Abstract
Background
Neutrophil-to-lymphocyte ratio (NLR) has been demonstrated a significant association with the prognosis of hepatocellular carcinoma (HCC). The current study aimed to evaluate the prognostic value of NLR at different time points in HCC patients receiving liver resection.
Methods
Data were retrospectively collected from 195 HCC patients receiving liver resection. The preoperative NLR (pre-NLR), postoperative NLR (post-NLR) and corresponding changes of NLR (NLRc) at different time points were calculated. The disease-free survival (DFS) and overall survival (OS) was calculated by the Kaplan-Meier method and compared by the log-rank test. Both univariate and multivariate analyses were performed to evaluate their prognostic values for DFS and OS. And the prognostic significance of pre-NLR, post-NLRs, and NLRcs were further evaluated with subgroup analysis and with early and late recurrence of HCC.
Results
Pre-NLR was not significantly correlated with DFS or OS (both P>0.05), whereas higher post-NLR at 4–8 weeks [NLR (4–8 w)] and 3–6 months [(NLR (3–6 m)] predicted worse DFS (P=0.023 and P<0.001, respectively) and OS (P=0.012 and P=0.001, respectively). The value of area under the curve (AUC) of NLR (3–6 m) were higher than NLR (4–8 w) for DFS (0.656 vs. 0.572) and OS (0.650 vs. 0.621). Multivariate analyses showed that NLRc (4–8 w) was not a significant predictor of DFS (P=0.369) or OS (P=0.173), while the NLRc (3–6 m) with 25% increase was found to be an independent factor for adverse DFS in patients with HCC (P=0.041). The AUC of NLRc (3–6 m) for DFS was 0.600. Subgroup analysis showed NLR (3–6 m) was significantly corrected to DFS (P<0.001) and OS (P=0.001) in patients with cirrhosis. And NLR (3–6 m) also showed with significant correlation with early recurrence (P<0.001), while NLR (4–8 w) was found with significant association both with early and late recurrence (P=0.037 and P=0.027, respectively).
Conclusions
The post-NLRs are significant predictors of clinical outcome in HCC patients receiving liver resection, and post-NLR and NLRc with a relatively long-term interval after operation have better prognostic values.
Keywords: Disease-free survival (DFS), hepatocellular carcinoma (HCC), neutrophil-to-lymphocyte ratio (NLR), overall survival (OS), prognosis
Introduction
Hepatocellular carcinoma (HCC), the most common type of malignant liver tumors, ranks sixth in incidence and third in mortality among all cancers, which causes a serious medical burden worldwide (1,2). The risk factors of HCC are diverse with different etiologies, while the prevalence of chronic liver disease accounts for more than 80%, especially with viral hepatitis-related cirrhosis (hepatitis B and/or C) (3). Despite the progression either in diagnostic modalities or surveillance programs of HCC, the proportion of patients available to curative treatments with early stage is still less than 30% (3,4). And according to the Barcelona clinic liver cancer (BCLC) system, liver resection, transplantation, and locoregional ablation are the potential curative therapies widely accepted (4). But the prognosis of these patients remains unsatisfactory with 5-year survival rates of 50–70%, and the high recurrence rate of HCC is also an important problem that cannot be ignored (3). Therefore, it’s essential to find novel biomarkers to recognize patients with high risk of tumor recurrence and then take preventive measures to prolong the recurrence-free survival and overall survival.
Recently, the relationship between systematic inflammation and tumor biology has been demonstrated by an increased evidence in many cancers (5,6). And studies have shown that systemic inflammatory responses can promote angiogenesis, DNA damage, and tumor invasion through the upregulation of cytokines. The neutrophil-to-lymphocyte ratio (NLR), which was considered as a credible indicator of systemic inflammatory response, has shown an association with prognosis in numerous malignancies. In addition, NLR has also been investigated for its prognostic role in HCC. Most of these studies showed that an elevated pretreatment NLR predicted a poor disease-free and overall survival of HCC after different treatments (7-13), while other studies failed to demonstrate such an association (14-16). Therefore, the prognostic role of NLR in HCC needs further elucidation.
NLR usually changes along with the course of disease, especially after treatment, which may reflect the status shift of inflammatory response. Recently, several studies have focused on the significance of dynamics of NLR after treatment in several solid malignancies, such as renal cancer (17,18), non-small cell lung cancer (19), and gastric cancer (20), which showed a better prognostic role of the change in NLR than pretreatment NLR and could act as a biomarker for efficacy. But the data of postoperative NLR differs from the timing after surgery in different studies and the optimal duration of postoperative NLR remains unclear. Moreover, the roles of postoperative NLR and change of NLR in HCC were also evaluated in several studies (15,16,21-23). But there exist some limitations: (I) most studies chose the NLR at one month after surgery as the postoperative NLR and carried the subsequent analysis; (II) the HCC patients were restricted to small HCCs or early stages HCCs in most studies; (III) there were few comparisons among preoperative NLR, postoperative NLR, and change of NLR in the prognostic value of HCC.
At present study, we performed a prognostic analysis in HCC patients who received liver resection as the first treatment, and subsequently evaluate the prognostic value of NLR at different time and the change of NLR after operation to find the optimal predictive parameter for HCC patients.
Methods
Patients
Patients, who were first diagnosed with HCC and treated in our department during March 2005 to May 2013, were collected from the inpatient database retrospectively. And the inclusion criteria were set as below: (I) adults with ages no less than eighteen years old; (II) received hepatectomy as the first treatment; (III) no pre-adjuvant therapies for HCC (TACE/ablation/sorafenib); (IV) pathologically proven HCC; (V) without extrahepatic or distant metastases; (VI) without other concurrent malignant tumors or hematological diseases; (VII) true, effective and complete inpatient data; (VIII) adequate follow-up. After reviewing the medical records, a total of 195 HCC patients met the criteria and were enrolled in our study.
Clinicopathological data
Personal basic information was collected with gender, age. Tumor-related data were reviewed by contrast-enhanced computed tomographic (CT) scans and/or magnetic resonance imaging (MRI) series and reports, including tumor size, nodule number and existence of vascular invasion. Besides, the presence of cirrhosis was evaluated both with radiological images and the histopathological reports. And the BCLC staging system was adopted to determine personalized staging for each patient.
Peripheral blood samples were obtained and examined within 1 week before surgery, 4–8 weeks and 3–6 months after surgery. The absolute neutrophil and lymphocyte counts were collected at each period. And the NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count. The pre-NLR, NLR (4–8 w) and NLR (3–6 m) represent NLR at different period mentioned above respectively. The NLR changes were calculated as % changes by the following calculation: NLRc = [(NLR after surgery/NLR before surgery) −1)*100%. Subsequently, we divided the NLRc into three groups with the change ratio [≥25% decrease, no change (<25% decrease to <25% increase), ≥25% increase]. The NLRc (4–8 w) and NLRc (3–6 m) represent the NLRc at 4–8 weeks and 3–6 months after surgery respectively.
Follow-up
Patients were followed up at the outpatient office with time sequences. The serum AFP levels, abdominal ultrasound, dynamic enhanced CT or MRI were performed according to the recommendations. And the follow-up programme began at the date of operation and ended with death or the time of last follow-up encompassed by this study (May 2013).
