Abstract
Aims
Patients with heart failure (HF) have a poor prognosis and are categorized by ejection fraction. We performed a meta‐analysis to compare baseline characteristics and long‐term outcomes of patients with heart failure with reduced (HFrEF), mid‐range (HFmrEF), and preserved ejection fraction (HFpEF).
Methods and Results
A total of 27 prospective studies were included. Patients with HFpEF were older and had a higher proportion of females, hypertension, diabetes, and insufficient neuroendocrine antagonist treatments, while patients with HFrEF and HFmrEF had a higher prevalence of coronary heart disease and chronic kidney disease. After more than 1‐year of follow‐up, all‐cause mortality was significantly lower in patients with HFmrEF 9388/25 042 (37.49%) than those with HFrEF 39 333/90 023 (43.69%) and HFpEF 24 828/52 492 (47.30%) (p < .001). Cardiovascular mortality was lowest in patients with HFpEF 1130/9904 (11.41%), highest in patients with HFrEF 3419/16 277 (21.07%) mainly coming from HF death and sudden cardiac death, and middle in patients with HFmrEF 699/5171 (13.52%) and the non‐cardiovascular mortality was on the contrary. Subgroup analysis showed that in high‐risk patients with atrial fibrillation, the all‐cause mortality of HFpEF was significantly higher than both HFrEF and HFmrEF (p < .001). HF hospitalization was lowest in patients with HFmrEF 1822/5285 (34.47%), highest in patients with HFrEF 12 607/28 590 (44.10%) and middle in patients with HFpEF 8686/22 763 (38.16%) and the composite of all‐cause mortality and HF hospitalization was also observed similar results.
Conclusions
In summary, patients with HFmrEF had the lowest incidence of all‐cause mortality and HF hospitalization, while the highest all‐cause mortality and HF hospitalization rates were HFpEF and HFrEF patients, respectively.
Keywords: heart failure with mid‐range ejection fraction, heart failure with preserved ejection fraction, heart failure with reduced ejection fraction, mortality
1. INTRODUCTION
Heart failure (HF) is a global pandemic affecting approximately 64.3 million people worldwide; 1 furthermore, the total number of patients living with HF is increasing. 2 At the same time, the poor prognosis of HF patients is another important and serious healthcare issue worldwide. Indeed, several studies have suggested similar mortality in patients with HF with reduced ejection fraction (HFrEF) and preserved ejection fraction (HFpEF), 3 whereas others have demonstrated HFpEF patients have a substantially better prognosis compared with patients with HFrEF. 4 The large meta‐analysis Global Group in Chronic Heart Failure (MAGGIC) study, pooling data from 30 cohort studies, showed that patients with HFpEF were at a significantly lower risk of death compared to their HFrEF counterparts. 5 However, this analysis included retrospective studies, which probably lead to higher mortality rates due to selection bias in trials that included patients with common serious comorbidities, and use left ventricular ejection fraction (LVEF) 40% as the cutoff value for HF classification (LVEF < 40% for HFrEF, LVEF ≥ 40% for HFpEF, respectively) ignoring of HF with mid‐range ejection fraction (HFmrEF), a novel category that was defined LVEF 40%–49% in the 2016 European Society of Cardiology heart failure guideline. 6 HFmrEF is considered as a transition between the HFpEF and HFrEF, it is imperative to investigate the differences between HFmrEF patients and those in the other two HF groups in terms of prognosis. More importantly, we need a better understanding of the causes of death in HF patients, which may contribute to better insights into the underlying pathophysiologic mechanisms and new treatments for improving patient outcomes.
Therefore, we conducted a meta‐analysis of prospective studies to compare clinical characteristics, assess the long‐term prognosis through all‐cause mortality and HF hospitalization of more than 1‐year follow‐up, and investigate the prevalence of cardiac/noncardiac causes of death among three categories of patients with HF.
2. METHODS
2.1. Ethics statement
As this study is a meta‐analysis, ethical approval was not required.
2.2. Search strategy
We performed a literature search in PubMed and Embase from the date of inception to March 2021. The following search formula (heart failure with reduced ejection fraction OR HFrEF) AND (heart failure with preserved ejection fraction OR HFpEF) AND (all‐cause mortality OR all‐cause death OR mortality OR death) was used in the English database. And language was restricted to English.
2.3. Study selection
Two independent reviewers screened the titles and abstracts of all selected articles. Only studies that were clearly irrelevant were excluded from this page. Any disagreements between the investigators were resolved by a third reviewer. Studies were included if they met the following criteria: (1) prospective studies; (2) providing numbers of events for all‐cause mortality in patients among three categories HF; (3) follow‐up period not less than 1 year. The definition of HF was made mainly based on 2016 ESC guideline, 6 categorizing HF as LVEF ≥ 50%, 40%–49%, <40% as HFpEF, HFmrEF, and HFrEF, respectively, or the American College of Cardiology and American Heart Association guideline, 7 which recommended LVEF ≥ 50%, 41%–49%, ≤40% as HFpEF, HFmrEF, and HFrEF, respectively. We excluded all retrospective studies or studies with unclear type, studies with a follow‐up period shorter than 1 year, and studies with insufficiently reported data.
2.4. Data extraction
Data were extracted by two independent reviewers. The extracted data included demographic features and key baseline clinical variables reported as means or medians with standard deviations (SD) or ranges from each study. We extracted absolute numbers for all‐cause and cardiovascular/non‐cardiovascular mortality and HF hospitalization. In addition, data on specific causes of cardiovascular mortality was also extracted. Disagreements were adjudicated by a third reviewer.
2.5. Statistical analysis
All statistical analyses were conducted by using Review Manager Version 5.4. The reported numbers of all‐cause and cardiovascular/non‐cardiovascular mortality and HF hospitalization in eligible studies were pooled for three categories of HF, followed by an estimation of an odds ratio (OR) with a 95% confidence interval (95% CI). The Q statistic was calculated and heterogeneity was quantified using the I2 statistic. Despite the significant heterogeneity between studies, we used a fix‐effects model to maintain the real sizes of the larger studies but beside that presented the results of a random‐effects methods wherever reasonable. A funnel plot was conducted to evaluate publication bias. We also conducted several subgroup analyses based on high‐risk patients, including acute HF, atrial fibrillation (AF), diabetes mellitus.
