Abstract
Oz virus is a novel thogotovirus isolated from ticks that causes lethal infection in mice. We conducted serosurveillance of Oz virus infection among humans and wild mammals in Japan using virus-neutralization tests and ELISAs. Results showed that Oz virus may be naturally infecting humans and other mammalian hosts.
Keywords: Oz virus, zoonoses, thogotoviruses, tick-borne viruses, vector-borne infections, arboviruses, viruses, Japan
The genus Thogotovirus, family Orthomyxoviridae, comprises viruses that are most frequently transmitted by a variety of hard and soft tick species (1). Although most thogotoviruses are associated with tick species, there are several exceptions, such as Sinu virus, which was isolated from mosquitoes (2); Dielmo orthomyxovirus, isolated from Culicoides midges (3); and Araguari virus, isolated only from vertebrates (4). Thogoto, Dhori, and Bourbon viruses have been associated with human disease. Thogoto and Dhori viruses have been reported to cause encephalitis, febrile illness, and death in humans (5,6), and Bourbon virus to cause febrile illness and death in humans (7). In addition, Thogoto virus has been reported to cause abortions in sheep (8), and many wild animals are positive for Bourbon virus antibodies (9).
Oz virus, a new member of the genus Thogotovirus, was first isolated from a pool of 3 Amblyomma testudinarium tick nymphs collected in Ehime prefecture, Japan (10). Phylogenetic analyses revealed that Oz virus is more closely related to Dhori, Batken, and Bourbon viruses than to other thogotoviruses (10). In addition, Oz virus has been shown to cause lethal infection in experimentally challenged suckling mice. To determine the potential of Oz virus as a zoonotic pathogen, we performed serosurveillance of Oz virus infection among mammals, including humans, in Japan.
The Study
To examine whether mammals are naturally infected with Oz virus, we collected serum samples from 24 hunters and 240 wild animals (40 Japanese macaques [Macaca fuscata], 124 wild boars [Sus scrofa], and 76 sika deer [Cervus nippon]) captured in Yamaguchi prefecture, Japan, during 2013–2019. Because Yamaguchi prefecture is close to Ehime prefecture and the environment in Yamaguchi is very similar to that in Ehime, we used stocked samples in Yamaguchi prefecture for the first surveillance of Oz virus infection. To test for the presence of Oz virus antibodies in the serum samples, we performed a PRNT80 (80% plaque-reduction neutralizing test) using Oz virus (Table 1). Among humans, 8.3% of the serum samples had Oz virus neutralization (VN) antibodies; VN titers were 1:40 and 1:80. In wild animals, serum from 47.5% of macaques, 60.5% of wild boars, and 73.7% of sika deer in Yamaguchi had Oz virus VN antibodies (Table 1).
Table 1. Serosurveillance of Oz virus infection by virus-neutralization test among mammals in Yamaguchi prefecture, Japan.
| Species | Genus and species | Years | Virus-neutralization titer |
||||||
|---|---|---|---|---|---|---|---|---|---|
| <1:10 | 1:10 | 1:20 | 1:40 | 1:80 | 1:160 | >1:160 | |||
| Human | Homo sapiens | 2015 | 22 | 0 | 0 | 1 | 1 | 0 | 0 |
| Macaque | Macaca fuscata | 2018–2019 | 21 | 0 | 2 | 3 | 3 | 6 | 5 |
| Wild boar | Sus scrofa leucomystax | 2013–2014 | 49 | 2 | 12 | 10 | 15 | 20 | 16 |
| Sika deer | Cervus nippon | 2014–2015 | 20 | 5 | 8 | 11 | 12 | 13 | 7 |
We applied ELISA protocol used for serosurveillance of many infectious diseases (11–13) to detect Oz virus antibodies in the serum samples from wild animals. We extracted proteins from Oz virus or mock-infected Vero cells and used the extracts as ELISA antigens. To prepare the primary antibody, we diluted serum samples 1:100 in phosphate-buffered saline containing 0.05% Tween 20 and 0.4% Block Ace. We used peroxidase conjugated recombinant protein A/G (Thermo Fisher Scientific, https://www.thermofisher.com) as the secondary antibody and KPL ABTS peroxidase substrate (SeraCare Life Sciences, https://www.seracare.com) as the detection reagent. We measured absorbance using a spectrophotometer (Bio-Rad Laboratories, https://www.bio-rad.com) with a 405 nm filter and subtracted the value of the corresponding control mock-infected cells from all values.
