Abstract
Background
Cancer-related fatigue (CRF) is a severe symptom in breast cancer survivors. We aimed to explore the effects of the solution-focused brief therapy (SFBT) on CRF in breast cancer patients after lumpectomy or mastectomy under adjuvant chemotherapy.
Methods
First, 196 patients with primarily diagnosed breast cancer were recruited, screened, and the qualified 160 patients were randomly assigned into the control (routine nursing interventions), and intervention (routine nursing interventions and SFBT) groups. CRF was evaluated using the Chinese version of revised Piper Fatigue Scale. Data were collected at baseline (T1), post-intervention (T2), and post-follow-up (T3).
Results
Mild to severe fatigue existed in all qualified participants at T1 and the fatigue symptom went worse with the chemotherapy in control group. In intervention group, the fatigue level decreased at T2 (P<0.05), and went to the similar level at T3 as that at T1. The levels of behavioral, affective, and sensory fatigues in the control group significantly increased at T3 (P<0.05), while no difference was found in the intervention group. This study showed that SFBT effectively decreased CRF in breast cancer survivors after surgery under adjuvant chemotherapy.
Conclusions
Hence, SFBT might be a beneficial non-pharmacological intervention alone or in combination with other interventions to improve patients’ quality of life.
Keywords: Breast cancer, cancer-related fatigue (CRF), adjuvant chemotherapy, solution-focused care model, intervention
Introduction
Breast cancer, the most common female malignancy and one of the three most common cancers worldwide, has an increasing morbidity and mortality in developing countries and remains the leading cancer-related cause of disease burden for women (1). Early breast cancer without distant metastases is now theoretically recognized to be curative. Conventional therapeutic strategies for early breast cancer include primary surgery, radiotherapy for local therapy and endocrine therapy, as well as chemotherapy for systemic therapy (1).
Cancer-related fatigue (CRF), a symptom generally experienced by cancer survivors throughout disease development and treatment, is now a severe, prevalent and unneglectable problem existing among cancer survivors, which has greatly affected their psychological conditions and life quality (2). While the etiology of CRF is extraordinarily complicated and many factors are considered to be involved in the generation, development and deterioration of CRF. The multifactorial processes of CRF might link with physiological, clinical, as well as psychological factors (2,3).
Cancer treatments including surgery, chemotherapy, radiotherapy and so on can cause severe CRF directly or indirectly (4-6). To alleviate CRF, strategies have been proposed to be applied in specific contexts. Evidences indicated that pharmacological and non-pharmacological measures can yield moderate or above benefits on CRF. Stimulants, antidepressants, and erythropoietin, are administered alone or in combination to relieve CRF (7). Physical exercises or therapies, such as yoga, auricular point therapy, as well as resistance exercises, can provide benefits for cancer survivors (8-10). Psychological interventions have been reported to be effective in cancer-related psychological symptoms, including stress, depression, and fatigue (11,12). Recently, Aminnasab et al. showed the solution-focused brief therapy (SFBT) effectively decreased depression and stress in breast cancer patients (13). The “solution-focused” was entitled according to the intervention. This therapy was initiated in the early 1980s at Brief Family Therapy Center in Milwaukee in order to investigate short-term therapeutic effects and techniques to help patients change. We hypothesized that this therapy might function in the relief of breast cancer-induced fatigue.
The present study aimed to explore the effects of SFBT on CRF in breast cancer patients after surgery and dedicated to adjuvant chemotherapy. We present the following article in accordance with the CONSORT reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-2734).
