update of the criteria to be considered for anticoagulation in patients with AF at discharge after COVID-19 pneumonia
To perform the first ANIBAL protocol,2 the following statements were considered: the ‘biological’ safety of DOACs over vitamin K antagonists (VKA);26 the potential of COVID-19 to promote liver injury, including the antiviral and immunomodulatory therapy;27–31 renal function at discharge;7 the high thrombogenicity of the COVID-19;32,33 the risk of new-onset AF amongst patients with COVID-19;34–36 and the use of DOACs with a specific reversal agent.37 Although the majority of variables analysed for the ANIBAL I protocol2 continue to be topical, new elements have been updated to be included in the new protocol (Box 1).
Box 1: Considerations for anticoagulation of patients with atrial fibrillation at discharge after COVID-19 pneumonia.
‘Biological’ safety of DOAC versus vitamin K antagonist as no anticoagulation control is required.
Hepatotoxicity of COVID-19 and antiviral therapy in short-term and long-term evolution as well as the presence of possible hepatic steatosis associated with metabolic syndrome.
Treatment with dexamethasone at discharge may reduce plasmatic levels of DOAC when they are used concomitantly.
Renal function at discharge (eGFR according to Cockroft–Gault).
Thrombogenicity of COVID-19: choose the most potent DOAC, preferably a twice daily dose.
Arrhythmogenicity of COVID-19: risk of new-onset atrial fibrillation that requires anticoagulation (choose DOAC to facilitate health education and adherence).
DOAC with specific reversal agent to reduce the impact of the underuse of the health system during the COVID-19 pandemic.
‘Biological’ safety of DOACs (versus VKA)
The introduction of DOACs in Spain has been restricted due to economic politics as reimbursement has been limited to some specific indications. This has led to a lower proportion of patients taking DOACs compared with other European countries.38 However, as patients taking VKA require periodic monitoring of anticoagulant activity (i.e. every 2–6 weeks, according to INR control) and this increases SARS-CoV-2 exposure, the Spanish Sanitary Authorities have reduced the restrictions of switching from VKA to DOACs, leading to a higher prescription of DOACs and, secondarily, to a reduction of outcomes. Of note, a recent study reported that, in Spain, the progressive higher prescription of DOACs has been associated with a decrease in the incidence of AF-related ischaemic stroke.39 It is not only important to reduce the risk of contagion for patients but also for healthcare professionals as they are an important risk group given their high exposure level. In fact, the main concern of healthcare professionals is contagion, particularly in the workplace.40 The use of DOACs instead of VKA thus reduces exposure to SARS-CoV-2 infection for both patients and professionals.
COVID-19-related hepatotoxicity, antiviral drugs and immunomodulatory therapy, previous hepatic disease such as MAFLD
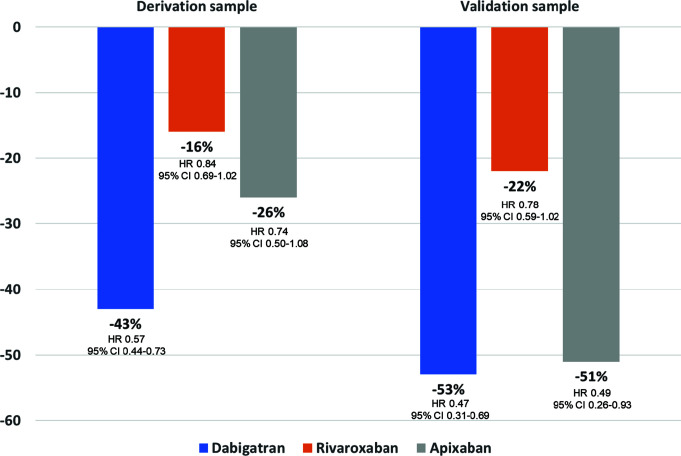
Although uncommon, drug-induced liver injury associated with the use of tocilizumab, remdesivir or baricitinib has been described.24,25,28,29 In this context, the use of those drugs with a lower risk of drug–drug interactions may be preferable. Thus, no clinically significant interaction is expected between dabigatran and tocilizumab, baricitinib, or remdesivir (Table 1).41 With regard to oral anticoagulants in patients with AF and liver injury, a study showed that after 1-year follow-up, hepatic injury hospitalization rates were lower in initiators of DOACs versus in those taking warfarin (HR 0.57, 95% CI 0.46–0.71) and, of available DOACs, dabigatran had the lowest risk compared with warfarin (Figure 3).31 However, not all studies have shown differences regarding the risk of acute liver injury and DOACs compared with VKA in patients with AF.42
Table 1.
