Abstract
Background
This study aimed to investigate whether the pretreatment platelet to lymphocyte ratio (PLR) is a significant prognostic factor in metastatic pancreatic ductal adenocarcinoma (PDAC).
Methods
A total of 134 histologically confirmed PDAC patients were included in our retrospective study. The data included treatment regimens, Karnofsky Performance Status (KPS), and PLR. Kaplan-Meier curves and univariate and multivariate Cox proportional hazards regression analyses were applied to identify the prognostic factors associated with overall survival (OS).
Results
We used the receiver operating characteristic (ROC) curve to set the cut-off value of the PLR. For the Kaplan-Meier analysis, the median overall survival in PDAC patients with a PLR of 123 or less was 19.7 months, whereas the values in those with a PLR greater than 123 was 13.7 months (P=0.014). PLR was a significant prognostic marker in the multivariate Cox model [hazard ratio (HR) =1.721, 95% CI: 1.162–2.550, P=0.007].
Conclusions
The PLR pretreatment had potential as prognostic indicator in patients with metastatic PDAC.
Keywords: Survival, lymphocyte, pancreatic ductal adenocarcinoma (PDAC), platelet to lymphocyte ratio (PLR)
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most devastating cancer types characterized by a 5-year patient survival rate of as low as 8% (1). By 2030, pancreatic cancer is predicted to surpass breast, prostate, and colorectal cancer and become the second leading cause of cancer-related death in the United States, next to lung cancer (2). The number of deaths caused by PDAC ranked top 6 among Chinese male population and top 7 among Chinese female population in all cancer-related mortalities (3). A paucity of efficient early detection method leads to advanced stages upon diagnosis, leaving more than 80% of all patients ineligible for radical resection. Moreover, the lack of effective therapeutic approaches only worsens the situation.
As one of the most lethal neoplasms, the resectability rate of PDAC is as low as 10–20%. So far, the only prognostic biomarker recommended by the NCCN guidelines for clinical use for PDAC patients is carbohydrate antigen 19-9 (CA 19-9). We focused on the cost-effectiveness and availability when searching for potential prognostic biomarkers. Hence, we focused on hematologic markers such as leukocyte, neutrophil, lymphocyte, platelet counts, neutrophil to lymphocyte ratio (NLR), and platelet to lymphocyte ratio (PLR) (4). These hematologic markers along with other systematic inflammatory markers like albumin and C-reactive protein affect varied solid tumors (5). The predicting ability for survival of PLR has been tested in numerous solid malignancies including gastric (6), non-small cell lung cancer (NSCLC) (7), breast (8), colorectal (9), esophageal (10), hepatocellular (11), ovarian (12), and pancreatic cancers (13). However, some literatures in PDAC are inconsistent. The relevance between PLR and metastatic PDAC remains enigmatic. The objective of our study is to investigate the prognostic role of PLR, especially in metastatic PDAC.
Methods
Patients
From 1 January 2010 to 1 June 2015, a total of 134 patients with cytologically or histologically confirmed metastatic PDAC were retrospectively included in our study for analysis. The study was approved by the ethics committee of the Chinese People’s Liberation Army (PLA) General Hospital. Patients were included using the following criteria: (I) treatment-naïve; (II) not eligible for operation; (III) sufficient bone marrow function; (IV) normal hepatic and renal function; and (V) Karnofsky Performance Status (KPS) score ≥70. A written informed consent from each patient was obtained prior to treatment. All patients were subjected to at least one cycle of gemcitabine monotherapy or combined therapy with no targeted therapies under the instruction of institutional guidelines and regulations. Patients with incomplete data of toxicities or those who were out of contact (loss of follow-up) were then excluded. We followed up on the patients on a 3-month basis until July 30, 2016 to obtain clinical and outcome information. The date of death was obtained from the China disease prevention and control information system or via follow-up.
Data collection
All clinical data for analysis, including age, sex, KPS, tumor location, and pretreatment laboratory peripheral blood tests, were collected from the medical records of the PLA General Hospital database. The blood tests were obtained 1 week prior to chemotherapy. The absolute lymphocyte count was calculated by the percentage of segmented neutrophils per WBC count. The PLR was determined by the absolute platelet count divided by the absolute lymphocyte count. The primary study endpoint was the overall survival (OS). Censoring occurred if patients were still alive at the last follow-up.