Methods
Study design
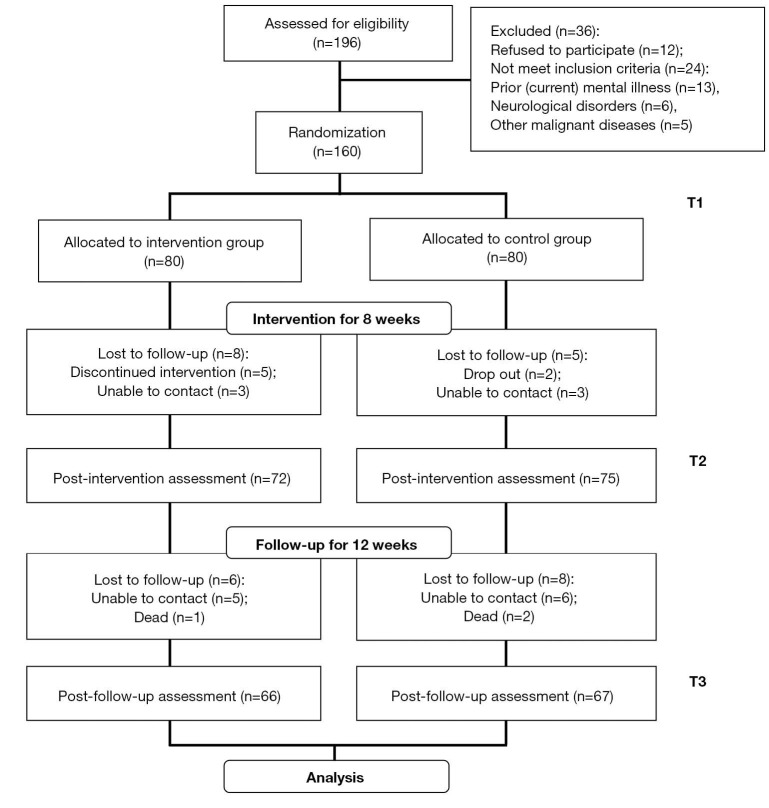
In the present study, 196 female breast cancer patients were consecutively recruited in Beijing Shijitan Hospital Affiliated to Capital Medical University, all of which were receiving adjuvant chemotherapy after surgery (lumpectomy or mastectomy) between June 2018 and June 2019. The recruited patients were then screened following the inclusion and exclusion criteria. The inclusion criteria were as follows: histologically diagnosed primary breast cancer; have been received lumpectomy or mastectomy; scheduled for adjuvant chemotherapy with the regimen of cyclophosphamide + hydrochloride epirubicin + fluorouracil injection; expected survival ≥1 year; body mass index (BMI) ≥18 kg/m2; able to understand and follow the study protocol. The exclusion criteria were: refused to participate; had prior or current mental illness; had a history of neurological disorders or concurrent malignant diseases (except carcinoma in situ of skin or cervix); already participating in other studies. According to the above two criteria, 36 patients were excluded, among which 12 refused to participate, 13 had prior or current mental illness, 6 had neurological disorders, and 5 had other malignant diseases (Figure 1). Then the eligible 160 patients were numbered in the order they were recruited and randomly classified into two groups, the intervention and control groups, each for 80 cases, using Stata 12.0 software (Figure 1). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. This study was reviewed and approved by the ethics commitment of Beijing Shijitan Hospital Affiliated to Capital Medical University (#2019-34). All patients enrolled completed the informed consent form.
Figure 1.
Research framework of this study. T1: Baseline; T2: Post-intervention; T3: Post-follow-up.
Interventions
The patients were randomly allocated to the experimental groups. For patients of control group, routine nursing interventions were performed including aerobic exercise (walking, swimming and many kinds of aerobics if circumstances permit), sleep management (resting regularly and sufficiently) and diet adjustment (light and healthy diet with moderate vegetables and fruits, etc.). For patients of intervention group, SFBT as well as routine nursing interventions were co-performed. SFBT in this study contained 5 sessions, introducing the problem, constructing feasible objectives (miracle exploration composed of setting positive goals and understanding the approaches selected to cope with problems encountered), probing exceptions (to eliminate disruptive behavior and cognitive patterns), controlling feedback (scaled questionnaire), and evaluating progress (scaled questionnaire), the similar with that described previously (13). Interventions for two groups were performed for 1 h weekly over 8 weeks, followed by a 12-week follow-up, under the supervision and guidance of experienced therapists. To assess the effects of SFBT on breast CRF, measurements of subjective fatigue were taken at 3 time points: baseline (T1, the time before the beginning of intervention), post-intervention (T2, the time after the termination of intervention), and post-follow-up (T3, the time after 12-week follow-up). Researchers who analyzed the data were blind to the grouping and intervention procedures.
