Abstract
Mitochondria are primarily involved in energy production through the process of oxidative phosphorylation (OXPHOS). Increasing evidence has shown that mitochondrial function impacts a plethora of different cellular activities, including metabolism, epigenetics, and innate immunity. Like the nucleus, mitochondria own their genetic material, but this organellar genome is circular, present in multiple copies, and maternally inherited. The mitochondrial DNA (mtDNA) encodes 37 genes that are solely involved in OXPHOS. Maintenance of mtDNA, through replication and repair, requires the import of nuclear DNA-encoded proteins. Thus, mitochondria completely rely on the nucleus to prevent mitochondrial genetic alterations. As most cells contain hundreds to thousands of mitochondria, it follows that the shear number of organelles allows for the buffering of dysfunction—at least to some extent—before tissue homeostasis becomes impaired. Only red blood cells lack mitochondria entirely. Impaired mitochondrial function is a hallmark of aging and is involved in a number of different disorders, including neurodegenerative diseases, diabetes, cancer, and autoimmunity. Although alterations in mitochondrial processes unrelated to OXPHOS, such as fusion and fission, contribute to aging and disease, maintenance of mtDNA integrity is critical for proper organellar function. Here, we focus on how mtDNA damage contributes to cellular dysfunction and health outcomes.
Keywords: cellular outcomes, DNA repair, mitochondria, mitochondrial genome, mtDNA damage
INTRODUCTION
The mitochondrial and the nuclear genomes have been evolving together for ∼2 billion years. Since then, the mitochondrial DNA (mtDNA) lost the majority of its genetic material, remaining with only 37 genes that are critical for ATP production. These genes, in addition to tRNA and rRNAs, encode 13 essential subunits of complexes I, III, IV, and V of the electron transport chain (ETC). Therefore, it is easy to foresee how loss of mtDNA integrity has the potential to alter mitochondrial function. However, the biogenesis of a functional ETC system requires a larger set of proteins (∼120), that are nuclear encoded (1, 2). This arrangement also establishes the active reliance of mitochondria on the nucleus to accomplish all of its functions, including maintaining the mtDNA through replication and repair. The mutation rate of the mitochondrial genome is higher than that of the nuclear DNA, which makes the cell prone to evolving compensatory means to maintain co-existing gene complexes (3). Furthermore, studies over the last decades have shown that the mtDNA is more prone to damage than the nuclear genome (4–11). Several reasons explain such increased sensitivity, including the lack of protective histones as well as the limited repertoire of DNA repair pathways operating in mitochondria relative to the nucleus. In addition, the proximity of the mtDNA to the ETC that generates potentially damaging reactive oxygen species (ROS) increases the probability of oxidative damage (12). Also, it is known that some genotoxins preferentially accumulate in mitochondria (13). Thus, exogenous and endogenous sources of DNA damage can affect both genomes, but with different outcomes.
The nucleus has an extensive variety of DNA damage-sensing signaling pathways and repair mechanisms that safeguard DNA integrity. These pathways frequently compete with each other to repair a lesion, overall assuring that those do not become established mutations. In contrast, only a few DNA repair pathways are functional in mitochondria and, despite the misconception that this organelle does not have repair, mitochondrial base excision repair (mtBER) is rather robust (12, 14). BER removes both oxidative damage and single-strand breaks (SSBs) whereas more complex lesions such as pyrimidine dimers (caused by UV radiation), DNA double-strand breaks (DSBs), or other bulky DNA adducts require different enzymatic properties. Thus, these complex lesions are not repaired from the mitochondrial genome although mammalian mitochondrial extracts have been shown to have DBS repair activity (15). Instead, strands having these non-BER substrates are digested, recombined, or the organelle is removed by mitophagy or autophagy (14, 16) (Fig. 1). Critically, to date there is little understanding of how the nucleus senses that the mtDNA is damaged and subsequently deploys the necessary and appropriate means to address it. For example, no p53-type signaling is observed when mtDNA alone is damaged (17) and neither are the levels of DNA polymerase γ (polγ), responsible for mtDNA replication and repair, nor mtBER activity decreased when mtDNA is lost (18). Also, some types of mtDNA changes, including DNA depletion and DSBs can be induced below thresholds that cause organellar dysfunction; yet they are sensed by the nucleus as judged by transcriptional remodeling (19, 20). Thus, it is likely that metabolic signaling sensitive to specific mtDNA changes is involved in communicating the mtDNA status to the nucleus. In support of this assumption, changes in serine biosynthesis seem to respond to loss of mtDNA but not to other types of mtDNA mutations (19, 21–23). Likewise, cellular levels of acetyl-CoA decrease upon progressive mtDNA depletion even before mitochondrial dysfunction, at which point histones are hypoacetylated (24). Conversely, despite complete loss of respiration, a mutation on the mtDNA-encoded gene cytochrome b (cytb) does not decrease cellular acetyl-CoA levels; in the nucleus histones are rather hyperacetylated (Santos, unpublished).
Figure 1.

Mitochondrial DNA damage and repair. Exogenous and endogenous sources can produce different lesions in mitochondrial DNA (mtDNA), like single-strand (SSB) and double-strand DNA breaks (DSB), complex bulky lesions, e.g., DNA protein crosslinks (DPCs), and DNA inter- and intrastrand crosslinks. Specialized mitochondrial DNA repair pathways and quality control systems safeguard mtDNA genome stability, as mitochondrial base excision repair (mtBER), mitophagy, degradation, or recombination. The lesion types and their respective means of removal are shown on the right.
The aim of this review is to contribute to our understanding of how mtDNA damage can impact cellular functions and human health, with or without inducing mitochondrial dysfunction. We will focus on the main studies in mammalian systems in which the mitochondrial genome was the initiating site of damage, with no detectable (or secondary) effects in the nucleus; we apologize for those whose work might not have been covered due to space constraints. We will discuss whether and how mtDNA damage affects the transcription of this genome, an area still poorly explored, and touch upon the emerging area demonstrating that mtDNA instability contributes to innate immunity and epigenetics. It is not our intent to review the literature about all the DNA repair pathways that operate in mitochondria as this topic has been extensively addressed in other excellent articles (12, 14, 16, 25–31). Nevertheless, we will briefly review the steps and enzymatic activities associated with mtBER.
MITOCHONDRIA, mtDNA, AND mtDNA REPAIR
Mitochondria house oxidative phosphorylation (OXPHOS), a key physiological process in eukaryotic cells that generates ATP necessary for many cellular activities. The OXPHOS system is an elegant, elaborate, and highly specialized multicomplex protein machinery that localizes to the inner mitochondrial membrane. The mitochondrial proteome consists of ∼1,500 proteins, mainly nuclear encoded, and with only 13 under mtDNA regulation (32). In 1963, Nass and Nass (33) described for the first time the presence of mitochondrial fibers in chick embryo with electron-staining properties, consistent with the presence of nucleic acids in mitochondria and the endosymbiotic origin of these organelles. MtDNA is transferred maternally, in contrast to the Mendelian inheritance of the nuclear genome. The replication of the mtDNA occurs continuously independently of the cell cycle, with different modes of replication being proposed (34, 35). The strand displacement model remains the most well established; whether mtDNA replicates like the nuclear genome and involves extensive incorporation of RNA as replication intermediates is still debatable. Irrespective, it is well known that DNA polγ is the mitochondrial replicase. In addition to working on mtDNA replication, polγ has also been touted as the protein responsible for mtDNA repair (36–38). Interestingly, recent evidence indicates that polymerase β (polβ), the key enzyme involved in nuclear BER, is also present in mitochondria and likely plays a role in the repair of mtDNA (39–41).
BER is primarily involved in the repair of oxidative lesions and abasic sites although it can also participate in the repair of SSBs (42). Two distinct forms of BER termed short- or long-patch repair exist in cells. In the former, a single nucleotide is inserted to fill the gap generated by the removal of the lesion, usually a base, which is then ligated by a DNA ligase. In long-patch repair, two or more nucleotides are inserted. This may displace downstream nucleotides that in turn need to be endonucleolytically cleaved. Generally, a glycosylase removes the damage generating an abasic site, an endonuclease cleaves the sugar backbone, a polymerase fills in the gap, and a DNA ligase seals the patch. Most of the nuclear BER enzymes are also targeted to the mitochondria while ligase III, instead of ligase I, is the one that seals the repair patch in the organelle. In the nucleus, polβ is involved in gap filling irrespective of the number (one or more) of nucleotides although, in proliferative cells, long-patch BER counts with polδ or polε for gap filling activity (43). In the mitochondria, up to recently, polγ was the enzyme thought to perform gap filling since it was the only recognized DNA polymerase in the organelle. However, identification of polβ in the mitochondria raises the possibility of overlapping, redundant, cooperative, or independent functions of these two enzymes in the context of mtBER. Although more studies are required to better understand their relationship and the effects on mtDNA integrity, it is notable that a direct comparison between polβ and polγ activities identified the former as more efficient in the repair of every DNA damage intermediate tested in vitro. Furthermore, mitochondrial extracts from polβ null cells were defective in gap filling activity (39), strongly suggesting that polγ may not be (solely) involved in this activity.
MtBER is robust but cannot handle all types of mtDNA damage. For example, DSBs, bulky products or other DNA adducts (like inter- and intrastrand crosslinks) are not repaired from the mtDNA given the absence of the enzymes required to process these types of lesions in mitochondria (4–8, 44, 45). The lack of complete repair pathways beyond BER has likely supported the misconstrued notion that mitochondria do not have repair. When the mtDNA is presented with these non-BER types of lesions, which can be caused by exogenous or endogenous factors including the replication of the mtDNA itself by polγ, their fate depends on several factors such as mitochondrial dysfunction that can indirectly “signal” their existence. For instance, mitophagy has been shown to efficiently help the removal of persistent UV-induced pyrimidine dimers from mtDNA (46). However, mitophagy that selectively target damaged mitochondria likely requires the mitochondrial membrane potential to collapse such that pink and parkin can accumulate in the organelles and help orchestrate their degradation (47). To date, mtDNA replication stalling emerges as the primary cause of endogenous DSBs in mitochondria. MtDNA DSBs can recombine or be degraded, the outcomes of which are mtDNA deletions and depletion, respectively (48–50). Although the enzyme(s) responsible for mtDNA recombination remains elusive, it has been suggested that polγ may be involved in digesting linear DNA generated after a mtDSB in turn making the mtDNA more prone to recombine (51, 52). Linear mtDNA, produced as a consequence of DSB, can be also digested by the mitochondrial genome maintenance exonuclease 1 (52, 53). Similarly, a role for the mitochondrial helicase Twinkle has been implied in mtDNA recombination by virtue of its NTP-dependent strand annealing and strand exchange activities (54, 55). Furthermore, Twinkle upregulation causes mtDNA replication stalling, accumulation of replicative intermediates, and mitochondrial genome destabilization linked to large-scale mtDNA deletions (56, 57). A representation of the fates of mtDNA damage is shown in Fig. 2.
Figure 2.