3. RESULTS
3.1. Search results
The flow chart of the search strategy is provided (Figure 1). The search strategy retrieved a total of 948 studies from PubMed (446) and Embase (505), with 214 duplicated studies, and the remaining 734 studies were performed for titles and abstracts screening, among which 266 irrelevant subjects and 85 narrative or systemic reviews were excluded. Ultimately, 383 relevant articles were reviewed in full text. A further 355 articles were excluded after careful review of full text, including 14 articles without all‐cause mortality for endpoint events, 111 articles that did not report the all‐cause mortality among three categories of HF patients, 18 articles with a follow‐up period of less than 1 year, 40 articles for retrospective studies or studies with unclear type, 72 articles that did not meet the definition of HF classification and 101 articles for the repeated trial database. Consequently, 27 studies 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 with a total of 167 557 patients met inclusion criteria and were included in the meta‐analysis.
Figure 1.
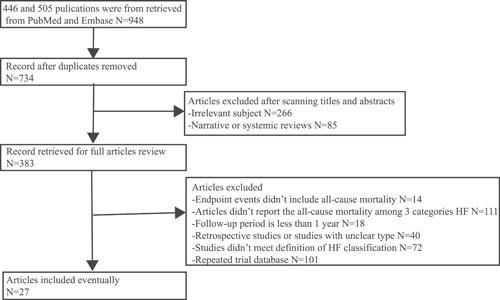
Flow chart of the search process result
3.2. Characteristics of included studies
The main characteristics of the included studies are summarized in Table 1. Among the included studies, only two were randomized controlled studies, 9 , 27 and the others were observational studies. The follow‐up duration varied from 1 to 6.3 years. In the included studies, 14 were from Asia, 9 from Europe, and 4 from North America. There were statistically differences in regard to baseline characteristics comparisons among three HF categories (Table 2). The baseline characteristics were as follows: age: 66.4 ± 12.5 versus 68.4 ± 12.9 versus 70.7 ± 12.8 years; male gender: 68.73% versus 61.48% versus 42.88%; coronary artery disease or ischemic HF: 55.41% versus 55.09% versus 42.13%; hypertension: 57.85% versus 65.11% versus 75.52%; diabetes: 32.24% versus 31.73% versus 34.72%; AF: 39.25% versus 47.50% versus 43.89%; chronic kidney disease: 23.09% versus 23.46% versus 20.47% among patients with HFrEF, HFmrEF, and HFpEF, respectively. Patients with HFpEF were significantly older than those with HFrEF and HFmrEF. The proportion of males and prevalence of coronary artery disease or ischemic HF and chronic kidney disease among HFpEF were significantly lower than those among HFrEF and HFmrEF, but hypertension and diabetes were more frequent in patients with HFpEF. The incidence of AF in patients with HFmrEF and HFpEF was significantly higher than that in patients with HFrEF. Drug applications, including ACEI or ARB, β‐blocker, aldosterone antagonists, and loop diuretics were the most used in HF patients with HFrEF, followed by HFmrEF, and the lowest application rate is HFpEF.
Table 1.
Study characteristics
| Study | Inclusion criteria | Country | Patients number (HFrEF/HFmrEF/HFpEF) | Outcomes (HFrEF/HFmrEF/HFpEF) | Follow up |
|---|---|---|---|---|---|
| Kawahira (2021) | Hospitalized patients with acute decompensated HF | Japan | 164/104/198 | ACM: 49/34/60 | 2.8 ± 1.5 years |
| SELFIE‐TR registry (2020) | Acute or chronic HF patients | Turkey | 780/170/72 | ACM: 155/31/17 | 1 year |
| Xu (2020) | Inpatients with HF | China | 202/94/109 | ACM: 21/8/2, HF hospitalization: 62/18/16, composite of ACM and HF hospitalization: 73/25/17 | 1 year |
| EXCEL trial (2020) | Hospitalized HF patients with left main coronary artery disease undergoing PCI or CABG | USA | 74/152/1578 | ACM: 13/14/96, CV mortality: 10/8/52 | 3 years |
| Song (2020) | Hospitalized HF patients | China | 215/80/110 | ACM: 36/8/13, HF hospitalization: 48/15/19, composite of ACM and HF hospitalization: 84/23/32 | Median: 12 months (IQR: 6–20 months) |
| KorAHF registry (2020) | Hospitalized patients with acute HF | South Korea | 3182/875/1357 | ACM: 1609/472/726, CV mortality: 530/115/161, composite of ACM and HF readmission: 2532/703/1088 | Median: 4.03 years (IQR: 1.39–5.58 years) |
| OPTIMIZE‐HF (2020) | Hospitalized HF patients | USA | 3688/NA/1848 | ACM: 2817/NA/1403, HF readmission: 2310/NA/959 | Median: 2 years |
| ASIAN‐HF registry (2020) | Inpatients and outpatient with symptomatic HF | 3 Asian regions | 4737/NA/1114 | ACM: 500/NA/60, CV mortality: 440/NA/46, non‐CV mortality: 170/NA/14 | 1 year |
| KCHF registry (2020) | Hospitalized patients with acute decompensated HF | Japan | 1383/703/1631 | ACM: 298/158/392, CV death: 203/97/223, (HF death: 128/65/131, SCD: 44/14/40, vascular death: 4/2/7, acute coronary syndrome: 5/0/4, stroke or intracranial hemorrhage: 8/9/21, other CV cause: 14/7/20), non‐CV death: 94/61/167, unknown death: 1/0/2 | Median: 470 days (IQR: 357–649 days) |
| Kanagala (2020) | HF patients | United Kingdom | 46/NA/140 | ACM: 6/NA/22 | Median: 1446 days (IQR: 1243–1613 days) |
| Gulf CARE registry (2020) | Hospitalized patients with acute HF | Seven Middle Eastern countries | 2683/962/932 | ACM: 548/152/181 | 1 year |
| Yee (2019) | Inpatients and outpatients with HF | USA | 516/NA/151 | ACM: 101/NA/13 | 16.6 ± 6.7 months |
