Abstract
Chlorinated disinfectants are widely used in hospitals, COVID-19 quarantine facilities, households, institutes, and public areas to combat the spread of the novel coronavirus as they are effective against viruses on various surfaces. Medical facilities have enhanced their routine disinfection of indoors, premises, and in-house sewage. Besides questioning the efficiency of these compounds in combating coronavirus, the impacts of these excessive disinfection efforts have not been discussed anywhere. The impacts of chlorine-based disinfectants on both environment and human health are reviewed in this paper. Chlorine in molecular and in compound forms is known to pose many health hazards. Hypochlorite addition to soil can increase chlorine/chloride concentration, which can be fatal to plant species if exposed. When chlorine compounds reach the sewer/drainage system and are exposed to aqueous media such as wastewater, many disinfection by-products (DBPs) can be formed depending on the concentrations of natural organic matter, inorganics, and anthropogenic pollutants present. Chlorination of hospital wastewater can also produce toxic drug-derived disinfection by-products. Many DBPs are carcinogenic to humans, and some of them are cytotoxic, genotoxic, and mutagenic. DBPs can be harmful to the flora and fauna of the receiving water body and may have adverse effects on microorganisms and plankton present in these ecosystems.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11356-021-18316-2.
Keywords: Environmental disinfection, Ecotoxicity, Hospital wastewater, Human health impacts, Surface water quality, Trihalomethanes, Chlorine, Natural organic matter
Introduction
In December 2019, the first confirmed case of COVID-19, a contagious respiratory disease caused by a newly discovered coronavirus (SARS-CoV-2), was reported from Wuhan, China (Huang et al. 2020). In a short period, the virus spread to almost every continent across the globe, and on 11 March 2020, the World Health Organization (WHO) declared COVID-19 a global pandemic. Consequently, numerous strategies have been adopted to contain the spread of the virus including nationwide lockdowns, sealing of borders between nations, social distancing, work-from-home, frequent cleaning, sanitization and disinfection, and use of face shields and masks. Among them, regular disinfection of public places and high-volume retail locations, indoor areas, and hospitals with the aid of chlorine-based disinfectants (CBDs) is the most widely practised approach. Wastewater treatment facilities/plants (WWTPs) were advised to enhance their disinfection routine (Kataki et al. 2021). Increased use of CBDs was recommended because enveloped viruses like SARS-CoV-2 are generally vulnerable to rapid inactivation by CBDs (La Rosa et al. 2020). As a result, many countries opted for large-scale disinfection of public places using liquid chemicals. Sodium hypochlorite (bleach), calcium hypochlorite (bleaching powder), sodium dichloroisocyanurate (NaDCC), chloramine, and chlorine dioxide are the most common CBDs used for reducing the transmission of SARS-CoV-2. These strategies have led to a colossal surge in the sales of these disinfecting agents (Future Market Insights 2020; Klemeš et al. 2020).
The efficacy of CBDs against coronaviruses is well-documented in the scientific literature (Bakhtiyari et al. 2020; Probst et al. 2021; Shimabukuro et al. 2020). However, once sprayed on streets and public places, these compounds can enter the sewer system or stormwater drains in the form of surface runoff, eventually leading to severe contamination of surface waters. Increased residual chlorine content up to 0.4 mg/L was observed in Wuhan lakes during the early months of COVID-19 affirming the transportation of CBDs to surface waters (Chu et al. 2021). Upon discharge into the aquatic environment, most CBDs release free chlorine which reacts with natural organic matter (NOM) to form potentially harmful organochlorine compounds called “disinfection by-products” (DBPs). Depending on the type of natural organic matter (NOM), inorganic constituents, and other physicochemical characteristics like pH and temperature, several classes of DBPs may form such as trihalomethanes (THMs), haloacetic acids (HAAs), haloacetonitriles (HANs), haloketones (HKs), and trihalophenols (THPs). A vast majority of them act as potential human carcinogens and mutagens and are often associated with rectal and colon cancers, as well as developmental and reproductive disorders (Andrzejewski and Nikolaou 2003). Additionally, DBPs can infiltrate underlying soils and groundwater over time, with far-reaching impacts on both ecosystems and human health. While the use of CBDs may have a reassuring effect on populations in the fight against COVID-19, their harmful effects on the environment are not clear.
The potential impacts of the widespread use of CBDs to combat COVID-19 on both human health and the environment are presented in this paper. In particular, the DBP formation potential in public sewers and hospital wastewaters (HWW) as a result of extensive disinfectant spraying activities in the wake of the COVID-19 pandemic is comprehensively examined. In addition, the transport of DBPs to various environmental compartments is analyzed, and risks associated with them are assessed.
Fate and transport of CBDs during and after application
COVID-19 and disinfectant application
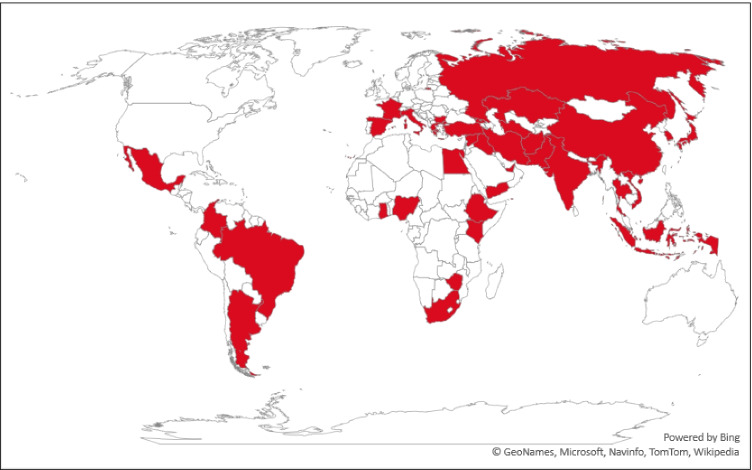
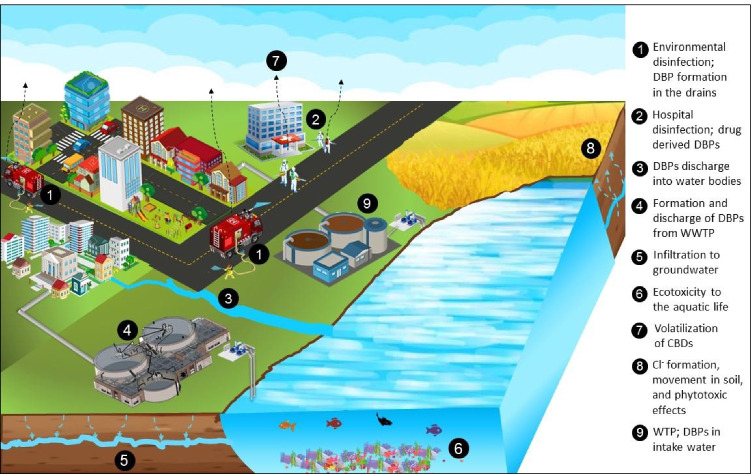
In general, outdoor application of CBDs and the smell of chlorine inside residential complexes and medical facilities are frequent occurrences since the beginning of 2020. Several Asian and European countries and parts of America and Africa opted for outdoor environmental disinfection. A sharp increase in the use of CBDs for household disinfection was observed as it increased from 19.9% before the pandemic to 48.7% after the COVID-19 outbreak (Guo et al. 2021). Fig. 1 shows the extent of environmental disinfection across the world. This tendency was severe at the beginning of the pandemic and later started to decline as some scientists indicated that the strategy was not effective. However, it is still practiced in several parts of the world, especially in and around medical facilities. Concerns regarding the application of CBDs for mass disinfection have been arising ever since the COVID-19 outbreak (Bhat et al., 2021; B. Chen et al., 2021a, b; Z. Chen et al., 2021a, b; Chu et al., 2021; Collins and Farmer, 2021; Dhama et al., 2021; Liu et al., 2021; Luan et al., 2020; Mohanan et al., 2021; Pan et al., 2022; Zhang et al., 2020a, b; T. Zhang et al., 2021c, a, b). Experiments were conducted by applying chlorine to wastewater and waterbodies to determine the extent to which DBPs were formed (Cui et al., 2021; Zhang et al., 2022). A summary of previous studies that explored the disinfection trends, health problems faced during or after application of disinfectants, risks associated with CBDs, and occurrence of DBPs in aquatic and air environment during COVID-19 is given in Table 1. The application of CBDs has induced direct health problems such as respiratory issues, skin irritation, and psychological discomfort and indirect concerns such as DBP formation and ecosystem disruptions. Based on the previous studies, the possible fate and transport of CBDs as well as the impacts are illustrated in Fig. 2. A detailed discussion of these impacts is presented in the following sections.
Fig. 1.
Countries that have opted for large-scale environmental disinfection practices (Map is prepared based on media reports)
Table 1.
