Abstract
Breast cancer is the leading cause of death from cancer in women worldwide. Chemotherapy represents one key treatment modality for the clinical management of breast cancer. However, ATP-binding cassette (ABC) transporter mediated active efflux of structurally and mechanistically different cytotoxic compounds results in multidrug resistance (MDR), eventually leading to failure of chemotherapy. The concept of combining anti-cancer drugs and transport inhibitors has been advocated as a concept for re-sensitization of resistant breast cancer to chemotherapy. Whether inhibition of efflux transporters may have the potential to improve therapeutic outcomes is discussed controversially. In this review we discuss challenges in the treatment of breast cancer, the role of MDR in development and the potential of natural products to overcome MDR.
Keywords: Breast cancer, multidrug resistance (MDR), ATP-binding cassette transporters (ABC transporters), inhibitors, natural products
Introduction
Breast cancer represents a malignant disease, which seriously affects female physical and mental health. Although morbidity and mortality of breast cancer have declined over the past 20 years, it still represents the leading cause of cancer deaths in women worldwide (1). According to the World Health Organization fact sheet, 571,000 breast cancer related deaths were reported in 2015 (2). Chemotherapy has developed into an effective way of treating breast cancer, but multidrug resistance (MDR) frequently occurs in the course of cancer chemotherapy and eventually results in its failure (3). MDR refers to a condition, in which cancer cells acquire cross-resistance to anti-cancer drugs of different chemical structure and mechanism of action. The phenotype of MDR can have different reasons, including overexpression of drug efflux transporters, induction of detoxifying enzyme systems, suppression of apoptotic pathways and in a more narrow sense, mutation of target structures. Efflux transporter mediated MDR is typically caused by upregulation of members of the ABC protein family, primarily P-glycoprotein (P-gp) (ABCB1), multidrug resistance protein (MRP; ABCC1) and breast cancer resistance protein (BCRP, ABCG2).
The concept of overcoming MDR caused by overexpression of these efflux transporters envisioned co-administration of standard chemotherapy regimens in conjunction with inhibitors of these efflux transporters. Candidate compounds from the arsenal of approved drugs in the market were initially identified serendipitously and first characterized in in vitro model systems in the early 1990’s. Clinical use of these first generation P-gp inhibitors was limited by intrinsic pharmacological properties. Second generation inhibitors were thus designed to be devoid of these intrinsic pharmacological effects. These compounds, however, turned out to suffer from interfering anticancer drugs with changing pharmacokinetics at the level of cytochrome P450 isoenzymes. Only third generation inhibitors such as tariquidar, elacridar and zosuquidar, which did not show this interference, were ultimately used in numerous clinical studies. Disappointingly, third generation inhibitors, either failed to show a clinical benefit in the verum group, or they had severe side effects due to inhibition of P-gp in tissues, which under cytostatic therapy required P-gp function for cell survival. Frequently they led to bone marrow aplasia related deaths.
Generally, many novel classes of P-gp inhibitors showed adverse effects in preclinical studies, or they required drug concentrations, which could not be reached in patients (4). Although results from clinical trials were disappointing, our understanding of drug resistance has become more nuanced. A role of ABC transporters in the failure of drug delivery to tumors has unequivocally been demonstrated. Even low expression of P-gp was shown to lead to pronounced decreases in cellular accumulation of cytotoxic drugs (5). Thus, the main question to be asked is, if the concept of resensitization of tumor cells to drugs is invalid, or if clinical studies have failed because of conceptual shortcomings. It can certainly not be denied that many of the clinical studies have not shown a benefit for patients. While the role of multidrug efflux transporters in drug resistance is unequivocally established, further discussion on the aspect of MDR inhibition in cancer cell resistance is thus required. Apart from their role in MDR, ABCB1, ABCC1 and ABCG2 play an important role in drug disposition and drug-drug interactions (DDIs). Thus for biology, clinical pharmacology and drug development, MDR transporters remain a research field of major interest.
Natural products are produced by living organisms. They are not essential for survival, but nevertheless provide organisms that produce them with an evolutionary advantage. Many of the compounds that are used in the treatment of human diseases are natural products or derivatives of them. One remarkable example for the use of a natural product in medicine is the compound class of artemisinin first line drug treatment of malaria (6). Thus natural products are deemed indispensable for the pharmacological treatment of human diseases, as pharmacology heavily relies on the use of natural product drugs.
As results with the first three generations of inhibitors at the clinical trial stage were disappointing, researchers turned their attention to potent and relatively non-toxic natural products as inhibitors for blocking ABC transporters. In this review, we focus on the clinical management of breast cancer, MDR transporters and inhibitors that have been used in in vitro, mouse models and clinical studies. A short discussion of natural products, including alkaloids, saponins and flavonoids is included. These compounds may have the potential to overcome transporter mediated breast cancer MDR in a clinical setting without having the severe side effects observed in earlier clinical studies.
Clinical management of breast cancer
Breast cancer is treated by combined modality. This includes surgery, radiation therapy, chemotherapy, hormone therapy, and targeted therapy with biologicals. Different types of breast cancer may require a different extent of surgical treatment from breast-conserving surgery to total mastectomy and modified radical mastectomy. Radiotherapy to the region of the tumor bed and regional lymph nodes is often following surgery, in order to destroy tumor cells that may have escaped or been spread by surgery (7). Radiation can reduce the risk of recurrence by 50–66%, when delivered in the correct dose (8). Drug treatment involves hormone blockers, chemotherapy and targeted therapy with biologicals. Hormonal therapy represents one of the major modalities of medical oncology (9). It involves the manipulation of the endocrine system through exogenous administration of specific hormones, particularly steroid hormones or drugs, which inhibit the production or activity of such hormones. The selective estrogen receptor modulator (SERM) tamoxifen is currently first-line treatment for pre-menopausal women with hormone receptor (HR)-positive breast cancer (10). Aromatase inhibitors such as anastrozole or letrozole are given in postmenopausal women (11). Chemotherapy mainly works by destroying fast-growing or fast-replicating cancer cells, either by causing DNA damage upon replication, or by mechanisms targeted at cell division (12). Chemotherapy can either be systemic or regional. Usually regimens contain combinations of different compounds, e.g., a combination of cyclophosphamide and doxorubicin, termed the “AC” regimen (13). However, medications may damage normal cells and thereby cause serious side effects. Thus targeted therapy with biopharmaceuticals or nanoengineered enzymes, which specifically identify and attack cancer cells, while minimizing damage to normal cells has evolved as a promising treatment strategy (14). The arsenal of targeted therapeutics currently includes monoclonal antibodies and small molecules [tyrosine kinase inhibitors (TKI), cyclin-dependent kinase inhibitors, mechanistic target of rapamycin (mTOR) inhibitors and poly ADP ribose polymerase (PARP) inhibitors. The anti HER2 monoclonal antibody trastuzumab has for example significantly improved the 5-year disease free survival of HER2-positive breast cancer (15). Recently, the FDA approved the cyclin dependent kinase 4/6 inhibitors ribociclib, and abemaciclib, which are administered in different combinations for the treatment of HR-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer (16). Targeted therapy can also be combined with chemotherapy as adjuvant therapy (17). Treatments with biologicals was demonstrated to improve prognosis and quality of life for patients, but at the same time caused an increase in the number of patients that experience adverse drug effects. These include metabolic toxicity (hyperglycaemia, hypertriglyceridemia, hypercholesterolemia), dermatologic and cardiovascular toxicity and immune suppression (18-20).