To determine ELISA cutoff values, we tested serum samples from the 40 macaques, 124 wild boars, and 76 sika deer captured in Yamaguchi prefecture. We compared the optical density values of the ELISA to the results of the VN test by 2-graph receiver-operating characteristic (ROC) curve analysis as described elsewhere (14). In macaques, the correlation coefficient between the ELISA and VN test was 0.9163, and an ELISA cutoff value of 0.2245 produced 100% sensitivity and specificity. In wild boars, the correlation coefficient was 0.8807, with 88.0% sensitivity and 89.8% specificity at an ELISA cutoff value of 0.1965. In sika deer, the correlation coefficient was 0.7569, sensitivity 78.6%, and specificity 80.0% at an ELISA cutoff value of 0.3165 (Figure 1).
Figure 1.
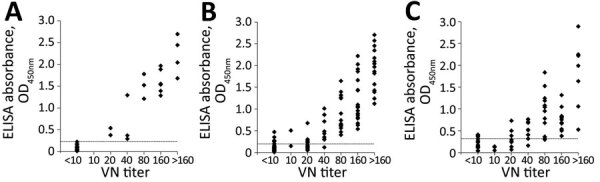
Dot plot comparison between VN test and ELISA against Oz virus in serum samples from wild animals in Yamguchi prefecture, Japan. A) Macaques (n = 40); B) wild boar (n = 124); C) sika deer (n = 76). The correlation coefficient between VN test and ELISA from macaques was 0.9163, from wild boars was 0.8807, and from sika deer was 0.7569. The optimal cutoff value of ELISA was calculated by 2-graph receiver-operating characteristic curve. The optimal cutoff values were set at 0.225 for macaques, 0.197 for wild boar, and 0.317 for sika deer serum samples and are indicated by dotted lines.
Next, we surveyed Oz virus infection among macaques, wild boars, and sika deer in many prefectures in Japan using the established ELISA (Table 2; Figure 2). Among 197 macaques captured during 2007–2019, seropositivity rates were 47.5% in Yamaguchi, 33.3% in Wakayama, and 6.3% in Mie prefectures. Among 879 wild boars captured during 2007–2014, seropositivity rates were 10.3% in Oita, 55.8% in Yamaguchi, 34.8% in Wakayama, 10.5% in Gifu, and 0% in both Toyama and Tochigi prefectures. Among 450 sika deer, seropositivity rates were 37.8% in Yamaguchi, 11.1% in Wakayama, 8.3% in Gifu, and 30% in Chiba prefectures.
Table 2. Serosurveillance of Oz virus infection by ELISA among wild animals, Japan .
| Species | Prefecture | Years | Cutoff | No. serum samples examined | No. (%) positive serum samples |
|---|---|---|---|---|---|
| Macaque |
Yamaguchi | 2018–2019 | 0.225 |
40 | 19 (47.5) |
| Wakayama | 2012–2013 | 15 | 5 (33.3) | ||
| Mie |
2007 |
142 |
9 (6.3) |
||
| Wild boar |
Oita | 2012 | 0.197 |
58 | 6 (10.3) |
| Yamaguchi | 2010–2014 | 344 | 192 (55.8) | ||
| Wakayama | 2007–2013 | 89 | 31 (34.8) | ||
| Gifu | 2014 | 19 | 2 (10.5) | ||
| Toyama | 2014 | 20 | 0 | ||
| Tochigi |
2010–2012 |
349 |
0 |
||
| Sika deer | Yamaguchi | 2010–2015 | 0.317 | 407 | 154 (37.8) |
| Wakayama | 2010–2014 | 9 | 1 (11.1) | ||
| Gifu | 2014 | 24 | 2 (8.3) | ||
| Chiba | 2014 | 10 | 3 (30.0) |
Figure 2.