Usage trends, ill effects, and risks of disinfectants observed during COVID-19
| Location | Assessment method | Usage trend/ill effects/risks/other observations about disinfectants | References |
|---|---|---|---|
| China | Questionnaire survey of general population (n = 3667) | Out of 3667 respondents, 99.5% chose their disinfectant based on the disinfecting power of the chemical while only 12.3% considered environmental impact as a factor while choosing. Use of CBDs for household and environmental cleaning increased from 3.6 to 65.9% since COVID-19 outbreak; 81.3% tend to throw the unused disinfectants along with garbage, and 15.1% either flushed it through toilet/sink. | (Guo et al. 2021) |
| Iran | Questionnaire survey of general population (n = 1090) | Approximately 77.9% of responders sanitized their hands at an average frequency of 10.74 times a day using either alcohol solutions (51% respondents) or sodium hypochlorite bleach (4.8% respondents). Bleach was preferred by low-income people for hand sanitizing since alcohol was costlier; 57.3% preferred bleach for surface disinfection. About 41.1% of respondent faced health issues such as skin dryness, skin itching, coughing, obsession, eye irritation, breath shortness, and headache while using disinfectants. | (Dindarloo et al. 2020) |
| Turkey | Questionnaire survey of general population (n = 674) | The amount of hand sanitizer, soap, and bleach increased around 92.7%, 87.4%, and 70.4%, respectively in the post-COVID-19 period. About 46.9% faced at least one health problem that they relate to the disinfectant use. The major health issue was on skin (itching/redness/scaling) followed by respiratory problems (shortness of breath/wheezing/burning in the chest, and asthma attack), and poisoning through skin or inhalation. | (Koksoy Vayisoglu and Oncu 2021) |
| Morocco | Chemical risk assessment and health survey (n = 40) of dairy industry employees. | One hundred percent of survey participants used alcohol-based sanitizers, and about 60% used bleach as well. Alcohol-based sanitizers caused dermatological problems to 47% and psychological discomfort such as fear and stress to 10.5% of the population. The group that used CBDs experienced respiratory issues, dermatological problems, dizziness, memory problems, eye problems, psychological discomfort, and other health issues. | (Rachidi et al. 2021) |
| China | Ecotoxicity assessment on freshwater phytoplankton community | Phytoplankton Scenedesmus sp., M. aeruginosa, and Cyclotella sp. were found to have inhibited growth under the influence of DBPs including THMs, HANs, and HAAs. THMs had the lowest toxicity as their growth inhibition percentage was less than 50% even at the highest test concentration. HAAs induced drifting in the phytoplankton community by changing the inter-algal relationships. These changes can cause cyanobacterial blooms that produce harmful toxins in the water body and disrupt the aquatic ecosystem. Authors indicated that intensified disinfection due to COVID-19 may lead to disturbances in the microalgal community. | (Cui et al. 2022) |
| China | Bacterial analysis of MRSAa infection in a hospital. | A sharp increase in MRSA detection compared to 4 years pre-COVID-19 was observed in a mental health facility for elders in China. The samples collected from staff hands and environment showed an increase from 2.3% in 2019 before COVID-19 outbreak to 20.6% in 2020. Further, MRSA detection was found to increase with concentration and frequency of disinfectant. A reduction in chlorine application from 1000 to 500 mg/L showed significant reduction in the MRSA detection. | (Yang et al. 2021) |
| China | Occurrence of DBPs and pharmaceuticals in aquatic environment | Grab samples of effluent discharges collected after 2 weeks, 3 months, and 8 months showed comparatively similar concentrations (55.9 µg/L, 74 µg/L, and 57 µg/L, respectively) of 21 DBPs. However, the presence of pharmaceutical products, especially the ones used for COVID-19 treatments, was seen to be increasing. | (Z. Zhang et al. 2021c, a, b) |
| China | Air sample analysis and health risk assessment | Air samples collected from 40 places before and 30 min after CBDs spraying showed at least 1.6 times higher concentrations of DBPs in the samples collected after spraying of CBDs. This shows that even in the air, there is DBP formation additional to that of aquatic environments. Chloroform was the most abundant DBP in the air. The median concentration of all DBPs was 34.1 µg/m3, 2.8 µg/m3, and 1.0 µg/m3 for hypochlorite spray doses of 100–200 mg/m3, 3–20 mg/m3, and 0 mg/m3, respectively. The health risk assessment showed a hazard index value of 0.5 (1 being the threshold for non-cancerous risks) for children indicating that this group may face inhalation-related risks. | (Lou et al. 2021) |
| China | DBP occurrence study | Water samples of rivers and lakes from Wuhan collected during COVID-19 pandemic contained THMs (2.2 µg/L), HANs (0.02 µg/L), and N-nitrosamines (60.8 ng/L). Prior to the pandemic outbreak, the DBPs concentration in Chinese surface waters was steady and at low levels. For example, the total nitrosamines were reported to be in the range of 1.6–62.4 ng/L. | (Wang et al. 2021) |
aMethicillin-resistant Staphylococcus aureus
Fig. 2.
Overview of possible fate and transport of CBDs during and after application
Evaporation of chlorine from hypochlorite solutions
When chlorine is applied to an aqueous solution, the following reactions occur simultaneously.
| 1 |
Addition of chlorine to water results in the formation of hypochlorous [HOCl] and hydrochloric acids [HCl]. This reaction is governed by Henry’s law. HOCl partly dissociates into protons and hypochlorite ions (OCl−) depending on the pH of the solution. The immediate production of HOCl in a water-chlorine mixture also lowers the pH of the aqueous solution for the same reason.
| 2 |
The OCl− ions then degrade to a mixture of chloride and chlorate ions; chlorate ions are considered to be toxic while chloride is considered to be non-toxic.
| 3 |
It is well known that chlorine is released from hypochlorite solutions to the surrounding atmosphere, thereby reducing disinfectant residuals over time. This gas-liquid exchange reaction can be understood from Henry’s law for the volatilization of gases from the liquid phase (Eq. 4):
| 4 |
where pCl2 is the partial pressure of chlorine in the atmosphere (atm), KH is Henry’s constant (L atm/mol), and [Cl2] is the concentration of chlorine in aqueous solution (mol/L).
This relationship is valid only in the case of volatilization of molecular chlorine (Cl2) from water. Henry’s constant (KH) is a function of temperature, pH, and the concentration of salts and acids in solution. When chlorine is added to water, several chlorine species are formed which together increase the concentration (as well as solubility) of chlorine in water; hence, the rate of volatilization of chlorine varies substantially. In addition, the aqueous chemistry and physical conditions of the solutions also greatly influence the escape of chlorine gas into air. Therefore, Henry’s law is not adequate for describing all these conditions. Instead, modified equations based on mass transfer experiments can be used. In general, it is expected that volatilization of toxic chlorine gas and associated compounds will occur during the use of CBDS and subsequent exposure to them is possible. Since the COVID-19 outbreak, an exponential increase in the number of calls daily regarding exposure to bleach and other CBDs in the USA indicates the same. Further, the US Department of Health and Human Services reported several cases of accidental inhalation poisoning of chlorine gas from CBDs during COVID-19 (Ha et al. 2021). Most city sanitizing agencies are employing sprayers and mist cannons. This equipment discharges high volume and highly concentrated sodium hypochlorite droplets into the environment at high pressures. Exposure to chlorine gas or CBDs may pose severe health issues for children and those who are sensitive to such toxic substances (Ha et al. 2021). While the general public is not likely to be exposed regularly to such concentrations of chlorine gas, the risk to healthcare and public sanitation workers who use CBDs frequently for cleaning and disinfection cannot be ignored.
CBDS in drainage system and subsequent formation of DBPs
Continuous application of CBDs can result in free chlorine residuals in the freshwater bodies. Even at low levels of chlorine application, free residual chlorine was found to alter the composition of the microbial community of the freshwater ecosystems (Zhang et al. 2022). CBDs may seep into soil, react with organic or inorganic substances, or eventually reach the drains or sewer lines, and end up in a wastewater treatment facility or surface waters without any treatment. In all cases, chlorine compounds can react with a vast range of organic and inorganic materials and other anthropogenic chemicals. This leads to the formation of DBPs in different types of aquatic systems like wastewater, surface water, and tap water due to elevated CBD application (Li et al. 2021; Meade et al. 2021; Paul et al. 2021; Wang et al. 2021).
The reactions of chlorine compounds in sewer lines and subsequent DBP formation are similar to reactions during wastewater chlorination. Therefore, DBP formation in wastewater is taken as the reference phenomenon for this study as the present scenario has not been addressed before. Several factors affect DBP formation and include source water characteristics like pH, type and amount of organic matter, presence of inorganics like bromide and iodide, or any other chlorine-demanding compounds; disinfectant properties: type, quantity of residual chlorine; and physical factors of the reaction media such as temperature and contact time. NOM is considered as the primary precursor for DBP formation in drinking water. NOM consists of humic and fulvic acids and humus. Humic and fulvic acids are soluble while hummus is the insoluble fraction of NOM and similar to peat. Humic acids and fulvic acids are known to react with chlorine and account for 50–90% of the dissolved organic carbon (DOC) present in surface waters (Thurman 1985). Currently, more than 600 DBPs have been identified across various aqueous environments, and only some compounds are regulated in drinking water (Goel 2019; Richardson et al. 2007). WHO provides guideline values for individual DBPs, while countries like the USA regulate DBPs based on their class, such as trihalomethanes, haloacetic acids, and haloacetonitriles. Individual DBPs, their chemical formulae, and human health risk indices are summarized in Table 2.
Table 2.