In addition to the above treatments, high-dose chemotherapy and subsequent autologous stem cell transplantation represents a way of escalating dose regimens that lead to otherwise intolerable bone marrow toxicity (21). However, proof of a beneficial effect that exceeds standard chemotherapy regimens has yet to be provided.
Inhibitors of ABC transporters in overcoming breast cancer resistance
In vitro studies
ABC transporters thus have been documented to play an important role in MDR of cancer. Thus inhibitors may still be considered to enhance chemotherapeutic efficacy of standard anticancer drugs (22). To date, a larger number of different chemical inhibitor scaffolds have been reported. The majority has been characterized in vitro, while a larger number of compounds have also been studied in animal models. Selected compounds have progressed to the stage of clinical phase trials. First generation inhibitors included the calcium channel blocker verapamil, calmodulin antagonists, protein C kinase inhibitors, immune suppressive drugs such as cyclosporine A, antibiotics (e.g., erythromycin), antimalarial drugs (e.g., quinine) and steroid hormones (23-25). Verapamil was reported to not only increase the concentration of doxorubicin in drug-resistant human breast cancer cells (MCF-7/ADR) by inhibition of P-gp, but also to induce apoptosis of tumor cells (26). However, first generation P-gp inhibitors suffered from the limitation of having inherent pharmacological properties (27). Second-generation inhibitors were in part obtained by modifying first-generation inhibitors. PSC-833 was developed as a non-immunosuppressive analog of cyclosporine A with an improved safety profile and higher affinity. Studies showed that PSC-833 can be used as an efficient MDR modulator to reverse DOX-resistance in the human breast cancer cell line T47D/TAMR-6 (28,29). Another example for a second generation inhibitor of P-gp and BCRP was VX710 (biricodar). Second-generation inhibitors thus had higher potency, specificity, and lower cytotoxicity than the first generation inhibitors (30). However, second-generation inhibitors suffered from the initially unrecognized limitation that they interfered with pharmacokinetics (and thus cytotoxicity) of anticancer agents in co-administration protocols at the level of cytochrome P450 isoforms. In consequence, third generation modulators were developed to design out pharmacokinetic interference with anticancer drugs. These inhibitors were primarily directed against P-gp, including the derivatives of o-aminobenzamide tariquidar (XR9576), zosuquidar (LY335979), elacridar (GF120918) and laniquidar (R101933) (31-34). Compared to the first and the second generation inhibitors, third generation inhibitors exhibited a reversal potential of 200-fold or more at nanomolar concentrations, but did not show a significant influence on pharmacokinetics of co-administered chemotherapeutic agents (30,35), as these inhibitors are not metabolized by the cytochrome P450 isoform 3A4. LY335979 (zosuquidar) was found to not only inhibit P-gp, but also to down regulate its expression in breast cancer MCF-7/ADR and MT-3/ADR cells. In addition some tyrosine kinase inhibitors (TKIs) have been found to be not only substrates, but to also act as inhibitors of ABC transporters (36,37).
Inhibitor action in mouse models in vivo
Several breast cancer mouse models for preclinical intervention trials such as genetically engineered mouse models (PDX models, organoids, non-germline models) have been established to investigate the progression of breast cancer and drug resistance mechanisms. In particular genetically engineered mouse models have been used (38-40). The hereditary breast cancer K14cre; Brca1F/F; p53F/F mouse model of spontaneous breast tumors shows that doxorubicin and docetaxel resistance was associated with up-regulation of the two P-gp or thologs in mouse Mdr1a and Mdr1b. This confirmed the relevance of even moderate increases of P-gp in drug resistance development in vivo (41). Resistance can be overcome by inhibiting P-gp. For example, long time treatment with the poly (APD-ribose) polymerase (PARP) inhibitor AZD2281did result in drug resistance, which could be reversed by co-administration of the P-gp inhibitor tariquidar. This suggests that P-gp inhibition provides a feasible strategy in vivo (5,42). In addition, anti-cancer drug resistance was also reported to be associated with increased expression of ABCG2 in the mouse model. Tumor-specific genetic ablation of the mouse isoform of ABCG2 significantly increased the overall survival of BRCA1 breast cancer mice treated with topotecan, confirming the correlation of ABCG2 expression and topotecan resistance (43). Indeed, the specific ABCG2 inhibitor Ko143 in combination with topotecan increased the overall survival of mice in the Brca1/p53 mouse model. The benefit, however, was modest and the study failed to demonstrate an increased accumulation of topotecan. Conversely the PEGylated SN38 compound EZN-2208 circumvents ABCG2-mediated topotecan resistance. ABCG2-expressing tumors were highly sensitive to EZN-2208. A significant increase in survival was observed, suggesting that PEGylation of Top1 inhibitors may be useful to circumvent transporter-mediated resistance and improve clinical efficacy (44). Therefore, we can conclude that breast cancer MDR is related to expression of P-gp and ABCG2 in in vivo studies, and the combination of inhibitors and chemotherapeutic drugs can overcome MDR and increase the sensitivity of tumor tissue to drugs in vivo. Furthermore, the use of mouse models may help clinicians to derive real-time genotype-specific drug response profiles and design more effective and durable patient-specific regimens (38,40). Although MDR1 acts a potential resistance mechanism in breast cancer of mice, the role of MDR1 in mediating resistance has not been as clearly implicated in human breast cancer (45). The inability to convert MDR1 data derived from a mouse model into humans may be due to the fact that the basal levels of MDR1 in rodents are higher than in humans, which converted to MDR1 expression in response to anticancer drug therapy in rodents (40). Another explanation is that due to methodological limitations, such as techniques for determining MDR1 expression, a relatively small increase of MDR1 expression in clinically important may not have been detected (40). To overcome the limitations of animal models in preclinical target characterization, human mammary epithelial organs can be used to validate data from animal models (46).