Subjective fatigue measurement
The subjective fatigue level was measured using the Chinese version of the revised Piper Fatigue Scale (14), a commonly used self-assessment tool to multidimensionally measure fatigue in cancer research. This tool includes 27 items with fatigue assessments of subjective perception from four dimensions (22 items), 4 open-ended questions (the reason of fatigue, other symptoms, the description of fatigue, and measurements to alleviate fatigue; 4 items), and the assessment of fatigue duration (1 item). Patients were scored focusing the 22 items in the four-dimensional category, that is, 6 items in the Behavioral/Severity dimension (effects of fatigue on daily activities; Item 2 to 7), 5 items in the Affective Meaning dimension (emotional performances of fatigue; Item 8-12), 5 items in the Sensory dimension (physiological, psychiatric, and emotional symptoms of fatigue; Item 13 to 17), and 6 items in the Cognitive/Mood dimension (absorption and temper; Item 18 to 23). Each item is matched with a fixed word pair, such as strong/weak, awake/sleepy, and so on. For the 22 items, each was valued from 0 (no fatigue) to 10 (worst), and patients were asked to circle one single number that best reflects their current severity of fatigue. The above mentioned 22 items were calculated for the total and separate scores of four dimensions, and the other 5 items were not involved in the scoring. Thus, the total score of the whole scale and the score of each dimension should be assessed, with the higher score representing the more serious fatigue. The total score of fatigue ranges from 0 to 220, and the total score divided by 22 equaled the average. The scoring manner of each dimension was similar with that of the total score, that is, the total score divided by the number of contained items equaled the average score of each dimension. The fatigue was divided into 3 grades, that is, the none or mild (score 0 to 3), moderate (score 3 to 6), and severe (more than 6) grades.
Statistical analysis
Values were expressed as n (percentage, %) or mean ± SD. The collected data were analyzed using SPSS 23.0 (IBM Corp., Chicago, IL, USA). For data of demographic and clinical characteristics of two groups, P values for each group were derived from either unpaired t-test or Mann–Whitney test as appropriate. Chi-square test or Fisher’s exact test was used for assessing distribution of observations or phenomena between different groups. The effect of the SFBT on the fatigue measured at T1, T2, and T3 was tested using ANOVA. P<0.05 was considered to be statistically significant.
Results
As shown in Figure 1, 196 female breast cancer patients were recruited at the beginning of the present study. After screened according to inclusion and exclusion criteria, 36 patients were excluded, among which 12 refused to participate, 13 had prior or current mental illness, 6 had neurological disorders, and 5 had other malignant diseases. Then the remained 160 patients were randomly assigned into intervention and control groups (each for 80 cases). During the 8-week intervention, some patients quit and some were out of touch, which made the follow-ups of 27 patients were lost and those of 133 patients were obtained. Then the total of 133 patients with full-course subjective fatigue data at 3 time points were analyzed (66 in the Intervention group, 67 in the Control group). Table 1 displayed their demographic and clinical characteristics. No significant differences were discovered in each item between two groups at the baseline (P>005). The majority of participants were aged 40 to 60, and diagnosed with stage II or III. More patients with subjective fatigue were married with less than 2 children and relatively lower education level (high school and below, data not shown).
Table 1. Demographic and clinical characteristics of the breast cancer study participants.
