Lymphedema is an abnormal extracellular protein-rich fluid accumulation within the interstitial compartment of tissue. This is commonly caused by an impairment of lymphatic drainage which can have different causes. Possible etiologies are lymphatic malformation, infection, iatrogenic injury, metastatic Chron’s disease, amyloidosis, sarcoidosis and malignancy (1). Anyway, in the western world the majority of cases of limb lymphedema occur after cancer-related surgeries and adjuvant therapies (2). Being breast cancer the most frequent malignancy among women, breast cancer-related lymphedema (BCRL) is a rather common complication, in particular when axillary lymph node dissection and radiotherapy are required. Zou et al. showed that over a period of 2 years after breast cancer surgery almost one-third of the patients develop BCRL (3). It usually involves the ipsilateral arm but it is worth mentioning that also breast lymphedema (BLE) is a frequent complication of this treatment, especially when axillary lymph node removal is performed where it can reach an incidence up to 50% (4).
Lymphedema is a delicate condition that can deeply affect the quality of life of the patients and may lead to severe complications. The most common symptoms are swelling, redness, and a sensation of heaviness that can be very disturbing for the patient. Moreover, in the long term an irreversible fibrosis of the tissues might develop leading to chronic cellulitis and range of motion limitation (2). For this reason, it is important to face promptly this problem with the best possible therapy. Early and mild lymphedemas are primarily managed conservatively with compressive bandings and manual decongestion while more severe cases often require a surgical approach. However, conservative treatments do not solve the cause of the disease but simply try to limit its manifestations. The idea behind surgery, instead, is to try to restore a functional pathway for lymph drainage in the most physiological way. The first interventions involving lymphatic vessels were described back in 1962 by Jacobson and Suarez (5). Nowadays, lympho-lymphatic bypass, vascularized lymph node transfer (VLNT) and lymphovenous anastomosis (LVA) are the most credited options, but which one of these procedures is the most indicated is still widely debated (6). Lympho-lymphatic bypass uses healthy lymphatic grafts from another site, usually the lower extremity, to bypass the damaged area. For example, in the arm region the lymphatics of the upper extremity are anastomosed with the lymphatic vessels of the graft and the lymphatic graft is then anastomosed to healthy lymphatic ducts in the supraclavicular-neck area (7). This technique showed a concrete volume reduction but presents a high risk of developing lymphedema in the donor site. Then, VLNT involves harvesting the lymph nodes with their vascular supply from a healthy region and transferring them as a free tissue transfer performing a microsurgical anastomosis between the blood vessels of the flap and the recipient site vessels. Typical donor sites are axillar, inguinal, submental, supraclavicular, omental and mesenteric lymph nodes (8).
LVA consists in linking a lymphatic vessel to a nearby vein diverting the lymph flow into the systemic circulation bypassing a damaged area. The venous system can easily accommodate the extra volume offering a powerful drainage pathway for the lymphatic fluid of the affected limb. With the improvement of supermicrosurgical instruments, this technique is gaining more and more approval for the management not only of lymphedema but also of lymphoceles refractory to conservative treatments and other lymphatic diseases in many different body areas (9). One of the main critics addressed to LVA is the long-term patency of the anastomosis. In this perspective, Wolfs et al. (10) have done a particularly interesting work analyzing not only the efficacy of this procedure in terms of improvement of signs and symptoms, but also measuring the actual number of patients presenting a conserved patency after 12 months and its correlation with clinical improvements. They showed that more than 70% of the patients still had at least one patent anastomosis, and, even more interesting, that this percentage increased with increasing number of anastomoses, up to 100% for those who received three anastomoses. In the literature there is a significant variation from the technical point of view regarding the ideal number of anastomoses and the necessity of supplementary interventions (11), therefore this element may help in giving a clear indication about the adequate number of anastomoses to be performed in each patient. Previous studies tried to tackle this point but no consensus was obtained with some authors sustaining the importance of a high number of anastomoses and others stating that this does not significantly influence the efficacy of the treatment (12-14).