Clinical inhibitor studies
Although many studies have shown that multidrug transport inhibitors can work synergistically with anticancer drugs to reverse ABC transporter-mediated MDR in vitro, clinical results suggest that inhibitors do not benefit breast cancer patients (47). The epithelial tumors, breast cancer and advanced cancer of patients were selected to phase I studies. The safety, tolerability, pharmacokinetic and pharmacodynamics of MDR inhibitors co-administered with anti-breast cancer agents were assessed (48). Neurological or hematological toxicity emerged when P-gp inhibitors were administered in combination with anticancer drugs in phase I studies (49,50). Then phase II and III trials mainly studied in metastatic breast cancer patients. One randomized phase II clinical trial compared single-agent epirubicin treatment with or without lonidamine in metastatic breast cancer patients (51). The patients receiving lonidamine failed to show prolonged survival and reduced anthracycline-related toxicity (alopecia, nausea, vomiting, and stomatitis) compared to controls. Moreover, when different inhibitors were combined with the same anticancer drug, a significant improvement in the response rate could not be demonstrated (52,53). In a more recent phase III trial, both, relative improvement and absolute increase in response rate was shown for patients who received dofequidar plus cyclophosphamide, doxorubicin, and fluorouracil (CAF) (54). The 221 patients with metastatic breast cancer received six cycles of CAF (100 mg cyclophosphamide administered orally on days 1–14, 25 mg/m2 adriamycin and 500 mg/m2 fluorouracil administered intravenously on days 1 and 8) with or without 900 mg dofequidar administered orally on days 1–14). The treatment results showed the overall response rates of 42.6% and 53.1% (P=0.077) and median progression-free survivals of 241 and 366 days (P=0.145) for CAF alone and CAF plus dofequidar, respectively. Again, these results did not reach statistical significance. Nevertheless, the search for inhibitors of ABC transporters is still ongoing. A recent report showed that HM30181AK, a novel P-gp inhibitor, prevents the efflux of various chemotherapeutic agents from intestinal epithelial cells to the gastrointestinal tract (55).
Potential natural sources to overcome breast cancer
More than 70% of reported MDR inhibitors identified to date are natural products, while those inhibitors, which have been used in phase III studies are synthetic compounds. Although some third generation inhibitors have entered phase III clinical trials, the results of relevant clinical trials are not ideal. The search for high-efficiency, low-toxic P-gp inhibitors from chemically synthesized source compounds has encountered insurmountable barriers in real-world research. Therefore, it is feasible to find efficient and low-toxic P-gp inhibitors from novel and diverse natural extracts. In the process of evolution, natural plants synthesize and secrete secondary metabolites (SMs) that are used to protect natural enemies (herbivores). These SMs are not directly involved in plant growth, differentiation and regeneration, but it has a toxic effect on herbivorous animals. In the course of natural evolution, herbivores express a related substance transport system with exogenous toxicants, such as P-gp or other transport proteins, in order to protect against such toxic SMs and eliminate harmful substances in food. Therefore, the academic community speculated that there must be related SMs in the natural plants that inhibit the activity of transporters such as P-gp (56). Most of the mechanisms of action of P-gp inhibitors from chemically synthesized sources have been reported to reverse tumor MDR by inhibiting the function of P-gp efflux substrates. One of the major candidate reversal drug resistance agents is a competitive substrate of P-gp in chemical structure and can be mediated by P-gp. However, natural extraction and isolation of organic products not only inhibit P-gp efflux function but some of the reported natural candidate agents such as Ramified curcumin hydrolyzed (57), Honokiol (58), Tetramethylpyrazine (59), Triptolide (60), can also inhibit the expression level of P-gp in drug-resistant tumor cells, thereby mediating the reversal of tumor MDR (61). The candidate reversal compounds of natural origin show better reversal of tumor resistance in structure and mechanism of action. Natural products can be a reliable candidate compound source for finding efficient and low-toxic P-gp inhibitors.
Natural products that are administered in treatment regimens have been in the environment of evolutionary successful organisms for a long time and upregulation of MDR transporters has been one successful strategy to ensure survival of other species in the presence of these natural toxins. MDR transporters thus represent an Achilles heel for cancer treatment, as their presence allowed survival of extant species. On the other hand, organisms that produce xenotoxic compounds may also have developed strategies to keep these toxins effective, by co-releasing inhibitors of MDR transporters. Thus research on identification of compounds with MDR inhibitory action from plants may be considered a promising strategy for the identification of novel scaffolds.
Natural products have been the single most productive source of leads for the development of drugs (40). They can act as MDR inhibitors as they compete with the efflux of cytotoxic agents by binding to the active site. It is hoped that the drug discovery process will be spurred by the identification of more effective inhibitors or modulators originating from natural sources. Materials extracted from plants are most diverse and often have complex chemical scaffolds. Importantly, many natural products have low toxicity and are well tolerated (62). Curcumin, ginsenoside and alkaloids are examples of natural produces with MDR inhibitory properties (63). In addition, these compounds can also help to increase oral bioavailability or tissue penetration of therapeutic drugs. This may represent an additional concept for a clinical application of these natural products (64). For instance, quercetin was able to increase the peak plasma concentration and oral bioavailability of the P-gp substrate doxorubicin by 1.3- to 2.4-fold in rats (61).
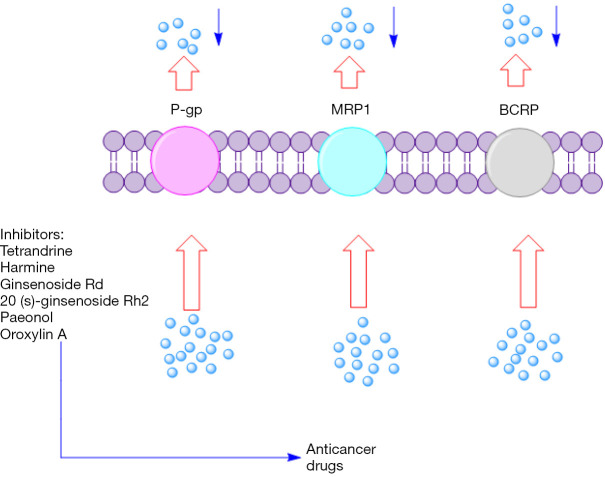
Notably, it should not be ignored that nearly 80% of all drugs approved by the FDA for cancer therapy during the past three decades were either natural products per se or based on a natural product scaffold. Others are mimetics of natural products in one form or another (65). Therefore, the interest of scientists in searching for candidates from natural sources that have a modulatory effect on the function of disease-associated ABC transporters persists. Below, we present a brief overview of potential natural products that can overcome ABC transporter mediated MDR in breast cancer (Figure 1).
