Abstract
Background
The relapse and metastasis of bladder cancer are due to its strong resistance to chemotherapeutic drugs after surgery as a result of the expansion and self-renewal of cancer stem cells (CSCs). However, the molecular mechanisms underlying the biology of bladder CSCs are unknown. This study aimed to investigate the role of interleukin 6 (IL6)/IL6 receptor (IL6R) in the stem-like characteristics of bladder CSCs.
Methods
Enzyme-linked immunosorbent assay (ELISA) and real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed to detect IL6 expression in the supernatant and cells of bladder CSCs, respectively. Following that, self-renewal, stem cell-associated gene expression, invasion, metastasis, and tumorigenicity were assessed by sphere-forming assay, qRT-PCR, invasion and transwell assays, and tumor-forming experiment in NOD/SCID mice, respectively. Finally, Western blot and qRT-PCR were employed to examine the IL6 signaling pathway in regulating the stem-like properties of bladder CSCs.
Results
The spheres, originating from the bladder cancer cell lines RT4 and J82, possessed a higher expression of stem-associated genes. The expression levels of IL6 were elevated in the supernatant and cells of the bladder CSCs. IL6R was also up-regulated in the bladder CSCs. Recombinant IL6 promoted the stem-like properties of the bladder CSCs, including self-renewal, expression of stem-associated genes, invasion, migration, and tumorigenicity. Mechanistically, IL6 exerted its biological effects by binding to IL6R, which enhanced the phosphorylation of STAT3 and triggered its activation. Furthermore, these effects were alleviated by the FDA-approved drug tocilizumab.
Conclusions
Our findings demonstrate that IL6/IL6R/STAT3 maintains the stem-like properties of bladder CSCs. Furthermore, IL6R may serve as a potential therapeutic target for CSCs in bladder cancer.
Keywords: Bladder cancer, cancer stem cells (CSCs), interleukin 6 (IL6), STAT3, IL6 receptor (IL6R)
Introduction
The incidence and mortality of bladder cancer is high, with an estimated 429,800 new cases of bladder cancer and 165,100 deaths occurring in 2012 worldwide (1). Surgery is the first choice for the treatment of bladder cancer, but relapse and metastasis after the operation are common (2). The prognosis of patients with bladder cancer is poor due to its strong resistance to chemotherapeutic drugs (3), which is due to the heterogeneity inside the tumor tissues (4).
Evidence shows that bladder tumor cells are heterogeneous and are assumed to include cancer stem cells (CSCs), which possess a special stem-like phenotype and strong abilities for self-renewal, tumorigenicity, invasion, and migration (5). Bladder CSC markers have been reported, including CD133 and CD44 (6,7). Furthermore, spheres have been referred to as tumor cell aggregations possessing CSC properties (8,9). Consistent with this assignment, sphere-forming bladder cancer cell lines are suitable models for bladder CSCs, a result that has been demonstrated by previous studies underlying bladder CSCs (10,11). Though many signaling networks, including the Wnt/β-catenin, Hedgehog, and PI3K/AKT pathways have been reported to be crucial in regulating bladder CSCs (12-14), the molecular mechanisms underlying CSCs in the relapse and metastasis of bladder cancer are unknown.
Interleukin 6 (IL6) signaling has been reported to mediate the proliferation, invasion, and metastasis of various types of cancers (15-17). In addition, the IL6-related pathway regulates the stemness properties of CSCs (18). Many reports have demonstrated that IL6 exerts its biological effects by binding to the IL6 receptor (IL6R), which triggers the downstream activation including STAT3 (19,20). However, it is still unknown whether IL6/IL6R maintains the stem-like characteristics of bladder CSCs and if the blockade of the IL6R suppresses the expansion of CSCs.
In this study, we demonstrated that IL6 and its receptor IL6R were up-regulated in bladder CSCs. In addition, IL6 promoted the stem-like characteristics of bladder cancer by binding to the IL6R and enhancing the phosphorylation of STAT3. Furthermore, the administration of tocilizumab (anti-IL6 receptor drug) inhibited the IL6 promotion of bladder CSCs.
Methods
Cell culture
The bladder cancer cell lines RT4 and J82 cells were obtained from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences. The RT4 and J82 cells were respectively maintained in McCoy’s 5A medium (Invitrogen, Carlsbad, CA, USA) and MEM medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA). The spheres were cultured in DMEM/F-12 (Gibco, Carlsbad, CA, USA) without FBS and supplemented with EGF recombinant human protein solution (Gibco, Carlsbad, CA, USA), FGF-Basic (AA 1–156) recombinant human protein (Gibco, Carlsbad, CA, USA) and ITS liquid media (Sigma-Aldrich, St Louis, MO, USA). Human recombinant IL-6 (50 ng/mL, R&D Systems, Minneapolis, MN, USA), an IL6 neutralizing antibody (20 µg/mL, Abcam, Cambridge, MA, USA) and a humanized anti-IL6 receptor inhibitor (5 µg/mL, tocilizumab, Genentech) were added in the indicated experiments to selected wells.