Fates of mitochondrial DNA damage. Mitochondrial DNA (mtDNA) damage can lead to different outcomes, depending on the type of lesion present on the genome. The three main outcomes associated with the presence of damage on the mtDNA are shown.
OUTCOMES OF MITOCHONDRIAL DNA DAMAGE
mtDNA Mutations
The classic outcome of nonrepaired DNA lesions is the fixation of mutations. The most frequent genotoxin-induced mutations on the mtDNA are those caused by oxidative damage, particularly the presence of 8-oxo-guanine (8-oxo-G) since it is a mutagenic lesion (58). mtDNA mutations may have consequences on mitochondrial function or not, depending on where they occur on the mtDNA, whether they are silent or, if translated, induce a change in the reading frame or amino acid sequence. Most importantly, because mtDNA is present in multiple copies within a mitochondrion and thus in the cell, the relative number of mutated versus normal mtDNA molecules dictates whether mitochondrial function is impaired or not at the cellular or tissue level. Even a detrimental mutation will have functional effects only when the number of mitochondrial genomes bearing such changes overcomes the wild-type (WT) mtDNA counterpart. This concept of mitochondrial heteroplasmy (different sequences of mtDNA within the same cell or tissue) is a well-established characteristic of mitochondrial diseases and has been extensively reviewed elsewhere (59–62). Thus, from here on we will focus on the outcomes of DNA damage to the mtDNA that are unrelated to their mutational properties or heteroplasmic levels. Readers are referred to an excellent recent short review and the references therein (63) for studies on mitochondrial diseases associated with mtDNA mutations caused by changes in the mitochondrial replisome (i.e., polγ, the mitochondrial single-stranded binding protein 1—mtSSB1—or Twinkle).
Effects of Genotoxin-Induced mtDNA Damage to Mitochondrial and Cellular Function
Seminal studies in the 70s through the 90s established that although oxidative damage was efficiently removed from the mtDNA, other types of lesions such as those induced by UV or nitrogen mustard were not (4–8, 11, 44, 45). Many of these studies also compared the levels of damage incurred on the mtDNA to those present in the nucleus under the same conditions, using techniques such as Southern analysis or HPLC, which while providing information about damage to both genomes was not ideal for comparisons purposes since they require isolation of the mtDNA—a procedure that is known to artifactually oxidize this genome (12). The establishment of PCR-based assays has greatly improved the ability to more faithfully estimate and compare damage levels to both genomes in samples where total genomic DNA is isolated and thus the entire sample is processed in one step. Using long-range quantitative PCR (QPCR), Yakes and Van Houten (11) showed that the mtDNA was more prone to oxidative damage than the nuclear DNA under the same treatment conditions and, more importantly, that the lesions on the mtDNA persisted longer than in the nuclear DNA despite active repair. This study laid the groundwork that suggested that mtDNA damage itself can initiate a cascade of events with implications to cell fate. Yet, it was not until recently that the use of elegant strategies provided unequivocal evidence to support this hypothesis; key studies are described below.
Hydrogen peroxide (H2O2) is freely diffusible, causing oxidative damage to various biomolecules including DNA (nuclear and mitochondrial), membranes, and proteins. Early work by the Van Houten laboratory by QPCR demonstrated that exposure to 200 µM H2O2 for 60 min led to mtDNA damage whereas no nuclear DNA lesions were identified in human fibroblasts. DNA repair kinetics showed that damage to mtDNA persisted for 24 h and was accompanied by loss of the mitochondrial membrane potential (ΔΨm) and eventually apoptosis. Sorting of the cells based on their ΔΨm 24 h after H2O2 treatment demonstrated that apoptotic cells were those bearing damaged mtDNA and whose ΔΨm had collapsed; cells that maintained ΔΨm had no mtDNA damage and continued to proliferate. Although by the 24-h time point apoptotic cells showed nuclear DNA damage (∼5 lesion per 100 kb of dsDNA), which is not surprising given the endonucleotic cleavage of the DNA during programmed cell death, the mtDNA still carried ∼5 lesions/molecule (9). These data not only reproduced the increased sensitivity of mtDNA to oxidative damage relative to the nucleus but, most importantly, suggested that mtDNA damage was the event that ultimately led to cell death. Interestingly, exposure of mouse embryonic fibroblasts to methylmethanesulfonate (MMS), an alkylating agent, damaged the mtDNA to levels similar to damage observed after H2O2 treatment. However, H2O2 but not MMS, led to mtDNA loss and mitochondrial dysfunction, leading the authors to suggest that it may be the loss of mtDNA, not the initial or persistent level of damage that causes mitochondrial dysfunction (64). Although this remains an intriguing possibility, it is conceivable that H2O2, unlike MMS, damaged other cellular components that contributed to its overall effects. Alternatively, the type and quality of DNA damage (i.e., the DNA ends generated by processing the lesion) rather than quantity might dictate subsequent outcomes.
Ionizing radiation (IR) can induce DSB and oxidative DNA damage to both nuclear and mitochondrial genomes; it is generally accepted that damage to the nucleus is responsible for the cellular effects of IR. Nevertheless, with the development of microbeams that can target precise positions inside the cells, accumulating evidence has shown that energy deposited by radiation in the cytoplasm can induce similar biological effects to targeting nuclear DNA (65). In this context, data indicate that mitochondria are a target of the cytoplasmic effects of radiation. Microbeam or target irradiation strategies have been extensively used to study bystander effects, which are those observed in the nonirradiated neighbors of directly irradiated cells. Activation of several signaling pathways, including those involving DNA damage such as the generation of p53 binding protein (53BP1) foci, are used to determine the effects of IR in bystander cells. Using a microbeam to irradiate solely the cytoplasm of cultured cells, Tartier et al. (66) identified 53BP1 foci in both directly hit and bystander cells. They also showed that inhibitors of ROS or reactive nitrogen species (RNS) prevented these effects, indicating that free radicals were involved. More importantly, the effects were abolished when the cytoplasm of cells devoid of mtDNA (ρ0) was irradiated, indicating that active ETC (or mitochondrial function) was needed to produce a bystander signal and that direct nuclear irradiation was not required to trigger 53BP1 foci. Subsequently, Zhang et al. (67) showed that targeted cytoplasmic irradiation by high linear energy transfer (LET) α particles induced oxidative DNA damage and DSBs in the nucleus of WT cells. This was followed by activation of autophagy, micronuclei formation, activation of nuclear factor kappa B (NF-kB), and mitochondrial inducible nitric oxide synthase (iNOS) signaling pathways. As in the previous studies, these outcomes were dampened if the cytoplasm of ρ0 cells was irradiated, further alluding to the contribution of damaged mitochondria, and likely damaged mtDNA, to these effects.
Direct evidence of mitochondrial dysfunction as a result of cytoplasmic irradiation was obtained with a precision microbeam of 1 μm width that assured no nuclear targeting. Using this system, cytoplasmic irradiation of cells was shown to reduce succinate dehydrogenase (complex II) and cytochrome c oxidase (complex IV) activities, increase superoxide production relative to nontreated controls, and induce dynamin-related protein 1 (DRP1)-dependent mitochondrial fragmentation. These phenotypes were observed as soon as 4 h after irradiation but were essentially resolved by 24 h, leading to the conclusion that they were an acute mitochondrial response to cytoplasmic irradiation. The authors proposed that this acute response resulted in the release of several stress mediators, which were necessary for mitochondria to preserve cellular homeostasis, although the exact signals remain unclear (68). Whether mtDNA damage was induced by this level of radiation was not examined, but it is possible that it was present and might have contributed to complex IV dysfunction. Three of the 14 subunits of complex IV are mtDNA-encoded whereas the remaining 11 subunits are coded in the nucleus, which was not irradiated. Alternatively, ROS generated by IR-driven radiolysis could have directly damaged mitochondrial proteins and/or membranes, causing the reduction in the activity of complex II and IV. Collectively, the data obtained with the microbeam approaches suggest that proper mitochondrial function is needed for the effects associated with cytoplasmic-only irradiation. The use of ρ0 infers that mtDNA is needed, but given that complete loss of mtDNA leads to mitochondrial dysfunction on its own, moving forward it would be useful to quantify mtDNA integrity in the samples to define if damage to mtDNA is needed and can drive the outcomes. It would also be important to monitor potential changes in mitochondrial copy number, which can help understand the means through which mitochondria are coping with radiation damage.
Recently, another elegant strategy provided unequivocal evidence that damage to mtDNA can trigger cellular effects. The authors used a chemoptogenic approach involving a mitochondrially targeted fluorogen-activating peptide (Mito-FAP) to deliver a photosensitizer (MG-2I dye) to the mitochondria that can be activated upon light exposure. Such strategy provided precise spatiotemporal control of generation of singlet oxygen exclusively to the mitochondria. Singlet oxygen reacts with DNA, preferentially oxidizing guanine, although all four DNA bases can be attacked (69). Light-mediated activation of the Mito-FAP-MG-2I complex for 5 min led to mtDNA damage, rapid loss of mitochondrial respiration, decreased ETC activity, and mitochondrial fragmentation whereas no nuclear DNA damage was observed. Notably, a secondary wave of superoxide and H2O2 production followed the initial mitochondrial damage, being first detected 4 h after light-mediated activation of the Mito-FAP-MG-2I complex and was sustained for 48 h. This secondary wave of ROS eventually reached the nucleus, as judged by cysteine oxidation of a nuclear redox probe, causing telomere DSBs and replication stress. Telomeric damage in turn activated the DNA damage-repair signaling kinase ataxia-telangiectasia mutated (ATM); inhibition of ATM exacerbated the Mito-FAP-induced mitochondrial dysfunction and the nuclear phenotype (70). Collectively, the data are consistent with a model in which singlet oxygen damaged mtDNA, leading to a secondary wave of ROS production that impacted transcription of the mtDNA-encoded subunits of the ETC that in turn sustained the effects. The concept of a feed-forward vicious cycle of mitochondrial dysfunction leading to other cellular phenotypes has been previously proposed (31). Nevertheless, defining the earliest time point in which mtDNA damage was first observed is still required as it is possible that damage to protein subunits of ETC, rather than mtDNA, occurred first. Irrespective, the findings from Qian and coworkers clearly demonstrate that not only mitochondria can initiate the damage cross talk with the nucleus but it can, subsequently, be influenced by it.