| Vicent (2019) | Hospitalized patients with acute HF | Spain | 583/227/610 | ACM: 117/55/118, composite of ACM and HF readmission: 253/109/255 | 1 year |
| Vergaro (2019) | Chronic HF patients from the outpatient clinic | Italy | 1539/623/629 | ACM: 631/166/144, cardiac mortality: 415/74/54 (HF death: 277/40/37, SCD: 59/11/6, AMI: 27/10/7) | Median: 39 months (IQR: 17–79 months) |
| WET‐HF registry (2019) | Hospitalized patients with acute decompensated HF | Japan | 1143/532/1277 | ACM: 271/123/287, cardiac deaths: 69/45/128 | Median: 724 days |
| Lin (2019) | Hospitalized patients with HF | Taiwan | 158/NA/108 | ACM: 27/NA/15 | 18 months |
| Norwegian HF registry (2019) | Ambulatory patients with stable chronic HF | Norway | 7080/2086/1146 | ACM: 3836/957/504 | Median: 66 months (IQR: 33–105 months) |
| KorHF registry (2019) | Hospitalized patients due to HF | South Korea | 1684/NA/727 | ACM: 467/NA/226, composite of ACM and HF readmission: 729/NA/344 | Median: 1121 days (IQR: 355–1887 days) |
| ESC‐HF‐LT registry (2018) | Outpatients with chronic HF and inpatients admitted for acute HF | 21 European and Mediterranean countries | 7476/2913/3672 | ACM: 1240/403/548 | 800 days to 2.2 years |
| CHARM study (2018) | Patients with symptomatic HF | Sweden | 4323/1322/1953 | ACM: 1296/209/325, CV death: 1079/167/214, HF hospitalization: 1115/216/343 | Median: 2.9 years |
| Lam (2018) | Patient in the hospital for primary diagnosis of HF or in the outpatient setting within 6 months of an episode of decompensated HF | New Zealand and Singapore | 1209/256/574 | ACM: 233/30/80, composite of ACM and HF hospitalization: 522/103/199 | 2 years |
| CHART‐2 study (2018) | Chronic HF patients from patient clinics or just before discharge | Japan | 742/666/2893 | ACM: 330/330/887 | Median: 6.3 years |
| Gu (2018) | Hospitalized patients with a clinical diagnosis of HF and T2DM | China | 481/131//290 | ACM: 160/35/75, composite endpoints of ACM and HF hospitalization: 311/73/161 | 42 months |
| GWTG‐HF program (2017) | Hospitalized patients with acute HF | USA | 18398/3285/18299 | ACM: 13 847/2487/13 843, HF readmission: 8505/1416/7072 | 5 years |
| SwedeHF registry (2017) | HF patients from outpatient visits or hospital discharge | Sweden | 22954/8897/9595 | ACM: 8926/3367/4169, Composite of ACM or HF hospitalization: 13006/4551/5375 | 1 year |
| Pascual‐Figal (2017) | Ambulatory patients with chronic HF | Spain | 2351/460/635 | ACM: 776/128/178, CV death: 621/93/110, (HF death: 386/54/70, SCD: 190/29//24, other CV death: 45/10/16), non‐CV death: 155/35/68 | 4 years |
| Farre (2017) | Ambulatory HF patients | Spain | 2232/504/844 | ACM: 1023/221/444, CV death: 492/100/188, (HF death: 269/58/131, SCD: 101/13/12, other CV death: 122/29/45), non‐CV death: 265/72/163, unknown death: 266/49/93, HF hospitalization: 724/157/378, composite of ACM and HF hospitalization: 1277/272/564 | Median: 3.36 years (IQR: 1.69–6.04 years) |
Abbreviations: AMI, acute myocardial infarction; CABG, coronary artery bypass grafting; CV, cardiovascular; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; IQR, interquartile range; NA, not available; PCI, percutaneous coronary intervention; SCD, sudden cardiac death; T2DM, type 2 diabetes mellitus.
Table 2.
Comparison of baseline characteristics among three categories of HF
| Values shown as weighted means ± SD or numbers (%) | p values | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Numbers of studies | HFrEF | HFmrEF | HFpEF | HFrEF versus HFpEF | HFrEF versus HFmrEF | HFmrEF versus HFpEF |
| Demographic and clinical characteristics | |||||||
| Age | 13 | 66.4 ± 12.5 | 68.4 ± 12.9 | 70.7 ± 12.8 | <.001 | <.001 | <.001 |
| Male gender | 17 | 46 125/67 112 (68.73) | 12 582/20 465 (61.48) | 18 202/42 451 (42.88) | <.001 | <.001 | <.001 |
| Coronary artery disease or ischemic HF | 16 | 37 145/67 038 (55.41) | 11 190/20 313 (55.09) | 17 218/40 873 (42.13) | <.001 | .83 | <.001 |
| Hypertension | 17 | 38 826/67 112 (57.85) | 13 325/20 465 (65.11) | 32 059/42 451 (75.52) | <.001 | <.001 | <.001 |
| Diabetes | 16 | 21 482/66 631 (32.24) | 6452/20 334 (31.73) | 14 638/42 161 (34.72) | .71 | .07 | .01 |
| Atrial fibrillation | 15 | 23 533/59 958 (39.25) | 8657/18 227 (47.50) | 17 436/39 727 (43.89) | <.001 | <.001 | .2 |
| Chronic kidney disease | 7 | 7649/33 121 (23.09) | 2105/8972 (23.46) | 5722/27 951 (20.47) | <.001 | .14 | <.001 |
| Medications used | |||||||
| ACEI or ARB | 16 | 54 080/66 897 (80.84) | 15 154/20 385 (74.34) | 25 925/42 341 (61.23) | <.001 | <.001 | <.001 |
| Beta‐blocker | 16 | 55 755/66 897 (83.34) | 16 190/20 385 (79.42) | 29 015/42 341 (68.53) | <.001 | <.001 | <.001 |
| Aldosterone antagnoists | 15 | 22 846/66 823 (34.19) | 5415/20 233 (26.76) | 8009/40 763 (19.65) | <.001 | <.001 | <.001 |
| Loop diuretics | 14 | 46 772/64 931 (72.03) | 12 888/19 455 (66.25) | 23 589/40 100 (58.83) | <.001 | <.001 | <.001 |
Abbreviations: ACEI, angiotensin enzyme inhibitor; ARB, angiotensin receptor blocker; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; SD, standard deviation.