Then they noticed a positive correlation between patent anastomosis and clinical improvements in terms of quality of life, arm circumference and discontinuation of compression stockings. This is a very relevant aspect because it offers a proof behind the effectiveness of this therapy already showed by other studies. Furthermore, the discontinuation of compressive stockings is another interesting point since, as they properly stated in the article, these are one of the most disturbing problems for the patients and being able remove them is a goal not to be underestimated.
Another valuable indication can be obtained analyzing the different patient characteristics between patent and non-patent anastomosis group. We notice that those who had a worse outcome were older patients with a longer history of lymphedema. This led to a much higher rate of preoperative infections and, as we said before, probably a higher degeneration of quality of tissue which makes the surgical treatment less effective. There is a well-established correlation between lymphedema and episodes of cellulitis and the fibrosis induced by recurrent inflammation causes an additional deterioration of the remaining lymphatic vessels that further exacerbates the disease (15,16). For this reason, once again, we can realize how important is to treat the lymphedema as soon as possible.
In addition, also the ICG lymphography deserves a careful consideration since it is a precious tool in all the steps of lymphatic diseases management. It is easy to execute and, depending on the pattern shown, it allows a definitive differential diagnosis between lymphatic or venous etiology of the edema and gives a reliable staging of the severity. It is also useful intraoperatively in order to identify the functional vessels for the anastomosis and postoperatively to check its patency, both immediately and during the follow up (12).
Our experience over the last 2 years consists in about 25 cases of BCRL treated by means of LVA performing a mean of 5 anastomoses. It is our practice to always proof intraoperatively the patency and function of the LVAs with ICG lymphography (Figures 1,2). Similarly, to what presented Wolfs and his colleagues we obtained in almost all cases an improvement of subjective symptoms and quality of life with a marked satisfaction from the patients. We also resorted to this procedure to successfully treat other pathologies such as iatrogenic lymphoceles, especially in the thigh. For a consistent number of patients our follow up period is longer than 12 months and we report very few cases of recurrences. This is further evidence of the efficacy of this technique for restoring the drainage of lymph fluid accumulation. Moreover, compared to the other surgical options we believe this procedure offers many advantages. First of all, it is much less invasive, it can be performed under local anesthesia and it is relatively quick. This is an important aspect also from the patient point of view since they have often already experienced a long and difficult series of interventions to defeat the cancer and they are not prone to undergone more demanding surgeries. VLNT and lympho-lymphatic anastomosis with tissue transfer, unlike LVA, require some tissue from a donor site inevitably causing some damage to an otherwise healthy region. Moreover, the mechanism behind their function remains unclear.
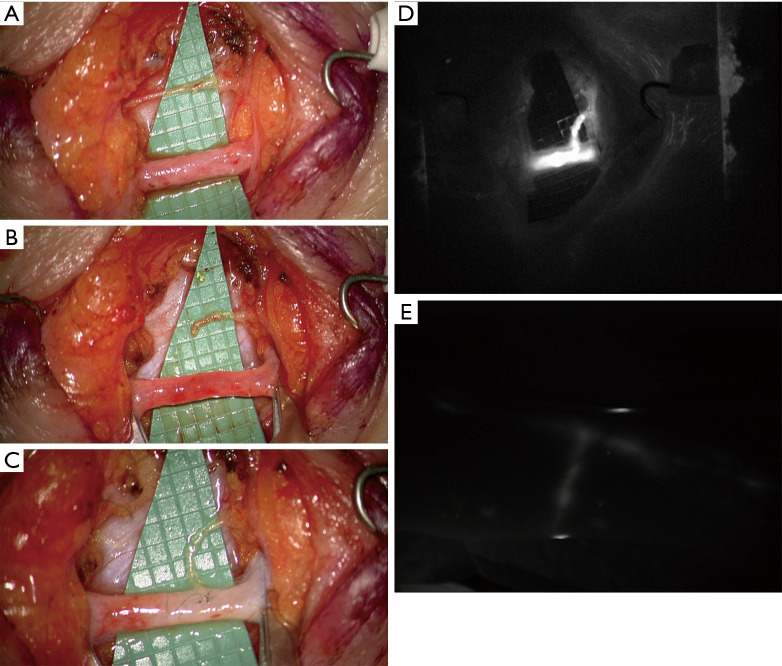
Figure 1.