Lastly, Geden et al. (17) established the outcomes of cisplatin-induced mtDNA damage to cellular function without affecting the nuclear genome by taking the advantage of the biology of neurons. In these cells, the cell body containing the nucleus is spatially distant from the axons that only contain mitochondria. Using microfluidic chambers that have been shown to maintain strict fluidic separation between the soma and axon, neuronal cultures were exposed to cisplatin. Exposure of soma and axons showed DNA damage in both compartments whereas exposure of axons alone induced only mtDNA lesions. Interestingly, the nuclear DNA damage sensing protein p53 was not activated upon axonal cisplatin treatment, indicating that canonical nuclear DNA damage sensors do not recognize mtDNA damage per se. Most notably, cisplatin-induced mtDNA damage triggered axonal degeneration that followed a sustained and progressive kinetics, which was complete by 120 h. Although mtROS was increased already at 24 h and remained overt for the duration of the experiments, no changes in ΔΨm or activation of apoptosis (as judged by cytochrome c release) were observed. Thus, the levels of mtDNA damage induced by cisplatin caused some, but not overt, mitochondrial dysfunction that sufficed to activate a degenerative pathway confined to the exposed axons. From a broader health perspective, chemotherapeutic agents like cisplatin frequently induce peripheral neuropathy (CIPN) that, intriguingly, often continue to impact long-term function and the quality of life of cancer survivors (71). The mechanisms underlying CIPN remain poorly understood, but the work from Geden et al. (17) brings to light the possibility that mtDNA damage and mitochondrial dysfunction can contribute to it. Considering the latest findings on the long-lasting epigenetic changes driven by mitochondrial dysfunction (72), it is feasible to speculate a model in which persistent mtDNA damage driven by chemotherapy influences long-term health outcomes of cancer patients through sustained epigenetic remodeling. Alternatively, it is possible that chronic aberrant innate immunity activation, also driven by mtDNA damage, may play a role (see Activation of DNA Damage by mtDNA and mtRNA). It will be interesting to address these possibilities in future studies.
Enzyme-Mediated mtDNA Damage and Cellular Outcomes
Many genotoxic agents have been shown to damage the mtDNA in addition to the nuclear genome, including those present in the environment such as UV light, IR, benzo[a]-pyrene, heavy metals, alcohol, cigarette smoke, etc., thus significantly expanding the types of lesions that can occur on the mtDNA (13, 26). However, because these agents are not specific to the mitochondrial genome, teasing out the extent to which mtDNA damage contributes to (or drives) cellular phenotypes is difficult. One way to address this challenge is to generate or inhibit the removal of damages unique to mtDNA by genetic or targeted means. For example, depleting mtBER proteins could in theory provide the means to increase DNA nicks or abasic sites. However, most BER proteins are dually targeted thus leading to effects in both nucleus and mitochondria. Defining the effects of polγ on mtDNA repair versus mtDNA replication is challenging and, in fact, has only been explored in vitro (37). Manipulation of ligIII has shown that, despite being dually targeted, its main function is on mtDNA metabolism so work on those models will be discussed below. We will also review work in which different enzymes that cut DNA causing abasic sites, SSB or DSBs, were targeted to the mitochondria and exclusively induced mtDNA damage.
A mouse model of ligIII deletion was created ∼15 years ago and caused early embryonic lethality. This phenotype was initially thought to result from increased sister chromatid exchange in the nucleus and thus a defect in nuclear DNA repair (73). However, a later study provided strong evidence that such lethality was due to the loss of ligIII in the mitochondria. By engineering versions of all cellular DNA ligases that would be targeted to the nucleus or the mitochondria, the authors showed that cells devoid of mitochondrial ligIII were not viable whereas loss of nuclear ligIII, ligI, or IV did not affect cell viability. Moreover, the effects on cell viability of ligIII deletion involved mitochondrial genome instability and required the ligase activity of the enzyme, ruling out an alternative mitochondrial function for ligIII (74). Contemporaneously, a mouse model of targeted ligIII deletion in the central nervous system (CNS) and in the heart reported mtDNA loss leading to profound mitochondrial dysfunction in the knockout (KO) animals. In the brain, these phenotypes disrupted cellular homeostasis causing incapacitating ataxia whereas loss of ligIII in the heart led to defective myocardial pumping activity and heart failure (75). As no nuclear defects in DNA ligation or genomic instability were observed in these models (74, 75), these two studies established loss of mtDNA integrity caused by impaired ligation of mtDNA ends as a driver for decreased mitochondrial function and cell/tissue homeostasis.
We and others later expanded these findings to demonstrate that decreased ligIII levels, loss of mtDNA integrity, and mitochondrial dysfunction were features of human disorders, including ataxia telangiectasia (A-T) (76), Alzheimer’s disease (77) and, most recently, a mitochondrial neurogastrointestinal encephalomyopathy (78). Conversely, high levels of ligIII found in cancers seem to provide an Achilles heel that can be exploited for therapy through its effects on mitochondria. Pharmacological inhibition of ligIII in cancer cells resulted in mitochondrial dysfunction as judged by altered mitochondrial morphology, mtDNA depletion, and increased levels of mitochondrial ROS, effects that were not observed in normal cells. More importantly, these effects sensitize the cells to apoptosis (79). Why normal cells did not have a mitochondrial phenotype, similar to the models of ligIII deletion described earlier (74, 75) is unclear but it might be associated with the efficacy of ligIII inhibition through pharmacological means. Irrespective, these results give further support to the notion that disruption of mtDNA metabolism, in this case through inhibition of ligIII, affect mitochondrial and cell function, which could also be exploited for therapeutic purposes.
At about the same time that the initial ligIII studies were performed, the loss of another enzyme activity in mitochondria exonuclease G (ExoG) was shown to increase the presence of persistent SSBs in the mtDNA. ExoG is a 5′-exo/endonuclease unique to the mitochondria implicated in repairing endogenous SSBs through the removal of the 5′-flap-blocking residue that is generated through long-patch BER. Using different cells in culture, the authors showed that depletion of ExoG using siRNA caused apoptotic cell death. Detailed analysis of the mechanisms associated with this phenotype demonstrated that ExoG depleted HeLa cells had dysfunctional mitochondria as gauged by loss of ΔΨm and increased superoxide generation. Similarly, depletion of ExoG in MCF7 cells led to changes in mitochondrial respiration and decreased OXPHOS. The authors went on to show that cells depleted of ExoG had increased mtDNA damage, and based on several in vitro repair studies using mitochondrial extracts from control or knockdown cells, established ExoG as the major 5′-exonuclease working on long-patch mitochondrial BER. Collectively, these data provided unequivocal evidence that persistent SSBs in the mtDNA alone could provide the initial trigger for apoptotic signaling in mammalian cells (80).
Abasic sites and SSBs have also been induced on mtDNA by targeting a mutated form, uracil-N-glycosylase (UNG), which removes thymine from DNA, or Escherichia coli exonuclease III (exoIII) to the mitochondria. Upon doxycycline addition to the cell culture medium, the transgenes were induced and shown to increase the frequency of mtDNA damage. Significant mtDNA depletion was observed within 6 h of UNG induction whereas only 30% of mtDNA was lost 12 h after exoIII-induced DNA damage. Although apparently abasic sites induced by UNG were less tolerated on the mtDNA, thus causing mtDNA loss, it is more likely that mtDNA depletion was associated with the abundance of mtDNA damage caused by the two enzymes. The mutated form of UNG utilized removes thymines, which are abundant bases normally present on the mtDNA. Conversely, exoIII works on prior existing damage (abasic sites or SSBs); in the context of the study, these substrates occurred naturally (at baseline) and thus were likely not very frequent. Irrespective of their initial incidence, the outcome of those persistent lesions on mtDNA was eventually decreased respiration, loss of ΔΨm, and decreased cell proliferation. No concomitant increase in mutation load was observed on the mtDNA. Based on the kinetics of these phenotypes, the authors proposed that the loss of ΔΨm and of cell proliferation were secondary to the reduced expression of mtDNA-encoded polypeptides that resulted from mtDNA depletion (81). Although possible, it is worth noting that a previous study showed that the ΔΨm is required to maintain cell proliferation (82) and, more recently, even cells completely devoid of mtDNA were shown to proliferate as long as their ΔΨm was maintained (83). Thus, the cell proliferation defects observed by targeted exoIII or UNG-induced mtDNA damage might be secondary to loss of ΔΨm but not necessarily to loss of mtDNA.
Attempts to damage the mitochondrial genome using restriction enzymes to induce DSBs have been tried for a few decades, and had an initial aim to understand if these lesions were repaired within the mtDNA. More recently, enzymes that can cut DNA with precise sequence specificity such as zinc finger nucleases (ZFN) or transcription activator-like effector nucleases (TALENs) were also targeted to the mitochondria, but with the goal to change heteroplasmic levels. Irrespective of the system, together these strategies provided further evidence that mtDNA lesions themselves influence mitochondrial and cellular function. Early work targeted the restriction enzyme PstI to the mitochondria in cells and in mice (50, 84). In all cases, it was shown that PstI induced mtDNA DSBs, mtDNA depletion and, less frequently, recombination (50, 84). In skeletal muscle, large mtDNA deletions were also observed, recapitulating features of mitochondrial diseases (50). Similarly, EcoRI was targeted to mitochondria but instead of depletion or recombination it caused complete mtDNA loss (85). Total depletion of mtDNA was surprising given EcoRI has three recognition sites on the mtDNA whereas PstI has two; nevertheless, differences in the promoters of the constructs may have led to higher EcoRI expression levels and thus cutting of more mtDNA molecules within the cells. As expected for ρ0 cells, mitochondrial dysfunction and impaired proliferation rates were observed upon EcoRI targeting to the mitochondria (85). In more recent works, DSBs have been induced by mtZFN or mtTALENs in mitochondrial disease models to shift heteroplasmy levels. It was shown that by decreasing mutated versus WT mtDNA molecules, mitochondrial function was ameliorated and disease phenotypes were improved (86–88). Although not focusing on mtDNA damage per se, these studies support the notion that mtDNA integrity drives health outcomes.
From an mtDNA damage-driving health outcomes perspective, it would be interesting to use these approaches to determine whether long-term induction of DNA breaks solely on the mitochondrial genome can lead to disease outcomes in otherwise normal animals. Notably, accelerated aging was reported in animals in which mtDSBs were transiently induced in early adulthood (see mtDNA Damage and Epigenetic Changes), suggesting that damage to the mitochondrial genome may impact disease susceptibility, although the mechanisms and outcomes may be distinct based on length and age of exposure. The mitochondrial polγ mutator mice described ∼20 years ago has recently been shown to accrue a significant level of linear mtDNA, indicative of accumulation mtDSBs (89). Given the phenotype of the animals involved significant health defects in addition to premature aging (90), it seems that mtDNA breaks may indeed contribute to disease. Likewise, defining whether different tissues would have a distinct response to a sustained level of mtDNA breaks and the resulting mtDNA depletion could provide better understanding about the potential outcomes of exposures to mitochondrial toxicants. Finally, one could target endonucleases to the mitochondria early in development versus in young or late adulthood to interrogate whether induction of mtDNA damage at different life stages can lead to distinct health outcomes.