3.3. Publication bias
Funnel plots were drawn for assessment of meta‐analysis in regard to all‐cause mortality among studies examining HFrEF versus HFpEF (Figure S1A), HFrEF versus HFmrEF (Figure S1B), and HFmrEF versus HFpEF (Figure S1C). The funnel plots for both groups of studies (HFrEF vs. HFpEF) look asymmetrical as there appear to be more studies missing on the left‐hand side and were relatively symmetrical between the studies of HFrEF versus HFmrEF and between HFmrEF versus HFpEF. The source of risk of bias across studies can only be speculated and could be attributed to publication bias, substantial heterogeneity, or even chance.
3.4. Study outcomes
3.4.1. All‐cause mortality
Patients with HFmrEF had lower all‐cause mortality 9388/25 042 (37.49%) than those with HFrEF 39 333/90 023 (43.69%) and HFpEF 24 828/52 492 (47.30%). Pooled data of 21 studies using the fixed‐effects model showed that the risk of all‐cause mortality was significantly lower in patients with HFmrEF than in those with HFrEF (OR = 1.14, 95% CI: 1.10–1.18, p < .001) and HFpEF (OR = 0.94, 95% CI: 0.90–0.97, p < .001), and Pooled data of 27 studies indicated that patients with HFrEF had lower all‐cause mortality compared with those with HFpEF (OR = 1.03, 95% CI: 1.01–1.06, p = .01) (Figure 2). There was significant heterogeneity between the included studies (p < .001 and i2 > 50%). Running the analysis using the random‐effects model showed that the risk of all‐cause mortality was still significantly lower in patients with HFmrEF than in those with HFrEF (OR = 1.2, 95% CI: 1.07–1.36, p = .002), but not significant when compared with those with HFpEF (OR = 1.03, 95% CI: 0.90–1.17, p = .7).
Figure 2.
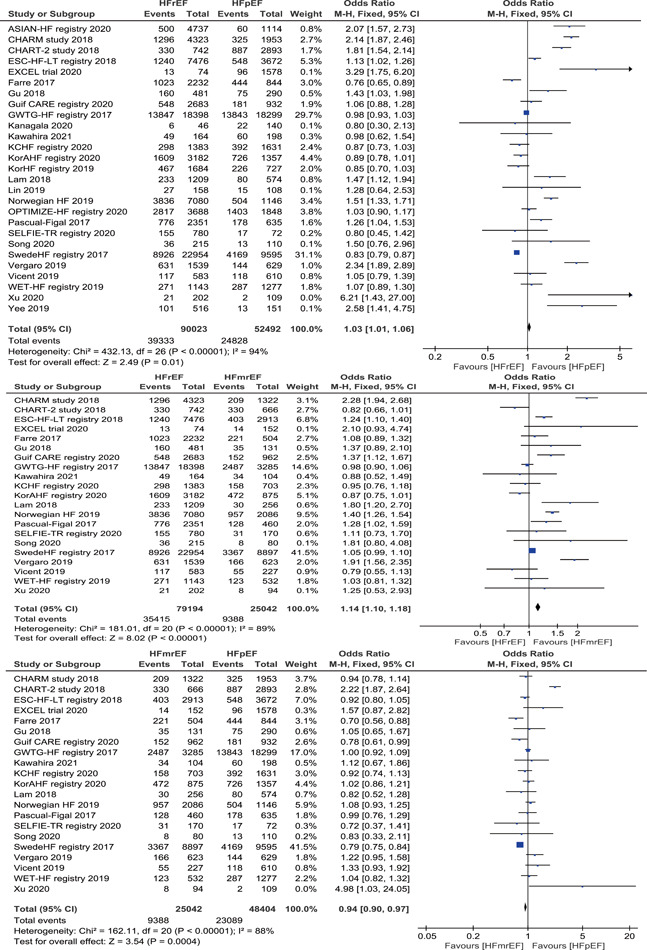
Forest plot of the odds ratio (OR) and 95% confidence interval (CI) for all‐cause mortality among three categories of HF. HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction
3.4.2. Causes of death
Eight studies provide data for cardiovascular mortality, which revealed that patients with HFrEF had higher cardiovascular mortality 3419/16 277 (21.07%) than those with HFmrEF 699/5171 (13.52%) and HFpEF 1130/9904 (11.41%), and meta‐analysis using the fixed‐effects model demonstrated a significantly higher risk of cardiovascular mortality in patients with HFrEF than in those with HFmrEF (OR = 1.60, 95% CI 1.46–1.74, p < .001) and HFpEF (OR = 1.64, 95% CI: 1.52–1.77, p < .001). In addition, a meta‐analysis from three studies indicated that patients with HFpEF had significantly higher non‐cardiovascular mortality 398/3110 (12.80%) than those with HFrEF 514/5966 (8.62%) and HFmrEF 168/1667 (10.08%) (Figure 3). Furthermore, we also analysis the cardiovascular‐specific death from four studies data, which displayed that patients with HFrEF were at significant higher risk of HF death 1060/7505 (14.12%) than those with HFmrEF 217/2290 (9.48%) and HFpEF 369/3739 (9.87%), and sudden cardiac death (SCD) were also significantly higher in patients with HFrEF 394/7505 (5.25%) than in those with HFmrEF 67/2290 (2.93%) and HFpEF 82/3739 (2.19%), but not significantly different between HFmrEF and HFpEF in regard to HF death and SCD (Figure S2). HF death accounted for 38.86%, 32.24%, 31.87% and SCD accounted for 14.44%, 9.96%, 7.08% of the total deaths in the three groups of HFrEF, HFmrEF, and HFpEF, respectively.
Figure 3.