Statistical analysis
Continuous variables are reported as medians and interquartile ranges, while categorical variables are presented as frequencies and percentages. For NLR, the optimal cut-off value was determined by using receiver operating characteristic (ROC) curves. The area under the curve (AUC) was applied to evaluate the prediction value with respective sensitivity and specificity. Survival curves were constructed using the Kaplan-Meier method, and log-rank tests were used for the univariate analysis. Variables that showed significant associations in the univariate analysis (P<0.05) were enrolled in a multivariate Cox regression model (forward stepwise method) to adjust the relationship between NLR (or NLRc) and DFS (or OS). All data analyses were carried out using the SPSS version 20.0 (IBM Crop., Chicago, IL, USA). All statistical tests were two-sided, and P<0.05 was defined as statistically significant.
Results
Patient characteristics
A total of 195 HCC patients with complete medical records and follow-up data were involved in this study, including 174 males and 21 females. The baseline characteristics of these patients are shown in Table 1. The median age was 51 years (range: 42–59 years). One hundred and twenty-six (64.6%) patients presented with cirrhosis, which were mainly related to hepatitis B and C. The median tumor size was 4.4 cm (range: 3.0–7.5 cm). One hundred and forty-seven patients (75.4%) presented with single tumor, while 48 (24.6%) patients have multiple nodules. And 80 (41.0%) patients were found with vascular invasion in contrast-enhanced radiology examinations. According to the BCLC staging system, the numbers of patients with stage 0~A, B, and C were 98 (50.3%), 77 (39.5%), and 20 (10.3%), respectively. All the patients received R0 resection for their tumor in liver with a surgical margin ≥1cm and confirmed with pathological examination. And well differentiation of the resected tumor was shown in 26 (13.3%) patients, and 169 (86.7%) patients presented with poor-moderate differentiation. During operation, 96 (49.2%) patients had blood transfusion. Alpha-fetoprotein (AFP) was found increased (>400 ng/mL) only in 65 (33.3%) patients, while 130 (66.7%) patients were with low level (≤400 ng/mL). The median levels of preoperative fibrinogen, platelet (PLT), alanine aminotransferase (ALT), albumin (ALB) and glutamyl transpeptidase (GGT) were 3.01 g/L (range: 2.43–3.65 g/L), 175×109/L (range: 124×109–231×109/L), 36 U/L (range: 26–53 U/L), 39.6 g/L (range: 37.5–42.5 g/L), and 63.0 U/L (range: 35.0–98.0 U/L), respectively. The median levels of pre-NLR, NLR (4–8 w), and NLR (3–6 m) were 1.95 (range: 1.12–2.12), 1.47 (range: 1.12–2.12), and 1.62 (range: 1.14–2.44).
Table 1. The baseline characteristics of 195 hepatocellular carcinoma patients.
| Variables | N (%) or median (range), n=195 |
|---|---|
| Gender (male/female) | 174 (89.2%)/21 (10.8%) |
| Age, years | 51 (42, 59) |
| Cirrhosis (absent/present) | 69 (35.4%)/126 (64.6%) |
| Tumor diameter (cm) | 4.4 (3.0, 7.5) |
| Tumor number (single/multiple) | 147 (75.4%)/48 (24.6%) |
| Vascular invasion (absent/present) | 115 (59.0%)/80 (41.0%) |
| BCLC stage (0~A/B/C) | 98 (50.3%)/77 (39.5%)/20 (10.3%) |
| Pathological differentiation (well/poor-moderate) | 26 (13.3%)/169 (86.7%) |
| Intraoperative blood transfusion (absent/present) | 99 (50.8%)/96 (49.2%) |
| AFP, ng/mL (≤400/>400) | 130 (66.7%)/65 (33.3%) |
| Fibrinogen, g/L | 3.01 (2.43, 3.65) |
| Platelet, ×109/L | 175 (124, 231) |
| Alanine aminotransferase, U/L | 36 (26, 53) |
| Albumin, g/L | 39.6 (37.5, 42.5) |
| Glutamyl transpeptidase, U/L | 63.0 (35.0, 98.0) |
| Pre-NLR | 1.95 (1.12, 2.12) |
| NLR (4–8 w) | 1.47 (1.12, 2.12) |
| NLR (3–6 m) | 1.62 (1.14, 2.44) |
BCLC, Barcelona clinic liver cancer; AFP, α-fetoprotein; NLR, neutrophil-to-lymphocyte ratio; Pre-NLR, preoperative NLR.
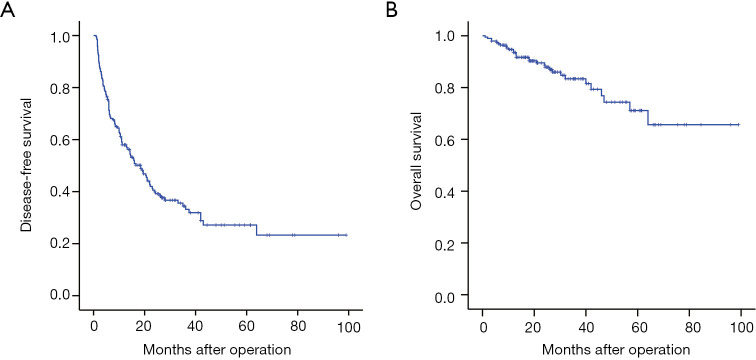
The median follow-up time was 25.5 months (range: 13.1–38.5 months). During the follow-up period, 121 (62.1%) patients experienced recurrence, whereas 30 (15.4%) patients died. The 1-, 3-, 5-year cumulative DFS rates was 58.0%, 34.4%, and 27.1% respectively, whereas the 1-, 3-, and 5-year OS rates was 93.5%, 83.4%, and 71.7%, respectively (Figure 1).
Figure 1.
Kaplan-Meier curves for disease-free survival and overall survival of 195 patients with HCC receiving liver resection. Diseases-free survival (A), and overall survival (B).
Evaluation of the NLRs and NLRcs for prognostic prediction
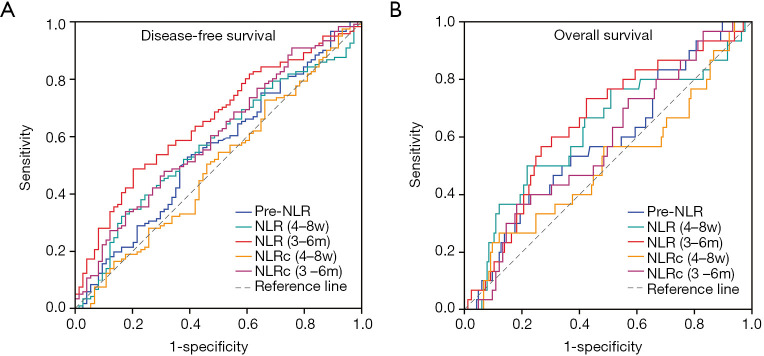
We analyzed survival using receiver operating characteristics (ROC) curves to evaluate the probability of pre-NLR, NLR (4–8 w), NLR (3–6 m), NLRc (4–8 w) and NLRc (3–6 m) (shown in Figure 2).
Figure 2.
Receiver operating characteristic (ROC) curves for disease-free survival and overall survival of NLRs and NLRcs in HCC patients. Diseases-free survival (A), and overall survival (B).
