Abstract
Background
Our aim is to report the incidence and risk factors of parastomal hernia (PH) after radical cystectomy (RC) and ileal conduit (IC) diversion with a cumulative analysis.
Methods
Various databases, including PubMed, the Cochrane Library, Embase and Web of Science, were retrieved electronically and manually to identify eligible studies from inception to August 20, 2020. Two reviewers independently searched the above databases and selected the studies using prespecified standardized criteria. The Newcastle-Ottawa Scale (NOS) was used to assess the risk of bias in the included studies, and the data was completed by STATA version 14.2.
Results
Fifteen studies were included in the final analysis. A pooled analysis of eight studies representing 1,878 patients reported the incidence of overall radiographic PH was 23% (95% CI: 17–29%). The 1-year PH incidence rate and 2-year incidence rate of RC and IC were 14% (95% CI: 6–22%) and 26% (95% CI: 14–38%), respectively. A pooled analysis of nine studies reported the incidence of clinically evident PH was 15% (95% CI: 10–19%). PH-related symptoms were reported in six studies, and the pooled result was 29% (95% CI: 24–33%), and a pooled analysis of ten studies showed that 20% (95% CI: 11–28%) of patients required surgical repair. However, it’s noteworthy that among symptomatic PH patients undergoing surgical repair, the pooled analysis of five studies showed that up to 26% (95% CI: 16–36%) of patients suffered PH recurrence. The most frequent risk factor was body mass index (BMI). Patients with BMI ≥22.9 kg/m2 experienced 2.92-fold higher risk of PH than their counterparts [hazard ratio (HR): 2.92; 95% CI: 1.65–5.19].
Conclusions
Our findings indicated that the PH incidence rate after RC and IC was significantly higher in radiographic evaluation than that of clinical examination, and the recurrence of repairment is considerable for patients requiring reconstruction.
Keywords: Parastomal hernia (PH), ileal conduit diversion (IC diversion), radical cystectomy (RC), bladder cancer
Introduction
Bladder cancer is one of the most pernicious urologic caners globally, with an estimated 549,000 new cases and 200,000 death each year (1). It is universally acknowledged that BC not only poses a great threat on patients’ existence and quality of life, but also increases drastically the economic burden of the national health care system (2). Radical cystectomy (RC) and ileal conduit (IC) diversion has currently been regarded as the mainstream treatment of muscle-invasive and high-risk non-muscle-invasive bladder cancer (3). However, this approach is usually associated with noteworthy complications and higher mortality concerning the complexity of the operation involving urinary and intestinal systems (4).
It is conceivable that the urinary diversion (UD) contributes to great risk of postoperative morbidity and complications other than cystectomy itself (4,5). Among the long-term complications reported, parastomal hernia (PH) is a frequent sequela resulting in cosmetic and functional concerns, and surgical repair is required in some cases, such as intestinal incarceration and strangulation (6,7). Concerning the incongruous incidence and risk factors, and deficiency of effective treatments, we determine to report the finding of this review with a cumulative analysis of incidence and risk factors of PH in patients undergoing RC and IC, and explore the potentially effective treatments for this medical problem. We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-3349).
Methods
Study selection
In accordance with the Preferred Reporting Items for Systematic Review and Meta-analyses guidelines (8), various databases including PubMed, the Cochrane Library, Embase and Web of Science were systematically searched through electronic retrieval from inception to August 20, 2020 without language limitations. The Medical Subject Heading (MeSH) terms related to this article included “Hernia” and “Cystectomy”, and the search strategy used in PubMed was as follows: ((Cystectomy[Title/Abstract]) OR (Cystectomies[Title/Abstract])) AND (((Hernia[Title/Abstract]) OR (Hernias[Title/Abstract])) OR (Enterocele[Title/Abstract])). The specific search strategy is showed in Appendix 1. We also artificially retrieved the reference lists of relevant reviews and articles to expand the search. Two independent authors identified the potential studies for full-text evaluation based on screening titles and abstracts. Subsequently, articles that met the inclusion criteria were included in the final analysis. Data were extracted by two independent authors, and any disagreement was resolved by consensus or a third party. By formulating a table previously, we extracted the following data: (I) the first author name and publication year; (II) details of the study design; (III) the characteristics of the included patients, and (IV) data associated with outcomes of interest.