Potential drug–drug interactions of anticoagulants with current COVID-19 therapies.
| Treatment for COVID-19 | Anticoagulant | Drug–drug interaction | Recommendation |
|---|---|---|---|
| Dexamethasone | VKA |
|
|
| Apixaban |
|
|
|
| Dabigatran |
|
||
| Edoxaban |
|
||
| Rivaroxaban |
|
||
| Enoxaparin |
|
|
|
| Tocilizumab | VKA |
|
|
| Apixaban |
|
|
|
| Rivaroxaban |
|
||
| Dabigatran Edoxaban Enoxaparin |
|
|
|
| Baricitinib | VKA Apixaban Dabigatran Edoxaban Rivaroxaban Enoxaparin |
|
|
| Remdesivir | VKA Apixaban Dabigatran Edoxaban Rivaroxaban Enoxaparin |
|
|
INR, International Normalized Ratio; LMWH, low-molecular-weight heparin; P-gp, P-glycoprotein; VKA, vitamin K antagonists.
Adapted from ref.41
Figure 3.
Risk of liver injury hospitalization by type of direct oral anticoagulant (versus warfarin).
CI, confidence interval; HR, hazard ratio.
Adapted from ref.31
On the other hand, we consider that the systematic search of MAFLD in patients with metabolic syndrome with imaging techniques, particularly echography, and if positive, to rule out the presence of hepatic fibrosis with indirect methods (i.e. FibroScan)43 may not be useful in routine practice. A better approach seems to be to consider that patients with AF and metabolic syndrome may have or at least are at high risk of MAFLD. In this context, it would be preferable to prescribe a first-line oral anticoagulant, such as dabigatran, that is not metabolized by CYP 450 and is less harmful to the liver.31,41
Use of DOACs with a low risk of dexamethasone interaction
Dexamethasone is a moderate CYP 3A4 inducer and an inducer of P-glycoprotein and may potentially have a clinically significant interaction with DOACs, leading to a reduction of serum concentrations of DOACs and, consequently, to a reduction of their efficacy.41 This is very relevant considering the high thrombogenicity of COVID-19.32,33 The EHRA update states the low risk of drug–drug interactions with those DOACs that are not preferentially metabolized by the CYP 450 pathway. In this context, the widespread use of dexamethasone in patients with COVID-19, either initially or later in case of progression, including at discharge, promotes the use of dabigatran or edoxaban due to the low risk of drug–drug interactions.41,44,45
Remarkably, this change allows the use of dabigatran or edoxaban immediately at discharge, without requiring low-molecular-weight heparin bridge therapy. This is the most important novelty compared to the first ANIBAL protocol.2
Renal function at discharge (according to Cockcroft–Gault formula)
First, it should be emphasized that the estimation of glomerular filtration rate should be performed by means of the Cockcroft–Gault formula as this was the method used in the pivotal clinical trials with DOACs (versus warfarin) to determine renal function, and approval by the regulatory agencies was performed considering this method.46–49
In addition, it has been reported that, in elderly patients, the use of other methods, such as the CKD-EPI equation instead of the Cockcroft–Gault formula, to calculate renal function could overestimate the glomerular filtration rate, leading to mistakes in the dosage of DOACs and a higher risk of side effects.50
Finally, it has been shown that the use of some DOACs, such as dabigatran and rivaroxaban but not of apixaban, could be associated with a lower risk of renal adverse events compared to warfarin.51 However, it should be also considered that, amongst patients with chronic kidney disease, the risk of stroke or bleeding could differ according to the DOAC used.52
Thrombogenicity and COVID-19
Patients with COVID-19 have a higher risk of pulmonary embolism and deep vein thrombosis.53,54 Although treatment with low-molecular-weight heparin reduced the risk of pulmonary embolism or venous thrombosis during COVID-19 hospitalization, the dose to be used remains a subject of debate, particularly in critically ill patients, as well as the identification of risk factors that may recognize those patients that would benefit more from high-dose low-molecular-weight heparin.55 Similarly, it remains unclear which is the best ambulatory thromboprophylaxis approach after discharge.56,57
The European Society of Cardiology recommends that, in patients with AF during COVID-19 pandemic, unless contraindicated, full therapeutic anticoagulation for the prevention of stroke should be administered to men and women with a CHA2DS2-VASc of ≥2/3, and anticoagulation should be considered in case of CHA2DS2-VASc 1/2.33 As a result, it is not clear whether anticoagulation should also be recommended during COVID-19 due to the high thrombogenicity of this period and whether this should be maintained over time after COVID-19 recovery.
