Abstract
Background
Colorectal cancer (CRC) is one of the most common cancers in the world, resulting in about 600,000 deaths every year. It is urgent to explore the molecular mechanism and find new effective therapy. Abnormal molecular expression in cancer is considered as a screening biomarker and therapeutic target for tumors, MicroRNA (miRNA) as one of the important molecules, plays an important role in the regulation of tumorigenesis.
Methods
In this study, we aimed to elucidate the molecular mechanism by which mir-138 regulates the development and progression of CRC, and to find new molecular targets for the diagnosis and therapy of CRC. We have used qRT-PCR to study the expression of miR-138 and SIRT1 in CRC cells and tissues, CCK8 assay was used to test the proliferation ability of CRC cells, and invasion and migration ability of CRC cells in vitro were studied by Transwell assay.
Results
We found that miR-138 was significantly decreased in CRC tissues and cell lines by qRT-PCR, the level of miR-138 was significantly correlated with lymph node metastasis and distant metastasis, the CRC patients with high miR-138 level whose overall survival and disease-free survival were significantly longer. We also found that the level of SIRT1 in CRC tissues and cell lines is higher, and through Dual-luciferase reporter assay, we found that SIRT1 is a new target of miR-138 in CRC, and SIRT1 knockdown could inhibit CRC proliferation, migration and invasion in vitro.
Conclusions
Thus, we found that miR-138 could inhibit CRC cell proliferation, migration and invasion by targeting SIRT1 firstly, and that will provide a new idea for the therapy of CRC.
Keywords: Colorectal cancer (CRC), miR-138, SIRT1, proliferation, migration, invasion
Introduction
Colorectal cancer (CRC) is one of the most common gastrointestinal malignancies worldwide. Due to its inapparent early symptoms and manifestations, most patients are already in the advanced stage of diagnosis, resulting in about 600,000 deaths every year (1). Postoperative recurrence and metastasis of CRC are easy to occur, and it is reported that approximately 7.5% to 16.4% of CRC will occur postoperative recurrence or metastasis, which lead to poor prognosis and low survival rate of patients (2). At present, the main treatment of CRC is surgery, adjuvant chemotherapy and radiation therapy. Its 5-year survival rate is about 60–95%, if accompanied by lymph node metastasis, it will be reduced to about 35%, and the early stage of CRC has no obvious symptoms and manifestations, the late stage is mainly manifested as changes in bowel habits, blood in the stool, abdominal pain, fatigue and weight loss and other symptoms, most patients have been in the late stage of diagnosis (3,4), early diagnosis is crucial for the treatment of CRC.
The previous study showed that the survival rate of early diagnosis in patients with CRC was about 60% (5). Distant metastasis has become an important cause of poor prognosis in patients with CRC, among which liver metastasis is the most common. According to previous study, about 30% of patients were diagnosed with liver metastasis, and 14.5% of patients also with liver metastasis even after receiving chemotherapy (6). With the in-depth discussion on the pathogenesis of CRC, studies have shown that by monitoring the dynamic changes of serum tumor markers in patients, the presence and degree of tumor in patients can be understood, which is of great significance for the diagnosis and prognosis evaluation of CRC (7). Detection of abnormal changes of tumor markers in patients’ body fluids or serum, can help diagnose primary tumors, screen high-risk populations of CRC, understand its morbidity, monitor recurrence or metastasis, and evaluate prognosis (8). Therefore, the study of accurate tumor markers plays an important role in the early detection of tumor control. In general, tumor markers are of great significance in individualized targeted therapy, disease control and prevention in addition to playing an important role in diagnosis of CRC and contributing to the assessment of prognosis.
With the rapid development of targeted therapy, the therapy of many human cancers, including CRC have made some progress, but the prevalence of CRC is still show a rising trend in developing countries (9). CRC is an adenoma carcinoma caused by mutagenesis of multiple oncogenes and tumor suppressor genes, the occurrence and development of CRC closely related to the factors such as genetic heterogeneity, environmental factors and eating habits (10). In recent years, the previous study found that a variety of gene mutation, abnormal DNA repair are closely related to the tumorigenesis and development of CRC, but the molecular mechanism of CRC pathogenesis is still unknown. therefore, to clarify the molecular mechanism of its pathogenesis is around the corner, it is of great significance to find the early diagnostic markers and to provide effective therapeutic targets for targeted therapy of CRC.
MicroRNA (miRNA) is a non-coding single-stranded RNA with a length of about 19-25 nucleotides. It is highly conserved and participates in the negative regulation of target genes at the post-transcription level by identifying specific sequences in the 3 'UTR of the target gene. miRNA is involved in many important biological processes such as cell development, proliferation, apoptosis and tumorigenesis (11,12). More than half of miRNA genes are located in fragile regions containing genes related to tumorigenesis and play an important role in the development and progression of tumors (13). previous studies have shown that a class of miRNAs was aberrantly expressed in tumors, including breast carcinoma (14), CRC (15), hepatic carcinoma (16), gastric cancer (17), glioma (18) and so on, which plays an important role in the occurrence and development of this human tumors.
So far, studies have confirmed many miRNA involved in the development and progression of CRC (19-22). miRNA can not only be used as a new biomarker for the early diagnosis or prognosis of CRC, but also as a target for targeted therapy of CRC in vivo (23). miR-138 was reported to play an important role in the tumorigenesis of CRC, and it has been confirmed to be decreased in CRC (24), miR-138 plays an important role in the tumorigenesis of CRC, but its specific molecular mechanism is still unclear, which requires further exploration.
In this paper, we found that miR-138 is decreased whether in CRC tissue or cell lines, and it’s up-regulated could inhibit CRC cell proliferation, migration, invasion by targeting SIRT1, and SIRT1 is the new target of miR-138 in CRC which has never been reported before. Taken together, the results of the present study implicated miR-138 as a novel tumor suppressor candidate in human CRC, and provided a novel molecular target for the treatment of human CRC. We present the following article in accordance with the MDAR checklist (available at https://dx.doi.org/10.21037/tcr-21-559).