Selection criteria
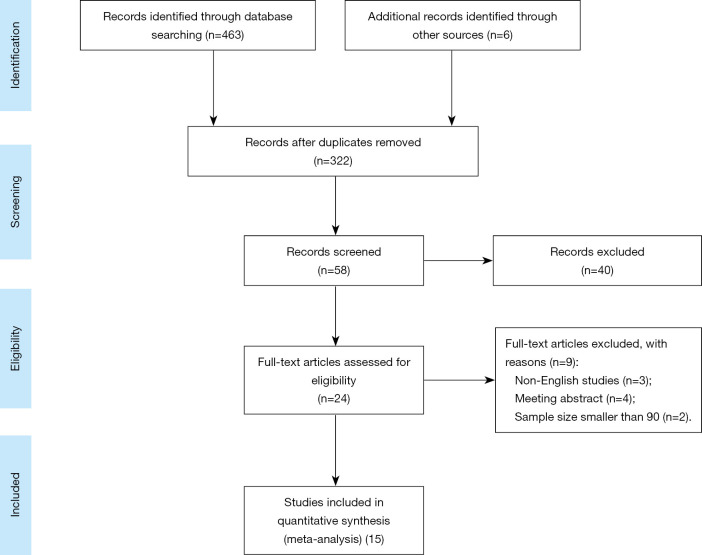
We used the following PICOS method to identify the qualification of included studies: (I) patients (P): patients undergoing RC and IC; sample size greater than 90; radiographically, PH was defined as evidence of the protrusion of abdominal contents through the abdominal wall defect created by forming the stoma. When present, PH grade was recorded using a previously published classification system of type 1-hernia sac that contains the prolapsed bowel loop forming the stoma, type 2-contains abdominal fat or omentum herniating through the abdominal wall defect created by the stoma and type 3-contains herniated loops of bowel other than that forming the stoma (9). Clinically, PH is defined as any protrusion in the vicinity of the urostoma with the patient straining in a supine and an erect position; (II) intervention (I): patients without any precaution for PH formation; (III) comparison; not applicable; (IV); outcomes (O): any study reporting incidence or risk factors of PH after RC and IC was collected. The main outcomes were the PH incidence rates of overall radiographic imaging and clinical examination; The secondary outcomes were the PH incidence of overall symptom, surgical repair and recurrence of reconstruction, and risk factors associated with PH. (V) Study design (S): full-text studies published with English. For articles with potentially overlapping population, only the largest report was included, unless they reported different outcomes of interest. Besides, exclusion criteria were as follows: (I) any study which did not satisfy the inclusion criteria; (II) single-case report, meeting abstracts, review or meta-analysis; (III) data not available. Figure 1 sketches the PRISMA flowchart of this meta-analysis.
Figure 1.
The PRISMA flowchart.
Quality assessment
The methodological quality of included studies was assessed by two independent reviewers using the Newcastle-Ottawa Scale (NOS) (10). The NOS applied a ‘star system’ to evaluate the quality of study from three perspectives: the selection of the studies, the comparability of studies, and the assessment of outcome. If seven or more stars were received, the study was considered as to be high-quality. Furthermore, two reviewers independently rated the level of evidence of the included articles through the Oxford Centre for Evidence-Based Medicine criteria (11). This scale graded studies from strongest (level 1) to weakest (level 5) strength of evidence according to study design and data quality.
Statistical analysis
The Q and I2 tests were calculated to assess the heterogeneity among studies. Pooled rates (PRs) or hazard ratio (HR) and corresponding 95% CIs were calculated through random-effect model unless the between-study heterogeneity was endurable (P>0.1 or I2≤50%). We quantified the asymmetry of funnel plots through the Egger’s test and Begg’s test to evaluate the underlying publication bias. A P value of less than 0.1 was regarded as significant. In addition, we conducted a sensitivity analysis through single factor analysis to evaluate the robustness of the pooled effects. For all statistical analyses, two-sided P<0.05 was considered statistically significant. Data from each study were pooled where possible, otherwise narrative review was conducted. This meta-analysis was accomplished through STATA version 14.2.
Results
Literature search results
After removing duplicates, screening titles and abstracts, and evaluating full texts, 15 (9,12-25) of 469 studies containing 4,252 patients were included to evaluate the incidence of PH after RC and IC. The studied population came from USA (9,12,14,15,18,20,24,25), China (22), Switzerland (23), Denmark (13), Japan (16,17), Spain (19), and Sweden (21). The vast majority of articles were reported retrospectively, and the span of median time to diagnosis of PH was from 11 to 28.8 months. Table 1 showed the characteristics of included studies in this meta-analysis.