Classes of DBPs, individual chemical composition, and human health risk indices
| DBPs | Chemical composition | Regulatory guidelinesa | Health risk indicesb | |||
|---|---|---|---|---|---|---|
| WHO | USEPA | Genotoxicity | Carcinogenicity | Cancer probability and critical organ (tumor site)c | ||
| Trihalomethanes (THMs) | ||||||
| Trichloromethane (chloroform) | CHCl3 | 300 µg/L | 80 µg/L for total THMs | − | + | B2. Hepatic (hepatic) |
| Bromodichloromethane | CHBrCl2 | 60 µg/L | + | + | B2. Urinary (urinary) | |
| Dibromochloromethane | CHBr2Cl | 100 µg/L | + | + | C. Hepatic (hepatic) | |
| Tribromomethane (bromoform) | CHBr3 | 100 µg/L | + | + | B2. Hepatic (gastrointestinal) | |
| Haloacetic acids (HAAs) | ||||||
| Chloroacetic acid | CH2ClCOOH | 60 µg/L for total of HHA5 | + | − | ||
| Bromoacetic acid | CH2BrCOOH | + | ||||
| Dichloroacetic acid | CHCl2COOH | 50 µg/L | + | + | Likely to be carcinogenic. Hepatic, nervous, and reproductive (hepatic). | |
| Dibromoacetic acid | CHBr2COOH | + | + | |||
| Trichloracetic acid | CCl3COOH | 100 µg/L | + | Suggestive evidence of carcinogenic potential. Hepatic (hepatic). | ||
| Bromodichloroacetic acid | CBrCl2OOH | + | ||||
| Dibromochloroacetic acid | CBr2ClOOH | + | ||||
| Bromochloroacetic acid | CHBrClCOOH | - | + | |||
| Tribromoacetic acid | CBr3COOH | + | ||||
| Haloacetonitriles (HANs) | ||||||
| Chloroacetonitrile | CH2ClCN | + | ||||
| Bromoacetonitrile | CH2BrCN | + | ||||
| Dichloroacetonitrile | CHCl2CN | 90 µg/L | + | |||
| Dibromoacetonitrile | CHBr2CN | 100 µg/L | + | |||
| Bromochloroacetonitrile | CHBrClCN | + | ||||
| Bromodichloroacetonitrile | CBrCl2CN | |||||
| Dibromochloroacetonitrile | CBr2ClCN | |||||
| Trichloroacetonitrile | CCl3CN | 1 µg/L | + | |||
| Tribromoacetonitrile | CBr3CN | |||||
| Aldehydes | ||||||
| Formaldehyde | CH2O | 900 µg/L | + | + | B1. Gastrointestinal and urinary (respiratory). | |
| Acetaldehyde | CH3CHO | + | + | B2. Nervous and respiratory (respiratory). | ||
| Chloroacetaldehydes | CH2ClCHO | + | ||||
| Bromoacetaldehydes | CH2BrCHO | |||||
| Dichloroacetaldehydes | CHCl2CHO | |||||
| Dibromoacetaldehyde | CHBr2CHO | |||||
| Bromochloroacetaldehyde | CHBrClCHO | |||||
| Bromodichloroacetaldehyde | CBrCl2CHO | |||||
| Dibromochloroacetaldehyde | CBr2ClCHO | |||||
| Trichloroacetaldehyde (chloral hydrate) | CCl3CH(OH)2d | 10 µg/L | + | + | C. Gastrointestinal and nervous. | |
| Tribromoacetaldehyde | CBr3CHO | |||||
| Haloketones (HKs) | ||||||
| 1,1-Dichloroacetone | CHCl2COCH3 | + | ||||
| 1,1,1-Trichloroacetone | CCl3COCH3 | + | ||||
| I-THMs | ||||||
| Dichloroiodomethane | CHCl2I | |||||
| Dibromoiodomethane | CHBr2I | |||||
| Chlorodiiodomethane | CHClI2 | |||||
| Bromodiiodomethane | CHBrI2 | |||||
| Bromochloroiodomethane | CHBrClI | |||||
| Iodoform | CHI3 | + | ||||
| Other I-DBPs | ||||||
| Iodoacetic acid | CH2ICOOH | + | ||||
| Bromoiodoacetic acid | CHBrICOOH | + | ||||
| Iodoacetonitrile | CH2ICN | + | ||||
| Haloacetamides (HAcAms) | ||||||
| Chloroacetamide | CH2ClCONH2 | + | ||||
| Bromoacetamide | CH2BrCONH2 | + | ||||
| Iodoacetamide | CH2ICONH2 | + | ||||
| Dichloroacetamide | CHCl2CONH2 | + | ||||
| Dibromoacetamide | CHBr2CONH2 | + | ||||
| Diiodoacetamide | CHI2CONH2 | + | ||||
| Bromochloroacetamide | CHBrClCONH2 | + | ||||
| Bromoiodoacetamide | CHBrICONH2 | + | ||||
| Chloroiodoacetamide | CHClICONH2 | + | ||||
| Bromodichloroacetamide | CBrCl2CONH2 | + | ||||
| Dibromochloroacetamide | CBr2ClCONH2 | + | ||||
| Trichloroacetamide | CCl3CONH2 | + | ||||
| Tribromoacetamide | CBr3CONH2 | + | ||||
| Nitrosamines | ||||||
| N-Nitrsodimethylamine (NDMA) | (CH3)2-N(NO) | + | + | B2. (Hepatic) | ||
| N-Nitrosopyrrolidine (NPYR) | C4H8-N(NO) | + | + | B2. (Hepatic) | ||
| N-Nitrosomorpholine (NMOR) | C4H8N2O2 | + | + | |||
| N-Nitrosopiperidine (NPIP) | C5H10-N(NO) | + | + | |||
| N-Nitrosodiphenylamine | C12H10N2O | + | + | B2. (Urinary) | ||
| N-Nitrosomethylethylamine | (CH3)C2H5-N(NO) | + | + | B2. (Hepatic) | ||
| N-Nitrosodiethylamin | (C2H5)2-N(NO) | + | + | B2. (Hepatic) | ||
aMaximum concentration allowed in drinking water; compounds unregulated are left as blank cells.
b+indicates positive evidence, − indicates negative evidence, and blank cell indicates lack of data.
cLetters denote weight of evidence classes as B1: probable human carcinogen based on limited evidence of carcinogenicity in humans, B2: probable human carcinogen based on sufficient evidence of carcinogenicity in animals, C: possible human carcinogen; human physiological system that is at risk to a particular compound, and possible tumor site is given in bracket—adopted from USEPA IRIS database.
dChemical formula of chloral hydrate
Speciation of DBPs
Increasing population and ever-mounting pollution result in wastewater discharges that are high in N and inorganic compounds. CBDs can enter these channels alone or along with rainwater and form DBPs before the stormwater or wastewater reaches the treatment plants or the waterbodies in the vicinity. Background concentrations of THMs, HAAs, and THPs in influent sewage of various WWTPs show that the formed DBPs are not readily biodegradable (Chen et al. 2008; Feng et al. 2019; Krasner et al. 2009a,b). Considering the quantity of CBDs applied in the present scenario, this background concentration may reach alarming levels. The DBPFP of untreated wastewater will be several times higher than that of the treated water due to the higher concentrations of organic and inorganic compounds. THMs and HAAs are commonly found in wastewater at concentrations far higher than that found in drinking water due to the higher concentrations of organic compounds (Feng et al. 2019). Besides carbonaceous DBPs (C-DBPs), common wastewater inorganics such as ammonia, nitrate, bromide, and iodide induce the formation of nitrogenous (N-DBPs), brominated (Br-DBPs), and iodinated (I-DBPs) compounds. These DBPs are several times more toxic and carcinogenic than their chlorinated counterparts. Similarly, industrial and medical wastewaters generate DBPs that are specific to these wastewaters depending upon the compounds present in them.
In a comparison between DBPs generated by chlorination of NOM versus effluent organic matter (EfOM), only two out of thirteen compounds (trichloromethane and dichloropropanone) had higher concentrations in NOM solutions, while the rest were higher in EfOM samples. Further, in cell toxicology experiments, substantial intracellular oxidative stress was observed in Chinese hamster ovary (CHO) cells with chlorinated NOM and EfOM samples. Oxidative stress leads to the formation of reactive oxygen species (free radicals) which can destroy the cell and damage macromolecules such as DNA, RNA, proteins, and lipids. The destructive action of a substance within a cell is denoted as cell toxicity, and it is measured by observing the quantity of base modification products that are formed when free radicals attack a cell. Chlorinated EfOM showed a higher tendency to induce oxidative stress in CHO cells compared to chlorinated NOM (Du et al. 2020). This observation clearly shows that the chlorine reaction in wastewater can lead to the formation of high concentrations of toxins. Similarly, in one of the largest WWTP-DBP surveys (23 WWTPs across the USA), THMs, HAAs, HANs, dihalogenated acetaldehydes (DHAs), trihalogenated acetaldehydes (THAs), and two nitrosamines (NDMA and NMOR) were detected in secondary effluents after disinfection with CBDs (Krasner et al. 2009b). Nitrosamines are highly toxic N-DBPs. NDMA is a non-halogenated DBP formed by nitrosation in water environments upon chlorination or even by various industrial activities (Mitch et al. 2003; Shah and Mitch 2012). NDMA is almost 600 times more toxic than any THM, and it has an estimated potency of 10−6 for lifetime cancer risk (LTCR) at an extremely low concentration of 0.7 ng/L (IRIS 1987). In another study, Krasner et al. (2009a, b) observed that WWTP effluents that were disinfected beyond breakpoint presented higher concentrations of halogenated DBPs, whereas the ones that did not reach breakpoint developed more N-DBPs. Therefore, it is clear that environmental disinfection may result in the production of highly toxic DBPs.
