Abstract
Background
Ras-associated domain family protein1 isoform A (RASSF1A) was significantly absent in clinical samples and many osteosarcoma (OS) cell lines. Overexpression of RASSF1A could suppress OS metastasis, which may be mediated by tumor-associated macrophages polarized M2 (M2-TAMs). However, the relationship between IL-1β secreted by M2-TAMs and RASSF1A remains unknown.
Methods
The expression levels of M2-TAMs markers CD68 and CD204 were measured by flow cytometry, and arginase-1 (Arg-1) and interleukin-1β (IL-1β) secreted by M2-TAMs were examined by real-time quantitative PCR (RT-qPCR). MTT assay was employed to determine the proliferation of OS cells, while scratch wound healing assay and Transwell assay were used to evaluate their migration and invasion, respectively. The level of miR-181α-5p was measured by RT-qPCR, while the levels of RASSF1A, GSK-3β, p-GSK-3β, β-catenin, MMP-2 and MMP-9 were evaluated by Western blot. The direct binding of miR-181α-5p and RASSF1A was identified using dual-luciferase reporter assay and RNA immunoprecipitation (RIP) assay.
Results
The levels of CD68, CD204, Arg-1 and IL-1β were elevated in M2-TAMs compared with control group. Overexpression of RASSF1A and knockdown of miR-181α-5p could both suppress invasion and migration of OS cells through Wnt pathway. IL-1β secreted by M2-TAMs facilitated the OS metastasis via RASSF1A/Wnt pathway, which could be targeted by miR-181α-5p and affected by nuclear factor-kappa B (NF-κB).
Conclusions
IL-1β secreted by M2-TAMs contributed to OS metastasis, which could be suppressed by knockdown of miR-181α-5p or overexpression of RASSF1A through NF-κB/miR-181α-5p/RASSF1A/Wnt pathway. These findings can guide new target discovery for drug development in OS treatment.
Keywords: Osteosarcoma (OS), Ras-associated domain family protein1 isoform A (RASSF1A), miR-181α-5p, interleukin-1β (IL-1β)
Introduction
Osteosarcoma (OS) is the most common primary bone tumor with poor prognosis and high incidence to develop metastasis, which accounts for 17% of the primary bone tumor and 42% of the malignant bone tumor (1). Recent decades, the treatment of OS is mainly surgery in combination with pre- and postoperative chemotherapy. The survival rate of primary OS varies between 60–70% (2,3). However, 80–90% of patients have had microscopic lesions in other parts of the body before diagnosis (4). Once recurrence or lung metastasis exists, the survival rate of OS is only 15–30%. OS metastasis has become one of the key prognosis factors affecting the survival outcomes of patients. Therefore, in-depth knowledge of the specific molecular mechanism of OS metastasis needs to be gained in order to find new targets with significant clinical importance and direct new drug discovery to effectively control the metastasis.
The Ras protein plays an active role in regulating cell cycle and apoptosis. It functions as a tumor suppressor and inhibits the migration and invasion of various cancer cells, and suppresses the phosphorylation of Akt/glycogen synthase kinase-3-β (GSK-3β), which locates in the upstream of the Wnt/β-catenin signaling pathway and promotes the degradation of β-catenin. Previous research has indicated that the Ras association domain family 1A (RASSF1A) was inactivated by hypermethylation of its promoter in OS clinical samples and various OS cell lines (5), the lowered expression of RASSF1A was also related with the clinical severity of OS (6). The Wnt/β-catenin signaling pathway modulates the stemness and differentiation in the cellular processes in stem cells, including skeletal development. It is also reported to be activated in different cancers, including bone cancer (7). Inhibition of this pathway by reducing β-catenin expression was reported to suppress OS cell migration and invasion (8). In addition, miR-181α-5p was reported to be abnormally overexpressed in OS cells, which facilitated the proliferation and invasion while suppressed apoptosis of OS cells (9). miR-181α-5p can specifically interact with RASSF1A, and down-regulate the expression of RASSF1A through using TargetScan tool. In recent work, it was also found that interleukin-1β (IL-1β)/nuclear factor-kappa B (NF-κB) signaling pathway was highly activated in OS cells, and the expression of IL-1β and NF-κB was positively correlated with miR-181α-5p level. Our recent studies have found that tumor-associated macrophages polarized M2 (M2-TAMs), the anti-inflammatory phenotype, promoted IL-1β expression, therefore activated the miR-181α-5p/RASSF1A/Wnt pathway and a series of activities during OS progression. To the best of our knowledge, the direct role of miR-181α-5p mediated RASSF1A/Wnt pathway has not been fully characterized in OS yet.
The current study is to investigate the molecular mechanism of NF-κB/miR-181α-5p/RASSF1A/Wnt signal pathway in M2-TAMs and OS cells, which can promote OS metastasis and provide a new perspective for the treatment of OS.
Methods
Cell lines and cell culture
U937, THP-1, MG63 and 143B cell lines were used in this study. All cell lines were obtained from Cell Bank of Type Culture Collection, Chinese Academy of Science (Shanghai, China) and supplemented with RPMI-1640 containing 10% fetal bovine serum (FBS) (both from Gibco, Grand Island, NY, USA) in 5% CO2 incubator until 80% confluence. Cells are cultured in a standard humidified incubator at 37 °C in a 5% CO2 atmosphere. Anakinra (Kineret; Amgen, Thousand Oaks, CA, USA) was used as IL-1β receptor inhibitor and Rocaglamide A (MedChemExpress, New Jersey, USA; Cat.no. HY-19356) was used as NF-κB inhibitor.
Mononuclear cell lines U937 and THP-1 (U937 10 ng/mL; THP-1 100 ng/mL) were induced by phorbol-12-myristate-13-acetate (PMA; MedChemExpress; Cat.no. HY-18739) for three days into macrophages. For induction of M2-TAMs, the cells were cultured in medium with 20 ng/mL interleukin-13 (IL-13) and interleukin-4 (IL-4) for 48 h. After treatment, cells were washed and cultured in serum-free medium for another 24 h.