Intraoperative picture of LVA performed in end-to-side fashion. Vessels isolated and prepared for the anastomosis (A,B); lymphatic vessel anastomosed to the side of the nearby larger-caliber vein (C); immediate intraoperative ICG lymphography proving the patency of the anastomosis (D); 12-month postoperative ICG lymphography showing conserved patency (E). LVA, lymphovenous anastomosis.
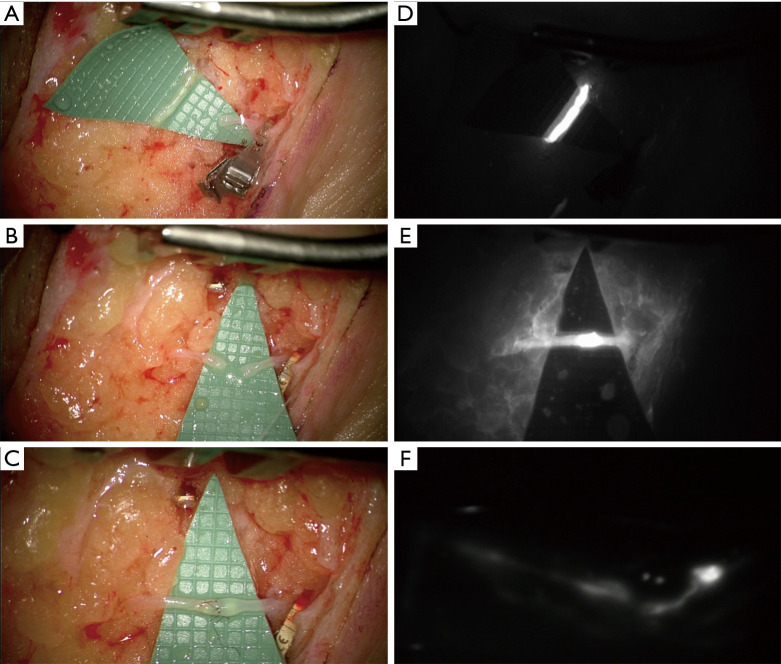
Figure 2.
Intraoperative picture of LVA performed in end-to-end fashion. Isolation of a functioning lymphatic vessel and a nearby similar-caliber vein (A); lymphatic vessel and vein prepared for the anastomosis (B); end-to-end anastomosis between the lymphatic vessel and the nearby vein (C); immediate intraoperative ICG lymphography before the anastomosis (D); intraoperative ICG lymphography proving the patency of the anastomosis (E); 12-month postoperative ICG lymphography showing conserved patency (F). LVA, lymphovenous anastomosis.
For all these reasons we believe that LVA could become in the near future the gold standard for the treatment of all those diseases caused by an impairment of lymph drainage pathway and it is important to keep on analyzing the growing amount of data about long term outcomes of patients that received this therapy.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Provenance and Peer Review: This article was commissioned by the Editorial Office, Translational Cancer Research. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.04.14). The authors have no conflicts of interest to declare.