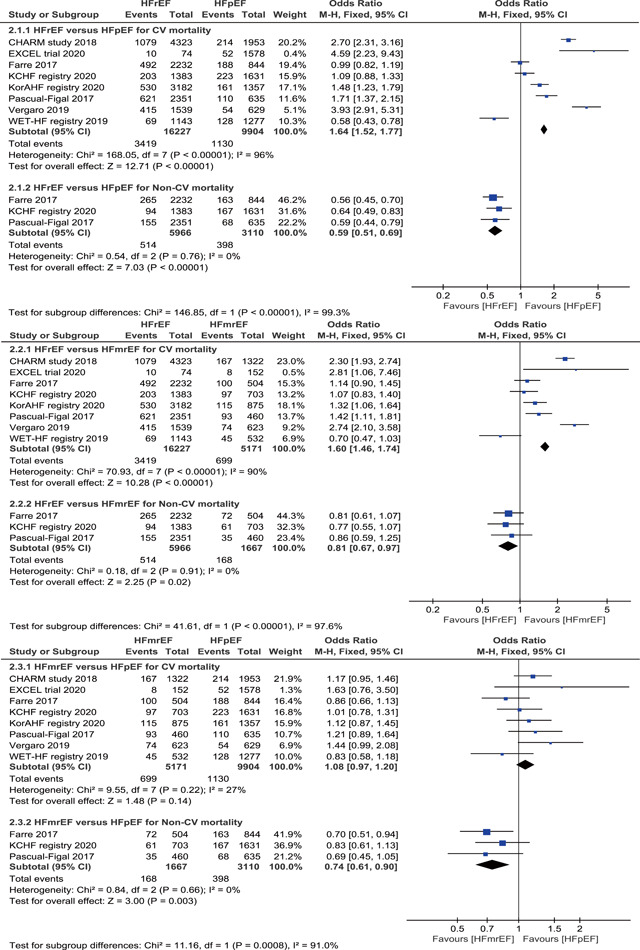
Forest plot of the odds ratio (OR) and 95% confidence interval (CI) for causes of death among three categories of HF. HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction
3.4.3. Subgroup analysis
The subgroup analysis was performed based on high‐risk patients with acute HF or AF or diabetes mellitus. Among high‐risk patients, the risk of all‐cause mortality was still lower in patients with HFmrEF than those with HFrEF and HFpEF, but a statistically significant difference was only observed in AF patients with HFrEF and HFmrEF compared with patients with HFpEF from three studies, and there was no statistically significant difference in patients with acute HF from eight studies or diabetes mellitus from two studies among three categories of HF patients, with low heterogeneity (Figure S3).
3.4.4. Other endpoints
Six studies provided data for HF hospitalization and nine studies for the composite of all‐cause mortality and HF hospitalization. There were 12 607/28 590 (44.10%), 1822/5285 (34.47%), and 8686/22 763 (38.16%) hospitalizations among HFrEF, HFmrEF, and HFpEF patients, respectively. When data are pooled using the fixed‐effects model, the risk of HF hospitalization was significantly lower in patients with HFmrEF than those with HFrEF and HFpEF, and significant differences were also observed between HFrEF and HFpEF. Similarly, the risk of composite of all‐cause mortality and HF hospitalization was significantly lower in patients with HFmrEF than those with HFrEF and HFpEF, but not significantly different between HFrEF and HFpEF (Figure S4).
4. DISCUSSION
This meta‐analysis consisting of recently published studies with substantial numbers of patients demonstrated marked differences in key baseline characteristics and long‐term prognosis, including all‐cause mortality, cardiovascular/non‐cardiovascular mortality, HF hospitalization, and composite of all‐cause mortality and HF hospitalization, among three HF categories. Patients with HFrEF were more often male, more frequently suffered from coronary artery disease or ischemic HF, and more often received the recommended medications, such as renin‐angiotensin system inhibitors and beta‐blockers. Baseline co‐morbidities, such as hypertension and diabetes, were more frequent in patients with HFpEF but AF was more common in patients with HFmrEF. Patients with HFmrEF had the lowest risk of all‐cause mortality, HF hospitalization and composite of all‐cause mortality and HF hospitalization. On the contrary, the highest incidence of all‐cause mortality was in patients with HFpEF, and patients with HFrEF had the highest HF hospitalization and composite of all‐cause mortality and HF hospitalization. Regarding the causes of death, HFrEF had the highest cardiovascular‐specific death, especially HF death and SCD.
HFmrEF is often termed as an “intermediate” phenotype between HFrEF and HFpEF but our findings challenge this. Based on our results, we observed that HFmrEF distinctly resembled HFrEF in coronary artery disease or ischemic HF, diabetes, and chronic kidney disease and was similar to HFpEF in AF except for age, sex, and hypertension, which was mostly different from a meta‐analysis consisting of 12 retrospective or prospective studies published 2018 whose results supported that demographics and comorbid conditions of HFmrEF were largely intermediate between those of HFpEF and HFrEF. 35 More importantly, we also noticed that patients with HFmrEF had the lowest risk of all‐cause mortality, HF hospitalization, and the composite of these two components, partially consistent with the other two meta‐analyses, 35 , 36 which proved similar results about the lowest all‐cause mortality in HFmrEF but different results with respect to the lowest risk of HF hospitalization in HFpEF. Why do we observe a favorable prognosis for patients with HFmrEF? The existing evidence suggests that HFmrEF is characterized by mixed pathophysiology and a recent expert consensus focuses more on the pathophysiological mechanisms of HF rather than LVEF. 37 As a subset of patients with HFmrEF appears to have more intense neurohormonal activation, therapies that block the neurohormonal axes may work in these patients, resembling the effects seen in HFrEF. Some observational studies and post hoc analyses of randomized controlled trials suggest that patients with HFmrEF benefit from medications that target the neurohormonal axes, including ACEI or ARB, β‐blocker, and aldosterone antagonists. Data from the Sweden HF registry suggested that ACEIs/ARBs were associated with a reduced risk of death irrespective of the presence or absence of coronary artery disease. 38 Another analysis of the CHARM data proved candesartan significantly reduced the primary composite outcome of cardiovascular death or first HF hospitalization compared to placebo in HFrEF and HFmrEF but not in HFpEF. 27 In an individual‐level meta‐analysis of 11 trials, β‐blockers halved cardiovascular mortality in patients with HFmrEF in sinus rhythm, regardless of ischemic or nonischemic etiology, which was similar to those observed in HFrEF, and β‐blockers helped to increase LVEF regardless of rhythm (sinus or AF) in the HFmrEF group, with a more pronounced benefit when the etiology was ischemic. 39 Data from the Swedish Heart Failure Registry indicated that the one‐year mortality benefit of β‐blockers in patients with HFmrEF was restricted to those with underlying coronary artery disease. 38 In our meta‐analysis, the characteristics of patients with HFmrEF, including comorbidities, such as coronary artery disease, diabetes, chronic kidney disease, and the medications they received were mostly similar to those of patients with HFrEF. From these results, treating HFmrEF with an evidence‐based therapy for HFrEF seems promising, and further studies should concentrate on this specific population with respect to the potential benefits of guideline‐directed medical therapy.