The ethics committee approval is not required by the local law, as the study involved no human tissues or animals. All cells lines used in this study require no ethics approval.
MTT assay
In brief, MG63 and 143B cells were seeded in 96-well plates. Then, 20 µL of MTT (MedChemExpress; 5 mg/mL stock) was added to each well containing 100 µL of medium. After 4 h of incubation at 37 °C, the culture medium was removed. Then, 100 µL of DMSO (Sigma) was added to each well to dissolve the crystal. The absorbance value at 490 nm was measured by a microplate reader (Bio-Rad, Hercules, CA, USA). Relative viability was expressed as the ratio of the viability of the treated cells relative to that of the control group.
Flow cytometry analysis
Cells (1×106 cells) with good growth status were selected and collected, then digested by trypsin and then washed with 1 the crystal. The absorbance value at 490 nm was measure FITC anti-human CD68 (BD Biosciences), PE anti-human CD204 (BD Biosciences) for 30 min at 4 °C. The cells were then washed with PBS buffer and subject to flow cytometry analyse. The analysis was performed on a FACS Calibur flow cytometer (BD Biosciences, San Diego, CA, USA).
Enzyme-linked immunosorbent assay (ELISA)
Culture medium was collected for the measurement of cytokine concentration by IL-1β ELISA kit (Sino biological, China) and arginase-1 (Arg-1) ELISA kit (Cusabio, China) according to manufacturer’s instructions. Standard curve were plotted by a series of cytokines concentrations, and concentrations within the culture medium collected from different experimental groups were determined by applying the optical density value to the standard curve.
Wound-healing migration assay
After adding MG63 or143B cells to a 6-well plates and incubating to a density of 70–80%, a wound was made by 10 µL tip. After replacing the fresh medium to remove the floating cells, different intervention media were added (protein, antibody or receptor inhibitor), photographed under a microscope (Carl Zeiss, Germany) and used as a control at 0 h. After culturing for 24 h, The width (W) of the scratch measured and the wound closure was calculated as (W0h–h24h)/W0h × hW width (W) of the scratch measured and the wound.
Transwell invasion assay
The cells were resuspended in serum-free medium and inoculated into the upper chamber (200 µL/well, 4×105 cells /mL). A layer of Matrigel was first placed on the upper chamber, and the lower chamber was added with 600 µL/well interventional media in different groups. It was placed in the incubator for 24 h. The upper and lower chambers were washed with PBS twice. Using cotton swabs to wipe off the upper chamber cells and fixing with methanol for 30 min, and finally staining with 0.1% crystal violet for 20 min. After washing with PBS, the cell invasion was observed under a microscope.
Real-time quantitative PCR (RT-qPCR)
Specific primers were designed and synthesized using PrimerExpress 3.0 software according to the nucleic acid sequences published by Gene Bank on NCBI. The total RNA extraction in each group of cell samples was carried out by using TRIzol reagent (Thermo Fisher Scientific) according to the procedure, and cDNA was reverse transcripted from total RNA using a PrimeScript RT reagent Kit (TaKaRa, Dalian, China). PCR reactions were performed using SYBR Premix Ex Taq II (Thermo Fisher Scientific) and the following primers were used: RASSF1A forward, 5'-AGT GCG CGC ATT GCA AGT T-3' and reverse, 5'-AAG GTC AGG TGT CTC CCA C-3'; miR-181α-5p forward, 5'-ACA CTC CAG CTG GGA ACA TTC AAC GCT GTC G-3' and reverse, 5'-CTC AAC TGG T GT CGT GGA GTC GGC AAT TCA GTT GAC TCA CCG-3'. The expression levels of RASSF1A and miR-181α-5p were calculated using the 2–ΔΔCt method. U6 or GAPDH acted as an internal control. All reactions were performed in triplicate.
Western blot
Protein extracts were prepared in radio immunoprecipitation assay (RIPA) lysis buffer (Sigma), resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto the polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA, USA). Blocking was performed using 5% non-fat milk, followed by incubation with corresponding primary and secondary antibodies. Primary antibodies from Abcam (Cambridge, UK) against RASSF1A (1:500, ab97749), GSK-3β (1:500, ab131356), p-GSK-3β (1:500, ab75745), β-catenin (1:2,000, ab16051), MMP-2 (1:1,000, ab97779), MMP-9 (1:300, ab38898) and p65 (1:1,000, ab16502) were obtained. It was normalized with β-actin (1:1,000, ab8227) and visualized by chemiluminescence.
Dual-luciferase reporter assay
The luciferase activity of mutant (Mut)- and wild-type (Wt)-RASSF1A 3'-untranslated region (3'UTR) was measured with the dual luciferase reporter assay in miR-181α-5p mimic negative control (NC) and miR-181α-5p mimic group. The miR-181α-5p mimic was purchased from Gene Pharma Co., Ltd. (Shanghai, China). The Wt-RASSF1A 3'UTR or Mut-RASSF1A 3'UTR was co-transfected with miR-181-5p mimic NC or miR-181-5p mimic into MG63 and 143B cells using Lipofectamine 3000 transfection reagent (Thermo Fisher Scientific). The dual luciferase reporter assay was performed using Dual Luciferase Reporter Assay System (Promega, Madison WI, USA) according to manufacturer’s protocol. Firefly luciferase activity was normalized to that of Renilla luciferase. All experiments were performed in triplicate.
RNA immunoprecipitation (RIP) assay
MG63 or 143B cells were co-transfected with pLV-MS2, pLV-RASSF1A-WT-MS2 or pLV-RASSF1A-Mut-MS2 and pMS2-FLAG by Lipofectamine 3000. Cells were harvested and subjected to RIP assays at 48 h post-transfection using the Magna RNA-binding protein immunoprecipitation kit (Millipore) according to the manufacturer’s instructions. The Flag antibody (Sigma) was used for RIP. The miR-181α-5p expression level in the precipitates was detected by RT-qPCR.