Risk of arrhythmias and COVID-19
COVID-19 affects both the respiratory system and the cardiovascular system. Patients with a prior cardiovascular condition have a higher risk of mortality and severe COVID-19. In addition, cardiovascular complications of COVID-19 include arrhythmias, particularly AF, cardiac injury, myocarditis, heart failure, arterial/venous thrombosis and acute coronary syndrome.58
A recent study that analysed 160 consecutive patients hospitalized due to COVID-19 showed that patients with new-onset AF had a higher incidence of thromboembolic events, bleeding, a combined endpoint of thrombosis and death, and longer hospital stays, indicating that an active search of AF should be performed in patients hospitalized with COVID-19 and the need of anticoagulation in case of development of AF.59
DOACs and specific reversal agents
During the COVID-19 pandemic, particularly during the lockdown period, there has been a reduction of hospital admissions for cardiovascular disease, including acute coronary syndrome, heart failure or AF, leading to an increase in adverse outcomes.60,61 This could be related, at least in part, to the lack of seeking for medical attention, despite symptoms, due to concerns about COVID-19 contagion. Thus, a study that analysed data from nationwide Danish registries showed a lower incidence of new-onset AF during the 3 weeks of lockdown compared with the previous year, with a 47% drop in total numbers, but with a higher risk of ischaemic stroke and death amongst patients with new-onset AF.61 As a result, during the COVID-19 pandemic, there has been an underdiagnosis of AF, increasing the risk of severe complications.
In order to reduce the risk of complications during the COVID-19 pandemic, and in some way to counteract the harmful effects of the underuse of health systems, the use of DOACs, such as dabigatran, which have a specific agent that rapidly reverses the effects of the drug in case of life-threatening bleeding, should be considered as first choice.37
Update of the protocol
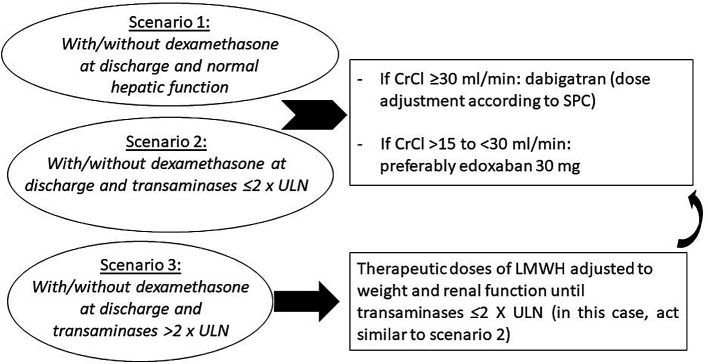
With all this new information, we have proposed an updated protocol, the ANIBAL II algorithm for the management of patients with AF at discharge after COVID-19 pneumonia (Figure 4). This algorithm has three scenarios: Scenario 1: with or without dexamethasone and normal hepatic function; Scenario 1: with or without dexamethasone and transaminases ≤2 × upper limit of normal (ULN); Scenario 1: with or without dexamethasone and transaminases >2 × ULN. In those patients with transaminases >2 × ULN, therapeutic doses of low-molecular-weight heparin are recommended as is switching to oral anticoagulation in case of transaminases ≤2 × ULN. In all other cases, dabigatran is recommended if the estimated glomerular filtration rate is ≥30 mL/min or edoxaban 30 mg is recommended if the estimated glomerular filtration rate >15 to <30 mL/min.