Sphere formation assay
Single-cell suspensions of 5,000 cells were incubated in 6-well ultra-low-attachment culture plates (Corning, NY, USA) for 4 days. The number of spheres was counted under a microscope.
Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was prepared from the cultured RT4 or J82 cells by using Trizol (Invitrogen, Grand Island, NY, USA) according to the manufacturer’s protocol. Reverse transcriptase PCR was performed using Superscript III RT (Invitrogen, Grand Island, NY, USA) in the presence of random primers. The amplification of the generated cDNA was carried out in SYBR Green PCR Master Mix (Takara, Otsu, Shiga, Japan) with the ABI PRISM 7300HT Sequence Detection System. The primer sequences are listed as follows:
CD133, 5'-GCCACCGCTCTAGATACTGC-3', 5'-TGTTGTGATGGGCTTGTCAT-3'; CD44, 5'-GAGCATCGGATTTGAGA-3', 5'-CATACTGGGAGGTGTTGG-3'; ABCG2, 5'-CAGGTTACGTGGTACAAGATGA-3', 5'-GATCAGTGATAAGCTCCATTCC-3'; Oct-4, 5'-AGTGAGAGGCAACCTGGAGA-3', 5'-ACACTCGGACCACATCCTTC-3'; Nanog, 5'-CTGCTGGACTGAGCTGGTTGCC-3', 5'-GCTGAGGCCTTCTGCGTCACA-3'; IL6, 5'-CTCAATATTAGAGTCTCAACCCCCA-3', 5'-GAGAAGGCAACTGGACCGAA-3'; IL6R, 5'-TCACTGTGTCATCCACGACG-3', 5'-CTGGATTCTGTCCAAGGCGT-3'; and β-actin, 5'-CCCTGGCACCCAGCAC-3', 5'-GCCGATCCACACGGAG-3'. Each measurement was performed in triplicate, and the results were normalized to the expression of the β-actin gene. The fold change relative to the mean value was determined by 2−ΔΔCt.
Enzyme-linked immunosorbent assay (ELISA)
Conditioned media of the adherent cells and spheres from RT4 and J82 cell lines were collected after 4 days of culturing, and then tested for the IL6 ELISA kit (R&D system, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Western blotting
The protein extracts were subjected to SDS-PAGE and were analyzed using the following primary antibodies: IL-6R; p-STAT3; STAT3 and β-actin (Abcam, Cambridge, MA, USA). The secondary antibodies were labeled with IRDye 700 (Rockland Immunochemicals, USA). The protein levels were detected by the Odyssey system (Li-Cor, Lincoln, NE, USA).
Flow cytometry assay
The flow cytometry assay was performed with a Cyan ADP Sorter (Beckman, CA, USA). Bladder cancer cell lines were magnetically labeled with a PE-conjugated-CD133 antibody (Miltenyi Biotec, Bergisch Gladbach, Germany). All the procedures were carried out according to manufacturer’s instructions.
Invasion and migration assay
The invasion and migration assays were carried out in a transwell chamber (Millipore, USA) with or without Matrigel (BD Biosciences, Bedford, MA, USA) according to manufacturer's instructions. Briefly, 500 µL conditioned medium from one of the different groups was added to the bottom chamber as a chemoattractant. A total of 2×104 cells suspended in 200 µL of serum-free culture medium were placed into the chamber with or without Matrigel, and the chambers were placed in 24-well microplates which contained 500 µL conditioned medium to allow the bottom of the chamber to touch the conditioned medium. Therefore, the upper chamber level contained bladder cancer cells suspended in serum-free culture medium, and the lower chamber level contained conditioned medium. Bladder cancer cells in upper chamber would invade through the Matrigel or the membrane of the chamber to the bottom level of the chamber. After 48 hours, the cells that invaded through the Matrigel or membrane to the bottom of the chamber were fixed with 1% paraformaldehyde, then photographed and counted after staining with 0.1% crystal violet. The experiments were independently performed for 3 times.
Tumor formation assay
The RT4 cells were transfected with the luciferase reporter gene. For each treatment, a total of 1×104 RT4-Luc-spheres in 50 µL of PBS were injected subcutaneously into 6-week-old NOD/SCID male mice (Chinese Science Academy, Shanghai, China). Tumor growth was monitored weekly by live-animal bioluminescence optical imaging with the IVIS Lumina II imaging system (PerkinElmer, Hopkinton, MA, USA) after an intraperitoneal injection of D-luciferin (150 mg/kg) (Gold Biotech, USA) in 100 µL of DPBS. The mice were sacrificed at the six weeks post-injection time point, and the tumors were harvested for further examination. Tumor volume was calculated based on three perpendicular measurements by using the formula: volume =1/2 × length × width × height. All experimental procedures were performed according to the guidelines of the Institutional Animal Care and Use Committee of the First Affiliated Hospital of Chongqing Medical University.