Effects of mtDNA Damage on Mitochondrial Transcription
One of the expected outcomes of lesions on mtDNA, whether persistent or not, would be an impact on transcription of mtDNA-encoded genes. Presumably, the presence of lesions on the mtDNA would block or slow down transcription of that genome by the mitochondrial RNA polymerase (POLRMT). Transcription-coupled repair (TCR) while active in the nucleus has not been shown to operate in mitochondria although Cockayne syndrome enzymes (CSA and CSB) that are associated with nuclear TCR have been shown to be present in mitochondria and impact their function—including transcription (91–93). Surprisingly, little is known about the outcomes of mtDNA damage to transcription, especially in vivo. The few available published studies were performed in vitro using purified POLRMT and lesion-bearing substrates, and overall showed that some types of damage were bypassed by the enzyme while others blocked transcription elongation. For example, DNA templates containing aldehyde adducts opposite a T or C significantly arrested POLRMT elongation complexes with some transcripts remaining associated with the enzyme after stalling at the adduct. This study also showed that the mtDNA strand bearing the lesion, i.e., the light or heavy strand may differently impact transcription (94), given most genes being transcribed from the heavy strand. Conversely, oxidative DNA damage, namely 8-oxo-G and thymine glycol, were bypassed by POLRMT although it paused at those lesions generating mutation-bearing transcripts. Like the aldehyde adducts, POLRMT was unable to bypass UV-induced DNA lesions [cyclobutane pyrimidine dimers and pyrimidine(6-4)pyrimidone photoproduct] and AP sites (95). Thus, at least in vitro, some oxidative lesions may not affect mitochondrial RNA transcription, while they could possibly induce mutations. More complex damage, however, is likely to affect transcription of mtDNA-encoded genes. However, additional studies are required to address these issues.
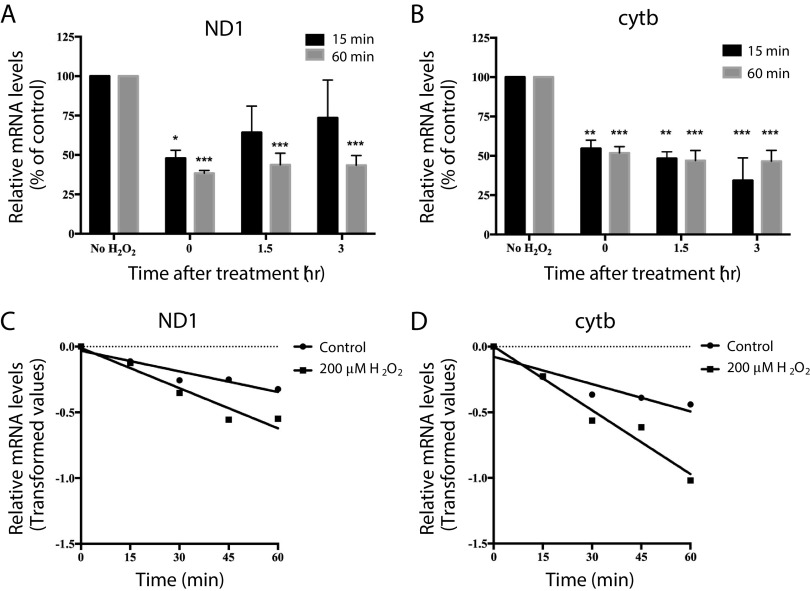
In trying to understand what happens to the transcription of mtDNA-encoded genes upon oxidative damage, we followed the expression levels of NADH:ubiquinone oxidoreductase core subunit I (ND1) and cyt b after treatment with 200 µM H2O2 for 15 or 60 min; conditions in which we had previously shown repairable or persistent damage to mtDNA (9). We found that levels of ND1 and cytb were dramatically decreased after both 15 and 60 min exposure to H2O2 relative to untreated controls (Fig. 3, A and B, time 0). Interestingly, expression of ND1 was restored to levels similar to untreated controls within 3 h of the short 15-min treatment, but not if cells were treated for 60 min (Fig. 3A, time 3). In contrast, levels of cytb did not returned to normal over time, irrespective of the initial length of H2O2 exposure (Fig. 3B). Given that mtDNA transcription gives rise to a polycistronic message (96), these differences are inconsistent with the reduction in transcripts simply reflecting changes in mtDNA copy number. Likewise, they do not seem fully explained simply by damaged mtDNA since H2O2 induces 8-oxo-G, which POLRMT seems to bypass at least in vitro (95), and the majority of damage generated by H2O2 are SSBs (97), which are blocking lesions. Unless there would be a preference from one type of damage in one gene versus the other, it is expected that damage induced by H2O2 on mtDNA would be randomly distributed. Given H2O2 is not specific to DNA, one explanation for our data would be that the RNA is also being oxidized (98), thereby decreasing its stability. To gain insights into this possibility, we measured steady-state levels of ND1 and cytb mRNAs in the presence of actinomycin D. Low doses of actinomycin D prevent transcription elongation of mtRNAs without affecting cytosolic transactions, thus providing an estimate of mtRNA stability (99). Exposure of cells to H2O2 in the presence of actinomycin D resulted in significant reduction of ND1 and cytb mRNA half-lives (Fig. 3, C and D), supporting the possibility that mtRNA (at the transcript or ribonucleotide level) is directly oxidized by H2O2. Although these experiments did not directly test mtRNA oxidation, they suggest that H2O2 exposure affected mtDNA transcription, at least partly, by altering mtRNA stability. Alternatively, damaged mtRNA might be destroyed by nonsense-mediated decay, which seems to be present in the organelle (100), whether the kinetics of decay would be different for these two genes is unclear. Additional experiments are required to tease out the contribution of mtDNA damage, oxidation of the ribonucleotide pool versus the mRNA as well as any potential effects of H2O2 directly on POLRMT.
Figure 3.
H2O2 causes a reduction of NADH:ubiquinone oxidoreductase core subunit I (ND1) and cytb mRNA levels and mRNA half-life in human fibroblasts. SV-40 transformed human fibroblasts were treated with 200 µM H2O2 for 15 or 60 min and harvested immediately after treatment (time 0), or allowed to recover for 1.5 and 3 h. Total RNA was isolated and analyzed by Northern blot. Signals are expressed as percentage of the controls after normalization to 18S ribosomal RNA; ND1 (A) and cytb (B). Data are presented as means ± SE of n = 3 independent biological replicates. Statistical analysis was performed by one-way ANOVA. *P < 0.05, **P < 0.01, and ***P < 0.001 versus control. Total RNA was isolated from cells cultured in the presence or absence of 200 µM H2O2 at 0, 15, 30, 45, and 60 min after incubation with 1 µg/mL actinomycin D. Linear regression analysis of the relative amounts of ND1 (C) and cytb (D) mRNA after normalization to 18S ribosomal RNA signal. Differences between the slopes are at the P < 0.05 (ND1) and P < 0.01 (cytb).
BEYOND MITOCHONDRIAL DYSFUNCTION: HOW mtDNA DAMAGE CAN AFFECT BROADER CELLULAR PHYSIOLOGY
Activation of Innate Immunity by mtDNA and mtRNA
DNA in the cytoplasm of cells is detected by the DNA sensing system that involves cyclic GMP-AMP synthase (cGAS). cGAS functions upstream of STING, stimulator of interferon genes, and normally is inactive. However, following binding to cytosolic DNA, cGAS undergoes a conformational change and becomes activated, producing the second messenger cyclic GMP-AMP (cGAMP) that in turn activates STING. Upon its activation, STING translocates to the Golgi apparatus where it stimulates TANK-binding kinase (TBK1). This cascade of events leads to a series of phosphorylation reactions that promote the dimerization of interferon regulatory factor 3 (IRF3), which translocates into the nucleus triggering the expression of interferon-stimulated genes (ISGs). Collectively, the gene products of ISG effectively resist and subdue infection by pathogens (101). Seminal work from the Shadel laboratory showed that endogenous mtDNA is able to leak into the cytosol activating cGAS and ISGs. When studying a mouse in which the levels of mitochondrial transcription factor A (TFAM) were reduced, West et al. showed activation of the cGAS-STING pathway, which resulted in IRF3-dependent expression of ISGs. Mechanistically, they found that aberrant mtDNA packaging by loss of TFAM promoted escape of mtDNA into the cytosol, which engaged cGAS. Notably, activation of this pathway by mtDNA potentiated type I interferon responses and conferred broad viral resistance. Overall, these observations suggested that mtDNA stress is a cell-intrinsic trigger of antiviral signaling that cooperates with canonical virus sensing mechanisms to fully engage antiviral innate immunity (102). Although the authors proposed that loss of TFAM led to altered nucleoid assembly, the recent data indicating that TFAM is required to process abasic sites during mtBER (103–105) raise the possibility that this cytosolic signaling was triggered by damaged mtDNA. It is possible that fragments of mtDNA, which would be more easily generated with strands bearing AP sites, were the ones that leaked. Nevertheless, it remains to be defined whether the mtDNA that leaks upon TFAM loss is damaged or not, whether the entire molecule is found in the cytosol, and the exact mechanism associated with the “export” of mtDNA molecules.
Subsequent work from the same group suggested that genotoxin-damaged mtDNA can effectively activate innate immunity. The authors showed that in cultured cells (both primary fibroblasts as well as cancer cells), treatment with the chemotherapeutic agent doxorubicin caused mtDNA damage and release, with consequent engagement of cGAS-STING and ISGs activation. Notably, TFAM-deficient mouse melanoma cells, which have innate immunity activated, produced tumors that were more resistant to doxorubicin in vivo. Similarly, TFAM+/− mice exposed to IR showed enhanced nuclear DNA repair in the spleen. These results led the authors to conclude that the release of damaged mtDNA elicited a protective signaling response that enhanced nuclear DNA repair in cells and tissues; this led to the proposal that mtDNA is a genotoxic stress sentinel (106). Further support for this hypothesis came from more recent work from Tigano et al. in which damage to mtDNA, specifically DSBs, was induced by mtTALENs. Surprisingly, however, it was not the mtDNA but rather mtRNA that accumulated in the cytosol and engaged the RNA-sensing protein RIG-I that ultimately led to expression of ISGs (20). Interestingly, the level of mtDSBs induced by mtTALENs was not sufficient to induce mitochondrial dysfunction, suggesting that subtle changes in mtDNA integrity suffice to trigger this cascade. How RNA leaked from the mitochondria when mtDNA DSBs were induced is unclear, but the authors proposed herniation of the inner membrane and across the outer mitochondrial membrane by the proapoptotic proteins BAX and BAK. Like the previous work (106), Tigano et al. (20) also showed that mtDNA breaks synergized with nuclear DNA damage caused by IR to mount a robust cellular immune response. Collectively, these studies suggest that cytoplasmic accumulation of mitochondrial nucleic acids are part of an intrinsic immune surveillance mechanism that help cells to cope with other stresses, including viral infection and nuclear DNA damage.