Table 1. The basic characteristics of included studies in this meta-analysis.
| Study | Year | Country | Study type | Samples | Duration | Time to PH | Surgical type | Overall incidence (%) | Risk factors | NOS* | LoE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Donahue et al. | 2014 | USA | Retrospective | 386 | 2006.1 to 2010.10 | Median: 1 year | ORC and IC | 35.4 | Female, BMI, preoperative albumin | 9 | 2b |
| Hussein et al. | 2017 | USA | Retrospective | 383 | 2005 to 2016 | Median: 13 months (IQR, 9–22) | RARC and IC | 19.5 | Operative time, postoperative eGFR less than 60 mL per minute, fascial defect size 30 mm or greater | 9 | 2b |
| Knap et al. | 2004 | Denmark | Retrospective | 195 | 1992.1 to 1998.12 | NR | RC and IC | 5.1 | NR | 7 | 4 |
| Kouba et al. | 2007 | USA | Retrospective | 137 | 2001 to 2005 | Mean: 8.4 months (range, 6–30) | RC and IC | 13.9 | NR | 8 | 4 |
| Liu et al. | 2014 | USA | Retrospective | 199 | 2001 to 2011 | Median: 14 months (range, 1–105) | RC and IC | 29.1 | BMI, prior exploratory laparotomy | 9 | 4 |
| Maruo et al. | 2020 | Japan | Retrospective | 194 | 2005 to 2016 | Median: 25.5 months | RC and IC | 10.3 | BMI, DPRAM | 8 | 4 |
| Rodriguez Faba et al. | 2011 | Spain | Retrospective | 405 | 2000 to 2006 | NR | RC and IC | 15.6 | NR | 7 | 2b |
| Miyake et al. | 2019 | Japan | Retrospective | 129 | 2000 to 2017 | Median: 11 months | RC and IC | 10.1 | NR | 7 | 4 |
| Movassaghi et al. | 2016 | USA | Prospective | 92 | 2003 to 2013 | Median: 11.5 months (range, 1–37.2) | ORC and IC | 22.8 | NR | 8 | 4 |
| Su et al. | 2020 | USA | Retrospective | 96 | 2011 to 2016 | NR | RC and IC | 28.1 | BMI, prior tobacco use | 8 | 4 |
| Pisters et al. | 2014 | USA | Retrospective | 496 | 1994.7 to 2010.8 | NR | RC and IC | 7.3 | Anterior fixation | 8 | 2b |
| Cheung MT | 1955 | China | Retrospective | 123 | NR | NR | RC and IC | 27.6 | NR | 7 | 2b |
| Madersbacher et al. | 2003 | Switzerland | Retrospective | 131 | 1971.3 to 1995.9 | NR | RC and IC | 13.7 | NR | 9 | 4 |
| Shimko et al. | 2011 | USA | Retrospective | 1,045 | 1980 to 1998 | Median: 2.4 years (range, 0.2–18.3) | RC and IC | 14.1 | NR | 8 | 2b |
| Liedberg et al. | 2020 | Sweden | RCT | 241 | 2012.8 to 2017.5 | NR | ORC and IC | 22.8 | BMI, prophylactic mesh, surgery in one of the hospitals | – | 1b |
*, The methodological quality of Liedberg et al. (21) was low, high, low, low, low, low, and unclear risk in turn according to the Cochrane Collaboration’s RoB tool in Review Manager software (https://community.cochrane.org/help/tools-and-software/revman-5). NR, not reported; PH, parastomal hernia; IQR, interquartile range; RC, radical cystectomy; RARC, robot-assisted RC; ORC, open RC; IC, ileal conduit; BMI, body mass index; DPRAM, diameter of the passage through the rectus abdominis muscle for the IC; NOS, Newcastle-Ottawa Scale; LoE, level of evidence; RoB, risk of bias.