| Characteristics | Study group | P | |
|---|---|---|---|
| Intervention (n=66) | Control (n=67) | ||
| Age (years) | 0.6427 | ||
| <40 | 2 (3.0%) | 5 (7.5%) | |
| 40–49 | 25 (37.9%) | 27 (40.3%) | |
| 50–60 | 30 (45.5%) | 28 (41.8%) | |
| >60 | 9 (13.6%) | 7 (10.4%) | |
| Body weight (kg) | 51.2±11.4 | 52.9±12.5 | 0.4937 |
| BMI | 24.1±4.4 | 22.4±5.1 | 0.2716 |
| Marital status | 0.2077 | ||
| Married | 49 (74.2%) | 56 (83.6%) | |
| Single/widowed/divorced | 17 (25.8%) | 11 (16.4%) | |
| No. of children | 0.2187 | ||
| ≤2 | 54 (81.8%) | 48 (71.6%) | |
| >2 | 12 (18.2%) | 19 (28.4%) | |
| Employment status | 0.4799 | ||
| No | 38 (57.6%) | 43 (64.2%) | |
| Yes | 28 (42.4%) | 24 (35.8%) | |
| Education level | 0.5657 | ||
| High school and below | 46 (69.7%) | 50 (74.6%) | |
| College and above | 20 (30.3%) | 17 (25.4%) | |
| Surgery type | 0.3755 | ||
| Mastectomy | 23 (34.8%) | 29 (43.3%) | |
| Lumpectomy | 43 (65.2%) | 38 (56.7%) | |
| Days since surgery | 62.3±30.4 | 58.4±34.6 | 0.1937 |
| Days since first chemotherapy | 16.7±12.8 | 19.2±14.2 | 0.3273 |
| Time of having breast cancer | 0.4889 | ||
| ≤1year | 29 (43.9%) | 34 (50.7%) | |
| >1 year | 37 (56.1%) | 33 (49.3%) | |
| Breast cancer stages | 0.7275 | ||
| I | 9 (13.6%) | 7 (10.4%) | |
| II | 30 (45.5%) | 32 (47.8%) | |
| III | 18 (27.3%) | 15 (22.4%) | |
| IV | 9 (13.6%) | 13 (19.4%) | |
Values were expressed as n (percentage, %) or mean ± SD. P values for each group were derived from either unpaired t-test or Mann-Whitney test as appropriate. Chi-square test or Fisher’s exact test was used for assessing distribution of observations or phenomena between different groups. BMI, body mass index.
As shown in Table 2, the total fatigue level in the control group increased at T3 time point compared with that at T1 baseline (P<0.05), indicating that the fatigued symptom went worse with the chemotherapy. While in the intervention group, the fatigue level decreased at the end of intervention (T2) compared with that at the T1 baseline (P<0.05), and went to the similar level at T3 with that at T1 baseline (P>0.05), suggesting that SFBT intervention prevents the decoration of fatigue along with the chemotherapy. Meanwhile, the fatigue level in the intervention group at the end of intervention (T2, P<0.01) or follow-up (T3, P<0.05) was significantly lower than that in the control group at the corresponding time point. The subclass scores of fatigue also showed time effects. The levels of behavioral, affective, and sensory fatigues in the control group significantly increased at the end of the follow-up (T3, P<0.05), while no difference was found in the intervention group (P>0.05), indicating that SFBT markedly prevents the decoration of the above fatigues with the chemotherapy. The group-time effects were represented in the affective, sensory, and cognitive fatigues, in which the control level was significantly higher than the invention level. Hence, SFBT effectively reduced the total as well as subclass fatigue levels of breast cancer patients, that is to say, SFBT possessed positive time and group-time effects in CRF.
Table 2. Comparison of subjective fatigue levels (Chinese version of the revised Piper Fatigue Scale).
| Items | Study group | P value | |
|---|---|---|---|
| Intervention group (n=66) | Control group (n=67) | ||
| Total fatigue | |||
| T1 | 4.34±0.83 | 4.58±0.91 | 0.481 |
| T2 | 3.24±0.74 | 5.11±0.85 | 0.005 |
| T3 | 4.26±0.86 | 5.94±0.75 | 0.023 |
| P value (T1 and T2) | 0.041 | 0.092 | |
| P value (T1 and T3) | 0.174 | 0.038 | |
| Behavioral fatigue | |||
| T1 | 5.02±0.79 | 4.86±0.88 | 0.386 |
| T2 | 4.95±0.92 | 5.67±0.96 | 0.072 |
| T3 | 5.43±0.84 | 5.91±0.83 | 0.163 |
| P value (T1 and T2) | 0.729 | 0.224 | |
| P value (T1 and T3) | 0.284 | 0.019 | |
| Affective fatigue | |||
| T1 | 3.89±0.77 | 4.02±0.86 | 0.208 |
| T2 | 3.13±0.69 | 4.48±0.91 | 0.029 |
| T3 | 4.01±0.91 | 5.13±1.04 | 0.016 |
| P value (T1 and T2) | 0.046 | 0.195 | |
| P value (T1 and T3) | 0.641 | 0.033 | |
| Sensory fatigue | |||
| T1 | 4.26±0.74 | 4.11±1.02 | 0.198 |
| T2 | 4.42±0.81 | 4.39±0.94 | 0.361 |
| T3 | 4.21±0.97 | 5.08±0.88 | 0.037 |
| P value (T1 and T2) | 0.392 | 0.118 | |
| P value (T1 and T3) | 0.217 | 0.024 | |
| Cognitive fatigue | |||
| T1 | 4.47±0.86 | 4.52±0.71 | 0.327 |
| T2 | 3.59±0.77 | 4.71±0.85 | 0.008 |
| T3 | 4.07±0.82 | 4.92±0.97 | 0.044 |
| P value (T1 and T2) | 0.041 | 0.682 | |
| P value (T1 and T3) | 0.224 | 0.291 | |
Values were expressed as mean ± SD.