Mitophagy is emerging as an important quality control mechanism that is essential to prevent inflammation by virtue of damaged mitochondria clearance. Knockdown of N-glycanase 1 (Ngly1), a deglycosylation enzyme that plays a role in immune homeostasis, activates DNA- and RNA-sensing pathways (107). Interestingly, the absence of Ngly1 triggered mitochondrial dysfunction and leakage of both mtDNA and mtRNA into the cytoplasm of the cells, which was coupled to impaired mitophagy. Ethidium bromide depletion of mtDNA in control and Ngly1−/− cells decreased interferon-induced immune response, supporting a role for cytosolic mtDNA in the activation of cGAS-STING signaling pathway in Ngly1−/− cells. Similarly, Sliter et al. used mutator mice that carry mutations in polγ to show accumulation of high levels of point mutations with age leading to development of a severe inflammatory phenotype following exhaustive exercise. In the absence of parkin, the inflammatory features were completely rescued to control levels by loss of STING (108), indicating that damage on mtDNA was the key inflammatory signal. Thus, it seems clear that by failing to clear damaged mitochondria, innate immunity is activated. The question that remains to be addressed is if accumulation of damaged mitochondria by the absence of mitophagy triggers an innate immune response due to a higher probability of mtDNA leakage into the cytosol.
Finally, another means through which mitochondria and mtDNA may crosstalk with innate immunity is through the NLRP3 (nucleotide-binding oligomerization domain leucine-rich repeat and pyrin domain-containing protein 3) inflammasome. NLRP3 inflammasomes are multiprotein complexes of the innate immune system that recruit pro-caspase-1 via the adaptor molecule apoptosis-associated speck-like protein (ASC), ultimately leading to the maturation of the proinflammatory cytokines IL-1β and IL-18 (109). Oxidized mtDNA has been shown in different contexts to be involved/required for activation of the NLRP3 inflammasome (110, 111). Nevertheless, whether the mtDNA is oxidized in the mitochondria and then leaks to the cytosol or whether it is first released and subsequently oxidized outside of the organelle remains unclear. Moving forward, it would be interesting to define whether damage initiated in the mtDNA can trigger this innate immunity pathway, further contributing to the increasing area of physiological outcomes impacted by loss or decreased mtDNA integrity. A summary of data demonstrating the role of damaged mtDNA in innate immunity activation is presented in Fig. 4.
Figure 4.
Cross talk between mitochondrial DNA (mtDNA) and immune response activation. Inflammation can be triggered by several stimuli that include mitochondrial nucleic acids release or low levels of mitochondrial transcription factor A (TFAM). Specific sensing mechanisms engage different immune responses, like the nucleotide-binding oligomerization domain leucine-rich repeat and pyrin domain-containing protein 3 (NLRP3) inflammasomes and the type I interferon response.
MtDNA Damage and Epigenetic Changes
Epigenetics is an emerging area in which mitochondrial function and metabolism are being increasingly recognized. Because many epigenetic relevant metabolites (acetyl-CoA, α-ketoglutarate, succinate, and fumarate) are primarily generated by mitochondria through the tricarboxylic acid (TCA) cycle, an increasing body of work has linked mitochondrial function and metabolism to nuclear epigenetic maintenance and cellular/tissue outcomes (112). Given the allosteric control that the ETC exerts over the TCA cycle, it follows that mtDNA instability, including mtDNA damage, likely affects broader metabolism and epigenetics. To date, no studies have been published demonstrating, specifically, that damage to the mitochondrial genome influences the epigenome. Nevertheless, since there are data demonstrating that mtDNA instability does, presumably loss of mtDNA integrity through, for example, genotoxic insult also has the capacity to impact epigenetics. For example, loss of TFAM decreases mtDNA content and might, as described earlier, also increase the abundance of abasic sites on this genome (105). In mice, decreased levels of TFAM in hematopoietic stem/progenitor cells (HSPCs) led to histone hyperacetylation in the nucleus, altered gene expression programs, and impaired erythropoiesis (113). Similarly, progressive mtDNA depletion, by expression of a dominant negative mutated polγ, led to significant epigenetic (i.e., genome-wide DNA methylation and histone acetylation changes) and transcriptomic remodeling. Of interest, the epigenetic changes caused by mtDNA depletion initiated before detection of mitochondrial dysfunction, when mtDNA was depleted to ∼40% (19, 24). These data not only suggest that the epigenome is sensitive to modulation of mitochondrial metabolism before the detection of overt organellar dysfunction, but also raise the possibility that damage to mtDNA that leads to depletion of this genome (like single- or double-strand DNA breaks) and can influence the epigenetic landscape.
Most recently, we showed pervasive and long-lasting nuclear DNA methylation changes in the liver of mice exposed perinatally to rotenone (72). Rotenone is a mitochondrial complex I inhibitor that by virtue of increasing the production of superoxide damages mtDNA (114). The mechanisms through which rotenone exposure led to epigenetic remodeling in the liver remain unclear, but could involve mtDNA damage since rotenone exposure increased superoxide production (72). Similarly, increased heteroplasmic levels of a mutant mtDNA altered nuclear histone marks (115). In addition, a mouse model expressing a mutant polγ, known to cause mutations on the mtDNA, showed altered DNA methylation patterns in the heart that were associated with cardiomyopathy (116). Lastly, use of an inducible system to systemically express mtPstI in young adult mice (3 mo old) for 5 days has shown that induction of mtDNA DSBs leads to mtDNA depletion in liver, thymus, and lungs; in other tissues, a small fraction of mtDNA deletions were observed. Notably, as the animals aged, no defects in mtDNA integrity were observed at the molecular level but, phenotypically, earlier aging-like phenotypes were identified (117). Although the authors conclude that these later effects could be attributed to loss of progenitor cells pools early by the induction of mtDNA DSBs, it is possible that those initial changes in mtDNA integrity impacted the nuclear epigenome and contributed to these late phenotypes.
Collectively, these data indirectly suggest that changes in mtDNA integrity/stability have the potential to remodel the epigenome, influencing transcription and health outcomes beyond the role of mitochondria in bioenergetics or redox homeostasis. Moving forward, it will be important to directly test the extent to which damage present on the mtDNA can initiate these events. This would be particularly relevant in the context of cancer, given the use of DNA damaging agents for therapeutic purposes and the long-term health outcomes associated with chemotherapy exposure in cancer survivors.
FINAL THOUGHTS AND PROSPECTS
It is clear based on the data discussed that the field has come a long way in understanding the relevance of mtDNA damage as a primary event to organellar and cellular function. Conceptually, different types of mtDNA lesions may also broadly affect organismal physiology (i.e., by engaging immunity or epigenetics) without causing mitochondrial dysfunction, but this remains poorly understood. The fact that cells and tissues are provided with many copies of the mtDNA may suggest that some genomes (and thus their integrity) may not be needed to maintain bioenergetics. The recent data on the role of mtDNA/RNA on triggering innate immunity supports this notion. Based on the data available, one common outcome of mtDNA damage is the loss of mitochondrial genomes. Thus, a fundamental question that remains unaddressed is whether mitochondrial dysfunction, when detectable after different types of mtDNA damage, is simply an indirect response to loss of that DNA. While this is possible, data from polγ mutator mice (90) suggest otherwise since mtDNA depletion is not a feature of that model. In the nucleus, the presence of different repair machineries and enzymatic activities that can compete for a lesion can—in effect—alter outcomes. For example, a DSB processed by nonhomologous end-joining will lead to different genomic results (and cellular phenotypes) than that handled by homologous recombination. As in the mitochondria DNA repair pathways are limited, it is possible that the various lesions that occur on the mitochondrial genome that are not substrates of mtBER essentially lead to the same effect: mtDNA depletion. Alternatively, the ability and kinetics of mtDNA damage processing, i.e., repair versus degradation or recombination, may provide windows in which physiological outcomes are distinct. Finally, whether mitochondria derived from different cell types and tissues react in a uniform fashion to the same type of mtDNA damage remains poorly understood. Clearly, more studies are required to address these points.
In broader physiological settings and disease contexts, common diseases such as cancer, diabetes, and neurodegenerative disorders along with the normal process of aging are characterized by mitochondrial dysfunction. Many of them also present with damaged mtDNA. Although it is unlikely that mtDNA instability is causative in their genesis, it is feasible to assume that loss of mtDNA integrity at least plays a role in disease phenotypes. Notably, DNA repair syndromes that count with nuclear genomic instability have increasingly identified mitochondrial dysfunction as a component of the disease, in many cases driving phenotypes that cannot be explained by the nuclear DNA repair defects (118–121). Thus, it seems that mtDNA damage in complex diseases is not just a simple bystander but an active contributor to altered cellular/tissue physiology. Finally, understanding the role of mtDNA damage/instability in triggering innate immunity and altered epigenetics to influence long-term health outcomes deserves attention. To date, a broad range of autoimmune and inflammatory diseases, including Aicardi-Goutières syndrome and systemic lupus erythematosus, have been associated to DNA damage and deficiency in cytosolic nucleic acid metabolizing enzymes. It will be interesting to define whether mtDNA or mtRNAs can contribute to these diseases. Clearly, the future holds exciting opportunities and challenges, many of which can now be tackled with the novel tools and strategies that can target the mitochondrial genome as means to initiate cellular outcomes and disease phenotypes.
GRANTS
This work was partially supported through intramural research support of the Division, National Toxicology Program at NIEHS, Z99-ES999999 (to J. H. Santos) and Grant 5SC1NS095380 (to S. Ayala-Peña from the Huntington Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.A.N. and S.A.-P. prepared figures; C.A.N. and J.H.S. drafted manuscript; C.A.N., S.A.-P., and J.H.S. edited and revised manuscript; C.A.N., S.A.-P., and J.H.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. William Copeland and Bruce Alex Merrick (NIEHS) as well as Gyorgy Hajnoczky (Thomas Jeffersion University) for critical comments on the manuscript.