Statistical analysis
The differences between the variables were assessed by a two-tailed Student’s t-test or an ANOVA. All the values were expressed as the means ± SD, and statistical significance was defined as P<0.05. The data analysis was performed with SPSS 16.0 (SPSS Inc., Chicago, Illinois, USA).
Results
Expression of IL6 and its receptor is up-regulated in bladder cancer spheres with CSC properties
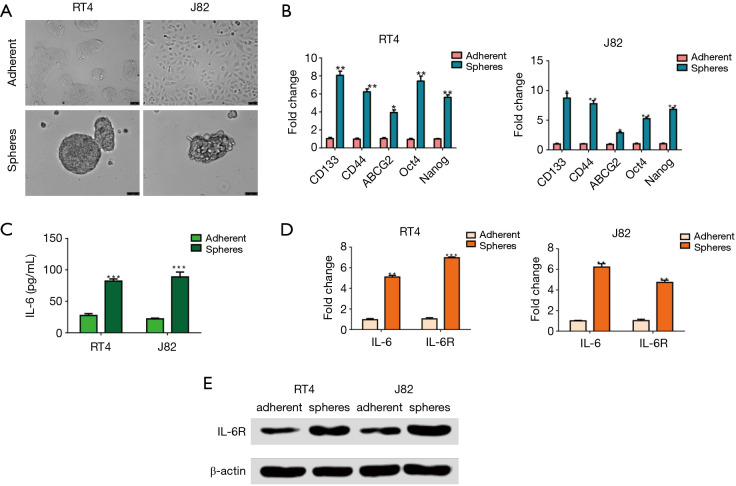
It has been reported that CSCs can be enriched in spheres (21,22), which are cultured in serum-free medium. In our study, single-cell suspensions of bladder cancer cells from the RT4 and J82 cells were incubated in 6-well ultra-low-attachment culture plates for 4 days to achieve the spheres. As expected, a higher expression of stem-associated genes was observed in the spheres from the RT4 and J82 cells via qRT-PCR, which has been reported to examine the stem-like associated genes (23,24) (Figure 1A,B), suggesting that CSCs are enriched in spheres.
Figure 1.
Expression of IL6 and its receptor is up-regulated in bladder cancer spheres with cancer stem cell (CSC) properties. (A) Morphology of bladder cancer cell lines RT4 and J82 grown as adherent cells (scale bar =75 µm) and spheres (scale bar =50 µm). (B) Expression of stem-like associated genes (CD133, CD44, ABCG2, Oct4 and Nanog) as assessed by qRT-PCR in the adherent cells and spheres, which were incubated in 6-well ultra-low-attachment culture plates for 4 days. The results are expressed as the fold change relative to the adherent cells. (C) The conditioned media from the adherent and sphere RT4 and J82 bladder cancer cell lines cells after 4 days of culturing were collected for the IL6 ELISA, which was performed to measure the IL6 level in the supernatants of the adherent cells and spheres (***P<0.001). (D) IL6 and IL6R expression were assessed by qRT-PCR in adherent and sphere RT4 and J82 cells. (E) Western blots were performed to detect IL6R expression in the adherent and sphere RT4 and J82 cells. Representative results from three independent experiments are shown, and all the values were expressed as the means ± SD, *P<0.05, **P<0.01 and ***P<0.001. qRT-PCR, real-time quantitative reverse transcription polymerase chain reaction; IL6, interleukin 6; IL6R, IL6 receptor; ELISA, enzyme-linked immunosorbent assay.
Cancer cells or CSCs are believed to evoke high IL6 secretion in an autocrine fashion, contributing to the malignant features of drug-resistance and metastasis. Therefore, to examine whether IL6 was secreted from bladder CSCs, an ELISA was performed to assess the IL6 expression in the supernatants of the adherent and sphere RT4 or J82 cells after 4 days of culturing. As expected, the expression of IL6 was robustly elevated in the supernatants of the spheres, in contrast to that of the adherent cells (Figure 1C). In addition, the expressions of IL6 and IL6R mRNA were both up-regulated in the spheres from the RT4 and J82 cells (Figure 1D). Consistently, the same results were confirmed by Western blot (Figure 1E). These results suggest that IL6 signaling may be crucial in regulating bladder CSCs.