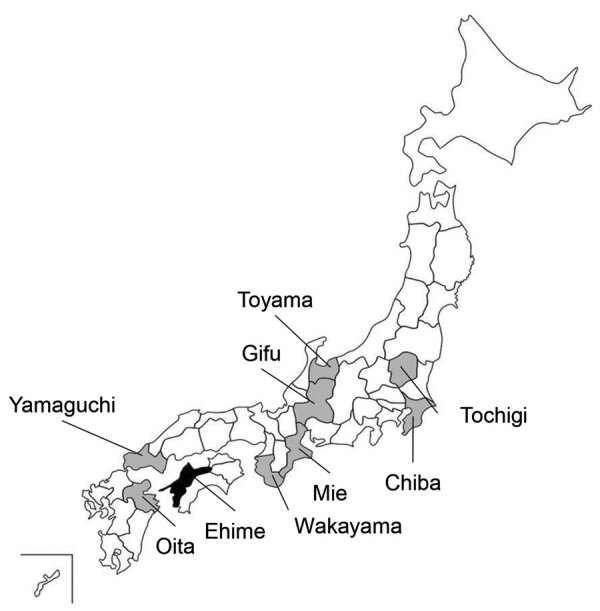
Collection sites of serum samples from macaques, wild boars, and sika deer for study of Oz virus seroprevalence in Japan. Gray shading indicates prefectures in which samples were collected; black shading indicates Ehime prefecture, where Oz virus was first isolated.
First, we applied PRNT80 to detect Oz virus antibodies in humans and wild animals in Yamaguchi prefecture. The results showed that 60.5% of wild boars, a major host of A. testudinarium, and 73.7% of sika deer in Yamaguchi prefecture during 2013 and 2015 had Oz virus VN antibodies, indicating that the virus was infecting wild animals in the western part of Japan. Next, we examined wild macaques for Oz virus infection; 48% were infected, and the antibody titers were high. In addition, 2 persons who hunted wild boars and sika deer in Yamaguchi prefecture had Oz virus antibodies. These results indicate that humans and macaques are also exposed to Oz virus.
We compared results from an ELISA, established for this study, using an Oz virus–infected cell extract for the surveillance of Oz virus infection among many mammalians, with those from the VN test to determine correlation between the 2 tests. The correlation coefficient was 0.9163 for macaques, 0.8807 for wild boars, and 0.7569 for sika deer, suggesting that the ELISA is effective for serosurveillance of Oz virus infection in samples from many animal species. However, because its sensitivity and specificity differed among animal species and values were lower for sika deer, in particular, cutoff values should be determined for each animal species. In addition, VN testing should be performed to confirm the presence of Oz virus antibodies.
Our nationwide surveillance of Oz virus infection in Japan indicated that many wild animals were positive for Oz virus antibodies. However, wild boars in Toyama and Tochigi prefectures did not have Oz virus antibodies, suggesting that the virus might not be distributed in the northern and eastern parts of Japan. A. testudinarium is the major tick species that infests humans in the southern and western parts of Japan (15), and because we found Oz virus mainly in those areas, it appears that the distribution of Oz virus–infected animals correlates with the habitat of the tick. In addition, because 2 hunters in Yamaguchi prefecture tested positive for Oz virus antibodies, further investigation is needed to determine whether Oz virus might be a zoonotic pathogen, especially because intracerebral inoculation of the virus in suckling mice causes lethal disease (10).
Acknowledgments
We thank Mr. Hashimura and Mr. Matsuzaki for the collection of macaque serum samples, Akiko Nakamura and Kishiko Matsumoto for the collection of human serum samples, Tsutomu Takeda for the collection of wild boar serum samples in Tochigi, Kazuo Suzuki for the collection of serum samples from wild animals, and Toshiya Kimura and Hiroto Shinomiya for advice on Oz virus infection in Ehime. We also acknowledge all of the hunters who provided support and cooperated with the collection of serum samples.
This study was supported by grants from AMED (Japan Agency for Medical Research and Development) (JP20wm0225009, JP21fk0108613, JP19fk0108097, JP20fk0108067 and JP16fk0108117). N.T.B.T. received a scholarship from the Government of Vietnam Ministry of Agriculture and Rural Development.
All animal samples used in this study were collected with the appropriate hunting permits issued by the respective local government. This article does not contain any studies with live animals. Human serum samples were collected from 24 hunters in Yamaguchi with the approval of the institutional review board of Yamaguchi University (number H26–116).
Biography
Mrs. Tran is a PhD student in the Joint Faculty of Veterinary Medicine, Yamaguchi University, Yamaguchi, Japan. Her research interest is zoonoses.