Methods
Cell culture
Human CRC cell line SW620, HT29, HCT-8, RKO, and SW480 was purchased from American Type Culture Collection (ATCC, Rockville, MD, USA). SW620, HCT-8, RKO, SW480 and normal colorectal epithelial cell lines CCD-18Co were cultured in RPMI 1640 medium which containing 10% fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO, USA), while the HT29 cells were cultured in McCoy’s 5A medium (Invitrogen; Life Technologies, Carlsbad, CA, USA), and all the cells were grown at 37 °C in a 5% CO2.
Human samples
Human CRC tissues and adjacent normal tissues were collected from the third hospital of Hebei medical university. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All the samples used in this study were informed consent of the patients and approved by the ethics committee of The Third Hospital of Hebei Medical University, fully meeting the ethical requirements. Patients in the study ranged in age from 34 to 73.
RNA isolation and qRT-PCR
We have isolated the total RNA of cells and tissues by using TRIzol reagent (TaKaRa, China). qRT-PCR was performed by using SYBR-green PCR Master Mix (TaKaRa, Japan), the reaction taken place in Bio-Rad iQ5 System (Bio-Rad, USA). The primers were in Table 1. Fold change of SIRT1 or miR-138 was calculated by the 2−∆∆Ct method.
Table 1. The primers sequence.
| Gene | Prime Sequence |
|---|---|
| miR-138 | Forward: CCCAGGGTCTGGTGCGGAGA |
| Reverse: CAGGGGCTGAGCGGTGAGGG | |
| SIRT1 | Forward: TGCTGGCCTAATAGAGTGGCA |
| Reverse: CTCAGCGCCATGGAAAATGT | |
| GAPDH | Forward: TGAAGGTCGGAGTCAACGGA |
| Reverse: CCTGGAAGATGGTGATGGGAT |
Western blot analysis
Cells were collected by RIPA lysis buffer (P0013C, Beyotime, Biotechnology Co., Ltd, Shanghai, China). Lysates were sonicated and centrifuged at 4 °C, then add 20% loading buffer with SDS, boil for 7–8 minutes until the protein is completely denatured, then stored at −20 °C. The proteins were separated by 10% SDS-PAGE, and then transferred onto a PVDF membrane, the membranes were incubated with blocking buffer for 2 h, and then incubated with the SIRT1 antibody (1:1,000, ab110304, Abcam, UK), or β-actin (1:10,000, ab179467, Abcam, UK), at 4 °C for 12 h. Then washed with TBST buffer and incubated with the Goat Anti-Rabbit IgG H&L (HRP) (1:20,000, ab97051, Abcam, UK). The expression of protein was assessed by Bio-Rad ChemiDoc XRS+ Chemiluminescence imaging system. The results were analyzed with LabWorks image analysis software.
Dual-luciferase reporter assay
We have constructed the wild and mutant plasmid called “pmiR-wild SIRT1” and “pmiR-wild SIRT1”, the wild plasmid containing pmiR luciferase reporter (Invitrogen) and with the putative complementary binding site for miR-138 of SIRT1 3′-UTR downstream of the luciferase gene. A site-directed mutagenesis kit (Takara Bio Inc., Kusatsu, Japan) was used to construct the mutant plasmid. For luciferase assays, the miR-138 (50 pmol/mL) or miR-NC (50 pmol/mL) and reporter plasmids were co-transfected to SW480 and SW620 cells, after 48 h, the Dual-Luciferase Reporter System Kit (Promega, Madison, WI) was used to measure the firefly and Renilla luciferase activity. Relative luciferase activity in the miR-138 groups was expressed as a firefly/Renilla luciferase ratio and normalized to the miR-NC groups.
Cell transfection and stable cell line establishment
Two human CRC cell line SW480, SW620 were seeded in a six-well plate and were transfected miR-138 mimics, negative control mimics (NC-mimics), miR-138 inhibitor and negative control inhibitor (NC-inhibitor) were purchased from FulenGen (Guangzhou, Guangdong, China) by Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) for miR-138 overexpression or downregulation, the cells were harvested after 48 h of transfection, then used for qPCR analysis. The LV-SIRT1-GFP overexpression lentivirus was purchased from Genchem (Shanghai, China), used according to the manufacturer’s instructions.
CCK8 assay
Cell activity was examined with Cell Counting Kit‐8 (CCK8, Sangon Biotech, Shanghai, China). cells were plated into 96‐well plates with 2×103 cells per well. 10 µL CCK8 regent was added to each well containing 100 µL medium at 0, 24, 48, 72, and 96 h time-points. 37 °C, 5% CO2 incubation for 1 h, then the plates were measured at 450 nm under a microplate reader.
Cell migration and invasion assays
To investigate the migration and invasion ability of SW480 and SW620 cells, the Transwell assay was conducted by using a 24-well insert with 8 µm pores (Corning Incorporated, Corning, NY, USA). SW480 and SW620 cells were hydrolyzed with 0.25% trypsin, then resuspended with 100 µL RPMI 1640 medium containing 1% FBS, and the cell density was controlled at 5×105 cells/mL. SW480 and SW620 cells were seeded in the upper chamber (with or without pre-coated with 500 ng/mL Matrigel solution (BD, Franklin Lakes, NJ, USA) for the migration and invasion assays respectively, 500 µL RPMI 1640 medium with 10% FBS was added to the lower chamber. Cells on the upper side of membrane were scraped off after incubation at 37 °C for 6 h, the migrated cells were fix with 20% ethanol solution, then stained with DAPI staining solution. And then, put the chamber under a microscope to observe and collect images. Each experiment and each measure were performed in triplicate.