Using DFS as the end point, the area under the ROC curve (AUC) for pre-NLR was 0.552 (95% CI: 0.468–0.635, P=0.226), while the AUCs for postoperative NLR (4–8 w) and NLR (3–6 m) were 0.572 (95% CI: 0.490–0.654, P=0.093) and 0.656 (95% CI: 0.578–0.733, P<0.001) respectively, which were both better than pre-NLR. Besides, the changes between postoperative and preoperative NLR, NLRc (4–8 w) and NLRc (3–6 m), showed different AUC with 0.498 (95% CI: 0.413–0.582, P=0.954) and 0.600 (95% CI: 0.519–0.682, P=0.042), respectively. So, NLRc (3–6 m) also presented with a higher AUC than pre-NLR.
As for OS, the AUC for pre-NLR was 0.582 (95% CI: 0.473–0.692, P=0.152), whereas the AUCs for NLR (4–8 w) and NLR (3–6 m) were 0.621 (95% CI: 0.504–0.739, P=0.035) and 0.650 (95% CI: 0.545–0.755, P=0.009) respectively, which were both higher than Pre-NLR. And both the NLRc (4–8 w) and NLRc (3–6 m) showed a lower AUC with 0.505 (95% CI: 0.386–0.625, P=0.924) and 0.579 (95% CI: 0.4729–0.686, P=0.055) than pre-NLR.
Prognostic values of NLRs and NLRcs for diseases-free and overall survival in HCC patients
The optimal cut-off values of NLRs was determined by using ROC curves with Youden index and adjusted to 2 as an integral number for convenient clinical application. So, the pre-NLR, NLR (4–8 w) and NLR (3–6 m) were all divided into two groups with ≤2 and >2. And the NLRc (4–8 w) and NLRc (3–6 m) were both divided into three groups as described above.
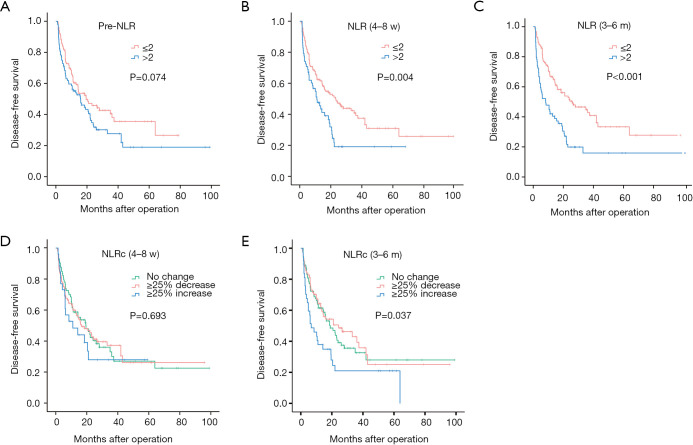
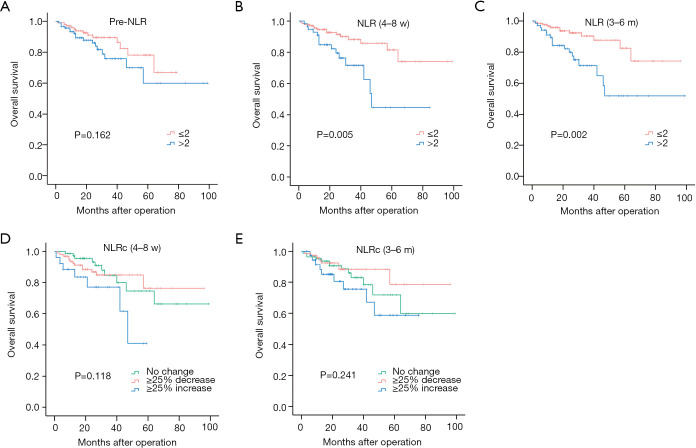
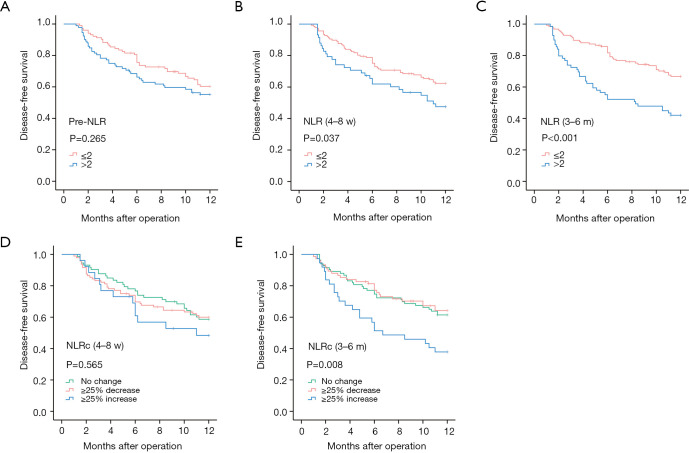
In univariate analyses, there was no significant relationship between pre-NLR and DFS (HR: 1.385, 95% CI 0.969–1.979, P=0.074; Figure 3A), while significant relationships were found with the NLR (4–8 w) (HR: 1.742, 95% CI: 1.193–2.544, P=0.004; Figure 3B) and NLR (3–6 m) (HR: 2.144, 95% CI: 1.494–3.077, P<0.001; Figure 3C). As for NLRcs, data showed that NLRc (4–8 w) was not significantly correlated with DFS (P=0.693; Figure 3D), while a significant relationship was found with the NLRc (3–6 m) (P=0.037; Figure 3E). Besides, no significant relationship between pre-NLR and OS (HR: 1.677, 95% CI: 0.813–3.460, P=0.162; Figure 4A) was found. However, the NLR (4–8 w) (HR: 2.838, 95% CI: 1.379–5.841, P=0.005; Figure 4B) and NLR (3–6 m) (HR: 3.122, 95% CI: 1.500–6.497, P=0.002; Figure 4C) were significantly related to OS. As for NLRcs, neither the NLRc (4–8 w) (P=0.118; Figure 4D) nor the NLRc (3–6 m) (P=0.241; Figure 4E) was found significant relationship with OS. And the DFS of ≥25% increase in NLRc (3–6 m) was significantly lower than no change (HR: 1.640, 95% CI: 1.037–2.594; P=0.034), whereas the DFS between ≥25% decrease and no change had no statistical significance (HR: 0.902, 95% CI: 0.598–1.361; P=0.623). Besides, tumor diameter (>5/≤5 cm; HR: 2.096, 95% CI: 1.457–3.016; P<0.001), tumor number (multiple/single; HR: 2.051, 95% CI: 1.397–3.012; P<0.001), vascular invasion (present/absent; HR: 1.972, 95% CI: 1.374–2.830; P<0.001), BCLC stage (B~C/0~A; HR: 2.141, 95% CI: 1.481–3.094, P<0.001), pathological differentiation (present/absent; HR: 1.941, 95% CI: 1.060–3.554, P=0.032) and intraoperative blood transfusion (present/absent; HR: 1.468, 95% CI: 1.024–2.105, P=0.037) were found to be related to DFS significantly, while tumor diameter (>5/≤5 cm; HR: 2.558, 95% CI: 1.229–5.325, P=0.012), vascular invasion (present/absent; HR: 2.724, 95% CI: 1.302–5.700, P=0.008), BCLC stage (present/absent; HR: 2.391, 95% CI: 1.123–5.089, P=0.024), AFP (>400/≤400 ng/mL; HR: 2.350, 95% CI: 1.146–4.820, P=0.020) and HBV-DNA (>1,000/≤1,000 copies/mL; HR: 2.295, 95% CI: 1.048–5.027, P=0.038) were found to be related to OS significantly (shown in Table 2).