If the water environment contains bromine (which occurs as bromide in natural conditions), the addition of chlorine may lead to the formation of hypobromous acid/hypobromite (HOBr/OBr−). HOBr acid is a weak acid (with a pKa of 8.7 at 25 °C) like HOCl (with a pKa of 7.5 at 25 °C). HOBr reacts with organic matter, is less stable, yet it forms highly toxic Br-DBPs. Bromide is usually present in natural waters; it can get into wastewater through industrial activities, seawater intrusion, and municipal wastes. Saline wastewater generates large amounts of polar and common Br-DBPs. Unlike common C-DBPs, detection of polar DBPs needs special methods such as precursor ion scan (PIS) using electrospray ionization-triple quadrupole mass spectrometry (ESI-tqMS). Thus, they often go undetected in most occurrence studies that use gas chromatography/mass spectrometry. Forty-eight known and six new polar Br-DBPs were identified in saline sewage effluents of two Hong Kong WWTPs after they were chlorinated (Ding et al. 2013). A steady increase in many polar Br-DBPs was observed in these experiments when primary saline effluent was dosed with increasing chlorine doses. This observation indicates that polar Br-DBPs once created do not decrease easily and can accumulate in aqueous environments. Cities that use seawater for flushing are at higher risk in the present scenario if their drainage systems have high amounts of bromine.
Similar to bromide, chlorine oxidizes iodide to hypoiodous/hypoiodite (HOI/OI−) which then reacts with organic and inorganic matter to generate I-DBPs (Dong et al. 2019). Six I-THMs that are listed in Table 2 are the most common iodinated by-products. The FP for I-THMs was higher in river water than in wastewater (Pantelaki and Voutsa 2018). I-THMs formed rapidly in wastewater, but the rate of production decreased with time. On the other hand, I-THMs had a slower but consistent generation rate in river water. This was attributed to differences in the reaction of HOI/OI− with larger river water NOM molecules and smaller but saturated wastewater organic matter. I-THMFP of treated wastewater was found to be almost 7 times higher than that of river water (Phatthalung and Musikavong 2019). Evidently, constituents of an aqueous medium will determine the types of DBPs generated. There are polar I-DBPs as well (Huang et al. 2019). As I-DBPs are emerging by-products, a grouping of compounds and their toxicity assessment are still in the initial stages. Although iodine occurs naturally in water, many industrial activities discharge huge amounts of iodine to sewers and wastewater facilities. Hospital wastes also contribute a good amount of iodine into aqueous environments which currently needs special attention.
DBP formation by disinfection of hospital surfaces and wastewater
Hospitals and healthcare facilities need to be extremely cautious to control the spread of the novel coronavirus. Measures taken by various countries and agencies for hospital or environmental disinfection are listed in Table 3. Use of hypochlorite is common and is being applied at many healthcare facilities (Achak et al. 2021; Lauritano et al. 2020; Sharafi et al. 2020). Normally, hospital wastewaters (HWWs) are treated under special rules. In some cases, the sewage generated from non-infectious patients is combined with municipal wastewater and sent to the WWTP (Wang et al. 2020a, b). However, the practicality of this approach, especially during a pandemic, is questionable.
Table 3.
Guidelines from various agencies regarding the use of CBDs for hospital disinfection during the COVID-19 pandemic
| Health agency | Suggested disinfection measures for healthcare settings and norms for environmental cleaning |
|---|---|
| World Health Organization |
a. Healthcare when COVID-19 is suspected: Clean environmental surfaces with water and detergent and apply common hospital disinfectants such as sodium hypochlorite. b. COVID-19 hospital: Environmental surfaces should be cleaned to remove organic matters; 70–90% ethanol, hypochlorite solution of 0.1% (1000 ppm) for general environments and 0.5% (5000 ppm) for high risk (blood and body fluid spills), or hydrogen peroxide having a concentration above 0.5% can be used as a disinfectant. The cleaning/disinfecting procedure should be repeated at least twice per day for screening areas, in-patient rooms, hallways, and private patient toilets/bathrooms, and at least thrice per day for shared patient toilets/bathrooms. Ambulances and outpatient rooms should be decontaminated after each patient visit. |
| Department of Health, Australian Government | Freshly made bleach solution of appropriate concentration is to be used to disinfect patient, and non-patient areas. In-patient areas should be frequently disinfected. Equipment should be cleaned and decontaminated after each use. Terminal cleaning of a room should be carried out when a patient is discharged or vacated. A 2-in-1 process, where a combined detergent and hospital grade disinfectant or sodium hypochlorite, or a 2-step process, where physical cleaning with detergent followed by sodium hypochlorite/hospital grade disinfectant is to be used. |
| Chinese Center for Disease Control and Prevention |
Disinfection of commonly contaminated objects in a medical institution/quarantine center/household where an infected person resided. a. Indoor air: Ultra-low volume spray of peracetic acid, chlorine dioxide, hydrogen peroxide, or other disinfectant can be used as indoor disinfection of an unoccupied room. b. Vomitus, blood, and other body fluids: Small spill should be removed with an absorbent material and a chlorinated disinfectant containing 5000–10,000 mg/L should be applied on the area. In case of large volume spill, cover the waste in chlorinated disinfectant powder (bleaching powder). Collect the vomitus and other body secretions of patients in a special container and soak it for 2 h in 20,000 mg/L chlorine disinfectant at a ratio of 1:2 for waste to disinfectant. Later, this container also needs to be disinfected by soaking it in 5000 mg/L chlorine disinfectant for 30 min. c. Feces and sewage: Separate septic tanks need to be installed to disinfect sewage of COVID-19 hospitals before discharging into municipal sewers. Enough chlorine disinfectant should add to the septic tank to leave an initial effective chlorine concentration of 40 mg/L and total residual chlorine of 10 mg/L after 1.5 h. In case the hospital lacks separate septic tanks, the procedure for disinfection of vomitus and body secretions can be followed for sewage as well. d. Floors, walls, and other subject surfaces: Remove any visible contaminants from floors, walls, furniture, door knobs, bed railings, treatment equipment, and household items, and spray/wipe with/soak in 1000 mg/L chlorine solution or 500 mg/L chlorine dioxide for at least 30 min. Clothing, beddings, and other textile items can be either boiled for 30 min or soaked in 500 mg/L chlorine disinfectant prior to standard washing process. Same procedure is applicable to tableware as well. e. Vehicles or transportation tools: interiors of trains, cars or ships which are used to transfer an infected person should be wiped with an absorbent material dipped in 5000–10,000 mg/L chlorine disinfectant. After this, spray disinfection with 1000 mg/L of chlorine solution and a final wipe with clean water also needs to be carried out. |
| Health Department, Republic of South Africa | For environmental cleaning frequent cleaning of 2–3 times (3–4 times in high-risk areas) per day followed by wiping with disinfectant containing 1000 ppm available chlorine or 70% alcohol is advised. Liquid and solid hypochlorite, chloramine, NaDCC tablets, or powder are recommended for disinfection. The predicted amount of 0.5% hypochlorite solution for a single healthcare worker is 10 L and 15 L in COVID-19 ward and ICU respectively. |
| Public Health Department, Government of UK |
a. Hospital room while the COVID-19 patient is there: Chlorine disinfectant containing minimum 1000 ppm available chlorine needs to be used after cleaning with a neutral detergent. This should be carried out at least twice daily for patient’s main isolation room. b. Cleaning the room once patient has left: After clearing all surfaces, clean with a neutral detergent followed by chlorine disinfectant of at least 1000 ppm. c. Equipment and the care environment: a combined detergent/disinfectant or a neutral detergent followed by disinfectant with a free available chlorine of 1000 ppm should be used. d. Environment: isolation rooms, cohort areas, PPE removing rooms, and clinical rooms should be decontaminated. |
A study from China reported the presence of SARS-CoV-2 virus RNA even after disinfection with 800 g/m3 of sodium hypochlorite in the septic tanks of a COVID-19 hospital (Zhang et al. 2020a, b). Influent and effluent samples from the septic tanks were collected on 26 February 2020 and 1 March 2020. On both sampling days, the free chlorine amount was reduced to non-detectable levels within 12 h of addition, and SARS-CoV-2 RNA was detected in the effluent. To eliminate the danger, the applied chlorine dose was increased to 6700 g/m3 of sodium hypochlorite on 6 March 2020 and thereafter, and subsequent tests were negative for the virus. On 6 March 2020, the free chlorine in the effluent after 12 h of contact time was 25 mg/L. Consequently, THMs formation, i.e., chloroform (332 ± 122 µg/L), bromodichloromethane (5.1 ± 3.1 µg/L), dibromochloromethane (0.6 ± 0.5 µg/L), and bromoform (1.9 ± 1.0 µg/L) was observed. Results of this study need to be considered along with previous analysis, which was carried out on wastewater collected from Beijing general hospital. They reported almost 15 times less THM concentration in the primary effluent (Luo et al. 2020). The increase in DBP concentration of Wuchang HWW can be attributed to the increased disinfection. In a subsequent study, Luo et al. reported background concentrations of THMs, HAAs, HANs, HKs, TCNM, CH, and haloacetoamides (HAcAms) in primary effluent before chlorination, which they correlated to the chlorinated flush water. These days, this background concentration can be expected to be several times higher than the reported cases since surface cleaning and disinfection of the entire medical facility can contribute a large amount of chlorine into the flow. This will then be added to those DBPs which are formed because of enhanced disinfection to remove viruses in the sewage. Once formed, most of the DBPs are difficult to remove. As a result, a huge load of ecotoxic compounds are being dumped in receiving water bodies. More studies need to be carried out to examine the breadth of this issue and to comprehend the effects on the ecosystems of surface water bodies.