Incidence
A total of eight studies (9,12,15,16,18-21) representing 1,878 patients reported the incidence of overall radiographic PH, and the rates ranged from 10% to 35%. As integrated, PH developed in 23% (95% CI: 17–29%; Figure 2) of patients who underwent RC and IC. The between-study heterogeneity was obvious (P<0.001, I2=90.8%; Figure 2). A pooled analysis of three studies (9,18,21) showed that the PH incidence rate of open RC (ORC) and IC was 27% (95% CI: 19–36%; Figure 2). The 1-year PH incidence rate and 2-year incidence rate of RC and IC were 14% (95% CI: 6–22%; Figure 2) and 26% (95% CI: 14–38%; Figure 2), respectively. Similarly, patients undergoing ORC and IC had a higher risk of 2-year radiographic PH (PR: 37%; 95% CI: 14–59%; Figure 2). The incidence of clinically evident PH after RC and IC was provided in nine studies including 2,433 patients (9,13,14,17,21-25), and the synthesized result was 15% (95% CI: 10–19%; Figure 2). The heterogeneity among studies was also detected (P<0.001, I2=90.2%; Figure 2). Only two studies (9,12) reported the incidence of type 1 PH and type 2 PH, and the corresponding PRs were 1% (95% CI: 0–2%; Figure 2) and 16% (95% CI: 4–29%; Figure 2), respectively. A total of three studies (9,12,21) provided the incidence of type 3 PH, and the pooled result was 10% (95% CI: 8–12%; Figure 2). Only Donahue et al. (9) reported the results of progression to a higher type of hernia. They observed that 80% (1/5) of initial type 1 hernias progressed to type 3 while 33% (30/90) of initial type 2 hernias progressed to type 3. In addition, Hussein et al. (12) reported that 32% patients developed PH after robot-assisted RC (RARC) and IC at 3 years.
Figure 2.
The pooled results of the primary outcomes in this study. PH, parastomal hernia; PR, pooled rate; RC, radical cystectomy; ORC, open RC; RARC, robot-assisted RC; IC, ileal conduit.
PH-related symptoms, such as pain, bowel incarceration, appliance difficulties, discomfort, or PH-induced leakage, were reported in six studies (9,12,14,16,19,20), and the incidence rate ranged from 15% to 44%. The pooled results indicated that 29% (95% CI: 24–33%; Figure 3) of PH patients were at risk of presenting symptoms without significant between-study heterogeneity (P=0.302, I2=17.2%; Figure 3). A pooled analysis of ten studies (9,12,14-21) including 455 symptomatic patients showed that 20% (95% CI: 11–28%; Figure 3) of patients who underwent RC and IC required surgical repair with significant heterogeneity among studies (P<0.001, I2=82.8%; Figure 3). Besides, the surgical repair rate of symptomatic patients after ORC and IC was provided in three studies (9,18,21), and the synthesized result was 7% (95% CI: 3–11%; Figure 3). We did not observe an evident between-study heterogeneity (P=0.163, I2=44.9%; Figure 3). However, it’s noteworthy that among symptomatic PH patients undergoing surgical repair, the pooled analysis of five studies (9,12,14,15,19) showed that up to 26% (95% CI: 16–36%; Figure 3) of patients suffered PH recurrence.
Figure 3.
The pooled results of the secondary outcomes in this study. PR, pooled rate; RC, radical cystectomy; ORC, open RC; RARC, robot-assisted RC; IC, ileal conduit.
Risk factors
A wide array of independent risk factors was reported. A pooled analysis of two studies (12,16) showed that patients with fascial defect size 24 mm or greater experienced 6.80-fold higher risk of PH than their counterparts (HR: 6.80; 95% CI: 3.54–13.06; Figure 3). A pooled analysis of two studies (9,21) showed that per increased unit of body mass index (BMI) was associated with the increasing risk of PH (HR: 1.05; 92% CI: 1.05–1.11). Furthermore, patients with BMI ≥22.9 kg/m2 had 2.17 times higher risk of PH than their counterparts (HR: 2.17; 95% CI: 0.81–5.80) (16). Other significantly reported risk factors included female (HR: 2.25; 95% CI 1.58–3.21) (9), preoperative albumin (HR: 0.43; 95% CI: 0.25–0.75) (9), longer operative time [odds ratio (OR): 1.25; 95% CI: 1.06–1.46] (12), postoperative eGFR less than 60 mL per minute (OR: 2.17, 95% CI: 1.23–3.90) (12), prior exploratory laparotomy (HR: 1.98; 95% CI: 1.97–3.36) (15), prior tobacco use (OR: 0.23; 95% CI: 0.09–0.63) (20), anterior fixation (OR: 2.3; 95% CI: 1.03–5.14) (24), prophylactic mesh (OR: 0.49; 95% CI: 0.26–0.95) (21) and surgery in one of the hospitals (OR: 3.34; 95% CI: 1.39–8.06) (21).