Figure 1.
Natural products as potential reversing agents of MDR. MDR, multidrug resistance.
Alkaloids
Alkaloids are a highly diverse group of compounds (66), which contain a ring structure and a nitrogen atom, and mainly exist in higher plants such as Leguminosae, Ranunculaceae and Magnoliaceae. Some of the alkaloids have been used as anticancer chemotherapeutics, including camptothecin (CPT) (67) and vinblastine (68). It was suggested that the active alkaloid may act as a competitive inhibitor of P-gp and BCRP (69). Tetrandrine (Tet), a benzyl isoquinoline alkaloid, is a good candidate for the development of new MDR-reversing agents due to the potent inhibition of P-gp (70). Similarly, Berberine, another isoquinoline alkaloid isolated from Berberis species, could suppress P-gp by down-regulating the AMPK-HIF-1α signalling pathway to enhance DOX chemosensitivity in MCF-7 drug-resistant cells (71,72). Ma et al. studied the effect of harmine on the overexpression of BCRP in MDA-MB-231 breast cancer cells (73). Twenty µM harmine could inhibit BCRP more effectively than FTC at identical concentration. Results indicated that harmine was able to inhibit BCRP-mediated drug efflux and reverse drug resistance, and suggested that harmine may be used as a lead compound for the development of BCRP reversal agents. In addition, it is reported that more than 15 alkaloid compounds exhibit reversal activity to drug-resistant breast cancer cells recently. However, these compounds need further investigation to convert into successful drug candidate (74).
Saponins
Saponins are a class of compounds found in various plant species. The pharmacological effects of saponins include antiviral (75), anti-inflammatory (76) and antitumor activity. These effects are proposed to be related to their cholesterol binding properties, polarity and hydrophobicity (77). A recent report has shown that saponins and anticancer drugs in combination can increase the sensitivity of P-gp-expressing tumors (78). Ginsenoside is the main active constituent of ginseng, which are highly valued owing to its enhanced pharmacology effects such as immunostimulating, antioxidant, anti-cancer and antiaging activity (79). Ginsenoside Rh2, a major pharmacological active component of ginseng, could influence the MAPK/NF-kB pathway to down-regulate Adriamycin-induced ABCB1 expression in previous study (80). Moreover, Ginsenoside Rh2 could mediate the miRNA expression to reduce the drug resistance of breast cancer (81). Similarly, Saikosaponin D, one of the major triterpenoid saponins derived from Bupleurum, significantly down-regulated MDR1 mRNA and P-gp expression without altering the pharmacokinetic profiles of DOX to involve in the reversal of MDR for breast cancer (82,83). Conversely, another saponin compound, 20(S)-protopanaxadiol, stimulated the activity of ABCB1 ATPase to enhance the efficacy of substrate drugs in drug-resistant breast cancer cells rather than suppress the expression of ABCB1 mRNA or protein (84).
Flavonoids
Flavonoids are a class of naturally occurring polyphenols, which are widely distributed in the leaves, flowers and stems of higher plants. When grapefruit juice was reported to affect therapeutic efficacy of nifedipine (a CYP3A substrate) (85), interest in the relevance of flavonoids in ABC transporter mediated MDR flared. Not surprisingly, flavonoids were found to inhibit ABC transporters (86-88). Interestingly, curcumin has broad-spectrum modulatory effects on all three major ABC drug transporters: ABCB1, ABCC1 and ABCG2 (89,90). Curcumin could reduce breast cancer stem cells population for sensitizing breast cancer cells to mitomycin C both in vitro and in vivo by suppressing ABCG2 (91,92). However, usually curcumin loaded nanoparticles combination with chemotherapy drugs for effectively overcoming MDR due to the low aqueous solubility and poor stability of curcumin (93,94). Furthermore, paeonol, another component extracted from the root cortex of the Paeonia suffruticosa, could reverse paclitaxel resistance in breast cancer with a superior 8.2-fold reversal index (95). The study found that the compound down-regulated transgelin 2-mediated paclitaxel resistance by reducing the expression of P-gp, MRP1 and BCRP in MCF-7/PTX cells. Also, Silibinin (96) and Rutin (97) have the potential to sensitize chemo-resistant breast cancer cells. Remarkably, a recent investigation confirmed that flavonoids are avid inhibitors of BCRP, which suggested that their presence at high levels in the diet could cause food-drug interactions (98).
Discussion and conclusions
Breast cancer is the second leading cause of death from malignant diseases in women. Chemotherapy is one important treatment option to slow the progress of the disease, but the emergence of MDR represents an impediment to successful treatment and ultimately to its failure. Transporter mediated drug efflux represents a critical factor in the development of drug resistance. Thus, clinical studies addressed synergistic effects of anti-cancer drugs and transport inhibitors based on preclinical studies. In vitro and in vivo studies demonstrated a benefit of the combination of anticancer drugs and transport inhibitors. However, these findings to date did not translate into beneficial clinical outcomes. Why has the majority of clinical trials trying to combine anti-cancer drugs with MDR inhibitors not been successful? Several factors may explain these disappointing results: (I) combinations of anticancer drugs and P-gp inhibitors can kill bone marrow stem cells in patients that receive combination therapy. However, this side effect may be circumvented by autologous bone marrow transplantation; (II) although inhibitors have been shown to be effective against tumors, in the absence of complete tumor eradication, survival of the fittest will lead to recurrence; (III) P-gp only contributes to tumor cell survival in vivo, but other factors contribute to a resistance phenotype and these effects may be dominant; (IV) the tumor microenvironment plays a crucial role in drug uptake; (V) the polymorphic variants of ABC transporters may influence the efficacy of inhibitors; (VI) patient recruitment will also affect clinical research results.
Although clinical studies with transport inhibitors did not live up to expectations that were tied to the advocation of a most simple concept, results from animal studies may warrant further attention to it. A balanced view on matters may be possible in retrospect from a distance in time. In this review, we attempted to discuss and summarize results with a focus on natural compounds from plants (alkaloids, saponins and flavonoids). As mentioned above, MDR may be caused by factors other than drug efflux transporters. Non-toxic, potent and selective natural product modulators of ABC transporter mediated MDRs may be identified by high-throughput screening and a subsequent combinatorial chemistry approach with extended quantitative structure-activity relationship studies (62).