References
- 1.Saha M, Edmonds E, Martin J, et al. Penile lymphoedema in association with asymptomatic Crohn's disease. Clin Exp Dermatol 2009;34:88-90. 10.1111/j.1365-2230.2008.02762.x [DOI] [PubMed] [Google Scholar]
- 2.Warren AG, Brorson H, Borud LJ, et al. Lymphedema: a comprehensive review. Ann Plast Surg 2007;59:464-72. 10.1097/01.sap.0000257149.42922.7e [DOI] [PubMed] [Google Scholar]
- 3.Zou L, Liu FH, Shen PP, et al. The incidence and risk factors of related lymphedema for breast cancer survivors post-operation: a 2-year follow-up prospective cohort study. Breast Cancer 2018;25:309-14. 10.1007/s12282-018-0830-3 [DOI] [PubMed] [Google Scholar]
- 4.Rönkä RH, Pamilo MS, von Smitten KA, et al. Breast lymphedema after breast conserving treatment. Acta Oncol 2004;43:551-7. 10.1080/02841860410014867 [DOI] [PubMed] [Google Scholar]
- 5.Jacobson JH, 2nd, Suarez EL. Microvascular surgery. Dis Chest 1962;41:220-4. 10.1378/chest.41.2.220 [DOI] [PubMed] [Google Scholar]
- 6.Leung N, Furniss D, Giele H. Modern surgical management of breast cancer therapy related upper limb and breast lymphoedema. Maturitas 2015;80:384-90. 10.1016/j.maturitas.2015.01.012 [DOI] [PubMed] [Google Scholar]
- 7.Baumeister RG, Siuda S. Treatment of lymphedemas by microsurgical lymphatic grafting: what is proved? Plast Reconstr Surg 1990;85:64-74; discussion 75-6. 10.1097/00006534-199001000-00012 [DOI] [PubMed] [Google Scholar]
- 8.Garza R, 3rd, Skoracki R, Hock K, et al. A comprehensive overview on the surgical management of secondary lymphedema of the upper and lower extremities related to prior oncologic therapies. BMC Cancer 2017;17:468. 10.1186/s12885-017-3444-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giacalone G, Yamamoto T, Hayashi A, et al. Lymphatic supermicrosurgery for the treatment of recurrent lymphocele and severe lymphorrhea. Microsurgery 2019;39:326-31. 10.1002/micr.30435 [DOI] [PubMed] [Google Scholar]
- 10.Wolfs JAGN, de Joode LGEH, van der Hulst RRWJ, et al. Correlation between patency and clinical improvement after lymphaticovenous anastomosis (LVA) in breast cancer-related lymphedema: 12-month follow-up. Breast Cancer Res Treat 2020;179:131-8. 10.1007/s10549-019-05450-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scaglioni MF, Fontein DBY, Arvanitakis M, et al. Systematic review of lymphovenous anastomosis (LVA) for the treatment of lymphedema. Microsurgery 2017;37:947-53. 10.1002/micr.30246 [DOI] [PubMed] [Google Scholar]
- 12.Chang DW, Suami H, Skoracki R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast Reconstr Surg 2013;132:1305-14. 10.1097/PRS.0b013e3182a4d626 [DOI] [PubMed] [Google Scholar]
- 13.Koshima I, Nanba Y, Tsutsui T, et al. Long-term follow-up after lymphaticovenular anastomosis for lymphedema in the leg. J Reconstr Microsurg 2003;19:209-15. 10.1055/s-2003-40575 [DOI] [PubMed] [Google Scholar]
- 14.Mihara M, Hara H, Tange S, et al. Multisite lymphaticovenular bypass using supermicrosurgery technique for lymphedema management in lower lymphedema cases. Plast Reconstr Surg 2016;138:262-72. 10.1097/PRS.0000000000002254 [DOI] [PubMed] [Google Scholar]
- 15.Simon MS, Cody RL. Cellulitis after axillary lymph node dissection for carcinoma of the breast. Am J Med 1992;93:543-8. 10.1016/0002-9343(92)90583-W [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto T, Koshima I. Supermicrosurgical anastomosis of superficial lymphatic vessel to deep lymphatic vessel for a patient with cellulitis-induced chronic localized leg lymphedema. Microsurgery 2015;35:68-71. 10.1002/micr.22327 [DOI] [PubMed] [Google Scholar]