IL6 sustains the stem-like characteristics of bladder cancer
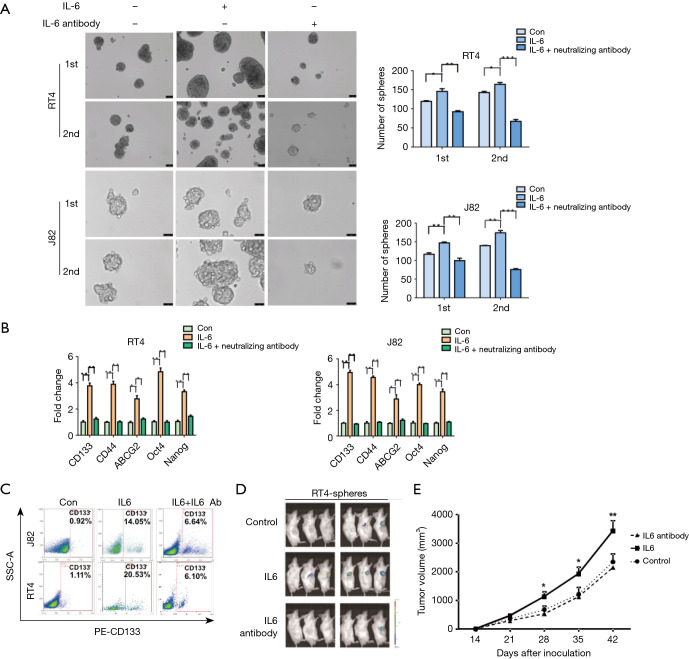
Given that the expressions of IL6 and IL6R are elevated in bladder CSCs, we next investigated whether IL6 promoted the self-renewal, stem-associated genes, invasion, migration, and tumorigenicity of the spheres from bladder cancer. First, single-cell suspensions of 5,000 bladder cancer cells were incubated in 6-well ultra-low-attachment culture plates for 4 days. As shown in Figure 2A, more spheres from the RT4 or J82 cells were observed in the group with human recombinant IL6 (50 ng/mL) than in the control group without the IL6 treatment. The same results were also found in the second generation of spheres. Additionally, the self-renewal of the spheres was alleviated by the administration of the IL6 neutralizing antibody (Figure 2A). Second, a higher expression of stem-associated genes was found in the IL6-treated bladder cancer cells than in the control cells, while the IL6 neutralizing antibody decreased the expression of the stem-associated genes (Figure 2B). Third, based on flow cytometry, a higher percentage of CD133 was observed in the IL6-treated bladder cancer cells than in the control cells, while the IL6 neutralizing antibody decreased the percentage of CD133 induced by IL6 (Figure 2C).
Figure 2.
IL6 sustains the stem-like characteristics of bladder cancer. (A) Primary and secondary spheroid assays were performed in the spheres treated with PBS, human recombinant IL6 (50 ng/mL) or the IL6 neutralizing antibody (20 µg/mL). The total numbers of spheroids from 5,000 cells were counted. The experiments were performed in triplicate (scale bar =50 µm). (B) The expression of stem-like associated genes (CD133, CD44, ABCG2, Oct-4 and Nanog) was assessed by qRT-PCR in the RT4 or J82 cells treated with PBS, human recombinant IL6 (50 ng/mL) or the IL6 neutralizing antibody (20 µg/mL). (C) The percentage of CD133 in the RT4 or J82 cells treated with PBS, human recombinant IL6 (50 ng/mL) or human recombinant IL6 (50 ng/mL) combined with the IL6 neutralizing antibody (20 µg/mL), as assessed by flow cytometry. (D) Male, non-obese, diabetic, severe combined immunodeficient (NOD-SCID) mice (n=6 in each group) were subcutaneously injected with 10,000 cells sorted from the bladder cancer spheres. Representative subcutaneous tumors from the mice after 6 weeks of transplantation are shown, as evaluated by bioluminescent imaging. (E) Tumor volume was measured once a week when tumors were palpable. Representative results from three independent experiments are shown, and all the values are expressed as the means ± SD, *P<0.05, **P<0.01 and ***P<0.001. qRT-PCR, real-time quantitative reverse transcription polymerase chain reaction; IL6, interleukin 6.
To further survey the role of IL6 in the stem-like properties of the bladder cancer cells, spheres without or with recombinant IL6 (50 ng/mL) or the IL6 neutralizing antibody (20 µg/mL) from the RT4 cells transfected with luciferase were injected subcutaneously into NOD/SCID male mice. Tumor growth was monitored weekly by live-animal bioluminescence optical imaging with the IVIS Lumina II imaging system. As expected, the subcutaneous injection of the IL6-treated spheres resulted in an increased incidence and tumor volume compared with that of the counterparts (Figure 2D,E), even when only 10,000 cells were implanted into the NOD/SCID mice. However, the IL6 neutralizing antibody decreased the tumorigenicity of the spheres and the tumor volume. (Figure 2D,E). These findings demonstrate that IL6 maintains the stem-like characteristics of bladder CSCs.