Acknowledgments
Funding: We acknowledge the financial support from the National Natural Science Foundation of China (81273707, 81173215), the Ministry of Education in the New Century Excellent Talents (NECT-12-0677), the Natural Science Foundation of Guangdong (S2013010012880,2016A030311037), the Science and Technology Program of Guangzhou (2014J4500005,201704030141), the Science Program of the Department of Education of Guangdong (2013KJCX0021, 2015KGJHZ012), the Science and Technology Program of Guangdong (2015A050502027), and the 2017 International Science and Technology Cooperation Project of Guangzhou Economic & Technological Development Zone (2017GH16).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.01.19). The authors have no conflicts of interest to declare.
References
- 1.What are the key statistics about breast cancer?[Online]. American Cancer Society. Available online: http://www.cancer.org/cancer/breastcancer/detailedguide/breastcancer-key-statistics
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 3.Holohan C, Van Schaeybroeck S, Longley DB, et al. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 2013;13:714-26. 10.1038/nrc3599 [DOI] [PubMed] [Google Scholar]
- 4.Szakacs G, Varadi A, Ozvegylaczka C, et al. The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME–Tox). Drug Discov Today 2008;13:379-93. 10.1016/j.drudis.2007.12.010 [DOI] [PubMed] [Google Scholar]
- 5.Pajic M, Iyer JK, Kersbergen A, et al. Moderate increase in Mdr1a/1b expression causes in vivo resistance to doxorubicin in a mouse model for hereditary breast cancer. Cancer Res 2009;69:6396-404. 10.1158/0008-5472.CAN-09-0041 [DOI] [PubMed] [Google Scholar]
- 6.Huang Y, Dong G, Hong X, et al. Studies on reversing MDR of K562/A02 by ramification of curcumin hydrolyzed. Zhongguo Zhong Yao Za Zhi 2010;35:2460-3. [PubMed] [Google Scholar]
- 7.Osuntokun O, Ojo R. Comparative Study on Breast Cancer. Available online: https://www.researchgate.net/publication/259482674_Comparative_Study_on_Breast_Cancer
- 8.Breastcancer.org. Radiation Therapy. 2017. Available online: http://www.breastcancer.org/treatment/radiation
- 9.Abraham J, Staffurth J. Hormonal therapy for cancer. Medicine 2016;44:30-3. 10.1016/j.mpmed.2015.10.01427336890 [DOI] [Google Scholar]
- 10.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013;381:805-16. 10.1016/S0140-6736(12)61963-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeVita VT, Hellman S, Rosenberg SA. Cancer: Principles and Practice of Oncology. Philadelphia: Lippincott, 2005. [Google Scholar]
- 12.Shen M, Duan WM, Wu MY, et al. Participation of autophagy in the cytotoxicity against breast cancer cells by cisplatin. Oncol Rep 2015;34:359-67. 10.3892/or.2015.4005 [DOI] [PubMed] [Google Scholar]
- 13.Biganzoli L, Cufer T, Bruning P, et al. Doxorubicin and paclitaxel versus doxorubicin and cyclophosphamide as first-line chemotherapy in metastatic breast cancer: The European Organization for Research and Treatment of Cancer 10961 Multicenter Phase III Trial. J Clin Oncol 2002;20:3114-21. 10.1200/JCO.2002.11.005 [DOI] [PubMed] [Google Scholar]
- 14.Huang M, Shen A, Ding J, et al. Molecularly targeted cancer therapy: some lessons from the past decade. Trends Pharmacol Sci 2014;35:41-50. 10.1016/j.tips.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 15.Jahanzeb M. Adjuvant trastuzumab therapy for HER2-positive breast cancer. Clin Breast Cancer 2008;8:324-33. 10.3816/CBC.2008.n.037 [DOI] [PubMed] [Google Scholar]
- 16.Hematology/Oncology (Cancer) Approvals & Safety Notifications . 2017. Available online: https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm279174.htm
- 17.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol 2014;15:640-7. 10.1016/S1470-2045(14)70080-4 [DOI] [PubMed] [Google Scholar]
- 18.Xu R, Wang Q. Large-scale automatic extraction of side effects associated with targeted anticancer drugs from full-text oncological articles. J Biomed Inform 2015;55:64-72. 10.1016/j.jbi.2015.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keefe DM, Bateman EH. Tumor control versus adverse events with targeted anticancer therapies. Nat Rev Clin Oncol 2011;9:98-109. 10.1038/nrclinonc.2011.192 [DOI] [PubMed] [Google Scholar]
- 20.Cleeland CS, Allen JD, Roberts SA, et al. Reducing the toxicity of cancer therapy: recognizing needs, taking action. Nat Rev Clin Oncol 2012;9:471-8. 10.1038/nrclinonc.2012.99 [DOI] [PubMed] [Google Scholar]
- 21.Breast Cancer Treatment (PDQ®)—Patient Version [Online] National Cancer Institute. Available online: https://www.cancer.gov/types/breast/patient/breast-treatment-pdq#section/_185
- 22.Chen Z, Shi T, Zhang L, et al. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett 2016;370:153-64. 10.1016/j.canlet.2015.10.010 [DOI] [PubMed] [Google Scholar]
- 23.Thomas H, Coley HM. Overcoming multidrug resistance in cancer: an update on the clinical strategy of inhibiting p-glycoprotein. Cancer Control 2003;10:159-65. 10.1177/107327480301000207 [DOI] [PubMed] [Google Scholar]
- 24.Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci 2000;11:265-83. 10.1016/S0928-0987(00)00114-7 [DOI] [PubMed] [Google Scholar]
- 25.Szakács G, Paterson JK, Ludwig JA, et al. Targeting multidrug resistance in cancer. Nat Rev Drug Discov 2006;5:219-34. 10.1038/nrd1984 [DOI] [PubMed] [Google Scholar]
- 26.Yang L, Wei DD, Chen Z, et al. Reversal of multidrug resistance in human breast cancer cells by Curcuma wenyujin and Chrysanthemum indicum. Phytomedicine 2011;18:710-8. 10.1016/j.phymed.2010.11.017 [DOI] [PubMed] [Google Scholar]