REFERENCES
- 1.Koopman WJH, Distelmaier F, Smeitink JA, Willems PH. OXPHOS mutations and neurodegeneration. EMBO J 32: 9–29, 2013. doi: 10.1038/emboj.2012.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Signes A, Fernandez-Vizarra E. Assembly of mammalian oxidative phosphorylation complexes I–V and supercomplexes. Essays Biochem 62: 255–270, 2018. doi: 10.1042/EBC20170098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chou J-Y, Leu J-Y. The Red Queen in mitochondria: cyto-nuclear co-evolution, hybrid breakdown and human disease. Front Genet 6: 187–187, 2015. doi: 10.3389/fgene.2015.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohr VA, Chu EH, van Duin M, Hanawalt PC, Okumoto DS. Human repair gene restores normal pattern of preferential DNA repair in repair defective CHO cells. Nucleic Acids Res 16: 7397–7403, 1988. doi: 10.1093/nar/16.15.7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clayton DA, Doda JN, Friedberg EC. The absence of a pyrimidine dimer repair mechanism in mammalian mitochondria. Proc Natl Acad Sci USA 71: 2777–2781, 1974. doi: 10.1073/pnas.71.7.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeDoux SP, Driggers WJ, Hollensworth BS, Wilson GL. Repair of alkylation and oxidative damage in mitochondrial DNA. Mutat Res 434: 149–159, 1999. doi: 10.1016/S0921-8777(99)00026-9. [DOI] [PubMed] [Google Scholar]
- 7.LeDoux SP, Wilson GL, Beecham EJ, Stevnsner T, Wassermann K, Bohr VA. Repair of mitochondrial DNA after various types of DNA damage in Chinese hamster ovary cells. Carcinogenesis 13: 1967–1973, 1992. doi: 10.1093/carcin/13.11.1967. [DOI] [PubMed] [Google Scholar]
- 8.Pettepher CC, LeDoux SP, Bohr VA, Wilson GL. Repair of alkali-labile sites within the mitochondrial DNA of RINr 38 cells after exposure to the nitrosourea streptozotocin. J Biol Chem 266: 3113–3117, 1991. doi: 10.1016/S0021-9258(18)49961-9. [DOI] [PubMed] [Google Scholar]
- 9.Santos JH, Hunakova L, Chen Y, Bortner C, Van Houten B. Cell sorting experiments link persistent mitochondrial DNA damage with loss of mitochondrial membrane potential and apoptotic cell death. J Biol Chem 278: 1728–1734, 2003. doi: 10.1074/jbc.M208752200. [DOI] [PubMed] [Google Scholar]
- 10.Santos JH, Meyer JN, Skorvaga M, Annab LA, Van Houten B. Mitochondrial hTERT exacerbates free-radical-mediated mtDNA damage. Aging Cell 3: 399–411, 2004. doi: 10.1111/j.1474-9728.2004.00124.x. [DOI] [PubMed] [Google Scholar]
- 11.Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci USA 94: 514–519, 1997. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandavilli BS, Santos JH, Van Houten B. Mitochondrial DNA repair and aging. Mutat Res 509: 127–151, 2002. doi: 10.1016/s0027-5107(02)00220-8. [DOI] [PubMed] [Google Scholar]
- 13.Meyer JN, Leung MC, Rooney JP, Sendoel A, Hengartner MO, Kisby GE, Bess AS. Mitochondria as a target of environmental toxicants. Toxicol Sci 134: 1–17, 2013. doi: 10.1093/toxsci/kft102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kazak L, Reyes A, Holt IJ. Minimizing the damage: repair pathways keep mitochondrial DNA intact. Nat Rev Mol Cell Biol 13: 659–671, 2012. [Erratum in Nat Rev Mol Cell Biol 13: 726, 2012]. doi: 10.1038/nrm3439. [DOI] [PubMed] [Google Scholar]
- 15.Lakshmipathy U, Campbell C. Double strand break rejoining by mammalian mitochondrial extracts. Nucleic Acids Res 27: 1198–1204, 1999. doi: 10.1093/nar/27.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Houten B, Hunter SE, Meyer JN. Mitochondrial DNA damage induced autophagy, cell death, and disease. Front Biosci (Landmark Ed) 21: 42–54, 2016. doi: 10.2741/4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geden MJ, Romero SE, Deshmukh M. p53 is required for nuclear but not mitochondrial DNA damage-induced degeneration. Cell Death Dis 12: 104, 2021. doi: 10.1038/s41419-020-03373-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stuart JA, Hashiguchi K, Wilson DM 3rd, Copeland WC, Souza-Pinto NC, Bohr VA. DNA base excision repair activities and pathway function in mitochondrial and cellular lysates from cells lacking mitochondrial DNA. Nucleic Acids Res 32: 2181–2192, 2004. doi: 10.1093/nar/gkh533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lozoya OA, Martinez-Reyes I, Wang T, Grenet D, Bushel P, Li J, Chandel N, Woychik RP, Santos JH. Mitochondrial nicotinamide adenine dinucleotide reduced (NADH) oxidation links the tricarboxylic acid (TCA) cycle with methionine metabolism and nuclear DNA methylation. PLOS Biol 16: e2005707, 2018. doi: 10.1371/journal.pbio.2005707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tigano M, Vargas DC, Tremblay-Belzile S, Fu Y, Sfeir A. Nuclear sensing of breaks in mitochondrial DNA enhances immune surveillance. Nature 591: 477–481, 2021. doi: 10.1038/s41586-021-03269-w. [DOI] [PubMed] [Google Scholar]
- 21.Bao XR, Ong S-E, Goldberger O, Peng J, Sharma R, Thompson DA, Vafai SB, Cox AG, Marutani E, Ichinose F, Goessling W, Regev A, Carr SA, Clish CB, Mootha VK. Mitochondrial dysfunction remodels one-carbon metabolism in human cells. eLife 5: e10575, 2016. doi: 10.7554/eLife.10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Q, Kirk K, Shurubor YI, Zhao D, Arreguin AJ, Shahi I, Valsecchi F, Primiano G, Calder EL, Carelli V, Denton TT, Beal MF, Gross SS, Manfredi G, D'Aurelio M. Rewiring of glutamine metabolism is a bioenergetic adaptation of human cells with mitochondrial DNA mutations. Cell Metab 27: 1007–1025.e5, 2018. doi: 10.1016/j.cmet.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikkanen J, Forsström S, Euro L, Paetau I, Kohnz RA, Wang L, Chilov D, Viinamäki J, Roivainen A, Marjamäki P, Liljenbäck H, Ahola S, Buzkova J, Terzioglu M, Khan NA, Pirnes-Karhu S, Paetau A, Lönnqvist T, Sajantila A, Isohanni P, Tyynismaa H, Nomura DK, Battersby BJ, Velagapudi V, Carroll CJ, Suomalainen A. Mitochondrial DNA replication defects disturb cellular dNTP pools and remodel one-carbon metabolism. Cell Metab 23: 635–648, 2016. doi: 10.1016/j.cmet.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Lozoya OA, Wang T, Grenet D, Wolfgang TC, Sobhany M, Ganini da Silva D, Riadi G, Chandel N, Woychik RP, Santos JH. Mitochondrial acetyl-CoA reversibly regulates locus-specific histone acetylation and gene expression. Life Sci Alliance 2: e201800228, 2019. doi: 10.26508/lsa.201800228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexeyev M, Shokolenko I, Wilson G, LeDoux S. The maintenance of mitochondrial DNA integrity–critical analysis and update. Cold Spring Harb Perspect Biol 5: a012641, 2013. doi: 10.1101/cshperspect.a012641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cline SD. Mitochondrial DNA damage and its consequences for mitochondrial gene expression. Biochim Biophys Acta 1819: 979–991, 2012. doi: 10.1016/j.bbagrm.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Copeland WC, Longley MJ. Mitochondrial genome maintenance in health and disease. DNA Repair (Amst) 19: 190–198, 2014. doi: 10.1016/j.dnarep.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dahal S, Raghavan SC. Mitochondrial genome stability in human: understanding the role of DNA repair pathways. Biochem J 478: 1179–1197, 2021. doi: 10.1042/BCJ20200920. [DOI] [PubMed] [Google Scholar]
- 29.Rong Z, Tu P, Xu P, Sun Y, Yu F, Tu N, Guo L, Yang Y. The mitochondrial response to DNA damage. Front Cell Dev Biol 9: 669379, 2021. doi: 10.3389/fcell.2021.669379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saki M, Prakash A. DNA damage related crosstalk between the nucleus and mitochondria. Free Radic Biol Med 107: 216–227, 2017. doi: 10.1016/j.freeradbiomed.2016.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Houten B, Woshner V, Santos JH. Role of mitochondrial DNA in toxic responses to oxidative stress. DNA Repair (Amst) 5: 145–152, 2006. doi: 10.1016/j.dnarep.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Formosa LE, Ryan MT. Mitochondrial OXPHOS complex assembly lines. Nat Cell Biol 20: 511–513, 2018. doi: 10.1038/s41556-018-0098-z. [DOI] [PubMed] [Google Scholar]
- 33.Nass MMK, Nass S. Intramitochondrial fibers with DNA characteristics. I. Fixation and electron staining reactions. J Cell Biol 19: 593–611, 1963. doi: 10.1083/jcb.19.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falkenberg M, Gustafsson CM. Mammalian mitochondrial DNA replication and mechanisms of deletion formation. Crit Rev Biochem Mol Biol 55: 509–524, 2020. doi: 10.1080/10409238.2020.1818684. [DOI] [PubMed] [Google Scholar]
- 35.Yasukawa T, Kang D. An overview of mammalian mitochondrial DNA replication mechanisms. J Biochem 164: 183–193, 2018. doi: 10.1093/jb/mvy058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graziewicz MA, Longley MJ, Copeland WC. DNA polymerase gamma in mitochondrial DNA replication and repair. Chem Rev 106: 383–405, 2006. doi: 10.1021/cr040463d. [DOI] [PubMed] [Google Scholar]
- 37.Longley MJ, Prasad R, Srivastava DK, Wilson SH, Copeland WC. Identification of 5′-deoxyribose phosphate lyase activity in human DNA polymerase γ and its role in mitochondrial base excision repair in vitro. Proc Natl Acad Sci USA 95: 12244–12248, 1998. doi: 10.1073/pnas.95.21.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Longley MJ, Ropp PA, Lim SE, Copeland WC. Characterization of the native and recombinant catalytic subunit of human DNA polymerase γ: identification of residues critical for exonuclease activity and dideoxynucleotide sensitivity. Biochemistry 37: 10529–10539, 1998. doi: 10.1021/bi980772w. [DOI] [PubMed] [Google Scholar]
- 39.Baptiste BA, Baringer SL, Kulikowicz T, Sommers JA, Croteau DL, Brosh RM Jr, Bohr VA. DNA polymerase β outperforms DNA polymerase γ in key mitochondrial base excision repair activities. DNA Repair (Amst) 99: 103050, 2021. doi: 10.1016/j.dnarep.2021.103050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prasad R, Çağlayan M, Dai D-P, Nadalutti CA, Zhao M-L, Gassman NR, Janoshazi AK, Stefanick DF, Horton JK, Krasich R, Longley MJ, Copeland WC, Griffith JD, Wilson SH. DNA polymerase β: a missing link of the base excision repair machinery in mammalian mitochondria. DNA Repair (Amst) 60: 77–88, 2017. doi: 10.1016/j.dnarep.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sykora P, Kanno S, Akbari M, Kulikowicz T, Baptiste BA, Leandro GS, Lu H, Tian J, May A, Becker KA, Croteau DL, Wilson DM 3rd, Sobol RW, Yasui A, Bohr VA. DNA polymerase beta participates in mitochondrial DNA repair. Mol Cell Biol 37: e00237-17, 2017. doi: 10.1128/MCB.00237-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hegde ML, Hazra TK, Mitra S. Early steps in the DNA base excision/single-strand interruption repair pathway in mammalian cells. Cell Res 18: 27–47, 2008. doi: 10.1038/cr.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krokan HE, Bjørås M. Base excision repair. Cold Spring Harb Perspect Biol 5: a012583, 2013. doi: 10.1101/cshperspect.a012583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prakash A, Doublié S. Base excision repair in the mitochondria. J Cell Biochem 116: 1490–1499, 2015. doi: 10.1002/jcb.25103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prakash L. Repair of pyrimidine dimers in nuclear and mitochondrial DNA of yeast irradiated with low doses of ultraviolet light. J Mol Biol 98: 781–795, 1975. doi: 10.1016/s0022-2836(75)80010-6. [DOI] [PubMed] [Google Scholar]