The IL6 signaling pathway is activated in bladder CSCs
IL6 is reported to regulate CSCs in various types of tumors, including liver cancer and breast cancer. By binding to its receptor IL6R, IL6 leads to the recruitment and activation of cytosolic STAT3, which then translocates into the nucleus and triggers the associated genes (25). To examine the molecular mechanism of IL6 promoting the stem-like properties of bladder CSCs, we detected whether IL6/STAT3 was activated in the IL6 treated-spheres from bladder cancer.
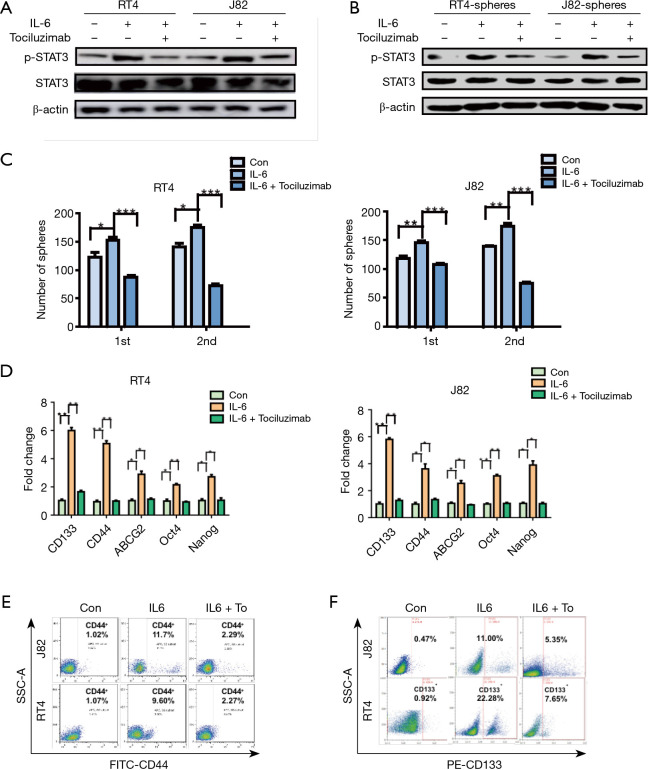
First, we assessed the protein expression of IL6R and the phosphorylation STAT3 in the IL6-treated bladder cancer cell lines and their spheres. As expected, the phosphorylated STAT3 was elevated in the IL6-treated bladder cancer cell lines and spheres compared with that of the control naïve cells (Figure 3A,B), suggesting that IL6/STAT3 activation was associated with the stem-like properties of bladder spheres. Then, to confirm the necessary role of IL6R in the IL6-promoted activation of STAT3, the FDA-approved drug tocilizumab (IL6R inhibitor) was applied. When tocilizumab (5 µg/mL) was added in the IL6-treated bladder cancer cell lines and spheres, the phosphorylation of STAT3 was decreased (Figure 3A,B).
Figure 3.
The IL6 signaling pathway is activated in bladder CSCs. (A,B) Western blots were performed to detect p-STAT3 and STAT3 expression in the adherent cells (A) and spheres (B) treated with PBS, human recombinant IL-6 (10 ng/mL) or human recombinant IL6 plus tocilizumab (5 µg/mL). (C) Primary and secondary spheroid assays were performed in the spheres treated with PBS, human recombinant IL6 (50 ng/mL) or human recombinant IL6 plus tocilizumab (5 µg/mL). The total numbers of spheroids from 5,000 cells were counted. (D) The expression of stem-like associated genes (CD133, CD44, ABCG2, Oct-4 and Nanog) was assessed by qRT-PCR in the RT4 or J82 cells treated with PBS, human recombinant IL6 or human recombinant IL6 (50 ng/mL) plus tocilizumab (5 µg/mL). (E,F) The percentage of CD44 or CD133 in the RT4 or J82 cells treated with PBS, human recombinant IL6 or human recombinant IL6 (50 ng/mL) plus tocilizumab (5 µg/mL), as assessed by flow cytometry. Representative results from three independent experiments are shown, and all the values are expressed as the means ± SD, *P<0.05, **P<0.01 and ***P<0.001. CSC, cancer stem cell; qRT-PCR, real-time quantitative reverse transcription polymerase chain reaction; IL6, interleukin 6.
Second, the effects of tocilizumab in the self-renewal and stem-associated gene expression of the bladder cancer cells were examined. According to the spheres-forming assay, when IL6R was inhibited by tocilizumab, the number of spheres was decreased (Figure 3C). As shown in Figure 3D, the elevated expression of the stem-associated genes was abated when tocilizumab was administered in the IL6-treated bladder cancer cells. In addition, based on flow cytometry, tocilizumab alleviated the increased CD44 or CD133 percentage in the IL6-treated bladder cancer cells (Figure 3E,F). These results indicate that IL6R is necessary for the IL6/STAT3 to promote the stem-like properties of bladder CSCs.