- 27.Choi YH, Yu AM. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr Pharm Des 2014;20:793-807. 10.2174/138161282005140214165212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baekelandt M, Lehne G, Tropé CG, et al. Phase I/II trial of the multidrug-resistance modulator valspodar combined with cisplatin and doxorubicin in refractory ovarian cancer. J Clin Oncol 2001;19:2983-93. 10.1200/JCO.2001.19.12.2983 [DOI] [PubMed] [Google Scholar]
- 29.Bajelan E, Haeri A, Vali AM, et al. Co-delivery of doxorubicin and PSC 833 (Valspodar) by stealth nanoliposomes for efficient overcoming of multidrug resistance. J Pharm Pharm Sci 2012;15:568-82. 10.18433/J3SC7J [DOI] [PubMed] [Google Scholar]
- 30.Li W, Zhang H, Assaraf YG, et al. Overcoming ABC transporter-mediated multidrug resistance: Molecular mechanisms and novel therapeutic drug strategies. Drug Resist Updat 2016;27:14-29. 10.1016/j.drup.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 31.Mistry P, Stewart AJ, Dangerfield W, et al. In vitro and in vivo reversal of P-glycoprotein-mediated multidrug resistance by a novel potent modulator, XR9576. Cancer Res 2001;61:749-58. [PubMed] [Google Scholar]
- 32.Naito M, Matsuba Y, Sato S, et al. MS-209, a quinoline-type reversal agent, potentiates antitumor efficacy of docetaxel in multidrug-resistant solid tumor xenograft models. Clin Cancer Res 2002;8:582-8. [PubMed] [Google Scholar]
- 33.Fracasso PM, Goldstein LJ, de Alwis DP, et al. Phase I study of docetaxel in combination with the P-glycoprotein inhibitor, zosuquidar, in resistant malignancies. Clin Cancer Res 2004;10:7220-8. 10.1158/1078-0432.CCR-04-0452 [DOI] [PubMed] [Google Scholar]
- 34.Chen LM, Liang YJ, Ruan JW, et al. Reversal of P-gp mediated multidrug resistance in-vitro and in-vivo by FG020318. J Pharm Pharmacol 2004;56:1061-6. 10.1211/0022357043879 [DOI] [PubMed] [Google Scholar]
- 35.Gupta P, Garg T, Tanmay M, et al. Polymeric Drug-Delivery Systems: Role in P-gp Efflux System Inhibition. Crit Rev Ther Drug Carrier Syst 2015;32:247-75. 10.1615/CritRevTherDrugCarrierSyst.2015011592 [DOI] [PubMed] [Google Scholar]
- 36.Shukla S, Chen Z, Ambudkar SV. Tyrosine kinase inhibitors as modulators of ABC transporter-mediated drug resistance. Drug Resist Updat 2012;15:70-80. 10.1016/j.drup.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anreddy N, Gupta P, Kathawala RJ, et al. Tyrosine Kinase Inhibitors as Reversal Agents for ABC Transporter Mediated Drug Resistance. Molecules 2014;19:13848-77. 10.3390/molecules190913848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Annunziato S, Barazas M, Rottenberg S, et al. Genetic Dissection of Cancer Development, Therapy Response, and Resistance in Mouse Models of Breast Cancer. Cold Spring Harb Symp Quant Biol 2016;81:141-50. 10.1101/sqb.2016.81.030924 [DOI] [PubMed] [Google Scholar]
- 39.Jaspers JE, Kersbergen A, Boon U, et al. Loss of 53BP1 Causes PARP Inhibitor Resistance in Brca1-Mutated Mouse Mammary Tumors. Cancer Discov 2013;3:68-81. 10.1158/2159-8290.CD-12-0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rottenberg S, Borst P. Drug resistance in the mouse cancer clinic. Drug Resist Updat 2012;15:81-9. 10.1016/j.drup.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 41.Rottenberg S, Nygren AO, Pajic M, et al. Selective induction of chemotherapy resistance of mammary tumors in a conditional mouse model for hereditary breast cancer. Proc Natl Acad Sci U S A 2007;104:12117-22. 10.1073/pnas.0702955104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rottenberg S, Jaspers JE, Kersbergen A, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci U S A 2008;105:17079-84. 10.1073/pnas.0806092105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zander SA, Kersbergen A, van der Burg E, et al. Sensitivity and Acquired Resistance of BRCA1;p53-Deficient Mouse Mammary Tumors to the Topoisomerase I Inhibitor Topotecan. Cancer Res 2010;70:1700-10. 10.1158/0008-5472.CAN-09-3367 [DOI] [PubMed] [Google Scholar]
- 44.Zander SA, Sol W, Greenberger L, et al. EZN-2208 (PEG-SN38) overcomes ABCG2-mediated topotecan resistance in BRCA1-deficient mouse mammary tumors. PLoS One 2012;7:e45248. 10.1371/journal.pone.0045248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robey RW, Pluchino KM, Hall MD, et al. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer 2018;18:452-64. 10.1038/s41568-018-0005-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu M, Jung L, Cooper AB, et al. Dissecting genetic requirements of human breast tumorigenesis in a tissue transgenic model of human breast cancer in mice. Proc Natl Acad Sci U S A 2009;106:7022-7. 10.1073/pnas.0811785106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruff P, Vorobiof DA, Jordaan JP, et al. A randomized, placebo-controlled, double-blind phase 2 study of docetaxel compared to docetaxel plus zosuquidar (LY335979) in women with metastatic or locally recurrent breast cancer who have received one prior chemotherapy regimen. Cancer Chemother Pharmacol 2009;64:763-8. 10.1007/s00280-009-0925-9 [DOI] [PubMed] [Google Scholar]
- 48.Amiri-Kordestani L, Basseville A, Kurdziel K, et al. Targeting MDR in breast and lung cancer: discriminating its potential importance from the failure of drug resistance reversal studies. Drug Resist Updat 2012;15:50-61. 10.1016/j.drup.2012.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trump DL, Smith DC, Ellis PG, et al. High-dose oral tamoxifen, a potential multidrug-resistance-reversal agent: phase I trial in combination with vinblastine. J Natl Cancer Inst 1992;84:1811-6. 10.1093/jnci/84.23.1811 [DOI] [PubMed] [Google Scholar]
- 50.Tolcher AW, Cowan KH, Solomon D, et al. Phase I crossover study of paclitaxel with r-verapamil in patients with metastatic breast cancer. J Clin Oncol 1996;14:1173-84. 10.1200/JCO.1996.14.4.1173 [DOI] [PubMed] [Google Scholar]