Unlike freshwater constituents, HWW contains many anthropogenic compounds which can contribute to DBP formation. These compounds are mainly from various medical practices such as diagnosis, treatment, medication, surgeries, and through research and development laboratories. Medicines, traces of pharmaceuticals and drugs, and other chemicals involved in healthcare can enter the sewage through excretion of patients. Since the outbreak of the pandemic, elevated consumption of umifenovir (an antiviral drug) has led to its presence at 1 µg/L in municipal wastewater. This drug forms a wide range of DBPs when it reacts with sodium hypochlorite, most of them being Br-DBPs with bromoform as the dominant species (Ul’yanovskii et al., 2022). It is well known that the consumption of various antibiotics and other life-saving medicines has been rising during the pandemic; these compounds will eventually end up in the WWTPs and waterbodies (Goel 2015).
Iodinated contrast media (ICM) is a well-explored HWW pollutant. ICM is commonly used in X-ray based imaging systems such as computer tomography scans and angiography. Traces of ICM have been found in water bodies; in one case, the concentration was as high as 100 µg/L (Ternes and Hirsch 2000). ICM is one of the largest contributors of organic iodine in water and wastewater (Duirk et al. 2011). Therefore, these compounds may be major precursors of I-DBPs in surface waters. Tramadol is another toxic drug-originated pollutant in HWW. Similar to ICM, traces of tramadol have also been found in wastewater and surface waters (Kasprzyk-Hordern et al. 2008; Rúa-Gómez and Püttmann 2012). This can be because of the complex metabolic fate of tramadol that leads to the excretion of partial amounts of the drug used by a patient. Chlorination degrades tramadol to some extent, but it also forms by-products that are more toxic than the parent compound. Seven such by-products were identified, and their toxicity was compared to Tramadol (Romanucci et al. 2019). A summary of this and other studies on medical/pharmaceuticals products that formed DBPs in various aquatic media and their toxicity status is shown in Table 4. Some of these studies concentrated on specific DBPs while ignoring the conventional ones. Therefore, in an HWW media, a mixture of all these by-products can be expected. The hazard of chlorine disinfection of medical wastes and by-product formation is poorly understood at this time. Most of the by-products formed are yet to be verified.
Table 4.
DBPs formed by chlorination of pharmaceutical/medical products and their toxicity. (Reference: (Wang et al. 2020a, b), (Negreira et al. 2016; 2015a,b,c), (Romanucci et al. 2019), (Duirk et al. 2011))
| Compounds that formed DBPs with chlorine | Reaction media used in the study | DBP analogues detected | Status of toxicity assaya |
|---|---|---|---|
|
Antibiotics i. Ciprofloxacin ii. Sulfamerazine iii. Chloramphenicol |
Milli-Q water | THMs, HAAs (BCAA, TCAA, DCAA, MBAA, and MCAA), HKs, HANs, and TCNM | + |
|
Anti-cancer drugs i. Etoposide |
River water, WWTP influent, and effluent |
By-product 1 and 2b (BP1: C28H29O13−, BP2: C26H25O13−) |
− |
| ii. Tamoxifen (TAM) | Ultra-pure water and wastewater |
OH-TAMc DBPs: DBP-438: C26H28O3NCl, DBP-472: C26H27O3NCl2, DBP-422A: C26H28O2NCl, DBP-422B: C26H28O2NCl, DBP-456: C26H27O2NCl2, DBP-440: C25H23O2NCl2, and DBP-454: C26H25O2NCl2 OH-D-TAMc DBP: DBP-424: C25H26O3NCl, DBP-458A: C25H25O3NCl2, DBP-458B: C25H25O3NCl2, DBP-458C: C25H25O3NCl2, DBP-408: C25H26O2NCl, DBP-442; C25H25O2NCl2, and DBP-440; C25H23O2NCl2 |
+ |
| iii. Erlotinibd | Ultra-pure water and wastewater | TP-444: C22H22O5N3Cl, TP-428A: C22H22O4N3Cl, TP-428B: C22H22O4N3Cl, TP-428C: C22H22O4N3Cl, TP-462A: C22H21O4N3Cl2, and TP-462B: C22H21O4N3Cl2 | − |
|
iv. Vinca alkaloids: vincristine, vinblastine, vinorelbine, and deacetyl vinorelbyne |
Ultra-pure water and wastewater | Total of sixty-five by-products were formed including twenty mono, 8 di-, and 2 tri-chlorinated compounds. | − |
| Tramadol (TRA) | Milli-Q water | BP1: C16H24ClNO2, BP2: C14H21NO3, BP3: C14H18O3, BP4: C16H23NO2, BP5: C17H23ClO5, BP6: C16H23NO3, and BP7: C15H20N2O3 | + |
| Iodinated contrast media (ICM); iopamidol, iohexol, and iopromide | Purified water and river watere |
Iodo-THMS: dichloroiodomethane, dibromoiodomethane, chlorodiiodomethane, bromochloroiodomethane, bromodiiodomethane, and iodoform. Iodo-acids: iodoacetic acid, bromoiodoacetic acid, (Z)-3-bromo-3-iodopropenoic acid, (E)-3-bromo-3-iodopropenoic acid, and (E)-2-iodo-3-methylbutenedioic acid. |
Iodo-THMs + iodo-acids + |
a+ indicates toxicity assessment has been done in the respective study and the by-products were found to be toxic, − indicates toxicity assessment has not been carried out in the respective study.
bProposed chemical formula.
cDBPs were formed from metabolites of TAM.
dOut of total 19 products formed, only chlorinated ones are listed.
eIohexol and iopromide generated I-DBPs only in purified water.
Cytotoxicity, carcinogenicity, and other human health impacts of DBPs
DBPs formed in the sewer, HWW, and municipal treatment plants reach the receiving water body along with sewage or effluent flow. During their transport and upon reaching the surface water, many of these by-products are transformed into new compounds. Thus, the flora and fauna of a receiving water body get exposed to a mixture of many DBPs and other pollutants. It is universal practice to withdraw water from surface water bodies for agriculture, industrial, and recreational activities. In some cases, drinking water intakes that are located downstream from the discharges of disinfected wastewaters may get large amounts of DBPs.
Human health risk assessment studies usually consider three routes of exposure, i.e., ingestion through drinking water, inhalation of volatile DBPs, and dermal absorbance while showering or swimming (Parveen et al. 2021). Among all DBPs, THMs have been extensively analyzed for their carcinogenicity (Cotruvo and Amato 2019). They are often associated with rectal cancer in population-based epidemiological studies (Jones et al. 2019). THM-human health risk assessment studies usually follow USEPA standard method. This was modified by Lee et al. (Lee et al. 2004) by incorporating an additional step for age adjustment. In 2017, this new approach for assessing cancer risk posed by THM exposure was applied in a study by Kumari and Gupta (2018) They considered six age groups (0–1 years, 1–4 years, 5–14 years, 15–44 years, 45–59 years, and 60–80 years) and assigned age-dependent adjustment factor (ADAF) values for each group, which was then added as ADAFi (age-dependent factor for ith age group) in each exposure routes. Oral ingestion had the highest risk followed by inhalation, whereas dermal contact posed negligible risk. Age group 60–80 years was found to be at the highest risk with a LTCR of 2.37×10−4 for total THMs. This value was more than the acceptable risk characterization value of LTCR ≤ 1×10−4. The authors attributed the higher risk to comparatively longer periods of exposure. Several studies have demonstrated the cytotoxicity, genotoxicity, and mutagenicity of regulated, unregulated and emerging DBPs on bacterial cells and mammalian cells. The potential health effects of some DBPs are summarized in the health indices section of Table 2.
Environmental impacts of excessive applications of CBDs
Effect on soil and vegetation
Small-scale environmental disinfection in many residential areas and cities involves the spreading of calcium hypochlorite or spraying bleach solution in alleys, open lands, roadside plants, lawns, gardens, and other vegetative areas. Hypochlorite applications on soil are likely to be detrimental to plants. Chlorine applied as disinfectant is unstable, and it easily gets converted to chloride (Cl−) ions. The Cl− ions move within and between ecosystems as they are stable in soil environments (Redon et al. 2011). Unlike other anions, chloride anion is least adsorbed on soil particles and is not easily altered by microorganisms. Hence, chloride flux in a soil mass is driven only by water flow.
The minimum level of chloride recommended for higher plants was 1 g/kg of the dry mass of the plant (White and Broadley 2001). As rainfall and atmospheric deposition are enough to provide sufficient chloride to soil, an additional chloride source is not necessary. At higher concentrations, chlorine as chloride is toxic to plants. Chloride accumulates in plant tissues, especially in leaves, and results in the death of the whole plant. A study carried out on California red bean (Phaseolus vulgaris, L.) plant recorded chloride accumulation in plant tissues even after its moribund point (Hajrasuliha 1980). Mustard (Sinapis alba L.) seedlings were allowed to germinate in NaOCl solution and two more commercial NaOCl-based disinfectants. Healthy looking mustard seeds of the same size were kept in petri dishes, each containing one of the CBDs at different concentration levels. All three CBDs reduced the growth of roots and shoots of the saplings while the roots were more sensitive than the shoots (Yildiz et al. 2012). This may be because the roots get exposed to chlorine well before the shoots do. The microbial population in the rhizosphere which is important for plant health are also destroyed by the CBDs which consequently affect the root’s health. As the chlorine flux across a plant is strongly associated with the water intake and translocation, when chlorine concentration increases, plants tend to reduce their water intake causing a reduction in fresh and dry biomass. Adverse effects of chlorine have been observed on species such as witchweed (Hsiao et al. 1981), flax seeds (Yildiz and Er, 2002), cereals, fruits and vegetables (Xu et al. 1999). In all these studies, chlorine-containing fertilizers and chlorinated irrigation water represented additional sources of chlorine toxicity for the plants. Direct application of hypochlorite on vegetation and public places amidst the new pandemic involves frequent spraying of concentrated solutions. This amount is several times higher than what can be expected from irrigation water and fertilizer run-offs. Rainwater or drainage flow can easily take these chloride ions to irrigation channels and then to agricultural fields where crops may get affected. As chlorine can even affect the regeneration of plant tissues (Yildiz and Er 2002), this issue needs to be examined further. It can be concluded based on these studies that direct application of hypochlorite on soil and vegetation and its consequent increase in water bodies is unfavorable for plants.