Figure 3.
Kaplan-Meier curves for disease-free survival of NLRs and NLRcs in HCC patients. Pre-NLR (A), NLR (4–8 w) (B), NLR (3–6 m) (C), NLRc (4–8 w) (D) and NLRc (3–6 m) (E).
Figure 4.
Kaplan-Meier curves for overall survival of NLRs and NLRcs in HCC patients. Pre-NLR (A), NLR (4–8 w) (B), NLR (3–6 m) (C), NLRc (4–8 w) (D) and NLRc (3–6 m) (E).
Table 2. Univariate analysis of prognostic factors for disease-free survival and overall survival in 195 hepatocellular carcinoma patients.
| Variables | N | DFS | OS | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |||
| Gender (female/male) | 21/174 | 1.063 (0.597, 1.892) | 0.836 | 0.860 (0.298, 2.481) | 0.781 | |
| Age, years (≤50/>50) | 95/100 | 0.979 (0.685, 1.399) | 0.906 | 0.498 (0.229, 1.045) | 0.065 | |
| Cirrhosis (absent/present) | 69/126 | 1.065 (0.727, 1.561) | 0.746 | 0.958 (0.448, 2.051) | 0.912 | |
| Tumor diameter, cm (≤5/>5) | 113/82 | 2.096 (1.457, 3.016) | <0.001 | 2.558 (1.229, 5.325) | 0.012 | |
| Tumor number (single/multiple) | 147/48 | 2.051 (1.397, 3.012) | <0.001 | 1.110 (0.475, 2.595) | 0.810 | |
| Vascular invasion (absent/present) | 115/80 | 1.972 (1.374, 2.830) | <0.001 | 2.724 (1.302, 5.700) | 0.008 | |
| BCLC stage (0~A/B/C) | 98/77/20 | <0.001 | 0.017 | |||
| BCLC stage (0~A/B) | 1.863 (1.259, 2.757) | 0.002 | 1.995 (0.884, 4.502) | 0.096 | ||
| BCLC stage(0~A/C) | 3.968 (2.288, 6.879) | <0.001 | 4.305 (1.564,11.850) | 0.005 | ||
| Pathological differentiation (well/poor-moderate) | 26/169 | 1.941 (1.060, 3.554) | 0.032 | 3.109 (0.730, 13.240) | 0.125 | |
| Intraoperative blood transfusion (absent/present) | 99/96 | 1.468 (1.024, 2.105) | 0.037 | 1.186 (0.576, 2.439) | 0.643 | |
| AFP, ng/mL (≤400/>400) | 130/65 | 1.431 (0.988, 2.074) | 0.058 | 2.350 (1.146, 4.820) | 0.020 | |
| HBV-DNA, copies/mL (≤1,000/>1,000) | 94/101 | 1.309 (0.914, 1.874) | 0.142 | 2.295 (1.048, 5.027) | 0.038 | |
| Pre-NLR (≤2/>2) | 103/92 | 1.385 (0.969, 1.979) | 0.074 | 1.677 (0.813, 3.460) | 0.162 | |
| NLR (4–8 w) (≤2/>2) | 137/58 | 1.742 (1.193, 2.544) | 0.004 | 2.838 (1.379, 5.841) | 0.005 | |
| NLR (3–6 m) (≤2/>2) | 126/69 | 2.144 (1.494, 3.077) | <0.001 | 3.122 (1.500, 6.497) | 0.002 | |
| NLRc (4–8 w) (no change/≥25% increase/≥25% decrease) | 73/26/96 | 0.693 | 0.118 | |||
| NLRc (4–8 w) (no change/≥25% increase) | 1.251 (0.716, 2.186) | 0.432 | 2.615 (0.985, 6.944) | 0.054 | ||
| NLRc (4–8 w) (no change/≥25% decrease) | 0.999 (0.678, 1.474) | 0.998 | 1.132 (0.494, 2.594) | 0.770 | ||
| NLRc (3–6 m) (no change/≥25% increase/≥25% decrease) | 83/37/75 | 0.037 | 0.241 | |||
| NLRc (3–6 m) (no change/≥25% increase) | 1.640 (1.037, 2.594) | 0.034 | 1.596 (0.681, 3.744) | 0.282 | ||
| NLRc (3–6 m) (no change/≥25% decrease) | 0.902 (0.598, 1.361) | 0.623 | 0.708 (0.293, 1.709) | 0.442 | ||
BCLC, Barcelona clinic liver cancer; AFP, α-fetoprotein; NLR, neutrophil-to-lymphocyte ratio; Pre-NLR, preoperative NLR; NLRc, changes of NLR.
In multivariate analyses, variables with P<0.05 in univariate analyses for DFS and OS were included as adjustment factors to evaluate the prognostic values of NLRs and NLRcs. After adjustment for confounders including tumor diameter, tumor number, vascular invasion, BCLC stage, pathological differentiation and intraoperative blood transfusion, there was no significant relationship between pre-NLR and DFS (HR: 1.178, 95% CI: 0.814–1.705, P=0.385), while both NLR (4–8 w) and NLR (3–6 m) showed as independent prognostic factors for DFS (HR: 1.570, 95% CI: 1.064–2.317, P=0.023; HR: 2.206, 95% CI: 1.518–3.206, P<0.001, respectively). And there was still no statistical significance between NLRc (4–8 w) and DFS (P=0.369) after the adjustment. As for NLRc (3–6 m) (P=0.013), the DFS of patients with ≥25% increase was significantly lower than no change (HR: 1.633, 95% CI: 1.020–2.615; P=0.041), whereas the DFS between patients with ≥25% decrease and no change had no statistical significance (HR: 0.784, 95% CI: 0.512–1.200; P=0.262) (shown in Table 3).
Table 3. The prognostic value of NLRs and NLRcs at different time points for disease-free survival in 195 hepatocellular carcinoma patients by multivariate analyses.