Statistical analysis
The SPSS 22.0 (SPSS Inc., Chicago, IL, USA) was used for all data in statistical analysis. Numerical data in the bar chart were presented as mean ± standard deviation (SD). For three or more group comparisons of one independent variable, each performed with one-way ANOVA. For two-group comparisons of data from one independent variable, each performed with Student’s t-test. P<0.05 was considered statistically significant. All experiments were performed for at least 3 times.
Results
Induction of M2-TAMs increases the expression level of Arg-1 and IL-1β
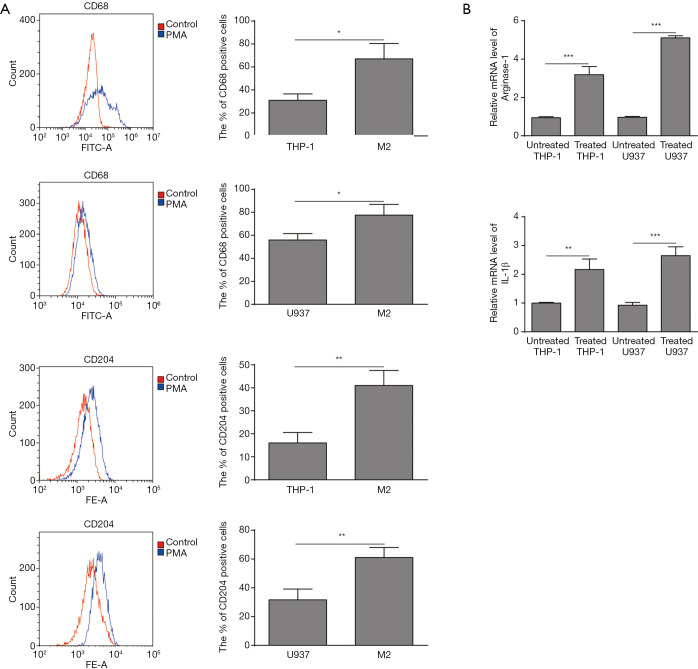
Mononuclear cell lines U937 and THP-1 (U937 10 ng/mL; THP-1 100 ng/mL) were induced by PMA into macrophages. In order to understand the effect of OS cells on the polarization process of TAMs, M2-TAMs were induced from THP-1 and U937 cell lines. The introduction and confirmation of polarized M2-TAMs was shown in Figure 1. CD204 and CD68 are both expressed on M2 macrophages. The flow cytometry analysis showed the level of CD204 (M2-TAMs) and CD68 (M2-TAMs) were both detected after PMA treatment (Figure 1A). In M2 induced group, the percentage of CD68+ and CD204+ cells were significantly higher than the control group for both U937 and THP-1 cells. Next, we demonstrated the expression levels of M2-TAMs related marker Arg-1 and IL-1β, and found that Arg-1 and IL-1β were obviously elevated (Figure 1B), which indicated that the macrophage polarization towards M2 phenotype from U937 and THP-1 cells was successful.
Figure 1.
Phorbol-12-myristate-13-acetate (PMA) induced the polarization of THP-1 and U937 cells to M2-polarized tumor-associated macrophages (TAMs) with increased expression level of Arg-1 and IL-1β. (A) Expressions of CD68 (M2-TAMs) and CD204 (M2-TAMs) were detected after PMA treatment by flow cytometry analysis and the percentage of CD68 and CD204 positive cells were increased in the induced M2-TAMs cells. (B) The mRNA level of Arg-1 and IL-1β were increased in induced M2-TAMs cells by RT-qPCR. Data are presented as the mean ± standard deviation (SD). All experiments were conducted in triplicate. *P<0.05; **, P<0.01; ***, P<0.001.
Overexpression of RASSF1A inhibited cell proliferation, migration and invasion through Wnt/β-catenin pathway
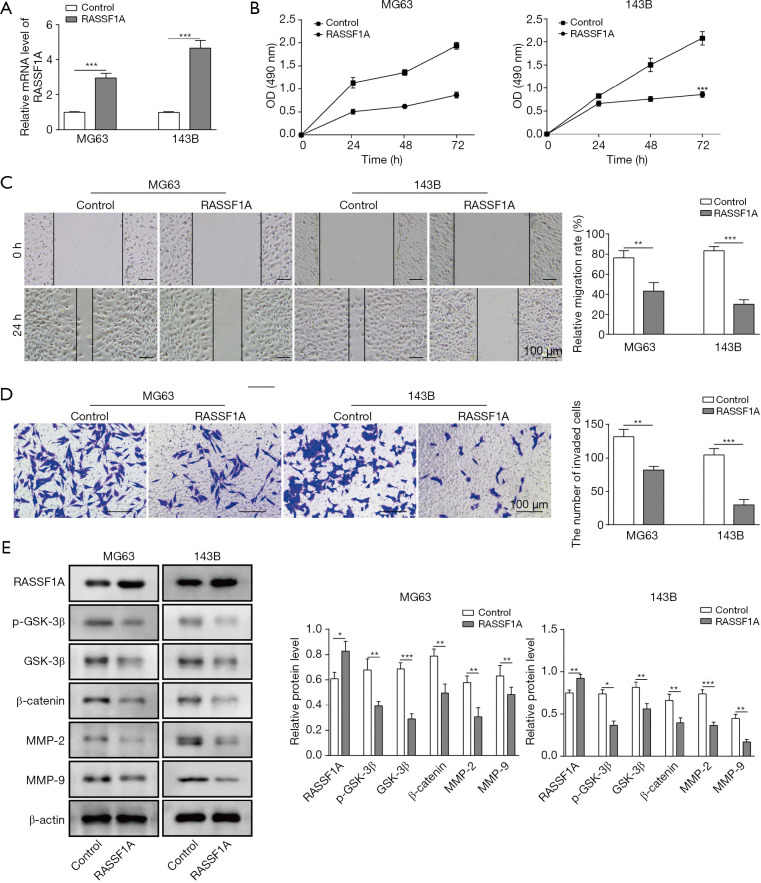
To understand the mechanism of RASSF1A in OS, cell proliferation, migration, invasion, and RASSF1A related downstream molecules were assessed. As shown in Figure 2A, we successfully constructed the RASSIF1A-overexpressed MG63 and 143B cells, with 2- and 4-fold increase, compared with the control groups, respectively. MTT assay showed that cell growth was significantly suppressed in RASSIF1A overexpression groups (Figure 2B). Additionally, the relative migration rate was inhibited in both cells with RASSF1A overexpression compared with the control (Figure 2C). Transwell invasion assay showed that relative invasion capacity was obviously reduced, indicating the overexpression of RASSF1A impeded the invasion of MG63 and 143B cells (Figure 2D). Finally, the expression of GSK-3β, p-GSK-3β, β-catenin, MMP-2 and MMP-9 was also assessed by Western blot. It was found their expression was suppressed by RASSF1A overexpression (Figure 2E). Thus, RASSF1A overexpression could down-regulate the OS metastasis related proteins through Wnt-β-catenin pathway, which positively participate in the invasion and migration of OS cells.