Of note, studies have shown that a considerable number of patients with HFmrEF transition to either HFrEF or HFpEF while on treatment, as do HFrEF and HFpEF. Among the included studies, only one study by Farre 34 provided changes in LVEF of patients with alive at 1 year, which shown that 62% of HFmrEF patients still remained LVEF 40~50% and 24% and 33% of HFmrEF patients transitioned to HFrEF and HFpEF, respectively, and there were no differences in mortality between patients who remained in HFmrEF group and those who changed to HFrEF, while survival was significantly higher in those patients who evolved to the HFpEF group. Unfortunately, other included studies failed to provide more information about this. A prospective cohort of 1821 chronic HF patients demonstrated that HF‐recovered patients, defined as LVEF enrollment ≥50% but prior LVEF < 50%, had the best prognosis in terms of death, cardiac transplantation, and ventricular assist device placement than HFrEF (LVEF < 50%) and HFpEF (LVEF always ≥ 50%) patients. 40 These suggest that HF‐recovered population may represent a distinct HF phenotype and we need to further investigate pathophysiological differences in these patient populations in an effort to better tailor therapy.
Unexpectedly, the highest risk of all‐cause mortality is in HFpEF patients, rather than HFrEF patients, which may be explained by a high proportion of higher age and females and the association of the markedly higher burden of co‐comorbidities, such as hypertension, diabetes, and AF, and our subgroup analysis confirmed the highest all‐cause mortality risk of HFpEF in the high‐risk population of AF. A multinational prospective observational study aimed at characterizing HFpEF (LVEF ≥ 45%) also confirmed that HFpEF was associated with higher age, female gender, hypertension, AF/flutter, and numerous non‐cardiovascular co‐morbidities, such as anemia, renal dysfunction, diabetes, lung disease, and cancer and the prognosis was determined by non‐cardiovascular co‐morbidities. 41 More critically, patients with HFpEF received application of renin–angiotensin system blockers and β‐blockers significantly less than those with HFrEF and HFmrEF from our results. Because the findings of randomized trials of neurohormonal modulation have been neutral in HFpEF and consistently positive in HFrEF, which results in the infrequent use of neuroendocrine antagonists in HFpEF. A recently published meta‐analysis consisting of randomized controlled trials involving patients with HFpEF revealed that β‐blockers, ACEI, ARB, and mineralocorticoid receptor antagonists treatment has little or no effect on all‐cause mortality, and β‐blockers maybe have a possible reduction in cardiovascular mortality, mineralocorticoid receptor antagonists probably reduces HF hospitalization, and other drugs have no observed benefits for cardiovascular mortality and heart hospitalization. 42 The PARAGON‐HF trial, including 4822 patients with HFpEF of LVEF ≥ 45%, demonstrated that sacubitril‐valsartan, a drug currently used to replace ACEI/ARB in the treatment of HFrEF, did not significantly lower the rate of total hospitalizations for HF, and death from cardiovascular causes compared with valsartan and sub‐group analysis identified lower risk reduction for the primary outcome among those with LVEF no more than 57%. 43 Thus, guidelines offer no specific treatment recommendations regarding the use of these therapies in HFpEF beyond the management of comorbidities. Furthermore, regarding the cause of death, our study indicated that the non‐cardiovascular deaths of patients with HFpEF were significantly higher than those with HFrEF and HFmrEF. In a KCHF study, 16 infection was the leading cause of non‐cardiovascular death, then followed by a malignant tumor. Regretfully, however, our results cannot add further information on non‐cardiovascular death causes of patients with HFpEF due to the lack of statistical power. Taken together, we should not only seek effective methods to treat HFpEF itself to improve prognosis but also pay more attention to the management of comorbidities.
HFrEF is the most commonly studied subgroup of HF and there are treatments proved to be effective in this phenotype, including ACEIs/ARBs or angiotensin receptor neprilysin inhibitor (ARNI) recently, β‐blockers, and aldosterone antagonists, which are definitely recommended as evidence‐based treatments by the ESC 6 and American College of Cardiology/American Heart Association (ACC/AHA) 44 yielding a reduction in mortality and morbidity, which are also confirmed in this article. The evidence‐based treatments were significantly higher in HFrEF patients than both HFmrEF and HFpEF patients, which may explain why the all‐cause mortality of patients with HFrEF was lower than those of patients with HFpEF, rather than the highest, in spite of the high prevalence of coronary artery disease or ischemic HF, which is one of the major contributing causes of death in HF populations. Hence, these drugs should be initiated as soon as possible, and they should be titrated up to the highest dose according to patient tolerability. Moreover, the cardiovascular mortality in patients with HFrEF was significantly higher than those with HFmrEF and HFpEF, especially HF death and SCD.
In addition, we conducted subgroup analyses of high‐risk populations and found that there was no difference in all‐cause mortality among the three categories of patients with acute HF or type 2 diabetes except for AF. This result suggested no association between the LVEF strata and the prognosis in patients with acute HF, which was not consistent with previous observations in chronic HF. 45 The differences may are attributed to dynamic LVEF changes as a result of correction of the underlying cardiac defect in the cases of hospitalization for acute HF, especially acute decompensated HF, and prognostic events occur during the vulnerable phase after hospital discharge, which is largely the results of insufficient treatments during the index hospitalization or nonadherence to the treatment associated with socioeconomic status or lack of education in this phase. 46 Thus, simply trying to evaluate the long‐term event rate in patients with acute HF according to the LVEF strata may be both difficult and inappropriate. AF was more common in patients with HFmrEF and HFpEF, and AF was more strongly associated with all‐cause mortality in the HFpEF group than in the HFrEF and HFmrEF group in our meta‐analysis, which was contrary to the result of a previous meta‐analysis in favor of significantly higher all‐cause mortality in AF patients with HFrEF compared with HFpEF. 47 However, A retrospective study supported that AF was associated with increased all‐cause mortality in patients with HFpEF but not in patients with HFrEF. 48 Furthermore, a recently published meta‐analysis evaluating the relationship between AF and mortality risk in HFpEF, showed that AF was associated with an 11% increased risk of all‐cause mortality in patients with HFpEF and AF was an independent predictor of HF hospitalization, cardiovascular death, and stroke. 49 Future studies should focus on the underlying mechanisms of these dual conditions and seek potential therapeutic strategies.