As shown in Table 3, all the qualified subjects had fatigue (mild to severe) and there was no difference of the fatigue distribution between the two groups at the baseline (T1, P<0.05). While at the end of intervention (T2) and follow-up (T3), the distribution pattern in these two groups were distinctly different. In the intervention group, mild fatigue cases were increased at T2 and T3 time points, and moderate and above cases at T3 were relatively less than the corresponding ones at T1. No significant changes were found in the distribution of control group throughout the whole study.
Table 3. Distribution of subjective fatigue occurrence (Chinese version of the revised Piper Fatigue Scale).
| Total fatigue | Study group | P value | |
|---|---|---|---|
| Intervention group (n=66) | Control group (n=67) | ||
| T1 | 0.5348 | ||
| Mild fatigue | 24 (36.4%) | 19 (28.4%) | |
| Moderate fatigue | 31 (46.9%) | 33 (49.2%) | |
| Severe fatigue | 11 (16.7%) | 15 (22.4%) | |
| T2 | 0.0048 | ||
| Mild fatigue | 37 (56.1%) | 22 (32.8%) | |
| Moderate fatigue | 24 (36.3%) | 28 (41.8%) | |
| Severe fatigue | 5 (7.6%) | 17 (25.4%) | |
| T3 | 0.0173 | ||
| Mild fatigue | 32 (48.5%) | 19 (28.4%) | |
| Moderate fatigue | 28 (42.4%) | 32 (47.7%) | |
| Severe fatigue | 6 (9.1%) | 16 (23.9%) | |
Values were expressed as n (percentage, %). Chi-square test or Fisher’s exact test was used for assessing distribution of observations or phenomena between different groups.
Discussion
In the present study, mild to severe fatigue existed in all qualified participants at T1 and the fatigue symptom went worse with the chemotherapy in control group. In intervention group, the fatigue level decreased at T2, and went to the similar level at T3 as that at T1. The levels of behavioral, affective, and sensory fatigues in the control group significantly increased at T3, while no difference was found in the intervention group. This study showed that SFBT effectively decreased CRF in breast cancer survivors after surgery under adjuvant chemotherapy.
In the recent decades, the mortality of breast cancer has been sharply decreased as a result of earlier detection, diagnosis, and advanced therapies. Thus, a large number of breast cancer patients survive after modern therapeutics and many of them suffer from severe effects of cancer treatments, including psychological symptoms, especially fatigue (15-17). CRF might affect efficacy of cancer therapy or even reduce survival at diagnosis, during and after treatment (2). Additionally, severe CRF is reported to prevent survivors from social reintegration (18). Thus, it is urgent to apply proper solutions to relive CRF during and after cancer treatment.
Multiple factors might add up to the occurrence of CRF. Potential disruptions caused by inflammation in the central nervous system, reduced energy metabolism is reported to molecularly underlie the development of CRF (19,20). Abrahams et al. reviewed 12,327 breast cancer survivors and concluded that advanced tumor stages, chemotherapy, as well as receiving surgery-radiotherapy-chemotherapy combination treatment were risk factors for severe fatigue of breast cancer survivors after treatment (21). Other studies further emphasized that chemotherapy appears to be the critic precipitating and risk factor for CRF in breast cancer patients (11,22). Hence, in the present study, we brought the risk factor surgery and adjuvant chemotherapy into the research system to promise a large ratio of CRF in the enrolled subjects facilitating the fluently conduction of following experiments. As predicted, all the enrolled subjects had fatigue at the beginning with the majority at mild to severe level, which is consistent with previous studies which showed that 30–60% patients scored their fatigue as moderate to severe extent during active treatment (23). This also signified that the sampling method in this study is appropriate.