Statistical analysis
Continuous variables were expressed as mean values ± standard deviation. Frequency or percentage was presented for categorical variables. We used the ROC curve to determine the best cut-off value for OS with pretreatment PLR. Survival data were analyzed using the Kaplan-Meier method followed by the log-rank test. Cox proportional hazards regression models were performed for univariate and multivariate survival analyses. All statistical analyses were performed using the statistical software packages R (http://www.R-project.org, The R Foundation). All tests were two sided and statistical significance was defined as P<0.05.
Results
A total 134 metastatic PDAC patients consisting of 89 males and 45 females who received at least one cycle of chemotherapy was eligible for the assessment (Table 1). The mean age at the time of diagnosis was 56.6 (95% CI: 48.14–64.46) years old. At the last follow-up, 122 (91.04%) patients had died. The median survival time was 7.50 months (95% CI: 6.23–8.77). We used over-all survival as the time point to generate the receiver operating characteristic (ROC) curve, the optimal cut-off value of 123 for pretreatment PLR, and area under the curve (AUC) of 0.586. The Kaplan-Meier cumulative survival curve for the metastatic PDAC patients stratified by pretreatment PLR is shown in Figure 1. The patients with PLR >123 had significantly shorter median survival (6.3 months; 95% CI: 4.97–7.63) compared with patients with PLR ≤123 (9.5 months; 95% CI: 7.26–11.74) (P=0.014). Univariate and multivariate analyses were performed to investigate the prognostic role of pretreatment PLR in pancreatic cancer patients who underwent chemotherapy. Male (P=0.010), higher PLR (P=0.015), and gemcitabine monotherapy compared with gemcitabine combined regimen (P=0.007) were poor prognostic factors for OS according to the univariate analysis in this study cohort (Table 2). A high level of pretreatment PLR was a robust prognostic factor for OS [hazard ratio (HR) =1.721, 95% CI: 1.162–2.550, P=0.007] according to the multivariable analysis adjusted for gender, KPS, location, and chemotherapy regimens (Table 3). Based on our analysis, PLR was an independent prognostic factor for metastatic PDAC patients.
Table 1. Demographics and pretreatment hematological results from patients with metastatic PDAC.
| Characteristics | Data |
|---|---|
| Age (year) | 56.60±8.46 [34–79] |
| Gender | |
| Male | 89 |
| Female | 45 |
| KPS | |
| 70 | 4 (3.0) |
| 80 | 19 (14.2) |
| 90 | 111 (82.8s) |
| Prediagnostic smoking status | |
| Never | 52 |
| Ever | 82 |
| Prediagnostic alcohol consumption | |
| 0 | 37 |
| >0 | 97 |
| Location | |
| Head | 44 |
| Body and tail | 90 |
| Chemotherapy | |
| Monotherapy | 42 |
| Combined therapy | 92 |
| Platelet (×109/L) | 196.76±69.05 [82–558] |
| Lymphocyte (×109/L) | 0.26±0.93 [0.04–0.49] |
| WBC (×109/L) | 7.03±2.48 [2.16–16.48] |
| PLR | 155.54±93.28 [39.25–725.55] |
Data are presented as number, mean ± SD, n (%), or [range]. PDAC, pancreatic ductal adenocarcinoma; KPS, Karnofsky Performance Status; WBC, white blood cell; PLR, platelet to lymphocyte ratio.
Figure 1.
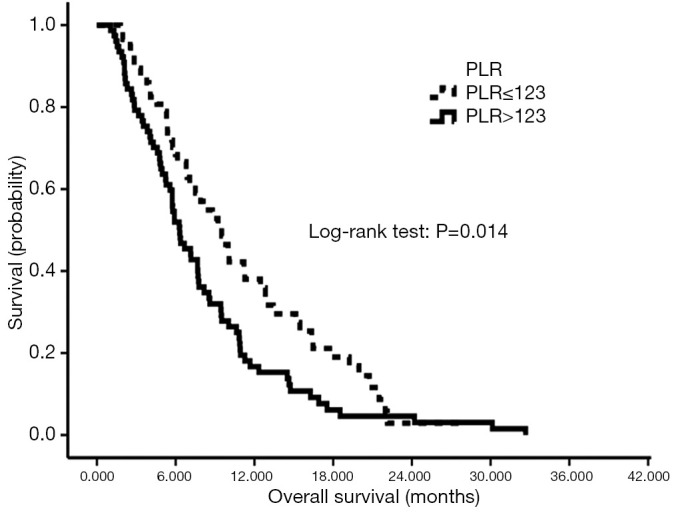
Kaplan-Meier cumulative survival curve for metastatic PDAC patients according to pretreatment PLR. PDAC, pancreatic ductal adenocarcinoma; HR, hazards ratio; PLR, platelet to lymphocyte ratio.