Blockade of IL6R signaling inhibits the invasion, migration and tumorigenicity of bladder CSCs
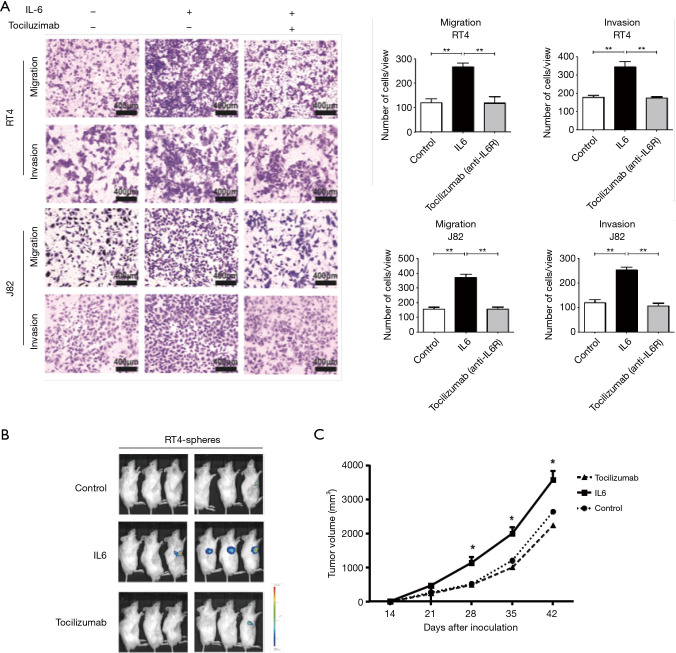
Based on the results above, we examined whether the blockade of the IL6R by tocilizumab inhibited the invasion, migration, and tumorigenicity of the bladder CSCs. First, invasion and migration assays were carried out in transwell chambers with or without Matrigel. A total of 2×104 bladder cancer cells were placed into the upper chamber, and the conditioned medium from one of the different groups was added to the bottom chamber as a chemoattractant. After 48 hours, the number of cells that invaded through the membrane or Matrigel was counted after staining with 0.1% crystal violet. As expected, the Matrigel invasion and transwell assays showed that the IL6-treated spheres exhibited a higher rate of cell invasion and migration than that of the control cells, whereas tocilizumab eliminated the effect of IL6 (Figure 4A). Finally, we determined whether tocilizumab inhibited the tumorigenicity of the IL6-treated spheres in vivo. A subcutaneous injection of the IL6-treated spheres resulted in an increased tumor incidence and tumor volume compared with the counterparts without the IL6 administration, while tocilizumab abrogated the enhanced tumorigenicity of the IL6-treated spheres and the tumor volume (Figure 4B,C). These findings demonstrate that tocilizumab inhibits the stem-like characteristics of bladder CSCs promoted by IL6.
Figure 4.
Blockade of IL6R signaling inhibits the invasion, migration and tumorigenicity of bladder CSCs. (A) A total of 2×104 cells were placed into the upper chamber, and for each experiment, conditioned medium from one of the different groups was added to the bottom chamber as a chemoattractant. After a 48-hour incubation at 37 °C, the cells that invaded through the membrane or Matrigel were photographed and counted after staining with 0.1% crystal violet for 15 min. Representative images of the invasion and migration assays in the spheres treated with PBS, human recombinant IL6 (50 ng/mL) or human recombinant IL6 plus tocilizumab (5 µg/mL). (B) Representative subcutaneous tumors from the mice after 6 weeks of transplantation are shown, as evaluated by bioluminescent imaging. (C) Tumor volume was measured once a week when tumors were palpable. Representative results from three independent experiments are shown, and all the values are expressed as the means ± SD, *P<0.05, **P<0.01. CSC, cancer stem cell; IL6, interleukin 6; IL6R, IL6 receptor.
Discussion
Despite advances in surgery and chemotherapy for bladder cancer treatment, the 5-year overall survival rate for bladder cancer patients remains poor (26). Invasion and metastasis are frequent in bladder cancer even after radical operation. However, the molecular mechanism of bladder cancer progression remains unknown. Recently, CSCs have been reported to be related to cancer progression, including chemoresistance, due to their expansion, self-renewal, and drug-resistant capacities. However, the mechanisms underlying CSCs in the progression of bladder cancer remain unclear and need to be elucidated.