| Variables | (1) DFS | (2) DFS | (3) DFS | (4) DFS | (5) DFS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |||||
| Tumor diameter | 1.492 (0.966,2.303) | 0.071 | 1.493 (0.967, 2.307) | 0.071 | 1.856 (1.210, 2.845) | 0.005 | 1.547 (1.002, 2.388) | 0.049 | 1.649 (1.064, 2.555) | 0.025 | ||||
| Tumor number | 1.847 (1.146, 2.978) | 0.012 | 1.921 (1.188, 3.106) | 0.008 | 1.864 (1.154, 3.012) | 0.011 | 1.919 (1.186, 3.106) | 0.008 | 1.928 (1.184, 3.141) | 0.008 | ||||
| Vascular invasion | 1.447 (0.716, 2.922) | 0.303 | 1.467 (0.725, 2.968) | 0.286 | 1.416 (0.704, 2.849) | 0.330 | 1.529 (0.757, 3.088) | 0.236 | 1.598 (0.780, 3.271) | 0.200 | ||||
| BCLC stage | 0.889 (0.399, 1.981) | 0.773 | 0.857 (0.385, 1.908) | 0.706 | 0.787 (0.353, 1.758) | 0.560 | 0.862 (0.386, 1.928) | 0.718 | 0.748 (0.330, 1.697) | 0.487 | ||||
| Pathological differentiation | 1.360 (0.710, 2.605) | 0.354 | 1.316 (0.686, 2.525) | 0.410 | 1.233 (0.641, 2.372) | 0.531 | 1.464 (0.755, 2.837) | 0.259 | 1.422 (0.740, 2.732) | 0.290 | ||||
| Intraoperative blood transfusion | 1.233 (0.849, 1.790) | 0.272 | 1.204 (0.830, 1.747) | 0.329 | 1.265 (0.872, 1.834) | 0.215 | 1.233 (0.851, 1.787) | 0.268 | 1.317 (0.906, 1.914) | 0.149 | ||||
| Pre-NLR | 1.178 (0.814, 1.705) | 0.385 | ||||||||||||
| NLR (4–8 w) | 1.570 (1.064, 2.317) | 0.023 | ||||||||||||
| NLR (3–6 m) | 2.206 (1.518, 3.206) | <0.001 | ||||||||||||
| NLRc (4–8 w) | 0.369 | |||||||||||||
| NLRc (4–8 w) ≥25% decrease | 0.786 (0.523, 1.182) | 0.248 | ||||||||||||
| NLRc (4–8 w) ≥25% increase | 1.079 (0.606, 1.920) | 0.797 | ||||||||||||
| NLRc (3–6 m) | 0.013 | |||||||||||||
| NLRc (3–6 m) ≥25% decrease | 0.784 (0.512, 1.200) | 0.262 | ||||||||||||
| NLRc (3–6 m) ≥25% increase | 1.633 (1.020, 2.615) | 0.041 | ||||||||||||
BCLC, Barcelona clinic liver cancer; AFP, α-fetoprotein; NLR, neutrophil-to-lymphocyte ratio; Pre-NLR, preoperative NLR; NLRc, changes of NLR.
After adjustment with tumor diameter, vascular invasion, BCLC stage, AFP and HBV-DNA, there was no significant association between pre-NLR and OS (HR: 1.508, 95% CI: 0.715–3.182, P=0.281), while both >2 NLR (4–8 w) and NLR (3–6 m) presented with lower OS than ≤2 NLR (4–8 w) and NLR (3–6 m) (HR: 2.601, 95% CI: 1.230–5.502, P=0.012; HR: 3.939, 95% CI: 1.807–8.583, P=0.001, respectively). Besides, neither NLRc (4–8 w) nor NLRc (3–6 m) showed statistical significance with OS (P=0.173; P=0.073, respectively) (shown in Table 4).
Table 4. The prognostic value of NLRs and NLRcs at different time points for overall survival in 195 hepatocellular carcinoma patients by multivariate analyses.
| Variables | (1) OS | (2) OS | (3) OS | (4) OS | (5) OS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |||||
| Tumor Diameter | 1.292 (0.535, 3.116) | 0.569 | 1.400 (0.594, 3.300) | 0.442 | 2.044 (0.859, 4.862) | 0.106 | 1.557 (0.643, 3.773) | 0.327 | 1.565 (0.647, 3.787) | 0.320 | ||||
| Vascular invasion | 3.074 (0.400, 23.613) | 0.280 | 2.684 (0.349, 20.645) | 0.343 | 3.109 (0.411,23.502) | 0.272 | 3.406 (0.449, 25.842) | 0.236 | 4.070 (0.534, 31.040) | 0.176 | ||||
| BCLC stage | 0.565 (0.070, 4.545) | 0.591 | 0.546 (0.068, 4.371) | 0.569 | 0.398 (0.050, 3.190) | 0.386 | 0.466 (0.057, 3.835) | 0.478 | 0.399 (0.049, 3.279) | 0.393 | ||||
| AFP | 2.137 (0.994, 4.594) | 0.052 | 2.289 (1.068, 4.905) | 0.033 | 2.493 (1.139, 5.456) | 0.022 | 2.027 (0.957, 4.296) | 0.065 | 2.304 (1.064, 4.986) | 0.034 | ||||
| HBV-DNA | 2.239 (0.998, 5.023) | 0.051 | 2.102 (0.925, 4.775) | 0.076 | 2.075 (0.920, 4.684) | 0.079 | 2.164 (0.962, 4.872) | 0.062 | 2.246 (1.001, 5.041) | 0.050 | ||||
| Pre-NLR | 1.508 (0.715, 3.182) | 0.281 | ||||||||||||
| NLR (4–8 w) | 2.601 (1.230, 5.502) | 0.012 | ||||||||||||
| NLR (3–6 m) | 3.939 (1.807, 8.583) | 0.001 | ||||||||||||
| NLRc (4–8 w) | 0.173 | |||||||||||||
| NLRc (4–8 w) ≥25% decrease | 0.899 (0.385, 2.101) | 0.806 | ||||||||||||
| NLRc (4–8 w) ≥25% increase | 2.162 (0.777, 6.013) | 0.140 | ||||||||||||
| NLRc (3–6 m) | 0.073 | |||||||||||||
| NLRc (3–6 m) ≥25% decrease | 0.632 (0.258, 1.544) | 0.314 | ||||||||||||
| NLRc (3–6 m) ≥25% increase | 1.978 (0.806, 4.856) | 0.136 | ||||||||||||
BCLC, Barcelona clinic liver cancer; AFP, α-fetoprotein; NLR, neutrophil-to-lymphocyte ratio; Pre-NLR, preoperative NLR; NLRc, changes of NLR.
Prognostic values of NLRs and NLRcs in HCC patients with and without cirrhosis
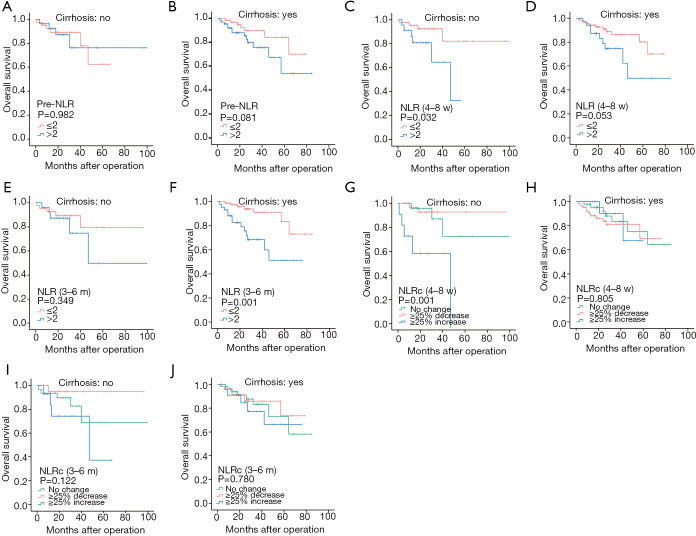
Furthermore, we made a subgroup analysis for the prognostic values of pre-NLR, NLR (4–8 w), NLR (3–6 m), NLRc (4–8 w), and NLRc (3–6 m) in specific HCC patients with and without cirrhosis. And the results showed that pre-NLR was not correlated to the DFS in HCC patients regardless of the existence of cirrhosis (all P>0.05, Figure 5A,B). The NLR (4–8 w) was also found no significant association with DFS in patients without cirrhosis (P=0.191, Figure 5C), while with significant association in patients with cirrhosis (P=0.010, Figure 5D). The similar findings were also found with NLR (3–6 m), which showed no relationship with DFS in patients without cirrhosis (P=0.131, Figure 5E) while with significant relationship in patients with cirrhosis (P<0.001, Figure 5F). NLRc (4–8 w) was found with no correlation with DFS both in patients with and without cirrhosis (both P>0.05, Figure 5G,H). As for NLRc (3–6 m), only significant correlation was found with DFS in patients without cirrhosis (P=0.001, Figure 5I), while no correlation in patients with cirrhosis (P=0.804, Figure 5J).