Figure 2.
Ras-associated domain family protein1 isoform A (RASSF1A) overexpression suppressed OS cell proliferation, migration and invasion in vitro. (A) Expression of RASSF1A in MG63 and 143B cells increased in test group compared with control. (B) MTT assay showed significant decrease of cells after 0, 24, 48 and 72 h. (C) Cell migration graph (left) and relative migration rate (%) (right) in wound-healing migration assay indicated impaired migration in test group compared with control. (D) Results of Transwell invasion assay showed the attenuated invasion abilities of MG63 and 143B cells. (E) Expression of p-GSK-3β, GSK-3β, β-catenin, MMP-2 and MMP-9 were reduced in RASSF1A overexpression group compared with control group by Western blot. Data are presented as the mean ± SD in bar chart. All experiments were conducted in triplicate. *, P<0.05; **, P<0.01; ***, P<0.001.
Knockdown of miR-181α-5p suppressed cell proliferation, migration and invasion through RASSF1A/Wnt pathway
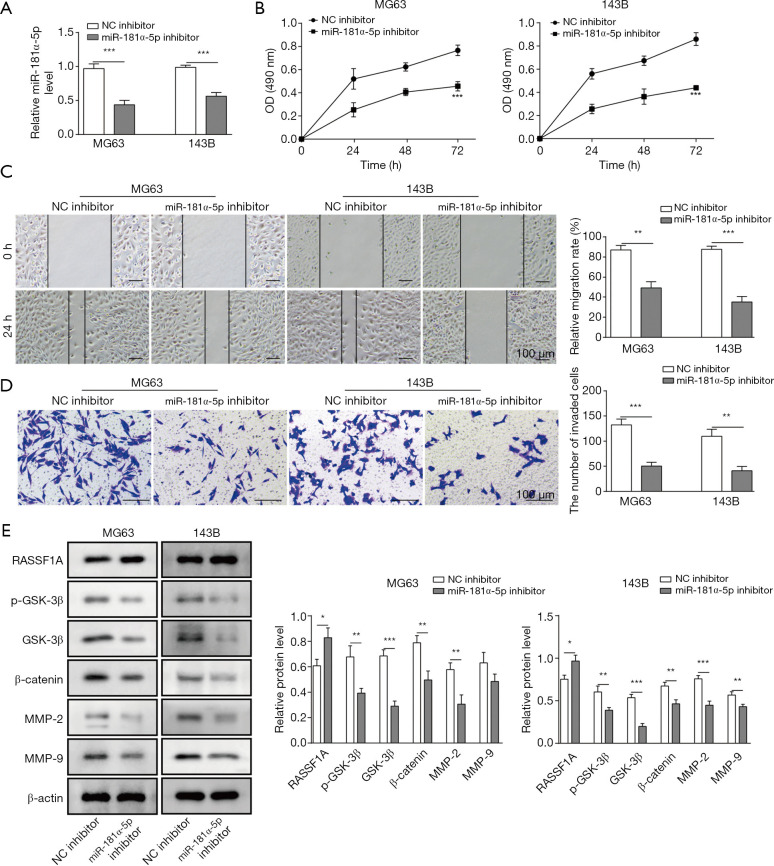
Following the above results, the biological role of miR-181α-5p in OS was also evaluated. The effect of miR-181α-5p knockdown on cell proliferation, migration and invasion were investigated. The relative level of miR-181α-5p was reduced around 50% compared with the NC inhibitor groups (Figure 3A). Cell growth, migration and invasion were significantly suppressed in miR-181α-5p knockdown MG63 and 143B cells by the MTT assay, wound-healing migration assay and Transwell invasion assay, respectively (Figure 3B,C,D). Finally, to investigate the possible signaling pathways involved in miR-181α-5p-mediated OS metastasis, we used Western blot to evaluate RASSF1A/Wnt pathway activation by measuring the protein levels of RASSF1A, GSK-3β, p-GSK-3β, β-catenin, MMP-2 and MMP-9. The results showed that the reduced level of miR-181α-5p led to the RASSF1A overexpression, and the knockdown of miR-181α-5p facilitated the suppressed expression of associated proteins, GSK-3β, p-GSK-3β, β-catenin, MMP-2 and MMP-9, due to the up-regulation of RASSF1A (Figure 3E).
Figure 3.
miR-181α-5p knockdown suppressed the OS cell proliferation, migration and invasion in vitro. (A) Expression of miR-181α-5p in MG63 and 143B cells in control and test group. (B) MTT assay showed significant decrease of cells after 0, 24, 48 and 72 h. (C) Cell migration graph (left) and relative migration rate (%) (right) in wound-healing migration assay indicated impaired migration in test group compared with control. (D) Results of Transwell invasion assay showed the attenuated invasion abilities of MG63 and 143B cells. (E) Expression of p-GSK-3β, GSK-3β, β-catenin, MMP-2 and MMP-9 were reduced while RASSF1A was enhanced in miR-181α-5p knockdown group compared with control group by Western blot. Data are presented as the mean ± SD in bar chart. All experiments were conducted in triplicate. *, P<0.05; **, P<0.01; ***, P<0.001. NC, negative control.