CBDs in air and their interaction with various components of the atmosphere
The release of CBDs into air results in an increase in HCl, HOCl, and Cl• in the atmosphere. Chlorine and chlorinated compounds are generally considered to be air polluting agents. The oxidation potential of chlorine gas, its hydrolysis in clouds, and heterogeneous chemical reactions with aerosols may indicate that Cl2 can be quickly washed out or deposited back to the ground by precipitation. However, photolytic reactions of chlorine molecules can lead to the production of chlorine atoms (Cl•), which leads to the formation of reactive halogen species (RHS). RHS have a lifespan of less than 180 days. RHS also lead to an increase in ozone (O3) formation in the atmosphere by oxidizing volatile organic compounds (VOCs). In Eq. 5–Eq. 9, VOCs are represented by R-OH (Tanaka et al. 2000; Li et al. 2020). O(3P) represents reactive oxygen species.
| 5 |
| 6 |
| 7 |
| 8 |
| 9 |
Ozone at ground level is a secondary air pollutant that is known to have adverse acute and chronic health impacts on humans and to decrease plant productivity significantly.
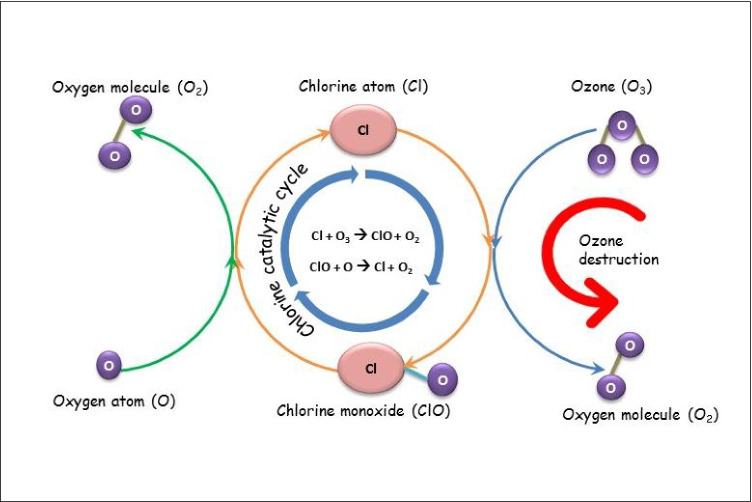
Further, at higher altitudes, the Cl• can form various NOx compounds and can destroy the ozone layer directly or indirectly by acting as a sink for NO2 (Eq. 10–Eq.15) (Li et al. 2020; Saiz-Lopez and von Glasow 2012; Simpson et al. 2015). Fig. 3 displays the chlorine catalytic cycle that leads to the destruction of the ozone layer. Chlorine acts as a catalyst in the net reaction of one ozone molecule (O3) reacting with an oxygen (O) atom to form two molecules of oxygen (O2). In each cycle, the Cl and ClO are regenerated while ozone is simply removed (NOAA 2010).
| 10 |
| 11 |
| 12 |
| 13 |
| 14 |
| 15 |
Fig. 3.
Ozone destruction cycle of chlorine. The chlorine catalytic cycle involves the cyclic formation of Cl atom and ClO and subsequent destruction of the ozone layer
Incineration of municipal waste that contains chlorine and/or HCl leads to the formation of chlorinated polycyclic aromatic hydrocarbons (ClPAHs) (Yoshino and Urano 1997). Mass disinfection practices lead to CBDs in open dump yards and community bins. Some of this waste containing CBDs is later incinerated. Further, incineration of clinical wastes from COVID-19 medical facilities and other hospitals is also treated with high concentrations of CBDs and are often incinerated. The exponential increase in clinical waste due to the COVID-19 outbreak has resulted in the co-incineration of clinical waste with municipal solid wastes as an emergency measure (Lan et al. 2022). This usually requires the clinical wastes to be disinfected with CBDs first and then transported to the incineration facility. As a result of this, the HCl concentration in the flue gases of mixed incineration facilities increased (Lan et al. 2022). These practices contributed to the chlorination of parental PAH molecules to form ClPAHs which are more toxic. Additionally, the photochemical reactions in air between the Cl• radical and parent PAHs also produce ClPAHs (Eq. 16–Eq. 18) (Vuong et al. 2020).
| 16 |
| 17 |
| 18 |
ClPAHs are hazardous air pollutants since they are proven to be carcinogenic and mutagenic in animal studies (Kamiya et al. 2016). These compounds are highly lipophilic and persistent in nature; therefore, they can be transferred through food chains and have the potential to bioaccumulate in animal tissues (Xie et al. 2021).
Another consequence of the increase in chlorine concentration in the air is the significant rise in PM2.5 (particulate matter of size less than 2.5-µm diameter) concentration. Anthropogenic chlorine emissions from burning agricultural wastes, biofuels, municipal waste incineration, and various other industrial sources across China were found to increase the total inorganic PM2.5 up to 3.2 µg/m3 per annum (Wang et al. 2020a, b). Another study from China also reported a similar finding with a monthly average increase in PM2.5 by 7.5 µg/m3 (9.1%) due to chlorine emissions into the atmosphere (Zhang et al. 2021c, a, b). Particulate matters are primary air pollutants that have numerous health as well as environmental impacts. Some of the human health impacts of particulate matter include premature death of people with lung or heart problems, aggravated asthma, decreased lung function, and nonfatal heart attack. PM2.5 is especially known to penetrate deep into the lungs, irritate and corrode the alveolar wall, and subsequently decrease lung functions (Xing et al. 2016).
Although most studies on halogen chemistry in the atmosphere consider marine water and polar ice as chlorine sources, they ascribed industrial point sources such as chlorine manufacturing factories, water and wastewater treatment plants, swimming pools, and cooling towers as anthropogenic sources. It is well known that these facilities involve gaseous chlorine or hypochlorite in their operations and involve the emission of chlorine in molecular or ion forms. Therefore, the impact of mass disinfection, especially the ones using fire hoses and nozzles, needs to be addressed and accounted for.
Ecotoxicity of DBPs to aquatic life of receiving water bodies
Aquatic organisms that thrive in receiving water bodies are the first to be exposed to the incoming DBPs. In a recent study, the additive toxicity of 44 DBPs to green algae, daphnid, and fish was evaluated. Major C-DBPs (THMs, HAAs, and HKs) and few N-DBPs (NAs, HANs, and HNMs) which are usually found in wastewater showed severe toxicity to green algae and a lower risk to fish and daphnids (Li et al. 2019). Among them, the N-DBP groups such as HANs and NAs accounted for the highest contribution in additive toxicity. This observation is alarming for two reasons. First, these two groups contributed only 10% and 1% respectively of the total DBPs in their study yet had the highest contribution to ecological risk. Second, as discussed in the preceding section, chlorination of partially/untreated wastewater results in the formation of large amounts of N-DBPs.
In another study, the effect of chlorinated WWTP discharge on DBP toxicity to three different bacterial species was monitored (Pignata et al. 2012). Influent to the WWTP had background concentrations of all four THMs and six other DBPs, and this posed some toxicity to the tested bacterial cells. After chlorination, acute toxicity was observed for most of the effluent samples. Eight samples out of 22 exhibited appreciable calculated toxicity with two of them exceeding the limit of acute toxicity unit (TU = 0.3) set by USEPA. These results successfully show that DBPs reaching a water body create lethal living conditions for aquatic life. Zebrafish embryos exposed to THMs, HAAs, bromate, haloacetamides (HAcAms), and several other chloro/bromo/iodo DBPs resulted in retarded hatching rates, growth inhibition, malformations, morphological changes (Ding et al. 2020; Hanigan et al. 2017; Teixidó et al. 2015). Embryo development under increasing concentrations of an emerging N-DBP class, monohaloacetamides, showed decreased hatching rate (from 93.2% hatching rate for control to 7.50% for maximum tested concentration of DBP), increased mortality rate, morphological deformities such as severe curvature in body axis and pericardial oedema, and decreased locomotor behavior of the embryo which stimulates the hatching process (Ding et al. 2020). Similar findings were obtained for marine organisms when they were in contact with DBPs (Liu and Zhang 2014; Yang and Zhang 2013). These findings prove that even higher vertebrates in aquatic environments were affected by DBPs. The toxicity and accumulation of chlorination by-products on planktons and fish in an aquatic environment may lead to chronic adverse effects on ecosystems. DBP toxicity to aquatic life is ill-defined when compared to the vast knowledge available regarding human health impact assessments. Although the latter helps in understanding the impact on humans, bioaccumulation of DBPs in aquatic organisms and their effects need to be explored. The stability of an aquatic system depends greatly on its biodiversity, especially on the microorganisms and plankton which are considered to form the basis of life in a water body.