Figure 4.
Algorithm of management of patients with atrial fibrillation at discharge after COVID-19 pneumonia. The ANIBAL II protocol.
CrCl, creatinine clearance; LMWH, low-molecular-weight heparin; SPC, summary of product characteristics; ULN, upper limit of normality
Conclusions
The application of the ANIBAL I protocol has had a positive impact in routine practice as it has allowed a holistic approach in the management of anticoagulation in patients with AF after COVID-19 hospitalization, considering different important variables with relevant impact on the clinical course of patients such as hepatic function and the risk of drug–drug interactions, drug-induced hepatotoxicity, or bleeding, as well as considering the availability of a specific reversal agent. The impact of the ANIBAL I protocol is currently being analysed in a Spanish multicentre study. However, as the management of COVID-19 pneumonia has been revised in the last months (i.e. using remdesivir, tocilizumab, baricitinib and dexamethasone) and as greater knowledge about the impact that COVID-19 may have on patients with AF and MAFLD has been obtained, the protocol has been updated as delineated herein.
Acknowledgements
Content Ed provided writing and editorial support, which was funded by the Fundación para la Formación e Investigación Sanitarias de la Región de Murcia, though an unrestricted grant from Boehringer Ingelheim Spain. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as it relates to Boehringer Ingelheim substances, as well as intellectual property considerations.
Footnotes
The name of this great Carthaginian military general has been chosen for the protocol as a tribute by the authors, as they carry out their healthcare work in Cartagena
Contributions: All authors contributed extensively to the work presented in this paper. All authors have contributed significantly to the conception, design, or acquisition of data, or analysis and interpretation of data. All authors have participated in drafting, reviewing, and/or revising the manuscript and have approved its submission. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Correct attribution: Copyright © 2022 Cerezo-Manchado JJ, Meca Birlanga O, García de Guadiana Romualdo L, Gil-Ortega I, Martínez Francés A, Iturbe-Hernandez T. https://doi.org/10.7573/dic.2021-9-4. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Submitted; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
DOAC, direct oral anticoagulant; eGFR, estimated glomerular filtration rate. Reproduced from ref.2 with permission.
Disclosure and potential conflicts of interest: JJCM reports non-financial support from Boehringer Ingelheim during the conduct of the study and personal fees from Boehringer Ingelheim, Pfizer, Bayer and Daichii-Sankyo outside the submitted work. The rest of the authors have nothing to disclose. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2021/12/dic.2021-9-4-COI.pdf
Funding declaration: Funded by the Fundación para la Formación e Investigación Sanitarias de la Región de Murcia, though an unrestricted grant from Boehringer Ingelheim Spain.