IL-1β secreted by M2-TAMs facilitated OS metastasis via NF-κB/miR-181α-5p/RASSF1A pathway
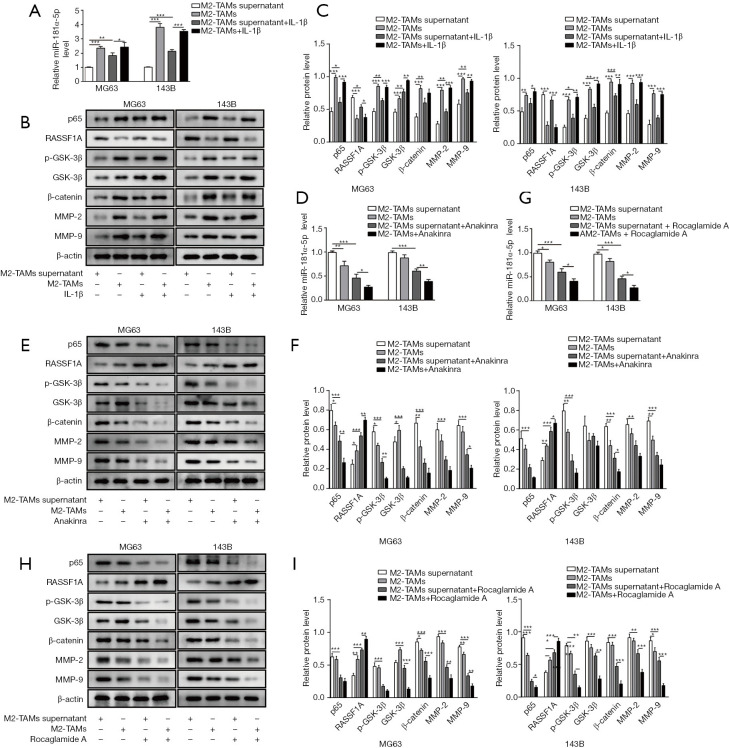
In order to understand the effect of M2-TAMs towards OS metastasis, IL-1β, IL-1β receptor inhibitor (Anakinra) and NF-κB inhibitor (Rocaglamide A) were used respectively in several culture compositions shown in Table 1 with either MG63 or 143B cells. RT-qPCR was used to assess the expression of miR-181α-5p after co-culture with M2-TAMs or M2-TAMs supernatant and OS cells with or without exogenous IL-1β. The expression of miR-181α-5p was promoted after co-culture M2-TAMs or M2-TAMs supernatant and OS cells with exogenous IL-1β (Figure 4A). Meanwhile, the addition of exogenous IL-1β to coculture system of M2-TAMs or M2-TAMs supernatant with the OS cells could promote the expression of NF-κB (p65), p-GSK-3β, GSK-3β, β-catenin, MMP-2 and MMP-9 and inhibit the expression of RASSF1A (Figure 4B,C), thus the IL-1β/NF-κB pathway and its downstream signaling can be activated through IL-1β or M2-TAMs or M2-TAMs supernatant. Next, following the addition of an Anakinra into co-culture system with M2-TAMs or M2-TAMs supernatant and OS cells, we repeated the above. RT-qPCR was used to measure the miR-181α-5p level and Western blot was to evaluate the expression of NF-κB (p65), RASSIF1A, p-GSK-3β, GSK-3β, β-catenin, MMP-2 and MMP-9, the results showed NF-κB/miR-181α-5p/RASSF1A/Wnt pathway remarkably suppressed, as indicated by the decreased level of miR-181α-5p (Figure 4D), and lower protein levels of NF-κB (p65) and p-GSK-3β, GSK-3β, β-catenin, MMP-2 and MMP-9 and higher protein levels of RASSIF1A (Figure 4E,F). Similar phenomenon was observed with the presence of Rocaglamide A (Figure 4G,H,I), indicating the IL-1β secreted by M2-TAMs exerted the effect via NF-κB signaling pathway. These results confirmed IL-1β secreted by M2-TAMs facilitated OS metastasis via NF-κB/miR-181α-5p/RASSF1A pathway.
Table 1. Co-culture compositions of M2-TAMs and osteosarcoma cells, with or without the addition of IL-1β, IL-1β receptor inhibitor or NF-κB inhibitor.
| Variables | IL-1β | IL-1β receptor inhibitor | NF-κB inhibitor |
|---|---|---|---|
| MG63 | M2-TAMs supernatant | M2-TAMs supernatant | M2-TAMs supernatant |
| M2-TAMs | M2-TAMs | M2-TAMs | |
| M2-TAMs supernatant + IL-1β | M2-TAMs supernatant + Anakinra | M2-TAMs supernatant + Rocaglamide A | |
| M2-TAMs + IL-1β | M2-TAMs + Anakinra | M2-TAMs + Rocaglamide A | |
| 143B | M2-TAMs supernatant | M2-TAMs supernatant | M2-TAMs supernatant |
| M2-TAMs | M2-TAMs | M2-TAMs | |
| M2-TAMs supernatant + IL-1β | M2-TAMs supernatant + Anakinra | M2-TAMs supernatant + Rocaglamide A | |
| M2-TAMs + IL-1β | M2-TAMs + Anakinra | M2-TAMs + Rocaglamide A |
TAMs, tumor-associated macrophages.
Figure 4.