CRF is a kind of subjective sense composed of tiredness or exhaustion from physical, emotional, and/or cognitive dimensions (24). There hasn’t been any universal standard to objectively evaluate CRF yet. Generally, CRF is generally evaluated by self-reporting using several validated questionnaires referring to severity, duration, dimensions and other aspects of fatigue performance, which were designed for different contexts (23,25). As instruments in the questionnaires exhibit obvious heterogeneity in different contexts, it is better to apply one that used in the similar context. The revised Piper Fatigue Scale has long been used to multidimensionally evaluated CRF in patients suffered from various cancers, such as melanoma, non-small cell lung cancer, prostate cancer and breast cancer (20,26-28). Additionally, increasing lectures are prone to use this scale to evaluate CRF in breast cancer patients under different contexts (20,29,30). Based on the above reasons, we applied its Chinese version in the present study.
Psychological interventions are recommended as one of the most effective non-pharmacological means to ameliorate CRF (31). In a systematic review with comparative meta-analyses of 245 studies for exercises and other non-pharmacological interventions indicated that many different exercises and nursing applications were able to reduce CRF during cancer treatment, suggesting that patients might choose suitable interventions to reduce CRF (32). SFBT was initiated in the early 1980s and now has been widely used in mental health field to rapidly change situations during treatment and improve quality of life. Aminnasab et al. reported that SFBT effectively improve the positive psychological state in breast cancer patients via decreasing depression and perceived stress (13). The present study illustrated that SFBT also significantly decreased CRF in breast cancer survivors after surgery and under adjuvant chemotherapy. This is a necessary supplement for the application of SFBT and the therapy for CRF during chemotherapy. In Biering et al.’s paper, fatigue in breast cancer patients after diagnosis and treatments would not return to normal level even after long time (33). It is worth thinking that whether the positive effect of SFBT in this study could be explained by placebo-effect. The present study aimed to find a useful solution to ease CRF caused by chemotherapy, and we found SFBT played significantly positive effect on fatigue during chemotherapy compared with the control group. To discriminate the SFBT-effect from placebo-effect, we should add another sham solution during chemotherapy, and we are considering this point in our future work.
There were still some limitations in the present study. This study didn’t analyze the effects of surgical options (lumpectomy or mastectomy, to present different shapes of body after surgery) on CRF and whether the SFBT-supplied relief on CRF of breast cancer patients differ between the two surgeries. Additionally, as many adult cancer survivors might chronically fatigued even years after active treatments, a long-term follow-up is needed to verify the beneficial effect of SFBT on CRF in breast cancer patients. Furthermore, the number of enrolled subjects should be enlarged to supply with more persuasive data. Corresponding studies will be conducted about the above points in our future research.
Conclusions
In short, this study showed that all the qualified subjects had mild to severe fatigue at the baseline time point. And SFBT effectively decreased CRF in breast cancer survivors after surgery and under adjuvant chemotherapy. Hence, SFBT might be a beneficial non-pharmacological intervention alone or in combination with other interventions to improve patients’ quality of life.
Acknowledgments
Funding: The study was supported by Youth Fund of Beijing Shijitan Hospital Affiliated to Capital Medical University (2018-q40), and the Special Project for Clinical Medicine Development of Beijing Municipal Hospital Administration (No. ZYLX201839).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. This study was reviewed and approved by the ethics commitment of Beijing Shijitan Hospital Affiliated to Capital Medical University (#2019-34). All patients enrolled completed the informed consent form.
Footnotes
Reporting Checklist: The authors have completed the CONSORT reporting checklist Available at http://dx.doi.org/10.21037/tcr-20-2734
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-2734
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-2734). The authors have no conflicts of interest to declare.