- 46.Bess AS, Leung MCK, Ryde IT, Rooney JP, Hinton DE, Meyer JN. Effects of mutations in mitochondrial dynamics-related genes on the mitochondrial response to ultraviolet C radiation in developing Caenorhabditis elegans. Worm 2: e23763, 2013. doi: 10.4161/worm.23763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin SM, Youle RJ. PINK1- and Parkin-mediated mitophagy at a glance. J Cell Sci 125: 795–799, 2012. doi: 10.1242/jcs.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bacman SR, Williams SL, Moraes CT. Intra- and inter-molecular recombination of mitochondrial DNA after in vivo induction of multiple double-strand breaks. Nucleic Acids Res 37: 4218–4226, 2009. doi: 10.1093/nar/gkp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moretton A, Morel F, Macao B, Lachaume P, Ishak L, Lefebvre M, Garreau-Balandier I, Vernet P, Falkenberg M, Farge G. Selective mitochondrial DNA degradation following double-strand breaks. PLoS One 12: e0176795, 2017. doi: 10.1371/journal.pone.0176795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Srivastava S, Moraes CT. Double-strand breaks of mouse muscle mtDNA promote large deletions similar to multiple mtDNA deletions in humans. Hum Mol Genet 14: 893–902, 2005. doi: 10.1093/hmg/ddi082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nissanka N, Bacman SR, Plastini MJ, Moraes CT. The mitochondrial DNA polymerase gamma degrades linear DNA fragments precluding the formation of deletions. Nat Commun 9: 2491, 2018. doi: 10.1038/s41467-018-04895-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peeva V, Blei D, Trombly G, Corsi S, Szukszto MJ, Rebelo-Guiomar P, Gammage PA, Kudin AP, Becker C, Altmüller J, Minczuk M, Zsurka G, Kunz WS. Linear mitochondrial DNA is rapidly degraded by components of the replication machinery. Nat Commun 9: 1727, 2018. doi: 10.1038/s41467-018-04131-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao L. Mitochondrial DNA degradation: a quality control measure for mitochondrial genome maintenance and stress response. Enzymes 45: 311–341, 2019. doi: 10.1016/bs.enz.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sen D, Nandakumar D, Tang GQ, Patel SS. Human mitochondrial DNA helicase TWINKLE is both an unwinding and annealing helicase. J Biol Chem 287: 14545–14556, 2012. doi: 10.1074/jbc.M111.309468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sen D, Patel G, Patel SS. Homologous DNA strand exchange activity of the human mitochondrial DNA helicase TWINKLE. Nucleic Acids Res 44: 4200–4210, 2016. doi: 10.1093/nar/gkw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ciesielski GL, Nadalutti CA, Oliveira MT, Jacobs HT, Griffith JD, Kaguni LS. Structural rearrangements in the mitochondrial genome of Drosophila melanogaster induced by elevated levels of the replicative DNA helicase. Nucleic Acids Res 46: 3034–3046, 2018. doi: 10.1093/nar/gky094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pohjoismäki JL, Goffart S, Spelbrink JN. Replication stalling by catalytically impaired Twinkle induces mitochondrial DNA rearrangements in cultured cells. Mitochondrion 11: 630–634, 2011. doi: 10.1016/j.mito.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 58.Bruner SD, Norman DP, Verdine GL. Structural basis for recognition and repair of the endogenous mutagen 8-oxoguanine in DNA. Nature 403: 859–866, 2000. doi: 10.1038/35002510. [DOI] [PubMed] [Google Scholar]
- 59.Li M, Rothwell R, Vermaat M, Wachsmuth M, Schröder R, Laros JFJ, van Oven M, de Bakker PIW, Bovenberg JA, van Duijn CM, van Ommen G-JB, Slagboom PE, Swertz MA, Wijmenga C; Genome of Netherlands Consortium, Kayser M, Boomsma DI, Zöllner S, de Knijff P, Stoneking M. Transmission of human mtDNA heteroplasmy in the Genome of the Netherlands families: support for a variable-size bottleneck. Genome Res 26: 417–426, 2016. doi: 10.1101/gr.203216.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nissanka N, Moraes CT. Mitochondrial DNA heteroplasmy in disease and targeted nuclease-based therapeutic approaches. EMBO Rep 21: e49612, 2020. doi: 10.15252/embr.201949612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van den Ameele J, Li AYZ, Ma H, Chinnery PF. Mitochondrial heteroplasmy beyond the oocyte bottleneck. Semin Cell Dev Biol 97: 156–166, 2020. doi: 10.1016/j.semcdb.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 62.Wallace DC, Chalkia D. Mitochondrial DNA genetics and the heteroplasmy conundrum in evolution and disease. Cold Spring Harb Perspect Biol 5: a021220, 2013. doi: 10.1101/cshperspect.a021220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gustafson MA, Sullivan ED, Copeland WC. Consequences of compromised mitochondrial genome integrity. DNA Repair (Amst) 93: 102916, 2020. doi: 10.1016/j.dnarep.2020.102916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Furda AM, Marrangoni AM, Lokshin A, Van Houten B. Oxidants and not alkylating agents induce rapid mtDNA loss and mitochondrial dysfunction. DNA Repair (Amst) 11: 684–692, 2012. doi: 10.1016/j.dnarep.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou H, Hong M, Chai Y, Hei TK. Consequences of cytoplasmic irradiation: studies from microbeam. J Radiat Res 50, Suppl A: A59–A65, 2009. doi: 10.1269/jrr.08120s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tartier L, Gilchrist S, Burdak-Rothkamm S, Folkard M, Prise KM. Cytoplasmic irradiation induces mitochondrial-dependent 53BP1 protein relocalization in irradiated and bystander cells. Cancer Res 67: 5872–5879, 2007. doi: 10.1158/0008-5472.CAN-07-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang B, Davidson MM, Hei TK. Mitochondria regulate DNA damage and genomic instability induced by high LET radiation. Life Sci Space Res (Amst) 1: 80–88, 2014. doi: 10.1016/j.lssr.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang B, Davidson MM, Zhou H, Wang C, Walker WF, Hei TK. Cytoplasmic irradiation results in mitochondrial dysfunction and DRP1-dependent mitochondrial fission. Cancer Res 73: 6700–6710, 2013. doi: 10.1158/0008-5472.CAN-13-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Agnez-Lima LF, Melo JT, Silva AE, Oliveira AH, Timoteo AR, Lima-Bessa KM, Martinez GR, Medeiros MH, Di Mascio P, Galhardo RS, Menck CF. DNA damage by singlet oxygen and cellular protective mechanisms. Mutat Res Rev Mutat Res 751: 15–28, 2012. doi: 10.1016/j.mrrev.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 70.Qian W, Kumar N, Roginskaya V, Fouquerel E, Opresko PL, Shiva S, Watkins SC, Kolodieznyi D, Bruchez MP, Van Houten B. Chemoptogenetic damage to mitochondria causes rapid telomere dysfunction. Proc Natl Acad Sci USA 116: 18435–18444, 2019. doi: 10.1073/pnas.1910574116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li T, Mizrahi D, Goldstein D, Kiernan MC, Park SB. Chemotherapy and peripheral neuropathy. Neurol Sci 42: 4109–4121, 2021. doi: 10.1007/s10072-021-05576-6. [DOI] [PubMed] [Google Scholar]
- 72.Lozoya OA, Xu F, Grenet D, Wang T, Grimm SA, Godfrey V, Waidyanatha S, Woychik RP, Santos JH. Single nucleotide resolution analysis reveals pervasive, long-lasting DNA methylation changes by developmental exposure to a mitochondrial toxicant. Cell Rep 32: 108131, 2020. doi: 10.1016/j.celrep.2020.108131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Puebla-Osorio N, Lacey DB, Alt FW, Zhu C. Early embryonic lethality due to targeted inactivation of DNA ligase III. Mol Cell Biol 26: 3935–3941, 2006. doi: 10.1128/MCB.26.10.3935-3941.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simsek D, Furda A, Gao Y, Artus J, Brunet E, Hadjantonakis A-K, Houten B, Shuman S, McKinnon P, Jasin M. Crucial role for DNA ligase III in mitochondria but not in XRCC1-dependent repair. Nature 471: 245–248, 2011. doi: 10.1038/nature09794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gao Y, Katyal S, Lee Y, Zhao J, Rehg JE, Russell HR, McKinnon PJ. DNA ligase III is critical for mtDNA integrity but not Xrcc1-mediated nuclear DNA repair. Nature 471: 240–244, 2011. doi: 10.1038/nature09773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sharma NK, Lebedeva M, Thomas T, Kovalenko OA, Stumpf JD, Shadel GS, Santos JH. Intrinsic mitochondrial DNA repair defects in Ataxia Telangiectasia. DNA Repair (Amst) 13: 22–31, 2014. doi: 10.1016/j.dnarep.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Canugovi C, Misiak M, Ferrarelli LK, Croteau DL, Bohr VA. The role of DNA repair in brain related disease pathology. DNA Repair (Amst) 12: 578–587, 2013. doi: 10.1016/j.dnarep.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bonora E, Chakrabarty S, Kellaris G, Tsutsumi M, Bianco F, Bergamini C, et al. Biallelic variants in LIG3 cause a novel mitochondrial neurogastrointestinal encephalomyopathy. Brain 144: 1451–1466, 2021. doi: 10.1093/brain/awab056. [DOI] [PubMed] [Google Scholar]
- 79.Sallmyr A, Matsumoto Y, Roginskaya V, Van Houten B, Tomkinson AE. Inhibiting mitochondrial DNA ligase IIIα activates caspase 1-dependent apoptosis in cancer cells. Cancer Res 76: 5431–5441, 2016. doi: 10.1158/0008-5472.CAN-15-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tann AW, Boldogh I, Meiss G, Qian W, Van Houten B, Mitra S, Szczesny B. Apoptosis induced by persistent single-strand breaks in mitochondrial genome: critical role of EXOG (5'-EXO/endonuclease) in their repair. J Biol Chem 286: 31975–31983, 2011. doi: 10.1074/jbc.M110.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shokolenko IN, Wilson GL, Alexeyev MF. Persistent damage induces mitochondrial DNA degradation. DNA Repair (Amst) 12: 488–499, 2013. doi: 10.1016/j.dnarep.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buchet K, Godinot C. Functional F1-ATPase essential in maintaining growth and membrane potential of human mitochondrial DNA-depleted rho degrees cells. J Biol Chem 273: 22983–22989, 1998. doi: 10.1074/jbc.273.36.22983. [DOI] [PubMed] [Google Scholar]