This meta‐analysis had several limitations. First, the populations of included studies were heterogeneous concerning the baseline characteristics and the size of the prevalence of comorbidities. Another source of heterogeneity is due to the different sizes of included studies, ranging from a few hundred to tens of thousands of samples. Thus, running the mortality and hospitalization analyses in the fixed‐effects model was more realistic. Second, some inculuded studies did not provide sufficient data for analyses regarding baseline characteristics and other endpoints, including cardiovascular/non‐cardiovascular mortality, HF hospitalization, and combination of all‐cause mortality and HF hospitalization, resulting in lacked statistical power. This article only took available key baseline characteristics into consideration and did not include body mass index, chronic kidney disease, chronic obstructive pulmonary disease, anemia, or HF‐related echocardiographic parameters other than LVEF in the analyses. Finally, the HFrEF population constituted almost of the whole analyzed population, while the HFmrEF and HFpEF population accounted for a small proportion, which may be attributed to imbalanced recruitment and registration. Thus, compared with well‐treated populations in randomized controlled trials, the all‐cause mortality estimates may be higher and a time effect is possible. Accordingly, the results of this study should be interpreted cautiously.
5. CONCLUSIONS
In conclusion, the long‐term prognoses, including all‐cause mortality, HF hospitalization, and composite of all‐cause mortality and HF hospitalization, for patients with HFmrEF were significantly lower than those for patients with HFpEF and HFrEF. Patients with HFpEF were associated with a higher risk of all‐cause mortality, which also has been observed in patients at high risk of AF and non‐cardiovascular mortality. Patients with HFrEF were related to a higher risk of cardiovascular mortality, especially HF death and SCD, and HF hospitalization and composite of all‐cause mortality and HF hospitalization. These findings should encourage more research on patient characteristics, mortality, and the effect of HF therapies to improve outcomes of patients, especially for the management of comorbidities of HFpEF.
Supporting information
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Liang M, Bian B, Yang Q. Characteristics and long‐term prognosis of patients with reduced, mid‐range, and preserved ejection fraction: a systemic review and meta‐analysis. Clin Cardiol. 2022;45:5‐17. 10.1002/clc.23754
Min Liang and Bo Bian contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990‐2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392:1789‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. 2020;22:1342‐1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee DS, Gona P, Vasan RS, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070‐3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Solomon SD, Anavekar N, Skali H, et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738‐3744. [DOI] [PubMed] [Google Scholar]
- 5. Pocock SJ, Ariti CA, McMurray JJ, et al. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34:1404‐1413. [DOI] [PubMed] [Google Scholar]
- 6. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891‐975. [DOI] [PubMed] [Google Scholar]
- 7. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2013;62:e147‐e239. [DOI] [PubMed] [Google Scholar]
- 8. Kawahira M, Tamaki S, Yamada T, et al. Prognostic value of impaired hepato‐renal function and liver fibrosis in patients admitted for acute heart failure. ESC Heart Failure. 2021;8:1274‐1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yılmaz MB, Aksakal E, Aksu U, et al. Snapshot evaluation of acute and chronic heart failure in real‐life in Turkey: a follow‐up data for mortality. Anatol J Cardiol. 2020;23:160‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu HX, Zhu YM, Hua Y, Huang YH, Lu Q. Association between atrial fibrillation and heart failure with different ejection fraction categories and its influence on outcomes. Acta Cardiol. 2020;75:423‐432. [DOI] [PubMed] [Google Scholar]
- 11. DJFM Thuijs, Milojevic M, Stone GW, et al. Impact of left ventricular ejection fraction on clinical outcomes after left main coronary artery revascularization: results from the randomized EXCEL trial. Eur J Heart Fail. 2020;22:871‐879. [DOI] [PubMed] [Google Scholar]
- 12. Song Y, Li F, Xu Y, et al. Prognostic value of sST2 in patients with heart failure with reduced, mid‐range and preserved ejection fraction. Int J Cardiol. 2020;304:95‐100. [DOI] [PubMed] [Google Scholar]
- 13. Son MK, Park JJ, Lim NK, Kim WH, Choi DJ. Impact of atrial fibrillation in patients with heart failure and reduced, mid‐range or preserved ejection fraction. Heart. 2020;106:1160‐1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malik A, Gill GS, Lodhi FK, et al. Prior heart failure hospitalization and outcomes in patients with heart failure with preserved and reduced ejection fraction. Am J Med. 2020;133:84‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. MacDonald MR, Tay WT, Teng THK, et al. Regional variation of mortality in heart failure with reduced and preserved ejection fraction across Asia: outcomes in the ASIAN‐HF registry. J Am Heart Assoc. 2020;9:e012199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kitai T, Miyakoshi C, Morimoto T, et al. Mode of death among Japanese adults with heart failure with preserved, midrange, and reduced ejection fraction. JAMA Network Open. 2020;3:e204296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kanagala P, Arnold JR, Singh A, et al. Characterizing heart failure with preserved and reduced ejection fraction: an imaging and plasma biomarker approach. PLoS One. 2020;15:e0232280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Al‐Jarallah M, Rajan R, Al‐Zakwani I, et al. Impact of digoxin on all‐cause mortality and re‐hospitalizations in acute heart failure patients. Annals of Clinical Cardiology. 2020;2:29‐35. [Google Scholar]
- 19. Yee D, Novak E, Platts A, Nassif ME, LaRue SJ, Vader JM. Comparison of the Kansas City Cardiomyopathy Questionnaire and Minnesota Living with Heart Failure Questionnaire in predicting heart failure outcomes. Am J Cardiol. 2019;123:807‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vicent L, Cinca J, Vazquez‐García R, et al. Discharge treatment with angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker after a heart failure hospitalisation is associated with a better prognosis irrespective of left ventricular ejection fraction. Intern Med J. 2019;49:1505‐1513. [DOI] [PubMed] [Google Scholar]