Statistical analysis
In this study, all statistical analyses were performed using SPSS (version 19.0) software. Continuous variables were shown as mean ± standard deviation (S.D), the differences in miR-138 and SIRT1 expression between CRC tissue and the adjacent normal tissues were evaluated by Student’s t-test. P≤0.05 were considered as statistically significant.
Results
miR-138 was low expressed in human CRC cell lines and tissues
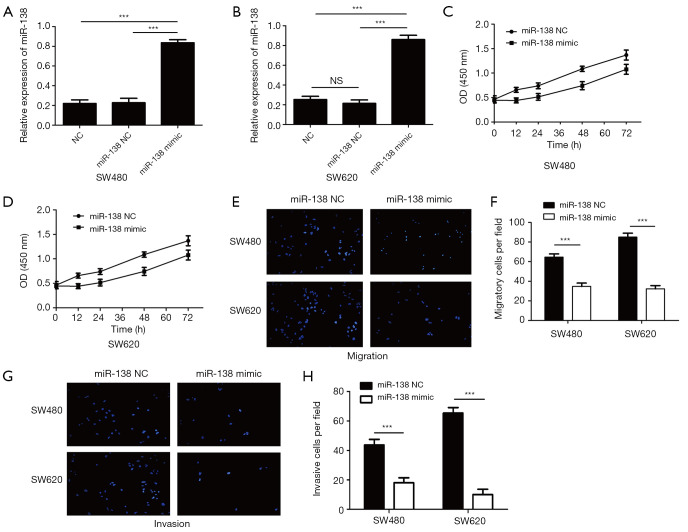
In order to investigate whether miR-138 was involved in the tumorigenesis of CRC, we detected the expression of miR-138 in CRC tissues and cell lines by qRT-PCR, the data was shown in Figure 1. The miR-138 expression was significantly decreased in the CRC tissue compared with the adjacent normal tissue (P<0.001) (Figure 1A). And the expression of miR-138 was largely decreased in CRC cell lines (SW480/SW620/HT-29/HCT-8/RKO) compared with the human normal cell lines (CCD-18Co) (Figure 1B). These results indicated that the expression of miR-138 was significantly decreased weather in human CRC tissues or cell lines.
Figure 1.
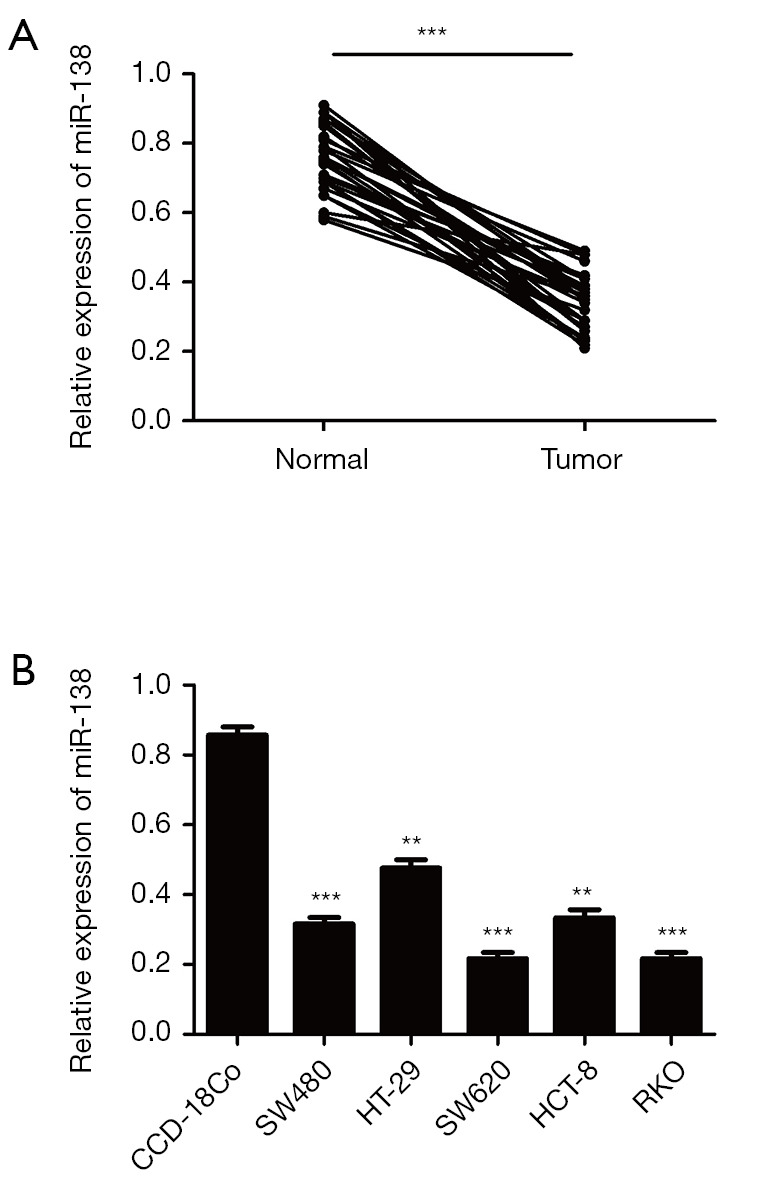
miR-138 is down-regulated in CRC tissues and cell lines. (A) Relative expression of miR-138 in 30 pairs of CRC tumors compared with their adjacent normal tissues; (B) relative expression of miR-138 in five CRC cell lines (SW480, HT-29, SW620, HCT-8, RKO) and the relative expression level of miR-138 in eight colon cancer cell lines and normal colorectal epithelial cell lines CCD-18Co by quantitative RT-PCR. Each experiment was repeated three times, then the deviations were eliminated and the average value was taken (**P<0.01, ***P<0.001).