- 83.Martínez-Reyes I, Diebold LP, Kong H, Schieber M, Huang H, Hensley CT, Mehta MM, Wang T, Santos JH, Woychik R, Dufour E, Spelbrink JN, Weinberg SE, Zhao Y, DeBerardinis RJ, Chandel NS. TCA cycle and mitochondrial membrane potential are necessary for diverse biological functions. Mol Cell 61: 199–209, 2016. doi: 10.1016/j.molcel.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Srivastava S, Moraes CT. Manipulating mitochondrial DNA heteroplasmy by a mitochondrially targeted restriction endonuclease. Hum Mol Genet 10: 3093–3099, 2001. doi: 10.1093/hmg/10.26.3093. [DOI] [PubMed] [Google Scholar]
- 85.Kukat A, Kukat C, Brocher J, Schäfer I, Krohne G, Trounce IA, Villani G, Seibel P. Generation of rho0 cells utilizing a mitochondrially targeted restriction endonuclease and comparative analyses. Nucleic Acids Res 36: e44, 2008. doi: 10.1093/nar/gkn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bacman SR, Kauppila JHK, Pereira CV, Nissanka N, Miranda M, Pinto M, Williams SL, Larsson N-G, Stewart JB, Moraes CT. MitoTALEN reduces mutant mtDNA load and restores tRNAAla levels in a mouse model of heteroplasmic mtDNA mutation. Nat Med 24: 1696–1700, 2018. [Erratum in Nat Med 24: 1940, 2018]. doi: 10.1038/s41591-018-0166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gammage PA, Viscomi C, Simard M-L, Costa ASH, Gaude E, Powell CA, Van Haute L, McCann BJ, Rebelo-Guiomar P, Cerutti R, Zhang L, Rebar EJ, Zeviani M, Frezza C, Stewart JB, Minczuk M. Genome editing in mitochondria corrects a pathogenic mtDNA mutation in vivo. Nat Med 24: 1691–1695, 2018. doi: 10.1038/s41591-018-0165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jackson CB, Turnbull DM, Minczuk M, Gammage PA. Therapeutic manipulation of mtDNA heteroplasmy: a shifting perspective. Trends Mol Med 26: 698–709, 2020. doi: 10.1016/j.molmed.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 89.Macao B, Uhler JP, Siibak T, Zhu X, Shi Y, Sheng W, Olsson M, Stewart JB, Gustafsson CM, Falkenberg M. The exonuclease activity of DNA polymerase γ is required for ligation during mitochondrial DNA replication. Nat Commun 6: 7303, 2015. doi: 10.1038/ncomms8303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly-Y M, Gidlöf S, Oldfors A, Wibom R, Törnell J, Jacobs HT, Larsson N-G. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429: 417–423, 2004. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 91.Berquist BR, Canugovi C, Sykora P, Wilson DM 3rd, Bohr VA. Human Cockayne syndrome B protein reciprocally communicates with mitochondrial proteins and promotes transcriptional elongation. Nucleic Acids Res 40: 8392–8405, 2012. doi: 10.1093/nar/gks565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Okur MN, Fang EF, Fivenson EM, Tiwari V, Croteau DL, Bohr VA. Cockayne syndrome proteins CSA and CSB maintain mitochondrial homeostasis through NAD+ signaling. Aging Cell 19: e13268, 2020. doi: 10.1111/acel.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scheibye-Knudsen M, Croteau DL, Bohr VA. Mitochondrial deficiency in Cockayne syndrome. Mech Ageing Dev 134: 275–283, 2013. doi: 10.1016/j.mad.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cline SD, Lodeiro MF, Marnett LJ, Cameron CE, Arnold JJ. Arrest of human mitochondrial RNA polymerase transcription by the biological aldehyde adduct of DNA, M1dG. Nucleic Acids Res 38: 7546–7557, 2010. doi: 10.1093/nar/gkq656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nakanishi N, Fukuoh A, Kang D, Iwai S, Kuraoka I. Effects of DNA lesions on the transcription reaction of mitochondrial RNA polymerase: implications for bypass RNA synthesis on oxidative DNA lesions. Mutagenesis 28: 117–123, 2013. doi: 10.1093/mutage/ges060. [DOI] [PubMed] [Google Scholar]
- 96.Bonawitz ND, Clayton DA, Shadel GS. Initiation and beyond: multiple functions of the human mitochondrial transcription machinery. Mol Cell 24: 813–825, 2006. doi: 10.1016/j.molcel.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 97.Shokolenko I, Venediktova N, Bochkareva A, Wilson GL, Alexeyev MF. Oxidative stress induces degradation of mitochondrial DNA. Nucleic Acids Res 37: 2539–2548, 2009. doi: 10.1093/nar/gkp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hofer T, Badouard C, Bajak E, Ravanat JL, Mattsson A, Cotgreave IA. Hydrogen peroxide causes greater oxidation in cellular RNA than in DNA. Biol Chem 386: 333–337, 2005. doi: 10.1515/BC.2005.040. [DOI] [PubMed] [Google Scholar]
- 99.González-Cadavid NF, Pérez JL. Identification of the products of mitochondrial transcription in the walker corcinosarcoma by the use of actinomycin D and ethidium bromide. Cancer Res 36: 1754–1760, 1976. [PubMed] [Google Scholar]
- 100.Ruzzenente B, Assouline Z, Barcia G, Rio M, Boddaert N, Munnich A, Rötig A, Metodiev MD. Inhibition of mitochondrial translation in fibroblasts from a patient expressing the KARS p.(Pro228Leu) variant and presenting with sensorineural deafness, developmental delay, and lactic acidosis. Hum Mutat 39: 2047–2059, 2018. doi: 10.1002/humu.23657. [DOI] [PubMed] [Google Scholar]
- 101.Schneider WM, Chevillotte MD, Rice CM. Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol 32: 513–545, 2014. doi: 10.1146/annurev-immunol-032713-120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, Bestwick M, Duguay BA, Raimundo N, MacDuff DA, Kaech SM, Smiley JR, Means RE, Iwasaki A, Shadel GS. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520: 553–557, 2015. doi: 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Canugovi C, Maynard S, Bayne AC, Sykora P, Tian J, de Souza-Pinto NC, Croteau DL, Bohr VA. The mitochondrial transcription factor A functions in mitochondrial base excision repair. DNA Repair (Amst) 9: 1080–1089, 2010. doi: 10.1016/j.dnarep.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chew K, Zhao L. Interactions of mitochondrial transcription factor A with DNA damage: mechanistic insights and functional implications. Genes (Basel) 12: 1246, 2021. doi: 10.3390/genes12081246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu W, Boyd RM, Tree MO, Samkari F, Zhao L. Mitochondrial transcription factor A promotes DNA strand cleavage at abasic sites. Proc Natl Acad Sci USA 116: 17792–17799, 2019. doi: 10.1073/pnas.1911252116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu Z, Oeck S, West AP, Mangalhara KC, Sainz AG, Newman LE, Zhang XO, Wu L, Yan Q, Bosenberg M, Liu Y, Sulkowski PL, Tripple V, Kaech SM, Glazer PM, Shadel GS. Mitochondrial DNA stress signalling protects the nuclear genome. Nat Metab 1: 1209–1218, 2019. doi: 10.1038/s42255-019-0150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang K, Huang R, Fujihira H, Suzuki T, Yan N. N-glycanase NGLY1 regulates mitochondrial homeostasis and inflammation through NRF1. J Exp Med 215: 2600–2616, 2018. doi: 10.1084/jem.20180783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sliter DA, Martinez J, Hao L, Chen X, Sun N, Fischer TD, Burman JL, Li Y, Zhang Z, Narendra DP, Cai H, Borsche M, Klein C, Youle RJ. Parkin and PINK1 mitigate STING-induced inflammation. Nature 561: 258–262, 2018. doi: 10.1038/s41586-018-0448-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 109.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440: 237–241, 2006. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 110.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, Arditi M. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36: 401–414, 2012. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhong Z, Liang S, Sanchez-Lopez E, He F, Shalapour S, Lin X-J, Wong J, Ding S, Seki E, Schnabl B, Hevener AL, Greenberg HB, Kisseleva T, Karin M. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 560: 198–203, 2018. doi: 10.1038/s41586-018-0372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Santos JH. Mitochondria signaling to the epigenome: a novel role for an old organelle. Free Radic Biol Med 170: 59–69, 2021. doi: 10.1016/j.freeradbiomed.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu X, Zhang Y, Ni M, Cao H, Signer RAJ, Li D, Li M, Gu Z, Hu Z, Dickerson KE, Weinberg SE, Chandel NS, DeBerardinis RJ, Zhou F, Shao Z, Xu J. Regulation of mitochondrial biogenesis in erythropoiesis by mTORC1-mediated protein translation. Nat Cell Biol 19: 626–638, 2017. doi: 10.1038/ncb3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sanders LH, McCoy J, Hu X, Mastroberardino PG, Dickinson BC, Chang CJ, Chu CT, Van Houten B, Greenamyre JT. Mitochondrial DNA damage: molecular marker of vulnerable nigral neurons in Parkinson’s disease. Neurobiol Dis 70: 214–223, 2014. doi: 10.1016/j.nbd.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kopinski PK, Janssen KA, Schaefer PM, Trefely S, Perry CE, Potluri P, Tintos-Hernandez JA, Singh LN, Karch KR, Campbell SL, Doan MT, Jiang H, Nissim I, Nakamaru-Ogiso E, Wellen KE, Snyder NW, Garcia BA, Wallace DC. Regulation of nuclear epigenome by mitochondrial DNA heteroplasmy. Proc Natl Acad Sci USA 116: 16028–16035, 2019. doi: 10.1073/pnas.1906896116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Koczor CA, Ludlow I, Fields E, Jiao Z, Ludaway T, Russ R, Lewis W. Mitochondrial polymerase gamma dysfunction and aging cause cardiac nuclear DNA methylation changes. Physiol Genomics 48: 274–280, 2016. doi: 10.1152/physiolgenomics.00099.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pinto M, Pickrell AM, Wang X, Bacman SR, Yu A, Hida A, Dillon LM, Morton PD, Malek TR, Williams SL, Moraes CT. Transient mitochondrial DNA double strand breaks in mice cause accelerated aging phenotypes in a ROS-dependent but p53/p21-independent manner. Cell Death Differ 24: 288–299, 2017. doi: 10.1038/cdd.2016.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.D'Errico M, Parlanti E, Pascucci B, Filomeni G, Mastroberardino PG, Dogliotti E. The interplay between mitochondrial functionality and genome integrity in the prevention of human neurologic diseases. Arch Biochem Biophys 710: 108977, 2021. doi: 10.1016/j.abb.2021.108977. [DOI] [PubMed] [Google Scholar]
- 119.Lee J-H, Paull TT. Cellular functions of the protein kinase ATM and their relevance to human disease. Nat Rev Mol Cell Biol 22: 796–814, 2021. doi: 10.1038/s41580-021-00394-2. [DOI] [PubMed] [Google Scholar]
- 120.Maynard S, Fang EF, Scheibye-Knudsen M, Croteau DL, Bohr VA. DNA damage, DNA repair, aging, and neurodegeneration. Cold Spring Harb Perspect Med 5: a025130, 2015. doi: 10.1101/cshperspect.a025130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Prates Mori M, de Souza-Pinto NC. Role of mitochondrial dysfunction in the pathophysiology of DNA repair disorders. Cell Biol Int 42: 643–650, 2018. doi: 10.1002/cbin.10917. [DOI] [PubMed] [Google Scholar]