Footnotes
Suggested citation for this article: Tran NTB, Shimoda H, Ishijima K, Yonemitsu K, Minami S, Supriyono, et al. Zoonotic infection with Oz virus, a novel thogotovirus. Emerg Infect Dis. 2022 Feb [date cited]. https://doi.org/10.3201/eid2802.211270
References
- 1.Hubálek Z, Rudolf I, Nowotny N. Arboviruses pathogenic for domestic and wild animals. Adv Virus Res. 2014;89:201–75. 10.1016/B978-0-12-800172-1.00005-7 [DOI] [PubMed] [Google Scholar]
- 2.Contreras-Gutiérrez MA, Nunes MRT, Guzman H, Uribe S, Suaza Vasco JD, Cardoso JF, et al. Sinu virus, a novel and divergent orthomyxovirus related to members of the genus Thogotovirus isolated from mosquitoes in Colombia. [Erratum in: Virology. 2017;503:114.]. Virology. 2017;501:166–75. 10.1016/j.virol.2016.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Temmam S, Monteil-Bouchard S, Robert C, Baudoin JP, Sambou M, Aubadie-Ladrix M, et al. Characterization of viral communities of biting midges and identification of novel Thogotovirus species and Rhabdovirus genus. Viruses. 2016;8:77. 10.3390/v8030077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Da Silva EV, Da Rosa AP, Nunes MR, Diniz JA, Tesh RB, Cruz AC, et al. Araguari virus, a new member of the family Orthomyxoviridae: serologic, ultrastructural, and molecular characterization. Am J Trop Med Hyg. 2005;73:1050–8. 10.4269/ajtmh.2005.73.1050 [DOI] [PubMed] [Google Scholar]
- 5.Moore DL, Causey OR, Carey DE, Reddy S, Cooke AR, Akinkugbe FM, et al. Arthropod-borne viral infections of man in Nigeria, 1964-1970. Ann Trop Med Parasitol. 1975;69:49–64. 10.1080/00034983.1975.11686983 [DOI] [PubMed] [Google Scholar]
- 6.Butenko AM, Leshchinskaia EV, Semashko IV, Donets MA, Mart’ianova LI. [Dhori virus—a causative agent of human disease. 5 cases of laboratory infection] [in Russian]. Vopr Virusol. 1987;32:724–9. [PubMed] [Google Scholar]
- 7.Kosoy OI, Lambert AJ, Hawkinson DJ, Pastula DM, Goldsmith CS, Hunt DC, et al. Novel thogotovirus associated with febrile illness and death, United States, 2014. Emerg Infect Dis. 2015;21:760–4. 10.3201/eid2105.150150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies FG, Soi RK, Wariru BN. Abortion in sheep caused by Thogoto virus. Vet Rec. 1984;115:654. 10.1136/vr.115.25-26.654 [DOI] [PubMed] [Google Scholar]
- 9.Jackson KC, Gidlewski T, Root JJ, Bosco-Lauth AM, Lash RR, Harmon JR, et al. Bourbon virus in wild and domestic animals, Missouri, USA, 2012–2013. Emerg Infect Dis. 2019;25:1752–3. 10.3201/eid2509.181902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ejiri H, Lim CK, Isawa H, Fujita R, Murota K, Sato T, et al. Characterization of a novel thogotovirus isolated from Amblyomma testudinarium ticks in Ehime, Japan: A significant phylogenetic relationship to Bourbon virus. Virus Res. 2018;249:57–65. 10.1016/j.virusres.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 11.Shimoda H, Inthong N, Noguchi K, Terada Y, Nagao Y, Shimojima M, et al. Development and application of an indirect enzyme-linked immunosorbent assay for serological survey of Japanese encephalitis virus infection in dogs. J Virol Methods. 2013;187:85–9. 10.1016/j.jviromet.2012.09.022 [DOI] [PubMed] [Google Scholar]
- 12.Suzuki J, Nishio Y, Kameo Y, Terada Y, Kuwata R, Shimoda H, et al. Canine distemper virus infection among wildlife before and after the epidemic. J Vet Med Sci. 2015;77:1457–63. 10.1292/jvms.15-0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yonemitsu K, Minami S, Noguchi K, Kuwata R, Shimoda H, Maeda K. Detection of anti-viral antibodies from meat juice of wild boars. J Vet Med Sci. 2019;81:155–9. 10.1292/jvms.18-0576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikawa-Yoshida A, Yoshii K, Kuwahara K, Obara M, Kariwa H, Takashima I. Development of an ELISA system for tick-borne encephalitis virus infection in rodents. Microbiol Immunol. 2011;55:100–7. 10.1111/j.1348-0421.2010.00296.x [DOI] [PubMed] [Google Scholar]
- 15.Okino T, Ushirogawa H, Matoba K, Hatsushika R. A bibliographical study of human cases of hard tick (Acarina: Ixodidae) bites received abroad and found in Japan. Kawasaki Med J. 2007;33:189–94. [Google Scholar]