References
- 1.Romiti GF, Corica B, Lip GYH, Proietti M. Prevalence and impact of atrial fibrillation in hospitalized patients with COVID-19: a systematic review and meta-analysis. J Clin Med. 2021;10:2490. doi: 10.3390/jcm10112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iturbe-Hernández T, García de Guadiana RL, Gil Ortega I, et al. Dabigatran, the oral anticoagulant of choice at discharge in patients with non-valvular atrial fibrillation and COVID19 infection: the ANIBAL protocol. Drugs Context. 2020;9:1–12. doi: 10.7573/dic.2020-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gavriatopoulou M, Ntanasis-Stathopoulos I, Korompoki E, et al. Emerging treatment strategies for COVID-19 infection. Clin Exp Med. 2021;21:167–179. doi: 10.1007/s10238-020-00671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rehman SU, Rehman SU, Yoo HH. COVID-19 challenges and its therapeutics. Biomed Pharmacother. 2021;142:112015. doi: 10.1016/j.biopha.2021.112015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee HW, Park J, Lee JK, Park TY, Heo EY. The effect of the timing of dexamethasone administration in patients with COVID-19 pneumonia. Tuberc Respir Dis. 2021;84:217–225. doi: 10.4046/trd.2021.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinzón MA, Ortiz S, Holguín H, et al. Dexamethasone vs methylprednisolone high dose for Covid-19 pneumonia. PLoS ONE. 2021;16:e0252057. doi: 10.1371/journal.pone.0252057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steffel J, Collins R, Antz M, et al. 2021 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace. 2021;23(10):1612–1676. doi: 10.1093/europace/euab065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bovolini A, Garcia J, Andrade MA, Duarte JA. Metabolic syndrome pathophysiology and predisposing factors. Int J Sports Med. 2021;42:199–214. doi: 10.1055/a-1263-0898. [DOI] [PubMed] [Google Scholar]
- 9.Grundy SM, Brewer HB, Cleeman JI, et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues related to Definiton. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 10.Beltrán-Sánchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J Am Coll Cardiol. 2013;62:697–703. doi: 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He D, Zhang X, Chen S, et al. Dynamic changes of metabolic syndrome alter the risks of cardiovascular diseases and all-cause mortality: evidence from a prospective cohort study. Front Cardiovasc Med. 2021;8:706999. doi: 10.3389/fcvm.2021.706999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahn HJ, Han KD, Choi EK, et al. Cumulative burden of metabolic syndrome and its components on the risk of atrial fibrillation: a nationwide population-based study. Cardiovasc Diabetol. 2021;20:20. doi: 10.1186/s12933-021-01215-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SY, Lee SR, Choi EK, et al. Association between change in metabolic syndrome status and risk of incident atrial fibrillation: a nationwide population-based study. J Am Heart Assoc. 2021;10:e020901. doi: 10.1161/JAHA.121.020901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohanty S, Mohanty P, Di Biase L, et al. Impact of metabolic syndrome on procedural outcomes in patients with atrial fibrillation undergoing catheter ablation. J Am Coll Cardiol. 2021;59:1295–1301. doi: 10.1016/j.jacc.2011.11.051. [DOI] [PubMed] [Google Scholar]
- 15.Eslam M, Sanyal AJ, George J International Consensus Panel. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 16.Dongiovanni P, Meroni M, Longo M, Fracanzani AL. MAFLD in COVID-19 patients: an insidious enemy. Expert Rev Gastroenterol Hepatol. 2020;14:867–872. doi: 10.1080/17474124.2020.1801417. [DOI] [PubMed] [Google Scholar]
- 17.Portincasa P, Krawczyk M, Smyk W, Lammert F, Di Ciaula A. COVID-19 and non-alcoholic fatty liver disease: two intersecting pandemics. Eur J Clin Invest. 2020;50:e13338. doi: 10.1111/eci.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji D, QWin E, Xu J, et al. Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol. 2020;73:451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Targher G, Mantovani A, Byrne CD, et al. Detrimental effects of metabolic dysfunction-associated fatty liver disease and increased neutrophil.-to-lymphocyte ratio on severity of COVID-19. Diabetes Metab. 2020;46(6):505–507. doi: 10.1016/j.diabet.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Targher G, Mantovani A, Byrne CD, et al. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545–1547. doi: 10.1136/gutjnl-2020-321611. [DOI] [PubMed] [Google Scholar]