Table 2. Univariate analysis for the association between clinical characteristics and survival in metastatic PDAC patients.
| Variables | Category | n | Univariate analysis | ||
|---|---|---|---|---|---|
| HR | 95% CI | P value | |||
| PLR | ≤123 | 57 | 1 | ||
| >123 | 77 | 1.581 | 1.092–2.287 | 0.015* | |
| Age | – | 1 | |||
| Increasing | 1.003 | 0.981–1.025 | 0.787 | ||
| Gender | Male | 89 | 1 | ||
| Female | 45 | 0.603 | 0.411–0.886 | 0.010* | |
| KPS | 90 | 111 | 1 | ||
| 80 | 19 | 1.103 | 0.673–1.809 | 0.696 | |
| 70 | 4 | 2.480 | 0.904–6.803 | 0.078 | |
| Location | Head | 44 | 1 | ||
| Body/tail | 90 | 1.150 | 0.786–1.684 | 0.471 | |
| Chemotherapy | Monotherapy | 42 | 1 | ||
| Combined therapy | 92 | 0.595 | 0.407–0.870 | 0.007** | |
*, P<0.05; **, P<0.01. PDAC, pancreatic ductal adenocarcinoma; HR, hazards ratio; PLR, platelet to lymphocyte ratio; KPS, Karnofsky Performance Status.
Table 3. Multivariate analysis for the association between clinical characteristics and survival in metastatic PDAC patients.
| Variables | Category | n | Multivariate analysis | ||
|---|---|---|---|---|---|
| HR | 95% CI | P value | |||
| PLR | ≤123 | 57 | 1 | ||
| >123 | 77 | 1.721 | 1.162–2.550 | 0.007** | |
| Age | – | 1 | |||
| Increasing | 0.993 | 0.971–1.016 | 0.564 | ||
| Gender | Male | 89 | 1 | ||
| Female | 45 | 0.616 | 0.413–0.918 | 0.017* | |
| KPS | 90 | 111 | 1 | ||
| 80 | 19 | 1.073 | 0.365–3.152 | 0.898 | |
| 70 | 4 | 1.082 | 0.641–1.825 | 0.769 | |
| Location | Head | 44 | 1 | ||
| Body/tail | 90 | 1.438 | 0.945–2.188 | 0.090 | |
| Chemotherapy | Monotherapy | 42 | 1 | ||
| Combined therapy | 92 | 0.588 | 0.388–0.890 | 0.012* | |
The multivariate Cox regression model adjusted for gender, KPS, location, and WBC, platelets, hemoglobin. *, P<0.05; **, P<0.01. PDAC, pancreatic ductal adenocarcinoma; HR, hazards ratio; PLR, platelet to lymphocyte ratio; KPS, Karnofsky Performance Status.
Discussion
In the last decade, a growing number of evidences showed the critical role of inflammation in tumor initiation, promotion, metastasis, and angiogenesis (14). Immune cells including macrophages, mast cells, natural killer cells, and T- and B-lymphocytes interweave with tumor cells and stoma cells to form a tumor complex. Various cells communicate with each other via autocrine and paracrine cytokines and chemokines to control tumor growth (15). A pancreatic cancer cell could trigger a systemic inflammation manifested as aberrant hematologic cell counts accompanied with elevated circulating cytokines, namely, interleukin (IL)-6, IL-10, IL-8, and IL-1RA (16). The concurrent overexpression of IL-6 and IL-1 stimulate megakaryocytes and results in thrombocytosis in PDAC. Moreover, platelets augment tumor growth, metastasis, and angiogenesis by secreting vascular endothelial growth factor (VEGF) (17) and platelet-derived growth factor (PDGF) (18). Platelets play a vital role in tumor cell-induced thrombosis. Tumor cells express a tissue factor that binds to factor VIIa, consequently initiating a coagulation cascade and promoting thrombosis (19). Therefore, tumor cells evade the host’s immune response and migrate to remote sites under the cloak of platelets (20). Brown et al. confirmed that elevated platelet counts are associated with shortened survival time in PDAC patients compared with non-elevated platelet counts (21). An in vivo study has also shown that anti-platelet drugs could reduce metastasis in PDAC. This information provides us a promising treatment option for metastatic PDAC (22).