IL-1β secreted by M2-polarised tumor-associated macrophages (M2-TAMs) facilitated OS metastasis via nuclear factor-kappa B (NF-κB)/miR-181α-5p/Ras-associated domain family protein1 isoform A (RASSF1A) pathway. (A) Relative level of miR-181α-5p in MG63 and 143B cells was increased by adding M2-TAMs supernatant, M2-TAMs to OS cells with exogenous IL-1β compared with control media with MG63 or 143B cells only. (B,C) The expression of NF-κB (p65), p-GSK-3β, GSK-3β, β-catenin, MMP-2 and MMP-9 was increased with the suppression of RASSF1A by adding M2-TAMs supernatant, M2-TAMs or exogenous IL-1β compared with control media. (D) Relative level of miR-181α-5p in MG63 and 143B cells was decreased by adding M2-TAMs supernatant, M2-TAMs to OS cells with or without exogenous IL-1β receptor inhibitor (Anakinra) treatment. (E,F) The expression of NF-κB (p65), p-GSK-3β, GSK-3β, β-catenin, MMP-2 and MMP-9 was decreased with the increased expression of RASSF1A by adding M2-TAMs supernatant, M2-TAMs to OS cells with or without Anakinra treatment. (G) Relative level of miR-181α-5p in MG63 and 143B cells was decreased by coculture M2-TAMs supernatant, M2-TAMs or exogenous IL-1β with OS cells after exogenous NF-κB inhibitor (Rocaglamide A) treatment. (H,I) The expression of NF-κB (p65), p-GSK-3β, GSK-3β, β-catenin, MMP-2 and MMP-9 was decreased with the increased expression of RASSF1A by coculture M2-TAMs supernatant, M2-TAMs or exogenous IL-1β with OS cells after Rocaglamide A treatment. Data are presented as the mean ± SD in bar chart. All experiments were conducted in triplicate. *, P<0.05; **, P<0.01; ***, P<0.001. NC, negative control.
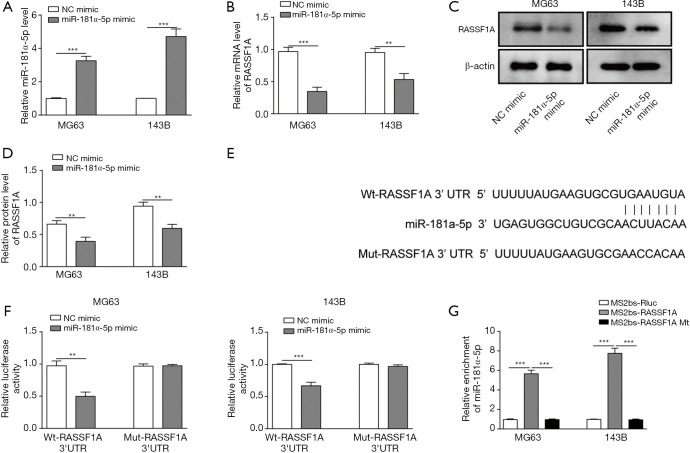
miR-181α-5p could target and down-regulate the RASSF1A expression
The interaction of miR-181α-5p and RASSF1A protein was assessed in OS cells. The relative expression level of miR-181α-5p was significantly higher, while the relative mRNA and protein level of RASSF1A was significantly lower in MG63 and 143B cells transfected with miR-181α-5p mimic compared with NC (Figure 5A,B,C,D). These results indicated that the miR-181α-5p could directly target and downregulate RASSF1A. The sequence in which miR-181α-5p bound to RASSF1A was detected according to TargetScan analysis (Figure 5E). The interaction between miR-181α-5p and RASSF1A was then assessed in dual-luciferase reporter assay. Relative luciferase activity in MG63 and 143B cells that were transfected with Wt-RASSF1A was significantly suppressed compared with NC, while it was not significant with Mut-RASSF1A (Figure 5F). These results indicate that there is a direct interaction between miR-181α-5p and RASSF1A through 3'UTR. To confirm the direct binding of RASSF1A and miR-181α-5p, the MS2-binding sequences-MS2-binding protein (MS2bs-MS2bp) based RIP assay was performed. The miR-181α-5p was mostly enriched in MS2bs-RASSF1A group as compared to the control and mutant group, indicating the interaction between RASSF1A and miR-181α-5p (Figure 5G).
Figure 5.
miR-181α-5p could target and downregulate Ras-associated domain family protein1 isoform A (RASSF1A). (A) The level of miR-181α-5p was increased in miR-181α-5p mimic compared with mimic control group. (B) The mRNA level of RASSF1A was decreased in miR-181α-5p mimic compared with mimic control group. (C) Western blot results showed the relative protein level of RASSF1A was decreased in miR-181α-5p mimic compared with mimic control group. (D) Relative protein level of RASSF1A decreased in miR-181α-5p mimic compared with mimic control group. (E) Interaction sequence between miR-181α-5p and RASSF1A indicated the specific binding site 3'-untranslated region (3'UTR) using TargetScan tool. (F) Relative luciferase activity was reduced with MG63 and 143B cells that were transfected with wild-type (Wt)-RASSF1A, but remained no change with mutant (Mut)-RASSF1A. Data were shown with and without addition of miR-181α-5p as control. (G) Relative enrichment of miR-181α-5p in cells transfected with wild-type MS2-binding sequences (MS2bs)-RASSF1A significantly increased compared with mutant MS2bs-RASSF1A or control group. Data are presented as the mean ± SD in bar chart. All experiments were conducted in triplicate. **, P<0.01; ***, P<0.001. NC, negative control.