- 51.Lopez M, Vici P, Di Lauro L, et al. Intrapatient comparison of single-agent epirubicin with or without lonidamine in metastatic breast cancer. Eur J Cancer 1995;31A:1611-4. 10.1016/0959-8049(95)00200-3 [DOI] [PubMed] [Google Scholar]
- 52.Mross K, Bohn C, Edler L, et al. Randomized phase II study of single-agent epirubicin +/- verapamil in patients with advanced metastatic breast cancer. An AIO clinical trial. Arbeitsgemeinschaft Internistische Onkologie of the German Cancer Society. Ann Oncol 1993;4:45-50. 10.1093/oxfordjournals.annonc.a058356 [DOI] [PubMed] [Google Scholar]
- 53.Wishart GC, Bissett D, Paul J, et al. Quinidine as a resistance modulator of epirubicin in advanced breast cancer: mature results of a placebo-controlled randomized trial. J Clin Oncol 1994;12:1771-7. 10.1200/JCO.1994.12.9.1771 [DOI] [PubMed] [Google Scholar]
- 54.Saeki T, Nomizu T, Toi M, et al. Dofequidar fumarate (MS-209) in combination with cyclophosphamide, doxorubicin, and fluorouracil for patients with advanced or recurrent breast cancer. J Clin Oncol 2007;25:411-7. 10.1200/JCO.2006.08.1646 [DOI] [PubMed] [Google Scholar]
- 55.Kim TE, Lee H, Lim KS, et al. Effects of HM30181, a P-glycoprotein inhibitor, on the pharmacokinetics and pharmacodynamics of loperamide in healthy volunteers. Br J Clin Pharmacol 2014;78:556-64. 10.1111/bcp.12368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Molnár J, Engi H, Hohmann J, et al. Reversal of multidrug resitance by natural substances from plants. Curr Top Med Chem 2010;10:1757-68. 10.2174/156802610792928103 [DOI] [PubMed] [Google Scholar]
- 57.Rocha Gda G, Oliveira RR, Kaplan MA, et al. 3β-Acetyl tormentic acid reverts MRP1/ABCC1 mediated cancer resistance through modulation of intracellular levels of GSH and inhibition of GST activity. Eur J Pharmacol 2014;741:140-9. 10.1016/j.ejphar.2014.07.054 [DOI] [PubMed] [Google Scholar]
- 58.Xu D, Lu Q, Hu X. Down-regulation of P-glycoprotein expression in MDR breast cancer cell MCF-7/ADR by honokiol. Cancer Lett 2006;243:274-80. 10.1016/j.canlet.2005.11.031 [DOI] [PubMed] [Google Scholar]
- 59.Wang XB, Wang SS, Zhang QF, et al. Inhibition of tetramethylpyrazine on P-gp, MRP2, MRP3 and MRP5 in multidrug resistant human hepatocellular carcinoma cells. Oncol Rep 2010;23:211-5. [PubMed] [Google Scholar]
- 60.Chen CY, Galluppi GR, Richardson JP. Transcription termination at lambda tR1 is mediated by interaction of rho with specific single-stranded domains near the 3' end of cro mRNA. Cell 1986;46:1023-8. 10.1016/0092-8674(86)90701-4 [DOI] [PubMed] [Google Scholar]
- 61.Eichhorn T, Efferth T. P-glycoprotein and its inhibition in tumors by phytochemicals derived from Chinese herbs. J Ethnopharmacol 2012;141:557-70. 10.1016/j.jep.2011.08.053 [DOI] [PubMed] [Google Scholar]
- 62.Wu CP, Ohnuma S, Ambudkar SV. Discovering natural product modulators to overcome multidrug resistance in cancer chemotherapy. Curr Pharm Biotechnol 2011;12:609-20. 10.2174/138920111795163887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu CP, Calcagno AM, Ambudkar SV. Reversal of ABC drug transporter-mediated multidrug resistance in cancer cells: evaluation of current strategies. Curr Mol Pharmacol 2008;1:93-105. 10.2174/1874467210801020093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shukla S, Ohnuma S, Ambudkar SV. Improving cancer chemotherapy with modulators of ABC drug transporters. Current Drug Targets 2011;12:621-30. 10.2174/138945011795378540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bishayee A, Sethi G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin Cancer Biol 2016;40-41:1-3. 10.1016/j.semcancer.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 66.Lu JJ, Bao JL, Chen XP, et al. Alkaloids isolated from natural herbs as the anticancer agents. Evid Based Complement Alternat Med 2012;2012:485042. 10.1155/2012/485042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang M, Gao H, Chen Y, et al. Chimmitecan, a novel 9-substituted camptothecin, with improved anticancer pharmacologic profiles in vitro and in vivo. Clin Cancer Res 2007;13:1298-307. 10.1158/1078-0432.CCR-06-1277 [DOI] [PubMed] [Google Scholar]
- 68.Li W, Shao Y, Hu L, et al. BM6, a new semi-synthetic vinca alkaloid, exhibits its potent in vivo anti-tumor activities via its high binding affinity for tubulin and improved pharmacokinetic profiles. Cancer Biol Ther 2007;6:787-94. 10.4161/cbt.6.5.4006 [DOI] [PubMed] [Google Scholar]
- 69.Wink M. Molecular modes of action of cytotoxic alkaloids: from DNA intercalation, spindle poisoning, topoisomerase inhibition to apoptosis and multiple drug resistance. Alkaloids Chem Biol 2007;64:1-47. 10.1016/S1099-4831(07)64001-2 [DOI] [PubMed] [Google Scholar]
- 70.Liu T, Liu X, Li W. Tetrandrine, a Chinese plant-derived alkaloid, is a potential candidate for cancer chemotherapy. Oncotarget 2016;7:40800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pan Y, Zhang F, Zhao Y, et al. Berberine Enhances Chemosensitivity and Induces Apoptosis Through Dose-orchestrated AMPK Signaling in Breast Cancer. J Cancer 2017;8:1679-89. 10.7150/jca.19106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pan Y, Shao D, Zhao Y, et al. Berberine Reverses Hypoxia-induced Chemoresistance in Breast Cancer through the Inhibition of AMPK- HIF-1α. Int J Biol Sci 2017;13:794-803. 10.7150/ijbs.18969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ma Y, Wink M. The beta-carboline alkaloid harmine inhibits BCRP and can reverse resistance to the anticancer drugs mitoxantrone and camptothecin in breast cancer cells. Phytother Res 2010;24:146-9. 10.1002/ptr.2860 [DOI] [PubMed] [Google Scholar]
- 74.Joshi P, Vishwakarma RA, Bharate SB. Natural alkaloids as P-gp inhibitors for multidrug resistance reversal in cancer. Eur J Med Chem 2017;138:273-92. 10.1016/j.ejmech.2017.06.047 [DOI] [PubMed] [Google Scholar]