miR-138 overexpression inhibited proliferation, migration and invasion of CRC in vitro
We investigate the effect of miR-138 overexpression on SW480 and SW620 cell lines proliferation, migration and invasion through CCK8 and Transwell assays, the increased expression of miR-138 in SW480 and SW620 cell lines following transfection were confirmed through qRT-PCR, data was shown in Figure 2A,B. we have used CCK8 assay to detected the proliferation of SW480 and SW620 cell lines, the SW480 and SW620 cell lines were seeded into 96-well plates, according to the manufacturer’s instructions, the OD 450 nm of each groups were detected at 24 h, 36 h and 48 h, the results showed that the proliferation of SW480 and SW620 cell lines overexpressing miR-138 was significantly reduced compared with the control groups, data was shown in Figure 2C,D. we have used Transwell assay to detected the migratory and invasive potential of the SW480 and SW620 cell lines which miR-138 overexpressed. The cells transfected with miR-138 mimics or control microRNA were seeded into the upper chambers with or without Matrigel, and their migratory and invasive potential were determined after 24 h culture. The migration of SW480 and SW620 cells which miR-138 overexpressed was significantly downregulated compared with the control group (Figure 2E,F), and the invasion of SW480 and SW620 cells were also downregulated (Figure 2G,H). These results shown that overexpression of miR-138 could significantly inhibit CRC proliferation, migration and invasion in vitro.
Figure 2.
Overexpression of miR-138 inhibited CRC proliferation, migration and invasion in vitro. (A) Relative expression of miR-138 in SW480 cells transfected with miR-138 mimic was detected by qRT-PCR (***P<0.001). (B) Relative expression of miR-138 in SW620 cells transfected with miR-138 mimic was detected by qRT-PCR (***P<0.001). (C) The proliferation of SW480 cell transfected with miR-138 mimic was detected by CCK8 assay. (D) The proliferation of SW620 cell transfected with miR-138 mimic was detected by CCK8 assay. (E,F) The migration of SW480 and SW620 cell transfected with miR-138 mimic was detected by transwell (Scale bars = 50 µm. Bars, s.e.m.; ***P<0.001). (G,H) The invasion of SW480 and SW620 cell transfected with miR-138 mimic was detected by transwell, each experiment was repeated three times (Scale bars = 50 µm. Bars, s.e.m.; ***P<0.001).
SIRT1 is a target of miR-138 in human CRC
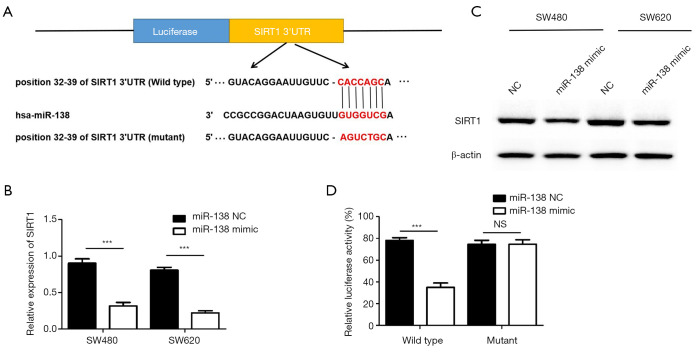
As we all known, miRNAs play their function by inhibiting their target genes expression through combine with its 3’UTR region. Through bioinformatics software prediction Targetscan (http://www.targetscan.org) and miRNA (http://www.microrna.org), we found that the 3’UTR region of SIRT1 contains the binding site of miR-138 (Figure 3A). We verified the effect of miR-138 on SIRT1 expression in in vitro by qRT-PCR and Western blot. the SIRT1 expression in SW480 and SW620 cells which transfected with either miR-138 mimics or control microRNA were detected (Figure 3B,C). we constructed a reporter vector consisting of the luciferase coding sequence followed by the 3′UTR of SIRT1, the data shown that the luciferase activity of wild type significantly decreased compared with mutant type when increase the miR-138 level, data were shown in Figure 3D, illustrating that SIRT1 is a direct target of miR-138 in CRC.
Figure 3.
SIRT1 is a target of miR-138. (A) Target sequences of miR-138 in SIRT1 mRNA were analysed by bioinformatics; (B) Relative expression of SIRT1 in SW480 and SW620 cells transfected with miR-138 mimic was detected by qRT-PCR (***P<0.001); (C) The expression of SIRT1 in SW480 and SW620 cells transfected with miR-138 mimic was detected by western blot; (D) The Luciferase activity of 293T cells after co-transfecting with SIRT1 WT or SIRT1 MUT plasmids as well as negative control (NC) mimic or miR-138 mimic, each experiment was repeated three times, then the deviations were eliminated and the average value was taken. (***P<0.001; NS, P>0.05).
Expression of SIRT1 in human CRC tissues and cell lines
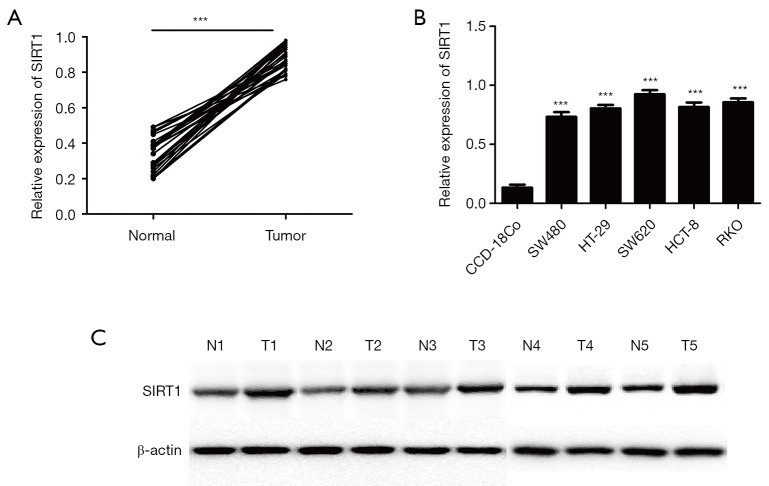
To study the level of SIRT1 in human CRC, we have used qRT-PCR and Western blot to detected the level of SIRT1 in CRC tissues and cell lines, data was shown in Figure 4A, B and C. the results indicated that SIRT1 is significantly upregulated in human CRC tissues and cell lines.