Publication bias and sensitivity analysis
We employed the Egger’s test and Begg’s test to quantify the asymmetry of funnel plots, and P value greater than 0.1 meant no obvious publication bias. The results of Egger’s test and Begg’s test related to the main outcomes were provided in Table S1. Generally speaking, we did not detect noticeable publication bias. We evaluated the impact of a single study on the combined effect size through excluding each study in sequence, and the results of the sensitivity analysis indicated that the pooled effects were robust (Figure S1).
Discussion
Perioperative outcomes and complications are the primary two aspects that patients are most concerned about. Postoperative complications are inevitable for complex procedures such as RC. As is known to all, ORC with extended pelvic lymph node dissection has been gold standard for patients with muscle-invasive and high-risk non-muscle-invasive bladder cancer for decades (3). However, conspicuous complications and higher mortality leave much to be desired (4). Although our previous studies indicated that minimally invasive approaches, scilicet RARC and laparoscopic RC (LRC), presented improved perioperative outcomes and comparable pathological and oncologic outcomes at the cost of longer operative time when compared to ORC (26,27), on no account can we ignore the fact that many of the perioperative complications following RC might derive from the reconstructive part of the procedure, and overwhelming majority of studies included in our meta-analysis were insufficient information of stomal-related complication, such as PH whose incidence was not low (PR: 23%; 95% CI: 17–29%).
RC is generally accompanied by the UD, which is closely related to the patient’s long-term quality of life. Since it was introduced by Seiffert and popularized by Bricker (23), the IC has been the gold standard for those with incontinent UD and has still been the primary choice for patients with contraindications to continent UD for over seven decades due to its simplicity and practicability (16,17). Extensively used as IC is, stoma-related complications are rarely reported in spite of relatively high incidence of 25% to 60% (14). PH is a relatively common complication with specific to IC. In our study, the synthesized rate was 23%, and the PH incidence seemed to be associated with length of follow up. The PH incidence rate at 1 and 2 years were 14% and 26%, respectively. Besides, the detection rate of radiographic evaluation is higher than that of clinical examination (23% vs. 15%). The reason was that clinical evaluation is enslaved to variation in terms of whether it is patient reported vs. documented on physical examination, and whether examination was performed with the patient supine or upright and with or without the Valsalva maneuver (6,12). On the other hand, radiological images are more objective, less prone to variation due to body habitus and not subject to bias while it also enables consideration of other aspects, including rectus muscle defects and abdominal wall thickness, which may contribute to PH (6,12).
In most cases, PH is symptomless and incidentally diagnosed during cancer surveillance. In our cumulative analysis, a total of 29% of PH patients suffered from symptoms. Symptoms related to PH included pain, discomfort, bowel incarceration. Moreover, if the hernia increases in size, distortion of the abdominal wall might obstruct the application of the stoma bag, leading to urine leakage. Additionally, our study indicated that a total of 20% of symptomatic patients with PH required surgical correction, but the recurrence of surgical repair was as great as 26%. There are currently conflicting results of risk factors associated with PH. Obesity is the most frequently reported factor among them. Based on our clinical experience, diameter of the passage through the rectus abdominis muscle for the IC (DPRAM), prophylactic mesh, and surgery in one of the hospitals are the most potential factors in addition to obesity. Several corrective techniques have been reported to repair PH, including translocation of the stoma (19), anterior fixation (24), and mesh usage (7,21,28-30). Prophylactic mesh placement has been demonstrated safe and effective in reducing PH rate for patients undergoing elective colorectal operation (31). However, there is a paucity of data alluding to the use of mesh in prevention or repairment of PH. Considering that a PH constitutes a significant clinical problem for the individual patient, it pays to be further investigated the role of a prophylactic mesh.
Our study did have the following limitations. First of all, there were inherent limitations in the included studies of this meta-analysis. The retrospective study may lead to selection bias, such as Berkson bias and Neyman bias. Secondly, the broad heterogeneity in study populations, designs and definitions of outcome measures. Finally, different follow-up duration detected among the included studies also affected the incidence rates, and we were unable to evaluate the long-term incidence rate of PH.