Discussion
TAMs are mainly derived from monocytes in circulating peripheral blood and are the largest group of immune cells in the tumor microenvironment. Similar to normal macrophages, TAMs can be polarized into two types. The M1 phenotype is correlated with inflammation and tumor cell apoptosis, while the M2 phenotype has been proved to enhance tumor progression through several mechanisms (10). The alternatively-activated M2 phenotype is the main phenotype of TAMs. These functionally polarized cells, in response to the micro-environmental signals, play an essential role in inflammatory circuits that can promote tumor growth and progression (11). By producing and secreting various immunosuppressive factors such as IL-1β, IL-4, IL-10, and TGF-β, M2-TAMs promote the growth and metastasis of tumor cells, blood vessel formation and extracellular matrix remodeling (12,13). Tumor cells can regulate the polarization process of TAMs by secreting cytokines, which induce the transformation to M2-TAMs, promote tumor growth and progression (14-17). It has been reported that TAMs are highly correlated with tumor metastasis and TAM-derived molecules such as IL-1β, cathepsin B, Wnt5a, and cerebral signaling protein 4D, which can significantly promote the progression and metastasis of different tumors (18,19). Recent studies have confirmed that M2-TAMs are closely associated with tumor cell proliferation, migration, invasion and poor prognosis in tumor metastasis, such as lung cancer (20) and colon cancer (21). However, little research of TAMs on OS metastasis has been reported yet. The effect of M2-TAMs on OS metastasis has not been clarified. The current research is to better understand the occurrence of M2-TAMs and its associated pathway NF-κB/miR-181α-5p/RASSF1A/Wnt promoting metastasis, which could facilitate the exploration of new strategy against OS metastasis.
The increase level of Arg-1 and IL-1β in polarized cells indicates the expression of those signals is associated with M2-TAMs. At the cellular level, IL-1β and NF-κB in OS cells were then intervened by exogenous proteins or antibodies such as exogenous IL-1β, M2-TAMs, IL-1β receptor inhibitors and NF-κB inhibitors, to investigate whether IL-1β and NF-κB promote OS cell metastasis via NF-κB/miR-181α-5p/RASSF1A/Wnt pathway. By establishing a variety of TAMs and OS cell co-culture systems, we found M2-TAMs releases IL-1β to activate NF-κB/miR-181α-5p/RASSF1A/Wnt pathway, release a series of inflammatory factors and promote OS metastasis.
The results of the present study reveal the role of miR-181α-5p in OS cells, indicating that miR-181α-5p may be an oncogenesis promoter in OS. Similar theories have been reported in colorectal cancer and esophageal cancer that miR-181α-5p is associated with cancer progression and recurrence, and miR-181α-5p may active oncogene in gastric cancer (22,23). Identifying the cancer-specific targets of miRNA is an essential step to find the mechanism of miRNAs in tumor development and progression. RASSF1A was found as a direct target of miR-181α-5p, as the luciferase activity was suppressed in 143B and MG63 cells co-transfected with Wt-RASSF1A and miR-181α-5p mimic compared with cells co-transfected with Mut-RASSF1A and control group. The assumed binding site 3’UTR of RASSF1A indicated that the mutation of this site affected the translation, which suggested that miR-181α-5p directly binds with the 3'UTR of RASSF1A. In addition, in the 143B and MG63 cell lines, the increase of miR-181α-5p level was associated with a decrease of RASSF1A protein level.
Increasing evidence has suggested that RASSF1A acts as a tumour suppressor in several types of cancer through multiple mechanisms thus its inactivation can lead to tumorigenesis and progression (24). The results of the current study suggest that RASSF1A can suppress cell proliferation, migration and invasion, which are consistent with a previous study where RASSF1A was reported to suppress cell progression in gastric cancer cells (25). RASSF1A also suppressed the expression of p-GSK-3β, GSK-3β, β-catenin, MMP-2 and MMP-9, which all have a role in various physiological and pathological processes in cancer cells (26-28). The current study also further demonstrated that miR-181α-5p can suppress RASSF1A through the miR-181α-5p/RASSF1A/Wnt pathway, by interacting with 3'UTR site. The downregulation of RASSF1A therefore promotes the activities of proliferation, migration and invasion through markers such as p-GSK-3β, GSK-3β, β-catenin, MMP-2 and MMP-9.
Conclusions
The current study provides a new understanding of the role of miR-181α-5p in macrophages by targeting RASSF1A and its downstream signaling pathway. The results suggest that M2-TAMs releases IL-1β, and in return activates the NF-κB/miR-181α-5p/RASSF1A/Wnt pathway to promote OS cell progression and metastasis. Meanwhile, miR-181α-5p can directly suppress the expression of RASSF1A, thus, promoting the OS progression. Therefore miR-181α-5p may contribute to oncogenesis and represent a potential molecular target for OS therapy. Further studies are required to fully understand the mechanism and signaling pathway of NF-κB/miR-181α-5p/RASSF1A/Wnt in OS.
Acknowledgments
Funding: This work was supported by Natural Science Foundation of Hunan Province (2019JJ40458).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The ethics committee approval is not required by the local law, as the study involved no human tissues or animals. All cells lines used in this study require no ethics approval.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.02.52). The authors have no conflicts of interest to declare.