Figure 4.
SIRT1 is up-regulated in CRC tissues and cell lines. (A) Relative expression of SIRT1 in 30 pairs of CRC tumors compared with their adjacent normal tissues. (B) Relative expression of SIRT1 in five CRC cell lines (SW480, HT-29, SW620, HCT-8, RKO) and the relative expression level of miR-138 in eight colon cancer cell lines and normal colorectal epithelial cell lines CCD-18Co by quantitative RT-PCR. Each experiment was repeated three times, then the deviations were eliminated and the average value was taken (***P<0.001). (C) The expression of SIRT1in 3 pairs of CRC tumors compared with their adjacent normal tissues was detected by western blot.
Konckdown of SIRT1 can suppress the proliferation, migration and invasion of human CRC cell lines
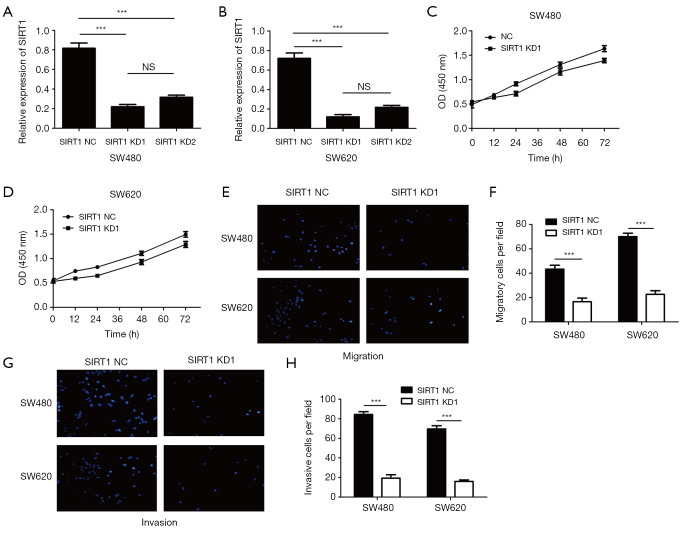
To investigate the function of human CRC cells, we have constructed SIRT1 stable knockdown SW480 and SW620 cell lines by LV-SIRT1-KD lentivirus, and the knockdown effect was shown in Figure 5A and B. we examine the proliferation, migration, and invasion of SIRT1 stable knockdown SW480 and SW620 cell lines through CCK8 and transwell assays. The SIRT1 stable knockdown SW480 and SW620 cell lines were seeded into 96 - well plates, the OD of each groups were detected at 24 h, 36 h and 48 h, the results showed that the proliferation of SW480 and SW620 cells knockdown SIRT1 was significantly reduced compared with the control groups, this is consistent with the results of overexpressing miR-138, data was shown in Figure 5C,D. The SIRT1 stable knockdown SW480 and SW620 cell lines were seeded into the upper chambers with or without Matrigel, and the migratory and invasive potential were detected after 24 h culture. The migration of SW480 and SW620 cells knockdown SIRT1 was significantly decreased compared with the control groups (Figure 5E,F), and so does the invasion (Figure 5G,H). The results indicated that SIRT1 knockdown could inhibit CRC proliferation, migration and invasion in vitro.
Figure 5.
Konckdown of SIRT1 can Suppressed the proliferation, migration and invasion of human CRC cell lines. (A) Relative expression of SIRT1 in SW480 cells stable knockdown SIRT1 was detected by qRT-PCR (***P<0.001; NS, P>0.05). (B) Relative expression of SIRT1 in SW620 cells stable knockdown SIRT1 was detected by qRT-PCR (***P<0.001; NS, P>0.05). (C) The proliferation of SW480 cell with stable knockdown SIRT1 was detected by CCK8 assay. (D) The proliferation of SW620 cell with stable knockdown SIRT1 was detected by CCK8 assay. (E,F) The migration of SW480 and SW620 cell with stable knockdown SIRT1 was detected by transwell (Scale bars =50 µm. Bars, s.e.m.; ***P<0.001). (G,H) The invasion of SW480 and SW620 cell with stable knockdown SIRT1 was detected by transwell (Scale bars =50 µm. Bars, s.e.m.; ***P<0.001), Each experiment was repeated three times, then the deviations were eliminated and the average value was taken.
Correlations of miR-138 expression and survival of CRC
To further investigate the Correlations of miR-138 expression and survival of CRC, the expression of in 152 tumor tissues and adjacent normal tissues were detected by qRT-PCR. As shown in Table 2, no significant differences were observed between the expression of miR-138 and age, sex, tumor site, tumor Size, CEA level, differentiation, and Local invasion. While, there is a significant difference between the miR-138 expression and the patient’s lymph node metastasis and distant metastasis. we also found that the overall survival (OS) of CRC patients with high miR-138 level were both significantly longer than those with low miR-138 level through Kaplan–Meier method and log-rank test (Figure 6).