- 21.Forlano R, Mullish BH, Mukherjee SK, et al. In-hospital mortality is associated with inflammatory reponse in MAFLD patients admitted for COVID-19. PLoS ONE. 2020;15:e0240400. doi: 10.1371/journal.pone.0240400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polyzos SA, Kechagias S, Tsochatzis EA. Review article: non-alcoholic fatty liver disease and cardiovascular diseases: associations and treatment considerations. Aliment Pharmacol Ther. 2021;54(8):1013–1025. doi: 10.1111/apt.16575. [DOI] [PubMed] [Google Scholar]
- 23.Aksu E, Sokmen A, Ispiroglu M, Gisi K, Celik E, Aykan AC. Early cardiac electrical and structural changes in patients with non-obese non-alcoholic fatty liver disease. Kardiologiia. 2021;61:51–58. doi: 10.18087/cardio.2021.5.n1416. [DOI] [PubMed] [Google Scholar]
- 24.Serviddio G, Villani R, Stallone G, Scioscia G, Foschino-Barbaro MP, Lacedonia D. Tocilizumab and liver injury in patients with COVID-19. Therap Adv Gastroenterol. 2020;13:1756284820959183. doi: 10.1177/1756284820959183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raschi E, Caraceni P, Poluzzi E, De Ponti F. Baricitinib, JAK inhibitors and liver injury: a cause for concern in COVID-19? Expert Opin Drug Saf. 2020;19:1367–1369. doi: 10.1080/14740338.2020.1812191. [DOI] [PubMed] [Google Scholar]
- 26.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Xiao SY. Hepatic involvement in COVID-19 patients: pathology, pathogenesis, and clinical implications. J Med Virol. 2020;92:1491–1494. doi: 10.1002/jmv.25973. [DOI] [PubMed] [Google Scholar]
- 28.Leegwater E, Strik A, Wilms EB, et al. Drug-induced liver injury in a COVID-19 patient: potential interaction of remdesivir with P-glycoprotein inhibitors. Clin Infect Dis. 2021;72:1256–1258. doi: 10.1093/cid/ciaa883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muhović D, Bojović J, Bulatović A, et al. First case of drug-induced liver injury associated with the use of tocilizumab in a patient with COVID-19. Liver Int. 2020;40:1901–1905. doi: 10.1111/liv.14516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lei F, Liu YM, Zhou F, et al. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology. 2020;72:389–398. doi: 10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alonso A, MacLehose RF, Chen LY, et al. Prospective study of oral anticoagulants and risk of liver injury in patients with atrial fibrillation. Heart. 2017;103:834–839. doi: 10.1136/heartjnl-2016-310586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Ani F, Chehade S, Lazo-Langner A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb Res. 2020;192:152–160. doi: 10.1016/j.thromres.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ESC guidance for the diagnosis and management of CVD during the COVID-19 pandemic – Updated 2021. https://www.escardio.org/Education/COVID-19-and-Cardiology .
- 34.Bhatla A, Mayer MM, Adusumalli S, et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17:1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inciardi RM, Adamo M, Lupi L, et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41:1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russo V, Rago A, Carbone A, et al. Atrial fibrillation in COVID-19: from epidemiological association to pharmacological implications. J Cardiovasc Pharmacol. 2020;76:138–145. doi: 10.1097/FJC.0000000000000854. [DOI] [PubMed] [Google Scholar]
- 37.Arellano-Rodrigo E, Fernandez-Gallego V, López-Vilchez I, et al. Idarucizumab, but not procoagulant concentrates, fully restores dabigatran-altered platelet and fibrin components of hemostasis. Transfusion. 2019;59:2436–2445. doi: 10.1111/trf.15259. [DOI] [PubMed] [Google Scholar]
- 38.Llisterri Caro JL, Cinza-Sanjurjo S, Polo Garcia J, Prieto Díaz MA. Use of direct-acting oral anticoagulants in Primary Care in Spain. Positioning statement by SEMERGEN on the current situation. Semergen. 2019;45:413–429. doi: 10.1016/j.semerg.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Díaz-Guzmán J, Freixa-Pamias R, García-Alegría J, et al. Epidemiology of atrial fibrillation-related ischemic stroke and its association with DOAC uptake in Spain: first national population-based study 2005 to 2018. Rev Esp Cardiol. 2021 doi: 10.1016/j.rec.2021.07.009. S1885-5857(21)00225-5. [DOI] [PubMed] [Google Scholar]