Conclusions
Our findings indicated that the PH incidence rate of radiographic evaluation and clinical examination were 23% and 15%, respectively. A total of 29% of PH patients suffered from symptoms, and a total of 20% of symptomatic patients with PH required surgical correction. However, the recurrence of surgical repair was as great as 26%. Given that a PH constitutes a noticeable clinical problem for the individual patient, it pays to be further investigated the PH-related risk factors and the role of a prophylactic mesh.
Acknowledgments
Funding: This work was supported by Department of Science and Technology of Sichuan Province (2020YFH0099) and the National Natural Science Foundation of China (No. 81370272, 30901621/C1705).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-3349
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-3349). The authors have no conflicts of interest to declare.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Leal J, Luengo-Fernandez R, Sullivan R, et al. Economic burden of bladder cancer across the European Union. Eur Urol 2016;69:438-47. 10.1016/j.eururo.2015.10.024 [DOI] [PubMed] [Google Scholar]
- 3.Witjes JA, Bruins HM, Cathomas R, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer. The Netherlands: European Association of Urology; 2020; Available online: https://uroweb.org/guideline/bladder-cancer-muscle-invasive-and-metastatic/ [Google Scholar]
- 4.Feng D, Tang Y, Yang Y, et al. Intracorporeal versus extracorporeal urinary diversion after robotic-assisted radical cystectomy: evidence from a systematic review and pooled analysis of observational studies. Minerva Urol Nefrol 2020;72:519-30. 10.23736/S0393-2249.20.03829-1 [DOI] [PubMed] [Google Scholar]
- 5.Patel HR, Santos PB, de Oliveira MC, et al. Is robotic-assisted radical cystectomy (RARC) with intracorporeal diversion becoming the new gold standard of care? World J Urol 2016;34:25-32. 10.1007/s00345-015-1730-1 [DOI] [PubMed] [Google Scholar]
- 6.Donahue TF, Bochner BH. Parastomal hernias after radical cystectomy and ileal conduit diversion. Investig Clin Urol 2016;57:240-8. 10.4111/icu.2016.57.4.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donahue TF, Cha EK, Bochner BH. Rationale and early experience with prophylactic placement of mesh to prevent parastomal hernia formation after ileal conduit urinary diversion and cystectomy for bladder cancer. Curr Urol Rep 2016;17:9. 10.1007/s11934-015-0565-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donahue TF, Bochner BH, Sfakianos JP, et al. Risk factors for the development of parastomal hernia after radical cystectomy. J Urol 2014;191:1708-13. 10.1016/j.juro.2013.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Ottawa Hosp Res Inst. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed August 22, 2020).
- 11.Centre for Evidence-Based. Oxford Centre for Evidence-Based Medicine: levels of evidence (March 2009). 2009. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009
- 12.Hussein AA, Ahmed YE, May P, et al. Natural history and predictors of parastomal hernia after robot-assisted radical cystectomy and ileal conduit urinary diversion. J Urol 2018;199:766-73. 10.1016/j.juro.2017.08.112 [DOI] [PubMed] [Google Scholar]
- 13.Knap MM, Lundbeck F, Overgaard J. Early and late treatment-related morbidity following radical cystectomy. Scand J Urol Nephrol 2004;38:153-60. 10.1080/00365590310020060 [DOI] [PubMed] [Google Scholar]
- 14.Kouba E, Sands M, Lentz A, et al. Incidence and risk factors of stomal complications in patients undergoing cystectomy with ileal conduit urinary diversion for bladder cancer. J Urol 2007;178:950-4. 10.1016/j.juro.2007.05.028 [DOI] [PubMed] [Google Scholar]
- 15.Liu NW, Hackney JT, Gellhaus PT, et al. Incidence and risk factors of parastomal hernia in patients undergoing radical cystectomy and ileal conduit diversion. J Urol 2014;191:1313-8. 10.1016/j.juro.2013.11.104 [DOI] [PubMed] [Google Scholar]