Table 2. The correlation between miR-138 expression and clinicopathological parameters of CRC.
| Clinical features | Case number (%) | miR-138 expression, n (%) | P | |
|---|---|---|---|---|
| High | Low | |||
| Age (years) | NS | |||
| <58 | 42 (27.63) | 18 (42.86) | 24 (57.14) | |
| ≥58 | 110 (72.37) | 53 (48.18) | 57 (51.82) | |
| Gender | NS | |||
| Male | 78 (51.32) | 38 (48.72) | 40 (51.28) | |
| Female | 74 (48.68) | 35 (47.30) | 39 (52.70) | |
| Tumor size (cm) | NS | |||
| <4 | 67(44.08) | 28 (41.79) | 39 (58.21) | |
| ≥4 | 85 (55.92) | 41 (48.24) | 44 (51.76) | |
| Tumor site | NS | |||
| Colon | 91 (59.87) | 53 (58.24) | 38 (41.76) | |
| Rectum | 61 (40.13) | 26 (42.62) | 35 (57.38) | |
| CEA Level | NS | |||
| 0–5 ng/mL | 59 (38.82) | 24(40.68) | 35 (59.32) | |
| >5 ng/mL | 93 (61.18) | 36 (38.71) | 57 (61.29) | |
| Differentiation | NS | |||
| Well/moderate | 102 (67.11) | 49 (48.04) | 53 (51.96) | |
| Poor | 50 (32.89) | 28 (56.00) | 42 (44.00) | |
| pN | 0.002* | |||
| 0 | 86 (56.58) | 41 (47.67) | 45 (52.33) | |
| ≥1 | 66 (43.42) | 25 (37.88) | 41 (62.12) | |
| pT | NS | |||
| T1 + T2 | 96 (63.16) | 23 (23.96) | 73 (76.04) | |
| T3 + T4 | 56 (36.84) | 13 (23.21) | 43 (76.79) | |
| Distant metastasis | 0.001* | |||
| M0 | 103 (67.76) | 56 (54.37) | 47 (45.63) | |
| M1 | 49 (32.24) | 37 (75.51) | 12 (24.19) | |
‘NS’ refers to the difference without statistical significance. *χ2 test.
Figure 6.
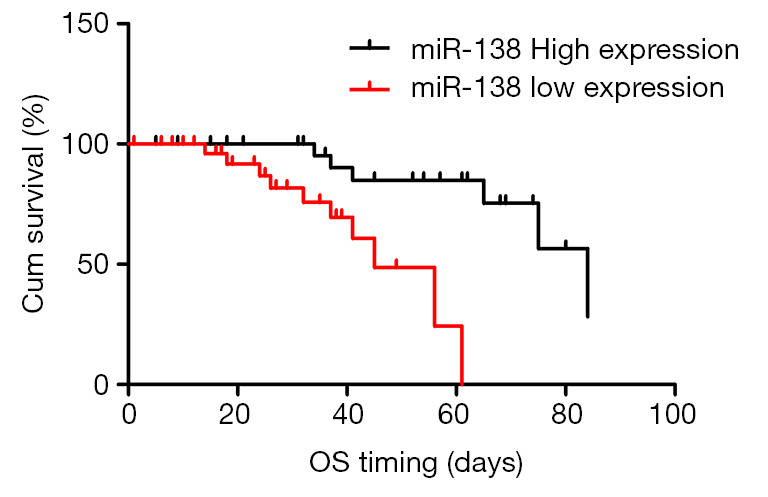
Kaplan-Meier analysis of overall survival for patients with miR-138 high or low expressions. Overall survival (OS) curves for two groups defined by low and high expression of miR-138 in patients with CRC.
Discussion
The pathogenesis of CRC is related to environment, heredity, disease and other factors, involving the activation of multiple oncogenes and the inactivation of tumor suppressor genes (25). Although some early-stage CRC patients can be founded through early screening technology, the patient’s survival rate is effectively improved, in addition, various new types of chemotherapy drugs and molecular targeted drugs continuously available can also improve the clinical curative effect, but the late-stage CRC patients still occupy higher proportion, with poor treatment effect, and the 5-year survival rate is still low (26). Therefore, it is of great clinical value and social benefit to search for new early diagnosis markers and new therapeutic targets of CRC, in order to improve the curative effect and improve the prognosis.
MicroRNAs (miRNAs) are important epigenetic regulators in the development and progression of human CRC, and function as oncogenes or tumor suppressors in CRC progression (27). Thus, identification of the exact biological role of miRNAs in CRC progression and development may helpful to finding novel diagnosis markers and therapy targets for CRC. Previous studies have shown that the abnormal expression of miRNAs is closely related to the molecular mechanism of CRC occurrence and development, Chen et al. Found that miR-203a-3p could promote CRC proliferation and migration by targeting PDE4D (28), Lu et al. Indicated that MicroRNA-124 could inhibit CRC cell proliferation and suppresses tumor growth by interacting with PLCB1 and regulating Wnt/β-catenin signaling pathway (29). Zheng et al. Found that overexpression of microRNA-98 could inhibit cell proliferation and promotes cell apoptosis via claudin-1 in human colorectal carcinoma (30). Zhang et al. Indicated that MicroRNA-296 could inhibit CRC cell growth and enhances apoptosis by targeting ARRB1-mediated AKT activation (31). miR-138 plays an important role in the tumorigenesis of CRC, but its specific molecular mechanism is still unclear, which requires further exploration. Ion et al. found that downregulation of miR-138 could rise the expression of Lcn-2 in CRC, and Lcn-2 overexpression significantly associated with liver metastasis in CRC (32). Long et al. also indicated that down-regulation of miR-138 promotes CRC metastasis via directly targeting TWIST2 (33). Xu et al. indicated that miR-138 could promotes CRC cell proliferation, migration, invasion and EMT by targeting PODXL (34). In our present study, we found that the expression level of miR-138 in CRC tissues and cell lines is significantly increased, and miR-138 could inhibit CRC cell proliferation, migration and invasion by targeting SIRT1.