- 40.Rivera-Izquierdo M, Valero-Ubierna MDC, Martínez-Diz S, et al. Clinical Factors, Preventive Behaviours and Temporal Outcomes Associated with COVID-19 Infection in Health Professionals at a Spanish Hospital. Int J Environ Res Public Health. 2020;17:4305. doi: 10.3390/ijerph17124305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Interaction report. Liverpool Drug Interactions Group. University of Liverpool; [Accessed August 28, 2021]. www.covid19-druginteractions.org . [Google Scholar]
- 42.Maura G, Bardou M, Billionnet C, Weill A, Drouin J, Neumann A. Oral anticoagulants and risk of acute liver injury in patients with nonvalvular atrial fibrillation: a propensity-weighted nationwide cohort study. Sci Rep. 2020;10(1):11624. doi: 10.1038/s41598-020-68304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oeda S, Tanaka K, Oshima A, Matsumoto Y, Sueoka E, Takahashi H. Diagnostic accuracy of FibroScan and factors affecting measurements. Diagnostics. 2020;10:940. doi: 10.3390/diagnostics10110940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Testa S, Paoletti O, Giorgi-Pierfranceschi M, et al. Switch from oral anticoagulant to parenteral heparin in SARS-CoV-2 hospitalized patients. Intern Emerg Med. 2020;15:751–753. doi: 10.1007/s11739-020-02331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schutgens RE. DOAC in COVID-19: yes or no? Hemasphere. 2020;5:e526. doi: 10.1097/HS9.0000000000000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 47.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 48.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus Warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 49.Giugliano RP, Ruff CT, Braunwald E, et al. ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 50.Simpson BH, Reith DM, Medlicott NJ, et al. Choice of renal function estimator influences adverse outcomes with dabigatran etexilate in patients with atrial fibrillation. TH Open. 2018;2:e420–e427. doi: 10.1055/s-0038-1676356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao X, Tangri Y, Gersh BJ, et al. Renal outcomes in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2017;70:2621–2632. doi: 10.1016/j.jacc.2017.09.1087. [DOI] [PubMed] [Google Scholar]
- 52.Feldberg J, Patel P, Farrell A, et al. A systematic review of direct oral anticoagulant use in chronic kidney disease and dialysis patients with atrial fibrillation. Nephrol Dial Transplant. 2019;34:265–277. doi: 10.1093/ndt/gfy031. [DOI] [PubMed] [Google Scholar]
- 53.Bompard F, Monnier H, Saab I, et al. Pulmonary embolism in patients with COVID-19 pneumonia. Eur Respir J. 2020;56:2001365. doi: 10.1183/13993003.01365-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suh YJ, Hong H, Ohana M, et al. Pulmonary embolism and deep vein thrombosis in COVID-19: a systematic review and meta-analysis. Radiology. 298(2):E70–E80. doi: 10.1148/radiol.2020203557. 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sussen S, Tacquard CA, Godon A, et al. Prevention of thrombotic risk in hospitalized patients with COVID-19 and hemostasis monitoring. Critical Care. 2020;24:364. doi: 10.1186/s13054-020-03000-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chowdhury JF, Moores LK, Connors JM. Anticoagulation in hospitalized patients with COVID-19. N Engl J Med. 2020;383:1675–1678. doi: 10.1056/NEJMclde2028217. [DOI] [PubMed] [Google Scholar]
- 57.Vivas D, Roldán V, Esteve-Pastor MA, et al. Recommendations on antithrombotic treatment during the COVID-19 pandemic. Position statement of the Working Group on Cardiovascular Thrombosis of the Spanish Society of Cardiology. Rev Esp Cardiol. 2020;73:749–757. doi: 10.1016/j.recesp.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116:1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pardo Sanz A, Salido Tahoces L, Ortega Pérez R, et al. New-onset atrial fibrillation during COVID-19 infection predicts poor prognosis. Cardiol J. 2021;28:34–40. doi: 10.5603/CJ.a2020.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Filippo O, D’Ascenzo F, Angelini F, et al. Reduced rate of Hospital Admissions for ACS during Covid-19 outbreak in Northern Italy. N Engl J Med. 2020;383:88–89. doi: 10.1056/NEJMc2009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holt A, Gislason GH, Schou M, et al. New-onset atrial fibrillation: incidence, characteristics, and related events following a national COVID-19 lockdown of 5.6 million people. Eur Heart J. 2020;41:3072–3079. doi: 10.1093/eurheartj/ehaa494. [DOI] [PMC free article] [PubMed] [Google Scholar]