In this study, we demonstrated that the IL6/IL6R/STAT3 axis was required for maintaining the stem-like properties of bladder CSCs. Furthermore, we found that tocilizumab, an FDA-approved drug targeting the IL6R, inhibited the effects of IL6/IL6R/STAT3 signaling on the expansion and progression of bladder CSCs. IL6 is a cytokine that provokes a broad range of cellular and physiological responses. In patient’s sera of different types of cancers, including bladder cancer, the IL6 level is elevated and is suggested to be associated with poor clinical outcome. However, whether IL6 plays a vital role in the expansion and self-renewal of bladder CSCs is unknown.
Our results showed that IL6 was up-regulated in the supernatants and CSCs, suggesting that the CSCs were the secreting origin of IL6 and that IL6 might regulate the stem-like characteristics of CSCs. Then, we demonstrated that recombinant IL6 promoted self-renewal, the expression of stem-associated genes, invasion, migration, and tumorigenicity. Consistently, we presented that IL6R was elevated in bladder CSCs. Although IL6R is reported to be required in the stem-like properties of CSCs, there are no studies about its role and related mechanisms in bladder CSCs. Therefore, we examined whether IL6/IL6R and the downstream STAT3 regulated bladder CSCs.
Our findings showed that either eliminating exogenous IL6 by a neutralizing antibody or targeting IL6R led to the reduced activity of self-renewal, invasion, migration, and tumorigenicity of bladder CSCs. IL6 is the most well-known traditional activator of STAT3, and IL6 exerts its biological effects by binding to the IL6 receptor on the cell surface, which leads to the phosphorylation and activation of cytosolic STAT3. This effect promotes the stem-like properties of bladder CSCs. In our model, the phosphorylation of STAT3 was up-regulated in the stem-like properties of bladder CSCs. However, when tocilizumab was added in the supernatant of the IL6-treated spheres, the phosphorylated STAT3 was decreased, which resulted in stem-like characteristics and even the tumorigenicity of bladder CSCs. Collectively, our findings demonstrate that IL6/IL6R/STAT3 promotes the stem-like characteristics of bladder CSCs. Furthermore, our study provides a novel target for bladder CSCs, IL6R. Future studies should make great efforts to explore the role of tocilizumab in clinical studies and examine the specific molecular mechanisms.
Acknowledgments
Funding: None.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was waived due to the nature of the study. The study was approved by the Institutional Animal Care and Use Committee of the First Affiliated Hospital of Chongqing Medical University (No. 2019016).
Footnotes
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.03.16). The authors have no conflicts of interest to declare.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Liu JY, Dai YB, Li X, et al. Solute carrier family 12 member 5 promotes tumor invasion/metastasis of bladder urothelial carcinoma by enhancing NF-κB/MMP-7 signaling pathway. Cell Death Dis 2017;8:e2691. 10.1038/cddis.2017.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SR, Roh YG, Kim SK, et al. Activation of EZH2 and SUZ12 Regulated by E2F1 Predicts the Disease Progression and Aggressive Characteristics of Bladder Cancer. Clin Cancer Res 2015;21:5391-403. 10.1158/1078-0432.CCR-14-2680 [DOI] [PubMed] [Google Scholar]
- 4.Glaser AP, Fantini D, Shilatifard A, et al. The evolving genomic landscape of urothelial carcinoma. Nat Rev Urol 2017;14:215-29. 10.1038/nrurol.2017.11 [DOI] [PubMed] [Google Scholar]
- 5.Yang Z, Li C, Fan Z, et al. Single-cell Sequencing Reveals Variants in ARID1A, GPRC5A and MLL2 Driving Self-renewal of Human Bladder Cancer Stem Cells. Eur Urol 2017;71:8-12. 10.1016/j.eururo.2016.06.025 [DOI] [PubMed] [Google Scholar]
- 6.Arum CJ, Anderssen E, Viset T, et al. Cancer immunoediting from immunosurveillance to tumor escape in microvillus-formed niche: a study of syngeneic orthotopic rat bladder cancer model in comparison with human bladder cancer. Neoplasia 2010;12:434-42. 10.1593/neo.91824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li C, Du Y, Yang Z, et al. GALNT1-Mediated Glycosylation and Activation of Sonic Hedgehog Signaling Maintains the Self-Renewal and Tumor-Initiating Capacity of Bladder Cancer Stem Cells. Cancer Res 2016;76:1273-83. 10.1158/0008-5472.CAN-15-2309 [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Xu W, Guo H, et al. NOTCH1 Signaling Regulates Self-renewal and Platinum Chemoresistance of Cancer Stem-likeCells in Human Non-small Cell Lung Cancer. Cancer Res 2017;77:3082-91. 10.1158/0008-5472.CAN-16-1633 [DOI] [PubMed] [Google Scholar]