Figure 5.
Kaplan-Meier curves for diseases-free survival of NLRs and NLRcs in HCC patients with and without cirrhosis. Without cirrhosis: pre-NLR (A), NLR (4–8 w) (C), NLR (3–6 m) (E), NLRc (4–8 w) (G) and NLRc (3–6 m) (I); With cirrhosis: pre-NLR (B), NLR (4–8 w) (D), NLR (3–6 m) (F), NLRc (4–8 w) (H) and NLRc (3–6 m) (J).
In addition, pre-NLR was also not correlated to the OS in patients regardless of the existence of cirrhosis (all P>0.05, Figure 6A-B). And NLR (4–8 w) was only found significant association with OS in patients without cirrhosis (P=0.032, Figure 6C), while not in patients with cirrhosis (P=0.053, Figure 6D). However, NLR (3–6 m) was found not correlated to OS in patients without cirrhosis (P=0.349, Figure 6E), while correlated with OS in patients with cirrhosis (P=0.001, Figure 6F). NLRc (4–8 w) was found with correlation with OS in patients without cirrhosis (P=0.001, Figure 6G), while not in patients with cirrhosis (P=0.805, Figure 6H). In regard of NLRc (3–6 m), no association was found with OS in patients regardless of the presence of cirrhosis (all P>0.05, Figure 6I,J).
Figure 6.
Kaplan-Meier curves for overall survival of NLRs and NLRcs in HCC patients with and without cirrhosis. Without cirrhosis: pre-NLR (A), NLR (4–8 w) (C), NLR (3–6 m) (E), NLRc (4–8 w) (G) and NLRc (3–6 m) (I); with cirrhosis: pre-NLR (B), NLR (4–8 w) (D), NLR (3–6 m) (F), NLRc (4–8 w) (H) and NLRc (3–6 m) (J).
Prognostic values of NLRs and NLRcs for early and late recurrence in HCC patients
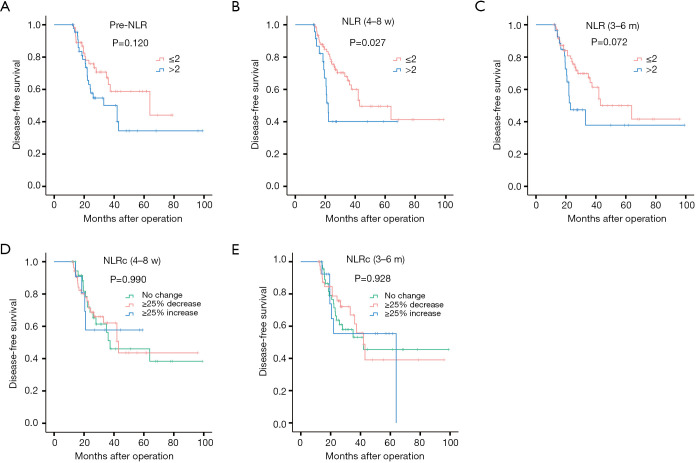
The recurrence of HCC could be divided into two patterns with early and late phase. In the present study, the cut-off time point was set at one year after surgery, and recurrence within one year postoperatively was classified as early recurrence, while recurrence occurred after one year of surgery was named to late recurrence. Of 121 HCC patients with recurrence, 81 (66.9%) cases belonged to early recurrence, while 40 (33.1%) cases were late recurrence. And the results of survival analysis for early recurrence showed that pre-NLR (Figure 7A) was not correlated to early DFS (P=0.265, Figure 7A), while the NLR (4–8 w) (P=0.037, Figure 7B) and NLR (3–6 m) (P<0.001, Figure 7C)were correlated to the early DFS. As for NLRcs, no correlation was found with NLRc (4–8 w) (P=0.565, Figure 7D) for early DFS, while NLRc (3–6 m) (P=0.008, Figure 7E) showed correlation. Patients with high NLR (4–8 w) and NLR (3–6 m), and with NLRc (3–6 m) ≥25% increase showed with worse early DFS. As for late recurrence, no significant association was found with pre-NLR (P=0.120, Figure 8A), while NLR (4–8 w) (P=0.027, Figure 8B) was found with significant association with late DFS. NLR (3–6 m) (P=0.072, Figure 8C) was also found no significance with late DFS. As for NLRcs, neither the NLRc (4–8 w) (P=0.990, Figure 8D) nor the NLRc (3–6 m) (P=0.928, Figure 8E) was found significant correlation with late DFS.
Figure 7.
Kaplan-Meier curves for early diseases-free survival of NLRs and NLRcs in HCC patients. Pre-NLR (A), NLR (4–8 w) (B), NLR (3–6 m) (C), NLRc (4–8 w) (D) and NLRc (3–6 m) (E).
Figure 8.
Kaplan-Meier curves for late diseases-free survival of NLRs and NLRcs in HCC patients. Pre-NLR (A), NLR (4–8 w) (B), NLR (3–6 m) (C), NLRc (4–8 w) (D) and NLRc (3–6 m) (E).
Discussion
Inflammation has been demonstrated as one of the hallmarks of cancers and plays an important role in tumor development and progression (6,24). And emerging evidence has shown that various inflammation-based parameters, including platelet to lymphocyte ratio (PLR), the lymphocyte to monocyte ratio (LMR), and NLR, are associated with prognosis of numerous tumors (25-30), but the underlying mechanisms have not been clarified clearly. NLR, a biomarker of systemic inflammation, has been regarded to reflect the balance between host inflammatory response and immune response in the context of a malignancy. A high NLR usually shows a significant association with unfavorable survival outcomes in patients with solid cancers (31-37), as well as in HCC (7-9,21). However, most previous investigations just focused on the pretreatment values of NLR, and only limited studies have evaluated the clinical significance of post-treatment NLR or the dynamic changes of NLR, which may reflect the new accomplished status after treatment or the shift of inflammation-immune balance. In the present study, we made a comparison among pre-NLR, post-NLRs, and the changes of NLR, to evaluate the optimal prognostic predictor of DFS and OS in HCC patients receiving liver resection. To our knowledge, this is the first study compared NLRs and NLRcs at different time points. And our results suggested that the pre-NLR wasn’t a significant prognostic marker of clinical outcomes for HCC patients after liver resection, whereas the higher postoperative NLRs (4–8 weeks and 3–6 months after operation) predicted worse DFS and OS in those patients. Besides, we also found that the change of NLR at 3–6 months after operation was superior to NLRc (4–8 w) on the prognostic value of recurrence. Patients with ≥25% increase of NLR at 3–6 months after operation had worse DFS. Subgroup analysis further demonstrated the superior prognostic significance of postoperative NLRs in HCC, especially with cirrhosis. And furthermore, postoperative NLRs also showed with better performance in predicting the early and late DFS than pre-NLR.