- 75.Xiang L, Yi X, Wang Y, et al. Antiproliferative and anti-inflammatory polyhydroxylated spirostanol saponins from Tupistra chinensis. Sci Rep 2016;6:31633. 10.1038/srep31633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Song G, Shen X, Li S, et al. Structure-activity relationships of 3-O-β-chacotriosyl oleanane-type triterpenoids as potential H5N1 entry inhibitors. Eur J Med Chem 2016;119:109-21. 10.1016/j.ejmech.2016.04.061 [DOI] [PubMed] [Google Scholar]
- 77.Ali R, Mirza Z, Ashraf GM, et al. New anticancer agents: recent developments in tumor therapy. Anticancer Res 2012;32:2999-3005. [PubMed] [Google Scholar]
- 78.Abdallah HM, Al-Abd AM, El-Dine RS, et al. P-glycoprotein inhibitors of natural origin as potential tumor chemo-sensitizers: A review. J Adv Res 2015;6:45-62. 10.1016/j.jare.2014.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiménez-Pérez ZE, Kim YJ, Castro-Aceituno V, et al. Novel application of cultured roots of mountain ginseng (panax ginseng meyer) and ginsenoside re as safe antimelanogenic cosmeceutical components. Afr J Tradit Complement Altern Med 2017;14:209-18. 10.21010/ajtcam.v14i5.2428480433 [DOI] [Google Scholar]
- 80.Zhang J, Lu M, Zhou F, et al. Key role of nuclear factor-κB in the cellular pharmacokinetics of adriamycin in MCF-7/Adr cells: the potential mechanism for synergy with 20(S)-ginsenoside Rh2. Drug Metab Dispos 2012;40:1900-8. 10.1124/dmd.112.045187 [DOI] [PubMed] [Google Scholar]
- 81.Wen X, Zhang HD, Zhao L, et al. Ginsenoside Rh2 differentially mediates microRNA expression to prevent chemoresistance of breast cancer. Asian Pac J Cancer Prev 2015;16:1105-9. 10.7314/APJCP.2015.16.3.1105 [DOI] [PubMed] [Google Scholar]
- 82.Li C, Guan X, Xue H, et al. Reversal of P-glycoprotein-mediated multidrug resistance is induced by saikosaponin D in breast cancer MCF-7/adriamycin cells. Pathol Res Pract 2017;213:848-53. 10.1016/j.prp.2017.01.022 [DOI] [PubMed] [Google Scholar]
- 83.Li C, Xue HG, Feng LJ, et al. The effect of saikosaponin D on doxorubicin pharmacokinetics and its MDR reversal in MCF-7/adr cell xenografts. Eur Rev Med Pharmacol Sci 2017;21:4437-45. [PubMed] [Google Scholar]
- 84.Chen G, Liu J, Chen W, et al. A 20 (S)-protopanoxadiol derivative overcomes multi-drug resistance by antagonizing ATP-binding cassette subfamily B member 1 transporter function. Oncotarget 2016;7:9388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bailey DG, Spence JD, Munoz C, et al. Interaction of citrus juices with felodipine and nifedipine. Lancet 1991;337:268-9. 10.1016/0140-6736(91)90872-M [DOI] [PubMed] [Google Scholar]
- 86.Morris ME, Zhang S. Flavonoid-drug interactions: effects of flavonoids on ABC transporters. Life Sci 2006;78:2116-30. 10.1016/j.lfs.2005.12.003 [DOI] [PubMed] [Google Scholar]
- 87.Tan KW, Li Y, Paxton JW, et al. Identification of novel dietary phytochemicals inhibiting the efflux transporter breast cancer resistance protein (BCRP/ABCG2). Food Chem 2013;138:2267-74. 10.1016/j.foodchem.2012.12.021 [DOI] [PubMed] [Google Scholar]
- 88.Alvarez AI, Real R, Perez M, et al. Modulation of the activity of ABC transporters (P-glycoprotein, MRP2, BCRP) by flavonoids and drug response. J Pharm Sci 2010;99:598-617. 10.1002/jps.21851 [DOI] [PubMed] [Google Scholar]
- 89.Chearwae W, Shukla S, Limtrakul P, et al. Modulation of the function of the multidrug resistance-linked ATP-binding cassette transporter ABCG2 by the cancer chemopreventive agent curcumin. Mol Cancer Ther 2006;5:1995-2006. 10.1158/1535-7163.MCT-06-0087 [DOI] [PubMed] [Google Scholar]
- 90.Obara M, Ohnuma S, Chufan EE, et al. Abstract 3242: Synthetic analogs of curcumin as modulators of multidrug resistance-linked ABC transporters. Cancer Res 2017;77:3242. 10.1158/1538-7445.AM2017-3242 [DOI] [Google Scholar]
- 91.Zhou Q, Ye M, Lu Y, et al. Curcumin Improves the Tumoricidal Effect of Mitomycin C by Suppressing ABCG2 Expression in Stem Cell-Like Breast Cancer Cells. PLoS One 2015;10:e0136694. 10.1371/journal.pone.0136694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou QM, Sun Y, Lu YY, et al. Curcumin reduces mitomycin C resistance in breast cancer stem cells by regulating Bcl-2 family-mediated apoptosis. Cancer Cell Int 2017;17:84. 10.1186/s12935-017-0453-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun X, Luo Y, Huang L, et al. A peptide-decorated and curcumin-loaded mesoporous silica nanomedicine for effectively overcoming multidrug resistance in cancer cells. RSC Advances 2017;7:16401-9. 10.1039/C7RA01128H [DOI] [Google Scholar]
- 94.Hong W, Shi H, Qiao M, et al. pH-sensitive micelles for the intracellular co-delivery of curcumin and Pluronic L61 unimers for synergistic reversal effect of multidrug resistance. Sci Rep 2017;7:42465. 10.1038/srep42465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cai J, Chen S, Zhang W, et al. Paeonol reverses paclitaxel resistance in human breast cancer cells by regulating the expression of transgelin 2. Phytomedicine 2014;21:984-91. 10.1016/j.phymed.2014.02.012 [DOI] [PubMed] [Google Scholar]
- 96.Molavi O, Narimani F, Asiaee F, et al. Silibinin sensitizes chemo-resistant breast cancer cells to chemotherapy. Pharm Biol 2017;55:729-39. 10.1080/13880209.2016.1270972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Iriti M, Kubina R, Cochis A, et al. Rutin, a Quercetin Glycoside, Restores Chemosensitivity in Human Breast Cancer Cells. Phytother Res 2017;31:1529-38. 10.1002/ptr.5878 [DOI] [PubMed] [Google Scholar]
- 98.Sjöstedt N, Holvikari K, Tammela P, et al. Inhibition of Breast Cancer Resistance Protein and Multidrug Resistance Associated Protein 2 by Natural Compounds and Their Derivatives. Mol Pharm 2017;14:135-46. 10.1021/acs.molpharmaceut.6b00754 [DOI] [PubMed] [Google Scholar]