SIRT1 an NAD+-dependent histone/protein deacetylase is the most extensively studied member among SIRT family (35), has diverse physiological actions. SIRT1 is involved in many processes such as cell energy metabolism, multiple inflammatory reactions, neural protection, reproductive aging, and tumorigenesis (36). The level of SIRT1 is up-regulated in a variety of human tumors and is associated with tumor chemotherapy resistance. But there are two diametrically opposed views on whether SIRT1 acts as an oncogene or tumor-suppressor. For example, there is a study showed that inhibiting SIRT1 expression could lead pancreatic cancer cells sensitive to gemcitabine by inducing DNA damage and apoptosis (37). However, some studies shown that SIRT1 could inhibit tumor progression (38,39). So we infer that the specific function of SIRT1 may depend on diverse biological signaling. In our study, we found that the expression of SIRT1 in CRC tissues and cell lines is significantly increased, and SIRT1 knockdown could inhibit CRC cell proliferation, migration and invasion. This suggesting its oncogenic potential, nevertheless, the molecular mechanisms of SIRT1 overexpression could either induce or inhibit the progression of CRC remains to be further studied.
Conclusions
In summary, this study mainly clarified the regulatory mechanism of miR-138 in the tumorigenesis of CRC, we found that miR-138 was decreased in CRC tissues and cell lines, and was closely related with lymph node metastasis, we also found that miR-138 could inhibit CRC cell proliferation, migration and invasion by targeting SIRT1 firstly. These findings revealed that miR-138 may function as a new diagnostic marker and a therapeutic target for CRC.
Acknowledgments
Funding: This work was supported by the Key Science and Technology Research project of Hebei Province (No. 20180444).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present study was approved by the Ethics Committee of the Third Hospital of Hebei Medical University. The pathological specimens and relevant information of the patients used in this study have been asked the patient for consent, and provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Footnotes
Reporting Checklist: The authors have completed the MDAR checklist. Available at https://dx.doi.org/10.21037/tcr-21-559
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tcr-21-559
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tcr-21-559). The authors have no conflicts of interest to declare.
References
- 1.Kolligs FT. Kolligs. Diagnostics and Epidemiology of Colorectal Cancer. Visc Med 2016;32:158-64. 10.1159/000446488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen CP, Ke TW, Cheng R, et al. Ramucirumab in the second-line treatment of metastatic colorectal cancer: a narrative review of literature from clinical trials. Transl Cancer Res 2020;9:5645-54. 10.21037/tcr-20-608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bensaada FZ, Cadi HO, Sahraoui T, et al. Colorectal Cancer: Epidemiological Study, Clinical, Histological and Immunohistochemistry Examination in Patient of West Algeria. J Cancer Ther 2017;8:26-36. 10.4236/jct.2017.81003 [DOI] [Google Scholar]
- 4.NaNa Keum, Edward L, Giovannucci. Epidemiology of Colorectal Cancer. Pathology and Epidemiology of Cancer 2017;391-407. [Google Scholar]
- 5.Wanebo HJ, LeGolvan M, Paty PB, et al. Meeting the biologic challenge of colorectal metastases. Clin Exp Metastasis 2012;29:821-39. 10.1007/s10585-012-9517-x [DOI] [PubMed] [Google Scholar]
- 6.Ambros V. The functions of animal microRNA. Nature 2004;431:350-55. 10.1038/nature02871 [DOI] [PubMed] [Google Scholar]
- 7.Chen D, Li Y, Li Y, et al. Tumor suppressive microRNA-429 regulates cellular function by targeting VEGF in clear cell renal cell carcinoma. Mol Med Rep 2016;13:1361-66. 10.3892/mmr.2015.4653 [DOI] [PubMed] [Google Scholar]
- 8.Stigliano V, Sanchez-Mete L, Martayan A, et al. Early-onset colorectal cancer: a sporadic or inherited disease. World J Gastroenterol 2014;20:12420-430. 10.3748/wjg.v20.i35.12420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson DE, Bensadoun RJ, Roila F, et al. Management of oral and gastrointestinal mucositis: ESMO Clinical Practice Guidelines. Ann Oncol 2011;22:vi78-84. 10.1093/annonc/mdr391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benson AB, Ajani JA, Catalano RB, et al. Recommended guidelines for the treatment of cancer treatment- induced diarrhea. J Clin Oncol 2004;22:2918-26. 10.1200/JCO.2004.04.132 [DOI] [PubMed] [Google Scholar]
- 11.Wu Q, Yang Z, Shi Y, et al. MiRNAs in human cancers: the diagnostic and therapeutic implications. Curr Pharm Des 2014;20:5336-47. 10.2174/1381612820666140128204914 [DOI] [PubMed] [Google Scholar]
- 12.Andrés-León E, Cases I, Alonso S, et al. Novel miRNA-mRNA interactions conserved in essential cancer pathways. Sci Rep 2017;7:46101. 10.1038/srep46101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tutar L, Özgür A, Tutar Y. Involvement of miRNAs and Pseudogenes in Cancer. Methods Mol Biol 2018;1699:45-66. 10.1007/978-1-4939-7435-1_3 [DOI] [PubMed] [Google Scholar]