- 21. Vergaro G, Ghionzoli N, Innocenti L, et al. Noncardiac versus cardiac mortality in heart failure with preserved, midrange, and reduced ejection fraction. J Am Heart Assoc. 2019;8:e013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takei M, Kohsaka S, Shiraishi Y, et al. Heart failure with midrange ejection fraction in patients admitted for acute decompensation: a report from the Japanese multicenter registry. J Card Failure. 2019;25:666‐673. [DOI] [PubMed] [Google Scholar]
- 23. Lin TK, Hsu BC, Li YD, et al. Prognostic value of anxiety between heart failure with reduced ejection fraction and heart failure with preserved ejection fraction. J Am Heart Assoc. 2019;8:e010739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hanna Froehlich H, Rosenfeld N, Taeger T, et al. Epidemiology and long‐term outcome in outpatients with chronic heart failure in north‐western Europe. Eur J Heart Fail. 2019;21:55. [DOI] [PubMed] [Google Scholar]
- 25. Chung J, Kim HL, Kim MA, et al. Sex differences in long‐term clinical outcomes in patients hospitalized for acute heart failure: a report from the Korean Heart Failure registry. Journal of Women's Health. 2019;28:1606‐1613. [DOI] [PubMed] [Google Scholar]
- 26. Zafrir B, Lund LH, Laroche C, et al. Prognostic implications of atrial fibrillation in heart failure with reduced, mid‐range, and preserved ejection fraction: a report from 14 964 patients in the European Society of Cardiology Heart Failure Long‐Term Registry. Eur Heart J. 2018;39:4277‐4284. [DOI] [PubMed] [Google Scholar]
- 27. Lund LH, Claggett B, Liu J, et al. Heart failure with mid‐range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail. 2018;20:1230‐1239. [DOI] [PubMed] [Google Scholar]
- 28. Lam CSP, Gamble GD, Ling LH, et al. Mortality associated with heart failure with preserved vs. reduced ejection fraction in a prospective international multi‐ethnic cohort study. Eur Heart J. 2018;39:1770‐1780. [DOI] [PubMed] [Google Scholar]
- 29. Kasahara S, Sakata Y, Nochioka K, et al. Comparable prognostic impact of BNP levels among HFpEF, Borderline HFpEF and HFrEF: a report from the CHART‐2 Study. Heart Vessels. 2018;33:997‐1007. [DOI] [PubMed] [Google Scholar]
- 30. Gu J, Pan JA, Fan YQ, Zhang HL, Zhang JF, Wang CQ. Prognostic impact of HbA1c variability on long‐term outcomes in patients with heart failure and type 2 diabetes mellitus. Cardiovasc Diabetol. 2018;17:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shah KS, Xu H, Matsouaka RA, et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5‐year outcomes. J Am Coll Cardiol. 2017;70:2476‐2486. [DOI] [PubMed] [Google Scholar]
- 32. Sartipy U, Dahlström U, Fu M, Lund LH. Atrial fibrillation in heart failure with preserved, mid‐range, and reduced ejection fraction. JACC: Heart Failure. 2017;5:565‐574. [DOI] [PubMed] [Google Scholar]
- 33. Pascual‐Figal DA, Ferrero‐Gregori A, Gomez‐Otero I, et al. Mid‐range left ventricular ejection fraction: clinical profile and cause of death in ambulatory patients with chronic heart failure. Int J Cardiol. 2017;240:265‐270. [DOI] [PubMed] [Google Scholar]
- 34. Farre N, Lupon J, Sole‐Gonzalez E, et al. Clinical characteristics and outcomes of patients with heart failure with mid‐range ejection fraction. Eur J Heart Fail. 2017;19:418. [Google Scholar]
- 35. Lauritsen J, Gustafsson F, Abdulla J. Characteristics and long‐term prognosis of patients with heart failure and mid‐range ejection fraction compared with reduced and preserved ejection fraction: a systematic review and meta‐analysis. ESC Heart Failure. 2018;5:687‐694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Altaie S, Khalife W. The prognosis of mid‐range ejection fraction heart failure: a systematic review and meta‐analysis. ESC Heart Failure. 2018;5:1008‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Triposkiadis F, Butler J, Abboud FM, et al. The continuous heart failure spectrum: moving beyond an ejection fraction classification. Eur Heart J. 2019;40:2155‐2163B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koh AS, Tay WT, Teng THK, et al. A comprehensive population‐based characterization of heart failure with mid‐range ejection fraction. Eur J Heart Fail. 2017;19:1624‐1634. [DOI] [PubMed] [Google Scholar]
- 39. Cleland JGF, Bunting KV, Flather MD, et al. Beta‐blockers for heart failure with reduced, mid‐range, and preserved ejection fraction: an individual patient‐level analysis of double‐blind randomized trials. Eur Heart J. 2018;39:26‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Basuray A, French B, Ky B, et al. Heart failure with recovered ejection fraction clinical description, biomarkers, and outcomes. Circulation. 2014;129:2380‐2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lund LH, Donal E, Oger E, et al. Association between cardiovascular vs. non‐cardiovascular co‐morbidities and outcomes in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16:992‐1001. [DOI] [PubMed] [Google Scholar]
- 42. Martin N, Manoharan K, Davies C, Lumbers RT. Beta‐blockers and inhibitors of the renin‐angiotensin aldosterone system for chronic heart failure with preserved ejection fraction. Cochrane Database Syst Rev. 2021;2021:CD012721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609‐1620. [DOI] [PubMed] [Google Scholar]
- 44. Yancy CW, Jessup M, Bozkurt B, et al. ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;2017(136):e137‐e161. [DOI] [PubMed] [Google Scholar]
- 45. Doughty RN, Cubbon R, Ezekowitz J, et al. The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta‐analysis: Meta‐analysis Global Group in Chronic Heart Failure (MAGGIC). Eur Heart J. 2012;33:1750‐1757. [DOI] [PubMed] [Google Scholar]
- 46. Cheema B, Ambrosy AP, Kaplan RM, et al. Lessons learned in acute heart failure. Eur J Heart Fail. 2018;20:630‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kotecha D, Chudasama R, Lane DA, Kirchhof P, Lip GY. Atrial fibrillation and heart failure due to reduced versus preserved ejection fraction: a systematic review and meta‐analysis of death and adverse outcomes. Int J Cardiol. 2016;203:660‐666. [DOI] [PubMed] [Google Scholar]
- 48. Jobs A, Schwind J, Katalinic A, et al. Prognostic significance of atrial fibrillation in acute decompensated heart failure with reduced versus preserved ejection fraction. Clin Res Cardiol. 2019;108:74‐82. [DOI] [PubMed] [Google Scholar]
- 49. Liu G, Long M, Hu X, Hu CH, Du ZM. Meta‐analysis of atrial fibrillation and outcomes in patients with heart failure and preserved ejection fraction. Heart Lung Circ. 2021;30:698‐706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