References
- 1.Harbeck N, Gnant M. Breast cancer. Lancet 2017;389:1134-50. 10.1016/S0140-6736(16)31891-8 [DOI] [PubMed] [Google Scholar]
- 2.Thong MSY, van Noorden CJF, Steindorf K, et al. Cancer-Related Fatigue: Causes and Current Treatment Options. Curr Treat Options Oncol 2020;21:17. 10.1007/s11864-020-0707-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Higgins CM, Brady B, O'Connor B, et al. The pathophysiology of cancer-related fatigue: current controversies. Support Care Cancer 2018;26:3353-64. 10.1007/s00520-018-4318-7 [DOI] [PubMed] [Google Scholar]
- 4.Guinan EM, Bennett AE, Doyle SL, et al. Measuring the impact of oesophagectomy on physical functioning and physical activity participation: a prospective study. BMC Cancer 2019;19:682. 10.1186/s12885-019-5888-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vardy JL, Dhillon HM, Pond GR, et al. Fatigue in people with localized colorectal cancer who do and do not receive chemotherapy: a longitudinal prospective study. Ann Oncol 2016;27:1761-7. 10.1093/annonc/mdw252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanguturi SK, Alexander BM. Neurologic Complications of Radiation Therapy. Neurol Clin 2018;36:599-625. 10.1016/j.ncl.2018.04.012 [DOI] [PubMed] [Google Scholar]
- 7.Mustian KM, Alfano CM, Heckler C, et al. Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue: A Meta-analysis. JAMA Oncol 2017;3:961-8. 10.1001/jamaoncol.2016.6914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armer JS, Lutgendorf SK. The Impact of Yoga on Fatigue in Cancer Survivorship: A Meta-Analysis. JNCI Cancer Spectr 2020;4:pkz098. 10.1093/jncics/pkz098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han Q, Yang L, Huang SY, et al. Effectiveness of auricular point therapy for cancer-related fatigue: A systematic review and meta-analysis. J Adv Nurs 2020. doi: . 10.1111/jan.14375 [DOI] [PubMed] [Google Scholar]
- 10.Pagola I, Morales JS, Alejo LB, et al. Concurrent Exercise Interventions in Breast Cancer Survivors with Cancer-related Fatigue. Int J Sports Med 2020;41:790-7. 10.1055/a-1147-1513 [DOI] [PubMed] [Google Scholar]
- 11.Kim YH, Choi KS, Han K, et al. A psychological intervention programme for patients with breast cancer under chemotherapy and at a high risk of depression: A randomised clinical trial. J Clin Nurs 2018;27:572-81. 10.1111/jocn.13910 [DOI] [PubMed] [Google Scholar]
- 12.Barre PV, Padmaja G, Rana S, et al. Stress and Quality of Life in Cancer Patients: Medical and Psychological Intervention. Indian J Psychol Med 2018;40:232-8. 10.4103/IJPSYM.IJPSYM_512_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aminnasab A, Mohammadi S, Zareinezhad M, et al. Effectiveness of Solution-Focused Brief Therapy (SFBT) on Depression and Perceived Stress in Patients with Breast Cancer. Tanaffos 2018;17:272-9. [PMC free article] [PubMed] [Google Scholar]
- 14.Piper BF, Dibble SL, Dodd MJ, et al. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol Nurs Forum 1998;25:677-84. [PubMed] [Google Scholar]
- 15.Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc 2008;67:253-6. 10.1017/S002966510800712X [DOI] [PubMed] [Google Scholar]
- 16.Sodergren SC, Copson E, White A, et al. Systematic Review of the Side Effects Associated With Anti-HER2-Targeted Therapies Used in the Treatment of Breast Cancer, on Behalf of the EORTC Quality of Life Group. Target Oncol 2016;11:277-92. 10.1007/s11523-015-0409-2 [DOI] [PubMed] [Google Scholar]
- 17.Juvet LK, Thune I, Elvsaas IKO, et al. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: A meta-analysis. Breast 2017;33:166-77. 10.1016/j.breast.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 18.Behringer K, Goergen H, Muller H, et al. Cancer-Related Fatigue in Patients With and Survivors of Hodgkin Lymphoma: The Impact on Treatment Outcome and Social Reintegration. J Clin Oncol 2016;34:4329-37. 10.1200/JCO.2016.67.7450 [DOI] [PubMed] [Google Scholar]