- 14.McGuire A, Brown JA, Kerin MJ. Metastatic breast cancer: the potential of miRNA for diagnosis and treatment monitoring. Cancer Metastasis Rev 2015;34:145-55. 10.1007/s10555-015-9551-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baltruskeviciene E, Schveigert D, Stankevicius V, et al. Down-regulation of miRNA-148a and miRNA-625-3p in colorectal cancer is associated with tumor budding. BMC Cancer 2017;17:607. 10.1186/s12885-017-3575-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song Y, Wang F, Huang Q, et al. MicroRNAs Contribute to Hepatocellular Carcinoma. Mini Rev Med Chem 2015;15:459-66. 10.2174/1389557515666150324125353 [DOI] [PubMed] [Google Scholar]
- 17.Shin VY, Chu KM. MiRNA as potential biomarkers and therapeutic targets for gastric cancer. World J Gastroenterol 2014;20:10432-39. 10.3748/wjg.v20.i30.10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue L, Wang Y, Yue S, et al. The expression of miRNA-221 and miRNA-222 in gliomas patients and their prognosis. Neurol Sci 2017;38:67-73. 10.1007/s10072-016-2710-y [DOI] [PubMed] [Google Scholar]
- 19.Hur K, Toiyama Y, Takahashi M, et al. MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut 2013;62:1315-26. 10.1136/gutjnl-2011-301846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moridikia A, Mirzaei H, Sahebkar A, et al. MicroRNAs: Potential candidates for diagnosis and treatment of colorectal cancer. J Cell Physiol 2018; 233: 901-13. 10.1002/jcp.25801 [DOI] [PubMed] [Google Scholar]
- 21.Heublein S, Albertsmeier M, Pfeifer D, et al. Association of differential miRNA expression with hepatic vs. peritoneal metastatic spread in colorectal cancer. BMC Cancer 2018;18:201. 10.1186/s12885-018-4043-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shirafkan N, Mansoori B, Mohammadi A, et al. MicroRNAs as novel biomarkers for colorectal cancer: New outlooks. Biomed Pharmacother 2018;97:1319-30. 10.1016/j.biopha.2017.11.046 [DOI] [PubMed] [Google Scholar]
- 23.Lee RC, Feinbaum RL, Ambros V. The Celegans hetero chronic gene lin-4 encodes small RNAs with antisense complement aritytolin-14. Cell 2009;89:1828-35. [Google Scholar]
- 24.Pang L, Li B, Zheng B, et al. miR-138 inhibits gastric cancer growth by suppressing SOX4. Oncol Rep 2017;38:1295-302. 10.3892/or.2017.5745 [DOI] [PubMed] [Google Scholar]
- 25.Eskandari E, Mahjoubi F, Motalebzadeh J. An integrated study on TFs and miRNAs in colorectal cancer metastasis and evaluation of three co-regulated candidate genes as prognostic markers. Gene 2018; 679: 150-59. 10.1016/j.gene.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 26.Yong L, YuFeng Z, Guang B. Association between PPP2CA expression and colorectal cancer prognosis tumor marker prognostic study. Int J Surg 2018;59:80-89. 10.1016/j.ijsu.2018.09.020 [DOI] [PubMed] [Google Scholar]
- 27.Moridikia A, Mirzaei H, Sahebkar A, et al. MicroRNAs: Potential candidates for diagnosis and treatment of colorectal cancer. J Cell Physiol 2018;233:901-13. 10.1002/jcp.25801 [DOI] [PubMed] [Google Scholar]
- 28.Chen L, Gao H, Liang J, et al. miR-203a-3p promotes colorectal cancer proliferation and migration by targeting PDE4D. Am J Cancer Res 2018;8:2387-401. [PMC free article] [PubMed] [Google Scholar]
- 29.Lu M L, Zhang Y, Li J, et al. MicroRNA-124 inhibits colorectal cancer cell proliferation and suppresses tumor growth by interacting with PLCB1 and regulating Wnt/β-catenin signaling pathway. Eur Rev Med Pharmacol Sci 2019;23:121-36. [DOI] [PubMed] [Google Scholar]
- 30.Zheng Y F, Luo J, Gan G L, et al. Overexpression of microRNA-98 inhibits cell proliferation and promotes cell apoptosis via claudin-1 in human colorectal carcinoma. J Cell Biochem 2019;120:6090-105. 10.1002/jcb.27895 [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Zhong X, Xiao Y, et al. MicroRNA-296 inhibits colorectal cancer cell growth and enhances apoptosis by targeting ARRB1-mediated AKT activation. Oncol Rep 2019;41:619-29. [DOI] [PubMed] [Google Scholar]
- 32.Cristóbal I, Torrejón B, González-Alonso P, et al. Downregulation of miR-138 as a Contributing Mechanism to Lcn-2 Overexpression in Colorectal Cancer with Liver Metastasis. World J Surg 2016;40:1021-22. 10.1007/s00268-015-3241-z [DOI] [PubMed] [Google Scholar]
- 33.Long L, Huang G, Zhu H, et al. Down-regulation of miR-138 promotes colorectal cancer metastasis via directly targeting TWIST2. J Transl Med 2013;11:275. 10.1186/1479-5876-11-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y, Pan Z G, Shu L, et al. Podocalyxin-like, targeted by miR-138, promotes colorectal cancer cell proliferation, migration, invasion and EMT. Eur Rev Med Pharmacol Sci 2018;22:8664-74. [DOI] [PubMed] [Google Scholar]
- 35.Schiedel M, Robaa D, Rumpf T, et al. The current state of NAD (+) -dependent histone deacetylases (Sirtuins) as novel therapeutic targets. Med Res Rev 2018;38:147-200. 10.1002/med.21436 [DOI] [PubMed] [Google Scholar]
- 36.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev 1999;13:2570-80. 10.1101/gad.13.19.2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oon CE, Strell C, Yeong KY, et al. SIRT1 inhibition in pancreatic cancer models: contrasting effects in vitro and in vivo. Eur J Pharmacol 2015;757:59-67. 10.1016/j.ejphar.2015.03.064 [DOI] [PubMed] [Google Scholar]
- 38.Firestein R, Blander G, Michan S, et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS One 2008;3:e2020. 10.1371/journal.pone.0002020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun L N, Zhi Z, Chen LY, et al. SIRT1 suppresses colorectal cancer metastasis by transcriptional repression of miR-15b-5p. Cancer Lett 2017;409:104-15. 10.1016/j.canlet.2017.09.001 [DOI] [PubMed] [Google Scholar]