Lymphocyte, as a vital element of host immune system, also predict a worse outcome in varied solid malignancies. Lymphocytopenia is a reflection of a generalized state of a depressed immune function. Lymphocyte alone has shown to be an independent survival factor of pancreatic cancers (22-25). Systemic inflammation induced by tumors is manifested by the secretion of a series of inhibitory immunologic mediators, remarkably, IL-10 and transforming growth factor-β (TGF-β). This secretion can result in a significant immunosuppression and impaired lymphocyte function (26). The aberrant overproduction of cytokines TGF-β and IL-10 inhibit proliferation and development of Th1-like responses in the peripheral blood mononuclear cell (PBMC) and result in T-cell cytokine production patterns in favor of a Th2 immunophenotype, thereby exerting an immunosuppressive effect and enabling tumor cells to survive in the host immune system (27).
By combining the two aforementioned elements, PLR has the potential to be a better biomarker in predicting PDAC prognosis. In our study, high PLR was significantly associated with shorter OS. This result is in accordance with the study conducted by Qi et al., which had a sample size of 321 locally advanced and metastatic pancreatic adenocarcinoma that showed a predictive value of PLR (HR =1.537, 95% CI: 1.114–2.122, P=0.009) for OS (4). Although inconsistencies exist among current literature, Templeton et al.’s meta-analysis found that a higher PLR is associated with worse OS in various solid tumors according to data from 22 studies that included a total of 12,754 patients (28). Besides, binary cutoffs were more advantageous than two cut-offs. Bhatti et al. reported an inclination toward a shorter survival in the highest category of PLR >200 compared with PLR <100 (14.4 vs. 8.0 months) (29). However, the study showed no statistical significance with respect to the relevance of PLR and survival. When compared with study populations with locoregional disease, the strongest association is between PLR and survival in metastatic or mixed groups of patients (by Templeton et al.) (28). Xu et al. summarized 14 studies analyzing PLR in pancreatic cancer and reached a conclusion of PLR as a good predictive biomarker (HR =1.24, 95% CI: 1.10–1.39, I2=74%) regardless of the cut-off for PLR, sample size, and treatment. Discrepancies may be derived from mixed treatment, the stratification of different stages of pancreatic cancer, and the inadequacy of follow-up (30).
In our study, an increased preoperative PLR was associated with prolonged OS. We specifically evaluated PLR in the metastatic stage of PDAC. The relatively homogeneous study sample enabled us to draw conclusions exempted from potential confounders. Yet, we need to be cautious when extrapolating the result to PDAC of earlier stages. The limitations of this study are as follows: (I) as we have performed a retrospective study that involved a relatively limited number of patients, large multicenter studies are required to further validate the clinical significance of PLR. (II) A consensus is needed to reach the suitable cut-off before using PLR as a clinical parameter for prognosis.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the Chinese People’s Liberation Army (PLA) General Hospital (No. S2016-062-02). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.12.20). The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. 10.1158/0008-5472.CAN-14-0155 [DOI] [PubMed] [Google Scholar]
- 3.Zheng R, Zeng H, Zhang S, et al. Estimates of cancer incidence and mortality in China, 2013. Chin J Cancer 2017;36:66. 10.1186/s40880-017-0234-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qi Q, Geng Y, Sun M, et al. Clinical implications of systemic inflammatory response markers as independent prognostic factors for advanced pancreatic cancer. Pancreatology 2015;15:145-50. 10.1016/j.pan.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 5.Dolan RD, McSorley ST, Horgan PG, et al. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: Systematic review and meta-analysis. Crit Rev Oncol Hematol 2017;116:134-46. 10.1016/j.critrevonc.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 6.Lee S, Oh SY, Kim SH, et al. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer 2013;13:350. 10.1186/1471-2407-13-350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu H, Wu Y, Wang Z, et al. Pretreatment platelet-to-lymphocyte ratio (PLR) as a predictor of response to first-line platinum-based chemotherapy and prognosis for patients with non-small cell lung cancer. J Thorac Dis 2013;5:783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koh CH, Bhoo-Pathy N, Ng KL, et al. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer 2015;113:150-8. 10.1038/bjc.2015.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon HC, Kim SH, Oh SY, et al. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers 2012;17:216-22. 10.3109/1354750X.2012.656705 [DOI] [PubMed] [Google Scholar]