Conclusions and outlook
CBDs are being used extensively amidst the COVID-19 pandemic for environmental cleaning and hospital disinfection. Chlorine is volatile and tends to leave hypochlorite solution in gaseous form under certain conditions. This tendency creates an inhalation risk for people near disinfectant sprayers and adds to air pollution. CBDs applied in public spaces will be carried along with sewage or stormwater where they will react with natural organic matter and form several DBPs. HWW-DBPs of medical products such as tramadol, antibiotics, anti-cancer drugs, and ICM are more toxic than the parent compounds and many carbonaceous DBPs. Elevated chlorine concentrations in soil and intake water can cause chlori(de)ne toxicity in plants. WW discharges from sewers and WWTPs into aquatic ecosystems are likely to have mixtures of several DBPs. In conclusion, environmental disinfection practices need to be reconsidered for their effectiveness as well as for potential environmental impacts. The following recommendations are noted here:
Workers spraying CBDs in virus risk areas such as hospitals and their premises should wear personal protective equipment designed to reduce the risk of inhalation of CBDs and their associated DBPs.
Unnecessary use of CBDs by spraying on vegetation and soil where direct human/animal exposure to COV-2 is not likely is undesirable since it is likely to destroy/inhibit the flora and fauna.
Mass disinfection by spraying huge amounts of CBDs in streets needs to be reduced unless it is essential because it makes people on the streets vulnerable to inhalation risk due to exposure to CBDs. Direct spraying of people with CBDs is also harmful, and alcohol-based disinfectants should be considered.
Proper guidelines need to be laid regarding the use of CBDs at household levels, institutes, industries, and hospitals.
Clinical wastes which are disinfected using CBDs should not be incinerated. Instead, alternate treatment procedures should be adopted.
Hospital wastewater treatment needs to be improved to reduce or eliminate discharges of DBPs into receiving water bodies.
Use of alternate disinfectants such as ozone, UV radiation, or sunlight/solar disinfection should be evaluated since no free disinfectant residual is required after wastewater disinfection. This will reduce the amount of DBPs formed and will mitigate human and ecological risks due to excessive use of CBDs.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
NP: conceptualization, literature review, data gathering, and writing—original draft; SG: literature review, supervising, and writing—review, editing, and correspondence; SC: supervising and writing—review and editing. All authors read and approved the final manuscript.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Achak M, Alaoui Bakri S, Chhiti Y, M’hamdiAlaoui FE, Barka N, Boumya W. SARS-CoV-2 in hospital wastewater during outbreak of COVID-19: a review on detection survival and disinfection technologies. Sci Total Environ. 2021;761:143192. doi: 10.1016/j.scitotenv.2020.143192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrzejewski, P., Nikolaou, A.D. (Eds.) (2003) Haloforms and related compounds in drinking water, The handbook of environmental chemistry water pollution. Springer, Berlin
- Bakhtiyari S, Mirzaei A, Jalilian M, Mazlomi S, Nourmoradi H, Kakaei H. The effects of personal, environmental, and genetic factors on epidemic of coronavirus disease-19: a review of the current literature. Open Access Maced J Med Sci. 2020;8:250–257. doi: 10.3889/oamjms.2020.5098. [DOI] [Google Scholar]
- Bhat SA, Sher F, Kumar R, Karahmet E, Haq SAU, Zafar A, Lima EC. Environmental and health impacts of spraying COVID-19 disinfectants with associated challenges. Environ Sci Pollut Res. 2021 doi: 10.1007/s11356-021-16575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, B., Westerhoff, P., Krasner, S.W. (2008) Fate and transport of wastewater-derived disinfection by-products in surface waters, in: Karanfil, T., Krasner, S.W., Westerhoff, P., Xie, Y. (Eds.), Disinfection by-products in drinking water, ACS Symposium Series. American Chemical Society, Washington, DC, pp. 257–273. 10.1021/bk-2008-0995.ch018
- Chen B, Han J, Dai H, Jia P. Biocide-tolerance and antibiotic-resistance in community environments and risk of direct transfers to humans: unintended consequences of community-wide surface disinfecting during COVID-19? Environ Pollut. 2021;283:117074. doi: 10.1016/j.envpol.2021.117074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Guo J, Jiang Y, Shao Y. High concentration and high dose of disinfectants and antibiotics used during the COVID-19 pandemic threaten human health. Environ Sci Eur. 2021;33:11. doi: 10.1186/s12302-021-00456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu W, Fang C, Deng Y, Xu Z. Intensified disinfection amid COVID-19 pandemic poses potential risks to water quality and safety. Environ Sci Technol. 2021;55:4084–4086. doi: 10.1021/acs.est.0c04394. [DOI] [PubMed] [Google Scholar]
- Collins DB, Farmer DK. Unintended consequences of air cleaning chemistry. Environ Sci Technol. 2021;55:12172–12179. doi: 10.1021/acs.est.1c02582. [DOI] [PubMed] [Google Scholar]
- Cotruvo JA, Amato H. Trihalomethanes: concentrations, cancer risks, and regulations. J - Am Water Works Assoc. 2019;111:12–20. doi: 10.1002/awwa.1210. [DOI] [Google Scholar]
- Cui H, Zhu X, Zhu Y, Huang Y, Chen B. Ecotoxicological effects of DBPs on freshwater phytoplankton communities in co-culture systems. J Hazard Mater. 2022;421:126679. doi: 10.1016/j.jhazmat.2021.126679. [DOI] [PubMed] [Google Scholar]
- Dhama K, Patel SK, Kumar R, Masand R, Rana J, Yatoo, Mohd I, Tiwari R, Sharun K, Mohapatra RK, Natesan S, Dhawan M, Ahmad T, Emran TB, Malik YS, Harapan H. The role of disinfectants and sanitizers during COVID-19 pandemic: advantages and deleterious effects on humans and the environment. Environ Sci Pollut Res. 2021;28:34211–34228. doi: 10.1007/s11356-021-14429-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G, Zhang X, Yang M, Pan Y. Formation of new brominated disinfection byproducts during chlorination of saline sewage effluents. Water Res. 2013;47:2710–2718. doi: 10.1016/j.watres.2013.02.036. [DOI] [PubMed] [Google Scholar]
- Ding X, Zhu J, Zhang J, Dong T, Xia Y, Jiao J, Wang X, Zhou W. Developmental toxicity of disinfection by-product monohaloacetamides in embryo-larval stage of zebrafish. Ecotoxicol Environ Saf. 2020;189:110037. doi: 10.1016/j.ecoenv.2019.110037. [DOI] [PubMed] [Google Scholar]
- Dindarloo K, Aghamolaei T, Ghanbarnejad A, Turki H, Hoseinvandtabar S, Pasalari H, Ghaffari HR. Pattern of disinfectants use and their adverse effects on the consumers after COVID-19 outbreak. J Environ Health Sci Eng. 2020;18:1301–1310. doi: 10.1007/s40201-020-00548-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Qiang Z, Richardson SD. Formation of iodinated disinfection byproducts (I-DBPs) in drinking water: emerging concerns and current issues. Acc Chem Res. 2019;52:896–905. doi: 10.1021/acs.accounts.8b00641. [DOI] [PubMed] [Google Scholar]
- Du Y, Wang W-L, He T, Sun Y-X, Lv X-T, Wu Q-Y, Hu H-Y. Chlorinated effluent organic matter causes higher toxicity than chlorinated natural organic matter by inducing more intracellular reactive oxygen species. Sci Total Environ. 2020;701:134881. doi: 10.1016/j.scitotenv.2019.134881. [DOI] [PubMed] [Google Scholar]
- Duirk SE, Lindell C, Cornelison CC, Kormos J, Ternes TA, Attene-Ramos M, Osiol J, Wagner ED, Plewa MJ, Richardson SD. Formation of toxic iodinated disinfection by-products from compounds used in medical imaging. Environ Sci Technol. 2011;45:6845–6854. doi: 10.1021/es200983f. [DOI] [PubMed] [Google Scholar]
- Feng H, Ruan Y, Wu R, Zhang H, Lam PKS. Occurrence of disinfection by-products in sewage treatment plants and the marine environment in Hong Kong. Ecotoxicol Environ Saf. 2019;181:404–411. doi: 10.1016/j.ecoenv.2019.06.034. [DOI] [PubMed] [Google Scholar]
- Future Market Insights (2020) Chlorine disinfectant sales soar at a promising pace as rise in COVID-19 caseloads accelerates demand at hospitals, finds a new FMI study (market research report). [WWW Document} URL https://www.futuremarketinsights.com/press-release/chlorine-disinfectant-market. Accessed 10.27.2021
- Goel S (2015) Antibiotics in the environment: a review. In: Kurwadkar S, Zhang X (Jackie), Ramirez D, Mitchell FL (eds) Emerging micro-pollutants in the environment: occurrence, fate, and distribution, ACS Symposium Series. American Chemical Society, Washington, DC, pp 19–42. 10.1021/bk-2015-1198.ch002
- Goel S (2019) Water and wastewater engineering, 1st edition. ed. Cambridge University Press, Cambridge, United Kingdom; New York, NY, USA
- Guo J, Liao M, He B, Liu J, Hu X, Yan D, Wang J. Impact of the COVID-19 pandemic on household disinfectant consumption behaviors and related environmental concerns: a questionnaire-based survey in China. J Environ Chem Eng. 2021;9:106168. doi: 10.1016/j.jece.2021.106168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha Y, Koo Y, Kwon J-H. Personal passive air samplers for chlorinated gases generated from the use of consumer products. Int J Environ Res Public Health. 2021;18:8940. doi: 10.3390/ijerph18178940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajrasuliha S. Accumulation and toxicity of chloride in bean plants. Plant Soil. 1980;55:133–138. doi: 10.1007/BF02149716. [DOI] [Google Scholar]