NLR is an easy and inexpensive parameter and could be determined by regular laboratory tests. Most previous studies have shown that pretreatment NLR is an important prognostic indicator in patients with HCC after various therapies, and elevated NLR usually predicts poor clinical outcomes in patients with different stages (7-13). Considering the potential bias from unbalanced groups with different NLR values, a method with propensity score matching (PSM) was applied by Yang et al. to adjust the clinical characteristics, and they found the preoperative NLR remained an independent predictor of recurrence for HCC patients receiving hepatectomy (30). And a recent meta-analysis also showed a positive correlation of elevated NLR with poor DFS and OS in patients with liver cancer (38). Although the molecular mechanisms associating high NLR and poor outcome remain poorly understood, the following may account for possible explanation: (I) Neutrophils play an important role in inflammation within the tumor. And neutrophilia, which could be induced by cytokines involved in cancer-associated inflammation, is able to inhibit the cytolytic activity of immune cells and exerts protumoral functions by enhancing tumor cell invasion and metastasis, angiogenesis, and extracellular matrix remodeling in cancers. (II) Evidence has proven that infiltrating lymphocytes suggest an anti-tumor immune response and associate with better response to cytotoxic treatment and prognosis in cancer patients. Thus, a lower lymphocyte count may indicate a deficient immunological defense against cancer. (III) A high NLR is also associated with a high infiltration of tumor-associated macrophages and high inflammatory cytokine production in the tumor, such as interleukin-6, interleukin-8, interleukin-17, matrix metalloproteinases, and elastases, which contribute to a stimulating tumor microenvironment.
Interestingly, in the present study, we found that pre-NLR was not an independent prognostic factor for neither DFS nor OS in HCC patients, and high pre-NLR did not predict a worse prognosis. Except for those studies with positive results, Dan et al. and Kinoshita et al. also found that pretreatment NLR wasn’t an independent predictor of recurrence-free survival (RFS)/DFS or OS in HCC patients (14,15). The various cut-off values of NLR in different studies may account for some reason. A high NLR was usually determined as more than 3 or 5 in most previous studies, but the cut-off value in our study, due to the relatively low distribution of NLR in HCC patients, was set with 2 by ROC curve, which was lower than other studies.
Of note, we found that the postoperative NLR was a superior parameter for the prognosis of HCC patients. Both high NLR (4–8 w) and NLR (3–6 m) were independently correlated with poor DFS and OS in HCC patients after liver resection. Nevertheless, Chen et al. also found that the higher post-RFA NLR predicted not only worse OS, but also higher tumor recurrence rate (21). In their study, the post-RFA NLR was obtained at first follow-up visit with a median time of 3 months after RFA (range: 10–16 weeks). And recently, Hung et al. collected the preoperative NLR and NLR at recurrence after operation in HCC patients. Their results showed that high NLR at recurrence predicted worse OS and recurrence-to- death survival, and persistence of high NLR or a new elevated of NLR at recurrence showed poor outcomes (23). A meta-analysis conducted by Qi et al. showed that low post-treatment NLR was significantly associated with better overall survival of HCC patients, and decreased NLR after treatment was significantly associated with better recurrence-free or disease-free survival of HCC patients (39). The postoperative NLR might reflect the newly achieved status of systematic inflammation after the removal of tumor. But given the influence on the alterations of inflammation caused by surgery, the time interval of recovery to new balance remains controversial. The majority of previous studies usually use one month as the interval of recovery, but we doubt whether it is the optimal time point because of the common backgrounds with cirrhosis in HCC patients. Thus, we conducted the comparative analyses of postoperative NLR at different time points after liver resection. We found that the postoperative NLR with a comparatively long-term interval had a better predictive value. Data showed that the AUC of NLR (3–6 m) for DFS and OS was 0.656 and 0.650, whereas the AUC of NLR (4–8 w) for DFS and OS was 0.572 and 0.621. Hence, the postoperative NLR at 3–6 months after operation might reflect more clinical significance than NLR at one month after operation, but further clinical and experimental investigations are needed. Besides, the median of NLR (4–8 w) was lower than NLR (3–6 m), which may be related to the medications used to avoid postoperative infection.
Based on the previous investigations on preoperative and postoperative NLR with significant results, some researchers have transferred their attention to the changes of NLR after different treatments. They believed the dynamic changes may reflect the transformation of balance between host inflammatory response and immune response after therapies, but its predictive role has not been adequately explored. In patients with small HCC after curative resection, Peng et al. found that neither pre-NLR nor post-NLR was an independent factor of DFS or OS, while the NLR change showed significant predictability for the prognostic value (22). And Dan et al. also found the postoperative NLR change was a predictor for both RFS and OS, which indicated a better predictive strength of postoperative NLR change than that of preoperative NLR (15). In the present study, we also made an analysis on the prognostic significance of NLRc in HCC patients. And notably, we compared the NLRc at different time points after operation, which could show a better landscape of inflammatory characteristics at different interval after operation. In addition, patients enrolled in our study were separated into three groups according to the change ratios, and we defined a group of no change with NLR change less than 25%, whereas most other studies only have two groups with an increase or decrease of NLR after treatment. Finally, we found that a 25% increase of NLR at 3–6 months after operation predicted a worse DFS than no change in HCC patients.
Nevertheless, there are several limitations in our study. Firstly, this is a retrospective analysis with data from a single center, which has some potential bias with limited cases. Secondly, the follow-up period was still limited with almost half of patients less than 24 months, which might also cause some biases. And the survival analyses need to be further evaluated in patients with long-term and valid follow-up. Thirdly, HCC patients enrolled in our study all received hepatectomy, and their BCLC stages range from 0 to C, which may have some influence on the overall DFS and OS. But on the contrast, this population characteristic may reflect the real-world practice of treatment selection in HCC patients.
Conclusions
In conclusion, the present study demonstrates that the post-NLRs and corresponding NLRcs have better prognostic significance than pre-NLR in HCC patients receiving liver resection, especially with a relatively long-term interval after operation.
Acknowledgments
Funding: This work was supported by the Guangdong Natural Science Foundation [2016A030313278, 2015A030313038, 2015A030312013]; Science and Technology Program of Guangzhou city [2014Y2-00200, 201604020001, 201508020262, 201400000001-3, 201607010024]; Science and Technology Program of Guangdong Province [2017B020209004, 20169013]; National 13th Five-Year Science and Technology Plan Major Projects of China [2017ZX10203205-006-001]; and Guangdong Key Laboratory of Liver Disease Research [2017B030314027].
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The Third Affiliated Hospital of Sun Yat-Sen University and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.11.29). The authors have no conflicts of interest to declare.