- 16.Maruo K, Tanaka T, Shindo T, et al. Incidence and risk factors of parastomal hernia after ileal conduit diversion in Japanese population. Int J Clin Oncol 2020;25:1830-4. 10.1007/s10147-020-01722-w [DOI] [PubMed] [Google Scholar]
- 17.Miyake M, Owari T, Tomizawa M, et al. Long-term changes in renal function, blood electrolyte levels, and nutritional indices after radical cystectomy and ileal conduit in patients with bladder cancer. Urol J 2019;16:145-51. [DOI] [PubMed] [Google Scholar]
- 18.Movassaghi K, Shah SH, Cai J, et al. Incisional and parastomal hernia following radical cystectomy and urinary diversion: the University of Southern California experience. J Urol 2016;196:777-81. 10.1016/j.juro.2016.03.150 [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez Faba O, Rosales A, Breda A, et al. Simplified technique for parastomal hernia repair after radical cystectomy and ileal conduit creation. Urology 2011;77:1491-4. 10.1016/j.urology.2010.11.047 [DOI] [PubMed] [Google Scholar]
- 20.Su JS, Hoy NY, Fafaj A, et al. The European Hernia Society classification applied to the rare cases of parastomal hernia after ileal conduit urinary diversion: a retrospective cohort of 96 patients. Hernia 2021;25:125-31. 10.1007/s10029-020-02230-6 [DOI] [PubMed] [Google Scholar]
- 21.Liedberg F, Kollberg P, Allerbo M, et al. Preventing parastomal hernia after ileal conduit by the use of a prophylactic mesh: a randomised study. Eur Urol 2020;78:757-63. 10.1016/j.eururo.2020.07.033 [DOI] [PubMed] [Google Scholar]
- 22.Cheung MT. Complications of an abdominal stoma: an analysis of 322 stomas. Aust N Z J Surg 1995;65:808-11. 10.1111/j.1445-2197.1995.tb00566.x [DOI] [PubMed] [Google Scholar]
- 23.Madersbacher S, Schmidt J, Eberle JM, et al. Long-term outcome of ileal conduit diversion. J Urol 2003;169:985-90. 10.1097/01.ju.0000051462.45388.14 [DOI] [PubMed] [Google Scholar]
- 24.Pisters AL, Kamat AM, Wei W, et al. Anterior fascial fixation does not reduce the parastomal hernia rate after radical cystectomy and ileal conduit. Urology 2014;83:1427-31. 10.1016/j.urology.2014.01.041 [DOI] [PubMed] [Google Scholar]
- 25.Shimko MS, Tollefson MK, Umbreit EC, et al. Long-term complications of conduit urinary diversion. J Urol 2011;185:562-7. 10.1016/j.juro.2010.09.096 [DOI] [PubMed] [Google Scholar]
- 26.Feng D, Liu S, Tang Y, et al. Comparison of perioperative and oncologic outcomes between robot-assisted and laparoscopic radical cystectomy for bladder cancer: a systematic review and updated meta-analysis. Int Urol Nephrol 2020;52:1243-54. 10.1007/s11255-020-02406-0 [DOI] [PubMed] [Google Scholar]
- 27.Feng D, Li A, Hu X, et al. Comparative effectiveness of open, laparoscopic and robot-assisted radical cystectomy for bladder cancer: a systematic review and network meta-analysis. Minerva Urol Nefrol 2020;72:251-64. 10.23736/S0393-2249.20.03680-2 [DOI] [PubMed] [Google Scholar]
- 28.Tully KH, Roghmann F, Pastor J, et al. Parastomal hernia repair with 3-D mesh implants after radical cystectomy and ileal conduit urinary diversion - a single-center experience using a purpose made alloplastic mesh implant. Urology 2019;131:245-9. 10.1016/j.urology.2019.05.006 [DOI] [PubMed] [Google Scholar]
- 29.Tenzel PL, Williams ZF, McCarthy RA, et al. Prophylactic mesh used in ileal conduit formation following radical cystectomy: a retrospective cohort. Hernia 2018;22:781-4. 10.1007/s10029-018-1801-5 [DOI] [PubMed] [Google Scholar]
- 30.Styrke J, Johansson M, Granåsen G, et al. Parastomal hernia after ileal conduit with a prophylactic mesh: a 10 year consecutive case series. Scand J Urol 2015;49:308-12. 10.3109/21681805.2015.1005664 [DOI] [PubMed] [Google Scholar]
- 31.Pianka F, Probst P, Keller AV, et al. Prophylactic mesh placement for the PREvention of paraSTOmal hernias: the PRESTO systematic review and meta-analysis. PLoS One 2017;12:e0171548. 10.1371/journal.pone.0171548 [DOI] [PMC free article] [PubMed] [Google Scholar]