References
- 1.Anderson ME. Update on Survival in Osteosarcoma. Orthop Clin North Am 2016;47:283-92. 10.1016/j.ocl.2015.08.022 [DOI] [PubMed] [Google Scholar]
- 2.Fuchs N, Bielack SS, Epler D, et al. Long-term results of the co-operative German-Austrian-Swiss osteosarcoma study group's protocol COSS-86 of intensive multidrug chemotherapy and surgery for osteosarcoma of the limbs. Ann Oncol 1998;9:893-9. 10.1023/A:1008391103132 [DOI] [PubMed] [Google Scholar]
- 3.Bacci G, Ferrari S, Bertoni F, et al. Long-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the istituto ortopedico rizzoli according to the istituto ortopedico rizzoli/osteosarcoma-2 protocol: an updated report. J Clin Oncol 2000;18:4016-27. 10.1200/JCO.2000.18.24.4016 [DOI] [PubMed] [Google Scholar]
- 4.Isakoff MS, Bielack SS, Meltzer P, et al. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J Clin Oncol 2015;33:3029-35. 10.1200/JCO.2014.59.4895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim S, Yang MH, Park JH, et al. Inactivation of the RASSF1A in osteosarcoma. Oncol Rep 2003;10:897-901. [PubMed] [Google Scholar]
- 6.Wang WG, Chen SJ, He JS, et al. The tumor suppressive role of RASSF1A in osteosarcoma through the Wnt signaling pathway. Tumour Biol 2016;37:8869-77. 10.1007/s13277-015-4660-z [DOI] [PubMed] [Google Scholar]
- 7.Tian J, He H, Lei G. Wnt/beta-catenin pathway in bone cancers. Tumour Biol 2014;35:9439-45. 10.1007/s13277-014-2433-8 [DOI] [PubMed] [Google Scholar]
- 8.Leow PC, Tian Q, Ong ZY, et al. Antitumor activity of natural compounds, curcumin and PKF118-310, as Wnt/beta-catenin antagonists against human osteosarcoma cells. Invest New Drugs 2010;28:766-82. 10.1007/s10637-009-9311-z [DOI] [PubMed] [Google Scholar]
- 9.Jianwei Z, Fan L, Xiancheng L, et al. MicroRNA 181a improves proliferation and invasion, suppresses apoptosis of osteosarcoma cell. Tumour Biol 2013;34:3331-7. 10.1007/s13277-013-0902-0 [DOI] [PubMed] [Google Scholar]
- 10.Goswami KK, Ghosh T, Ghosh S, et al. Tumor promoting role of anti-tumor macrophages in tumor microenvironment. Cell Immunol 2017;316:1-10. 10.1016/j.cellimm.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 11.Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002;23:549-55. 10.1016/S1471-4906(02)02302-5 [DOI] [PubMed] [Google Scholar]
- 12.Shen L, Li H, Shi Y, et al. M2 tumour-associated macrophages contribute to tumour progression via legumain remodelling the extracellular matrix in diffuse large B cell lymphoma. Sci Rep 2016;6:30347. 10.1038/srep30347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komohara Y, Ohnishi K, Kuratsu J, et al. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol 2008;216:15-24. 10.1002/path.2370 [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Zhou Q, Yuan G, et al. Notch signaling regulates M2 type macrophage polarization during the development of proliferative vitreoretinopathy. Cell Immunol 2015;298:77-82. 10.1016/j.cellimm.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 15.Yin J, Hu H, Li X, et al. Inhibition of Notch signaling pathway attenuates sympathetic hyperinnervation together with the augmentation of M2 macrophages in rats post-myocardial infarction. Am J Physiol Cell Physiol 2016;310:C41-53. 10.1152/ajpcell.00163.2015 [DOI] [PubMed] [Google Scholar]
- 16.Singla RD, Wang J, Singla DK. Regulation of Notch 1 signaling in THP-1 cells enhances M2 macrophage differentiation. Am J Physiol Heart Circ Physiol 2014;307:H1634-1642. 10.1152/ajpheart.00896.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang YC, He F, Feng F, et al. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res 2010;70:4840-9. 10.1158/0008-5472.CAN-10-0269 [DOI] [PubMed] [Google Scholar]
- 18.Gorelik E, Wiltrout RH, Brunda MJ, et al. Augmentation of metastasis formation by thioglycollate-elicited macrophages. Int J Cancer 1982;29:575-81. 10.1002/ijc.2910290514 [DOI] [PubMed] [Google Scholar]
- 19.Coffelt SB, Hughes R, Lewis CE. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochim Biophys Acta 2009;1796:11-8. [DOI] [PubMed] [Google Scholar]
- 20.Wei X, Nie S, Liu H, et al. Angiopoietin-like protein 2 facilitates non-small cell lung cancer progression by promoting the polarization of M2 tumor-associated macrophages. Am J Cancer Res 2017;7:2220-33. [PMC free article] [PubMed] [Google Scholar]
- 21.Piao C, Zhang WM, Li TT, et al. Complement 5a stimulates macrophage polarization and contributes to tumor metastases of colon cancer. Exp Cell Res 2018;366:127-38. 10.1016/j.yexcr.2018.03.009 [DOI] [PubMed] [Google Scholar]
- 22.Pichler M, Winter E, Ress AL, et al. miR-181a is associated with poor clinical outcome in patients with colorectal cancer treated with EGFR inhibitor. J Clin Pathol 2014;67:198. 10.1136/jclinpath-2013-201904 [DOI] [PubMed] [Google Scholar]
- 23.Xiang Z, Dong X, Sun Q, et al. Clinical significance of up-regulated miR-181a in prognosis and progression of esophageal cancer. Acta Biochim Biophys Sin (Shanghai) 2014;46:1007-10. 10.1093/abbs/gmu083 [DOI] [PubMed] [Google Scholar]
- 24.Dubois F, Bergot E, Zalcman G, et al. RASSF1A, puppeteer of cellular homeostasis, fights tumorigenesis, and metastasis-an updated review. Cell Death Dis 2019;10:928. 10.1038/s41419-019-2169-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu J, Qi J, Sun X, et al. MicroRNA181a promotes cell proliferation and inhibits apoptosis in gastric cancer by targeting RASSF1A. Oncol Rep 2018;40:1959-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaco-Levy R, Sharabi S, Benharroch D, et al. Matrix metalloproteinases 2 and 9, E-cadherin, and β-catenin expression in endometriosis, low-grade endometrial carcinoma and non-neoplastic eutopic endometrium. Eur J Obstet Gynecol Reprod Biol 2008;139:226-32. 10.1016/j.ejogrb.2008.01.004 [DOI] [PubMed] [Google Scholar]
- 27.Dwivedi A, Slater SC, George SJ. MMP-9 and -12 cause N-cadherin shedding and thereby beta-catenin signalling and vascular smooth muscle cell proliferation. Cardiovasc Res 2009;81:178-86. 10.1093/cvr/cvn278 [DOI] [PubMed] [Google Scholar]
- 28.Yang Z, Li K, Liang Q, et al. Elevated hydrostatic pressure promotes ameloblastoma cell invasion through upregulation of MMP-2 and MMP-9 expression via Wnt/β-catenin signalling. J Oral Pathol Med 2018;47:836-46. 10.1111/jop.12761 [DOI] [PubMed] [Google Scholar]