- 19.Lanser L, Kink P, Egger EM, et al. Inflammation-Induced Tryptophan Breakdown is Related With Anemia, Fatigue, and Depression in Cancer. Front Immunol 2020;11:249. 10.3389/fimmu.2020.00249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SH, Song YK, Han J, et al. Pro-inflammatory Cytokine Levels and Cancer-related Fatigue in Breast Cancer Survivors: Effects of an Exercise Adherence Program. J Breast Cancer 2020;23:205-17. 10.4048/jbc.2020.23.e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abrahams HJG, Gielissen MFM, Schmits IC, et al. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: a meta-analysis involving 12 327 breast cancer survivors. Ann Oncol 2016;27:965-74. 10.1093/annonc/mdw099 [DOI] [PubMed] [Google Scholar]
- 22.Schmidt ME, Wiskemann J, Schneeweiss A, et al. Determinants of physical, affective, and cognitive fatigue during breast cancer therapy and 12 months follow-up. Int J Cancer 2018;142:1148-57. 10.1002/ijc.31138 [DOI] [PubMed] [Google Scholar]
- 23.Joly F, Lange M, Dos Santos M, et al. Long-Term Fatigue and Cognitive Disorders in Breast Cancer Survivors. Cancers (Basel) 2019;11:1896. 10.3390/cancers11121896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells-Di Gregorio S, Porensky EK, Minotti M, et al. The James Supportive Care Screening: integrating science and practice to meet the NCCN guidelines for distress management at a Comprehensive Cancer Center. Psychooncology 2013;22:2001-8. 10.1002/pon.3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in long-term breast carcinoma survivors - A longitudinal investigation. Cancer 2006;106:751-8. 10.1002/cncr.21671 [DOI] [PubMed] [Google Scholar]
- 26.Trask PC, Paterson AG, Esper P, et al. Longitudinal course of depression, fatigue, and quality of life in patients with high risk melanoma receiving adjuvant interferon. Psychooncology 2004;13:526-36. 10.1002/pon.770 [DOI] [PubMed] [Google Scholar]
- 27.Hou L, Zhou C, Wu Y, et al. Transcutaneous electrical acupoint stimulation (TEAS) relieved cancer-related fatigue in non-small cell lung cancer (NSCLC) patients after chemotherapy. J Thorac Dis 2017;9:1959-66. 10.21037/jtd.2017.06.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsiao CP, Chen MK, Veigl ML, et al. Relationships between expression of BCS1L, mitochondrial bioenergetics, and fatigue among patients with prostate cancer. Cancer Manag Res 2019;11:6703-17. 10.2147/CMAR.S203317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li C, Yang G, Yu M, et al. Effects of traditional Chinese medicine Shu Gan Jian Pi granules on patients with breast cancer and cancer-related fatigue: study protocol for a randomized controlled trial. Trials 2015;16:192. 10.1186/s13063-015-0723-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou LY, Yang L, He XL, et al. Effects of aerobic exercise on cancer-related fatigue in breast cancer patients receiving chemotherapy: a meta-analysis. Tumour Biol 2014;35:5659-67. 10.1007/s13277-014-1749-8 [DOI] [PubMed] [Google Scholar]
- 31.Kangas M, Bovbjerg DH, Montgomery GH. Cancer-Related Fatigue: A Systematic and Meta-Analytic Review of Non-Pharmacological Therapies for Cancer Patients. Psychological Bulletin 2009;134:700-41. 10.1037/a0012825 [DOI] [PubMed] [Google Scholar]
- 32.Hilfiker R, Meichtry A, Eicher M, et al. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: a systematic review incorporating an indirect-comparisons meta-analysis. Br J Sports Med 2018;52:651-8. 10.1136/bjsports-2016-096422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biering K, Frydenberg M, Pappot H, et al. The long-term course of fatigue following breast cancer diagnosis. J Patient Rep Outcomes 2020;4:37. 10.1186/s41687-020-00187-9 [DOI] [PMC free article] [PubMed] [Google Scholar]