- 9.Portillo-Lara R, Alvarez MM. Enrichment of the Cancer Stem Phenotype in Sphere Cultures of Prostate CancerCell LinesOccurs through Activation of Developmental Pathways Mediated by the Transcriptional Regulator ΔNp63α. PLoS One 2015;10:e0130118. 10.1371/journal.pone.0130118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vantaku V, Donepudi SR, Ambati CR, et al. Expression of ganglioside GD2, reprogram the lipid metabolism and EMT phenotype in bladder cancer. Oncotarget 2017;8:95620-31. 10.18632/oncotarget.21038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su G, Zhao Y, Wei J, et al. The effect of forced growth of cells into 3D spheres using low attachment surfaces on the acquisition of stemness properties. Biomaterials 2013;34:3215-22. 10.1016/j.biomaterials.2013.01.044 [DOI] [PubMed] [Google Scholar]
- 12.Chan KS, Espinosa I, Chao M, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci U S A 2009;106:14016-21. 10.1073/pnas.0906549106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin K, Lim A, Odegaard JI, et al. Cellular origin of bladder neoplasia and tissue dynamics of its progression to invasive carcinoma. Nat Cell Biol 2014;16:469-78. 10.1038/ncb2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tatokoro M, Koga F, Yoshida S, et al. Potential role of Hsp90 inhibitors in overcoming cisplatin resistance of bladder cancer-initiating cells. Int J Cancer 2012;131:987-96. 10.1002/ijc.26475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibrahim SA, Gadalla R, El-Ghonaimy EA, et al. Syndecan-1 is a novel molecular marker for triple negative inflammatory breast cancer and modulates the cancer stem cell phenotype via the IL-6/STAT3, Notch and EGFR signaling pathways. Mol Cancer 2017;16:57. 10.1186/s12943-017-0621-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu B, Zhang K, Li S, et al. HIC1 attenuates invasion and metastasis by inhibiting the IL-6/STAT3 signalling pathway in human pancreatic cancer. Cancer Lett 2016;376:387-98. 10.1016/j.canlet.2016.04.013 [DOI] [PubMed] [Google Scholar]
- 17.Lee BR, Kwon BE, Hong EH, Shim A, et al. Interleukin-10 attenuates tumour growth by inhibiting interleukin-6/signal transducer and activator of transcription 3 signalling in myeloid-derived suppressor cells. Cancer Lett 2016;381:156-64. 10.1016/j.canlet.2016.07.012 [DOI] [PubMed] [Google Scholar]
- 18.Lin C, Liao W, Jian Y, et al. CGI-99 promotes breast cancer metastasis via autocrine interleukin-6 signaling. Oncogene 2017;36:3695-705. 10.1038/onc.2016.525 [DOI] [PubMed] [Google Scholar]
- 19.Pencik J, Schlederer M, Gruber W, et al. STAT3 regulated ARF expression suppresses prostate cancer metastasis. Nat Commun 2015;6:7736. 10.1038/ncomms8736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin KY, Ye H, Han BW, et al. Genome-wide screen identified let-7c/miR-99a/miR-125b regulating tumor progression and stem-like properties in cholangiocarcinoma. Oncogene 2016;35:3376-86. 10.1038/onc.2015.396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tominaga K, Shimamura T, Kimura N, et al. Addiction to the IGF2-ID1-IGF2 circuit for maintenance of the breast cancer stem-like cells. Oncogene 2017;36:1276-86. 10.1038/onc.2016.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Zhang Y, Du Y. Ovarian and breast cancer spheres are similar in transcriptomic features and sensitive to fenretinide. Biomed Res Int 2013;2013:510905. 10.1155/2013/510905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang D, Kong X, Li Y, et al. Curcumin inhibits bladder cancer stem cells by suppressing Sonic Hedgehog pathway. Biochem Biophys Res Commun 2017;493:521-7. 10.1016/j.bbrc.2017.08.158 [DOI] [PubMed] [Google Scholar]
- 24.Ojha R, Jha V, Singh SK, et al. Autophagy inhibition suppresses the tumorigenic potential of cancer stem cell enriched side population in bladder cancer. Biochim Biophys Acta 2014;1842:2073-86. 10.1016/j.bbadis.2014.07.007 [DOI] [PubMed] [Google Scholar]
- 25.Li L, Han R, Xiao H, et al. Metformin sensitizes EGFR-TKI-resistant human lung cancer cells in vitro and in vivo through inhibition of IL-6 signaling and EMT reversal. Clin Cancer Res 2014;20:2714-26. 10.1158/1078-0432.CCR-13-2613 [DOI] [PubMed] [Google Scholar]
- 26.Lee SR, Roh YG, Kim SK, et al. Activation of EZH2 and SUZ12 Regulated by E2F1 Predicts the Disease Progression and Aggressive Characteristics of Bladder Cancer. Clin Cancer Res 2015;21:5391-403. 10.1158/1078-0432.CCR-14-2680 [DOI] [PubMed] [Google Scholar]