- 10.Dutta S, Crumley AB, Fullarton GM, et al. Comparison of the prognostic value of tumour- and patient-related factors in patients undergoing potentially curative resection of oesophageal cancer. World J Surg 2011;35:1861-6. 10.1007/s00268-011-1130-7 [DOI] [PubMed] [Google Scholar]
- 11.Kinoshita A, Onoda H, Imai N, et al. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br J Cancer 2012;107:988-93. 10.1038/bjc.2012.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asher V, Lee J, Innamaa A, et al. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin Transl Oncol 2011;13:499-503. 10.1007/s12094-011-0687-9 [DOI] [PubMed] [Google Scholar]
- 13.Shirai Y, Shiba H, Sakamoto T, et al. Preoperative platelet to lymphocyte ratio predicts outcome of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Surgery 2015;158:360-5. 10.1016/j.surg.2015.03.043 [DOI] [PubMed] [Google Scholar]
- 14.Dougan M, Dranoff G. The immune response to tumors. Curr Protoc Immunol 2009;Chapter 20:Unit 20.11. [DOI] [PubMed] [Google Scholar]
- 15.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebrahimi B, Tucker SL, Li D, et al. Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis. Cancer 2004;101:2727-36. 10.1002/cncr.20672 [DOI] [PubMed] [Google Scholar]
- 17.Li N. Platelets in cancer metastasis: To help the "villain" to do evil. Int J Cancer 2016;138:2078-87. 10.1002/ijc.29847 [DOI] [PubMed] [Google Scholar]
- 18.Heldin CH, Westermark B, Wasteson A. Platelet-derived growth factor. Isolation by a large-scale procedure and analysis of subunit composition. Biochem J 1981;193:907-13. 10.1042/bj1930907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haas SL, Jesnowski R, Steiner M, et al. Expression of tissue factor in pancreatic adenocarcinoma is associated with activation of coagulation. World J Gastroenterol 2006;12:4843-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buergy D, Wenz F, Groden C, et al. Tumor-platelet interaction in solid tumors. Int J Cancer 2012;130:2747-60. 10.1002/ijc.27441 [DOI] [PubMed] [Google Scholar]
- 21.Brown KM, Domin C, Aranha GV, et al. Increased preoperative platelet count is associated with decreased survival after resection for adenocarcinoma of the pancreas. Am J Surg 2005;189:278-82. 10.1016/j.amjsurg.2004.11.014 [DOI] [PubMed] [Google Scholar]
- 22.Tzanakakis GN, Agarwal KC, Vezeridis MP. Prevention of human pancreatic cancer cell-induced hepatic metastasis in nude mice by dipyridamole and its analog RA-233. Cancer 1993;71:2466-71. [DOI] [PubMed] [Google Scholar]
- 23.Cheng H, Luo G, Lu Y, et al. The combination of systemic inflammation-based marker NLR and circulating regulatory T cells predicts the prognosis of resectable pancreatic cancer patients. Pancreatology 2016;16:1080-4. 10.1016/j.pan.2016.09.007 [DOI] [PubMed] [Google Scholar]
- 24.Clark EJ, Connor S, Taylor MA, et al. Preoperative lymphocyte count as a prognostic factor in resected pancreatic ductal adenocarcinoma. HPB (Oxford) 2007;9:456-60. 10.1080/13651820701774891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fogar P, Sperti C, Basso D, et al. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas 2006;32:22-8. 10.1097/01.mpa.0000188305.90290.50 [DOI] [PubMed] [Google Scholar]
- 26.Salazar-Onfray F, Lopez MN, Mendoza-Naranjo A. Paradoxical effects of cytokines in tumor immune surveillance and tumor immune escape. Cytokine Growth Factor Rev 2007;18:171-82. 10.1016/j.cytogfr.2007.01.015 [DOI] [PubMed] [Google Scholar]
- 27.Bellone G, Turletti A, Artusio E, et al. Tumor-associated transforming growth factor-beta and interleukin-10 contribute to a systemic Th2 immune phenotype in pancreatic carcinoma patients. Am J Pathol 1999;155:537-47. 10.1016/S0002-9440(10)65149-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2014;23:1204-12. 10.1158/1055-9965.EPI-14-0146 [DOI] [PubMed] [Google Scholar]
- 29.Bhatti I, Peacock O, Lloyd G, et al. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg 2010;200:197-203. 10.1016/j.amjsurg.2009.08.041 [DOI] [PubMed] [Google Scholar]
- 30.Xu ZS, Zhang FP, Zhang Y, et al. Prognostic role of the pre-treatment platelet-lymphocyte ratio in pancreatic cancer: a meta-analysis. Oncotarget 2017;8:99003-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