References
- 1.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301-14. 10.1016/S0140-6736(18)30010-2 [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2016;2:16018. 10.1038/nrdp.2016.18 [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology 2016;150:835-53. 10.1053/j.gastro.2015.12.041 [DOI] [PubMed] [Google Scholar]
- 5.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860-7. 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez D, Farid S, Malik HZ, et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg 2008;32:1757-62. 10.1007/s00268-008-9552-6 [DOI] [PubMed] [Google Scholar]
- 8.Halazun KJ, Hardy MA, Rana AA, et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg 2009;250:141-51. 10.1097/SLA.0b013e3181a77e59 [DOI] [PubMed] [Google Scholar]
- 9.Huang ZL, Luo J, Chen MS, et al. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization. J Vasc Interv Radiol 2011;22:702-9. 10.1016/j.jvir.2010.12.041 [DOI] [PubMed] [Google Scholar]
- 10.Mano Y, Shirabe K, Yamashita Y, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg 2013;258:301-5. 10.1097/SLA.0b013e318297ad6b [DOI] [PubMed] [Google Scholar]
- 11.Zheng YB, Zhao W, Liu B, et al. The blood neutrophil-to-lymphocyte ratio predicts survival in patients with advanced hepatocellular carcinoma receiving sorafenib. Asian Pac J Cancer Prev 2013;14:5527-31. 10.7314/APJCP.2013.14.9.5527 [DOI] [PubMed] [Google Scholar]
- 12.Son SH, Park EY, Park HH, et al. Pre-radiotherapy neutrophil-to-lymphocyte ratio as an independent prognostic factor in patients with locally advanced hepatocellular carcinoma treated with radiotherapy. Oncotarget 2017;8:16964-71. 10.18632/oncotarget.15209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taussig MD, Irene KME, Mouli SK, et al. Neutrophil to lymphocyte ratio predicts disease progression following intra-arterial therapy of hepatocellular carcinoma. HPB (Oxford) 2017;19:458-64. 10.1016/j.hpb.2017.01.013 [DOI] [PubMed] [Google Scholar]
- 14.Kinoshita A, Onoda H, Imai N, et al. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br J Cancer 2012;107:988-93. 10.1038/bjc.2012.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dan J, Zhang Y, Peng Z, et al. Postoperative neutrophil-to-lymphocyte ratio change predicts survival of patients with small hepatocellular carcinoma undergoing radiofrequency ablation. PLoS One 2013;8:e58184. 10.1371/journal.pone.0058184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parisi I, Tsochatzis E, Wijewantha H, et al. Inflammation-based scores do not predict post-transplant recurrence of hepatocellular carcinoma in patients within Milan criteria. Liver Transpl 2014;20:1327-35. 10.1002/lt.23969 [DOI] [PubMed] [Google Scholar]
- 17.Ohno Y, Nakashima J, Ohori M, et al. Followup of neutrophil-to-lymphocyte ratio and recurrence of clear cell renal cell carcinoma. J Urol 2012;187:411-7. 10.1016/j.juro.2011.10.026 [DOI] [PubMed] [Google Scholar]
- 18.Lalani AA, Xie W, Martini DJ, et al. Change in Neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J Immunother Cancer 2018;6:5. 10.1186/s40425-018-0315-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin F, Han A, Shi F, et al. The postoperative neutrophil-to-lymphocyte ratio and changes in this ratio predict survival after the complete resection of stage I non-small cell lung cancer. Onco Targets Ther 2016;9:6529-37. 10.2147/OTT.S117290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Min KW, Kwon MJ, Kim DH, et al. Persistent elevation of postoperative neutrophil-to-lymphocyte ratio: A better predictor of survival in gastric cancer than elevated preoperative neutrophil-to-lymphocyte ratio. Sci Rep 2017;7:13967. 10.1038/s41598-017-13969-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen TM, Lin CC, Huang PT, et al. Neutrophil-to-lymphocyte ratio associated with mortality in early hepatocellular carcinoma patients after radiofrequency ablation. J Gastroenterol Hepatol 2012;27:553-61. 10.1111/j.1440-1746.2011.06910.x [DOI] [PubMed] [Google Scholar]
- 22.Peng W, Li C, Wen TF, et al. Neutrophil to lymphocyte ratio changes predict small hepatocellular carcinoma survival. J Surg Res 2014;192:402-8. 10.1016/j.jss.2014.05.078 [DOI] [PubMed] [Google Scholar]
- 23.Hung HC, Lee JC, Cheng CH, et al. Impact of neutrophil to lymphocyte ratio on survival for hepatocellular carcinoma after curative resection. J Hepatobiliary Pancreat Sci 2017;24:559-69. 10.1002/jhbp.498 [DOI] [PubMed] [Google Scholar]
- 24.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Xu L, Luo Y, et al. The inflammation-based scores to predict prognosis of patients with hepatocellular carcinoma after hepatectomy. Med Oncol 2014;31:883. 10.1007/s12032-014-0883-x [DOI] [PubMed] [Google Scholar]
- 26.Pinato DJ, Shiner RJ, Seckl MJ, et al. Prognostic performance of inflammation-based prognostic indices in primary operable non-small cell lung cancer. Br J Cancer 2014;110:1930-5. 10.1038/bjc.2014.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang K, Lei J, Li C, et al. Comparison of the prognostic values of selected inflammation based scores in patients with medullary thyroid carcinoma: A pilot study. J Surg Oncol 2017;116:281-7. 10.1002/jso.24683 [DOI] [PubMed] [Google Scholar]
- 28.Song X, Zhu H, Pei Q, et al. Significance of inflammation-based indices in the prognosis of patients with non-metastatic colorectal cancer. Oncotarget 2017;8:45178-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeuchi H, Kawanaka H, Fukuyama S, et al. Comparison of the prognostic values of preoperative inflammation-based parameters in patients with breast cancer. PLoS One 2017;12:e0177137. 10.1371/journal.pone.0177137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang T, Zhu J, Zhao L, et al. Lymphocyte to monocyte ratio and neutrophil to lymphocyte ratio are superior inflammation-based predictors of recurrence in patients with hepatocellular carcinoma after hepatic resection. J Surg Oncol 2017;115:718-28. 10.1002/jso.24549 [DOI] [PubMed] [Google Scholar]
- 31.Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. 10.1093/jnci/dju124 [DOI] [PubMed] [Google Scholar]
- 32.Chua ML, Tan SH, Kusumawidjaja G, et al. Neutrophil-to-lymphocyte ratio as a prognostic marker in locally advanced nasopharyngeal carcinoma: A pooled analysis of two randomised controlled trials. Eur J Cancer 2016;67:119-29. 10.1016/j.ejca.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, Wang R, Chen W, et al. Prognostic significance of neutrophil to lymphocyte ratio in patients with gallbladder carcinoma. HPB (Oxford) 2016;18:600-7. 10.1016/j.hpb.2016.03.608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bar-Ad V, Palmer J, Li L, et al. Neutrophil to lymphocyte ratio associated with prognosis of lung cancer. Clin Transl Oncol 2017;19:711-7. 10.1007/s12094-016-1593-y [DOI] [PubMed] [Google Scholar]
- 35.Cho KM, Park H, Oh DY, et al. Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and their dynamic changes during chemotherapy is useful to predict a more accurate prognosis of advanced biliary tract cancer. Oncotarget 2017;8:2329-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, Zhang S, Song Y, et al. Prognostic role of neutrophil lymphocyte ratio in patients with glioma. Oncotarget 2017;8:59217-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin G, Liu Y, Li S, et al. Elevated neutrophil-to-lymphocyte ratio is an independent poor prognostic factor in patients with intrahepatic cholangiocarcinoma. Oncotarget 2016;7:50963-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Min GT, Li YM, Yao N, et al. The pretreatment neutrophil-lymphocyte ratio may predict prognosis of patients with liver cancer: A systematic review and meta-analysis. Clin Transplant 2018;32:e13151. 10.1111/ctr.13151 [DOI] [PubMed] [Google Scholar]
- 39.Qi X, Li J, Deng H, et al. Neutrophil-to-lymphocyte ratio for the prognostic assessment of hepatocellular carcinoma: A systematic review and meta-analysis of observational studies. Oncotarget 2016;7:45283-301. 10.18632/oncotarget.9942 [DOI] [PMC free article] [PubMed] [Google Scholar]