- Hanigan D, Truong L, Simonich M, Tanguay R, Westerhoff P. Zebrafish embryo toxicity of 15 chlorinated, brominated, and iodinated disinfection by-products. J Environ Sci. 2017;58:302–310. doi: 10.1016/j.jes.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Hsiao AI, Worsham AD, Moreland DE. Effects of sodium hypochlorite and certain plant growth regulators on germination of witchweed (Striga asiatica ) Seeds. Weed Sci. 1981;29:98–100. doi: 10.1017/S0043174500025911. [DOI] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Zhang Y, Zhou Q, Li A, Shi P, Qiu J, Pan Y. Detection, identification and control of polar iodinated disinfection byproducts in chlor(am)inated secondary wastewater effluents. Environ Sci Water Res Technol. 2019;5:397–405. doi: 10.1039/c8ew00718g. [DOI] [Google Scholar]
- IRIS (1987) Chemical assessment summary: N-nitrosodimethylamine; CASRN 62-75-9 (chemical assessment summary). U.S. Environmental Protection Agency, National Center for Environmental Assessment. [WWW Document] URL https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0045_summary.pdf. Accessed 07.28.2020
- Jones RR, DellaValle CT, Weyer PJ, Robien K, Cantor KP, Krasner S, Beane Freeman LE, Ward MH. Ingested nitrate, disinfection by-products, and risk of colon and rectal cancers in the Iowa Women’s Health Study cohort. Environ Int. 2019;126:242–251. doi: 10.1016/j.envint.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya Y, Iijima A, Ikemori F, Okuda T, Ohura T. Source apportionment of chlorinated polycyclic aromatic hydrocarbons associated with ambient particles in a Japanese megacity. Sci Rep. 2016;6:38358. doi: 10.1038/srep38358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ. The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales. UK. Water Res. 2008;42:3498–3518. doi: 10.1016/j.watres.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Kataki S, Chatterjee S, Vairale MG, Sharma S, Dwivedi SK. Concerns and strategies for wastewater treatment during COVID-19 pandemic to stop plausible transmission. Resour Conserv Recycl. 2021;164:105156. doi: 10.1016/j.resconrec.2020.105156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemeš JJ, Fan YV, Jiang P. The energy and environmental footprints of COVID-19 fighting measures—PPE, disinfection, supply chains. Energy. 2020;211:118701. doi: 10.1016/j.energy.2020.118701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koksoy Vayisoglu S, Oncu E (2021) The use of cleaning products and its relationship with the increasing health risks during the COVID‐19 pandemic. Int J Clin Pract 75. 10.1111/ijcp.14534 [DOI] [PMC free article] [PubMed]
- Krasner SW, Westerhoff P, Chen B, Rittmann BE, Amy G. Occurrence of disinfection byproducts in United States wastewater treatment plant effluents. Environ Sci Technol. 2009;43:8320–8325. doi: 10.1021/es901611m. [DOI] [PubMed] [Google Scholar]
- Krasner SW, Westerhoff P, Chen B, Rittmann BE, Nam S-N, Amy G. Impact of wastewater treatment processes on organic carbon, organic nitrogen, and DBP precursors in effluent organic matter. Environ Sci Technol. 2009;43:2911–2918. doi: 10.1021/es802443t. [DOI] [PubMed] [Google Scholar]
- Kumari M, Gupta SK. Age dependent adjustment factor (ADAF) for the estimation of cancer risk through trihalomethanes (THMs) for different age groups—a innovative approach. Ecotoxicol Environ Saf. 2018;148:960–968. doi: 10.1016/j.ecoenv.2017.11.067. [DOI] [Google Scholar]
- La Rosa G, Bonadonna L, Lucentini L, Kenmoe S, Suffredini E. Coronavirus in water environments: Occurrence, persistence and concentration methods—a scoping review. Water Res. 2020;179:115899. doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan D-Y, Zhang H, Wu T-W, Lü F, Shao L-M, He P-J. Repercussions of clinical waste co-incineration in municipal solid waste incinerator during COVID-19 pandemic. J Hazard Mater. 2022;423:127144. doi: 10.1016/j.jhazmat.2021.127144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritano D, Moreo G, Limongelli L, Nardone M, Carinci F. Environmental disinfection strategies to prevent indirect transmission of SARS-CoV2 in healthcare settings. Appl Sci. 2020;10:6291. doi: 10.3390/app10186291. [DOI] [Google Scholar]
- Lee SC, Guo H, Lam SMJ, Lau SLA. Multipathway risk assessment on disinfection by-products of drinking water in Hong Kong. Environ Res. 2004;94:47–56. doi: 10.1016/S0013-9351(03)00067-7. [DOI] [PubMed] [Google Scholar]
- Li Q, Badia A, Wang T, Sarwar G, Fu X, Zhang L, Zhang Q, Fung J, Cuevas CA, Wang S, Zhou B, Saiz-Lopez A. Potential effect of halogens on atmospheric oxidation and air quality in China. J Geophys Res Atmospheres. 2020;125:032058. doi: 10.1029/2019JD032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Liu X, Huang Z, Hu S, Wang J, Qian Z, Feng J, Xian Q, Gong T. Occurrence and ecological risk assessment of disinfection byproducts from chlorination of wastewater effluents in East China. Water Res. 2019;157:247–257. doi: 10.1016/j.watres.2019.03.072. [DOI] [PubMed] [Google Scholar]
- Li Z, Song G, Bi Y, Gao W, He A, Lu Y, Wang Y, Jiang G. Occurrence and distribution of disinfection byproducts in domestic wastewater effluent, tap water, and surface water during the SARS-CoV-2 pandemic in China. Environ Sci Technol. 2021;55:4103–4114. doi: 10.1021/acs.est.0c06856. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang X. Comparative toxicity of new halophenolic DBPs in chlorinated saline wastewater effluents against a marine alga: halophenolic DBPs are generally more toxic than haloaliphatic ones. Water Res. 2014;65:64–72. doi: 10.1016/j.watres.2014.07.024. [DOI] [PubMed] [Google Scholar]
- Liu X, Hong Y, Ding S, Jin W, Dong S, Xiao R, Chu W. Transformation of antiviral ribavirin during ozone/PMS intensified disinfection amid COVID-19 pandemic. Sci Total Environ. 2021;790:148030. doi: 10.1016/j.scitotenv.2021.148030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou J, Wang W, Lu H, Wang L, Zhu L. Increased disinfection byproducts in the air resulting from intensified disinfection during the COVID-19 pandemic. J Hazard Mater. 2021;418:126249. doi: 10.1016/j.jhazmat.2021.126249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Feng L, Liu Y, Zhang L. Disinfection by-products formation and acute toxicity variation of hospital wastewater under different disinfection processes. Sep Purif Technol. 2020;238:116405. doi: 10.1016/j.seppur.2019.116405. [DOI] [Google Scholar]
- Luan X, Liu X, Fang C, Chu W, Xu Z. Ecotoxicological effects of disinfected wastewater effluents: a short review of in vivo toxicity bioassays on aquatic organisms. Environ Sci Water Res Technol. 2020;6:2275–2286. doi: 10.1039/D0EW00290A. [DOI] [Google Scholar]
- Meade E, Slattery MA, Garvey M. Biocidal resistance in clinically relevant microbial species: a major public health risk. Pathogens. 2021;10:598. doi: 10.3390/pathogens10050598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitch WA, Sharp JO, Trussell RR, Valentine RL, Alvarez-Cohen L, Sedlak DL. N -nitrosodimethylamine (NDMA) as a drinking water contaminant: a review. Environ Eng Sci. 2003;20:389–404. doi: 10.1089/109287503768335896. [DOI] [Google Scholar]
- Mohanan PV, Sangeetha V, Sabareeswaran A, Muraleedharan V, Jithin K, Vandana U, Varsha SB. Safety of 05% hydrogen peroxide mist used in the disinfection gateway for COVID-19. Environ Sci Pollut Res. 2021 doi: 10.1007/s11356-021-15164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negreira N, López de Alda M, Barceló D. Degradation of the cytostatic etoposide in chlorinated water by liquid chromatography coupled to quadrupole-Orbitrap mass spectrometry: identification and quantification of by-products in real water samples. Sci Total Environ. 2015;506–507:36–45. doi: 10.1016/j.scitotenv.2014.10.097. [DOI] [PubMed] [Google Scholar]
- Negreira N, Regueiro J, López de Alda M, Barceló D. Reactivity of vinca alkaloids during water chlorination processes: identification of their disinfection by-products by high-resolution quadrupole-Orbitrap mass spectrometry. Sci Total Environ. 2016;544:635–644. doi: 10.1016/j.scitotenv.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Negreira N, Regueiro J, López de Alda M, Barceló D. Transformation of tamoxifen and its major metabolites during water chlorination: identification and in silico toxicity assessment of their disinfection byproducts. Water Res. 2015;85:199–207. doi: 10.1016/j.watres.2015.08.036. [DOI] [PubMed] [Google Scholar]
- Negreira N, Regueiro J, López de Alda M, Barceló D. Degradation of the anticancer drug erlotinib during water chlorination: non-targeted approach for the identification of transformation products. Water Res. 2015;85:103–113. doi: 10.1016/j.watres.2015.08.005. [DOI] [PubMed] [Google Scholar]
- NOAA (2010) NOAA CSL: Scientific assessment of ozone depletion: 2010 [WWW document]. Chemical Sciences Laboratory, National Oceanic Atmospheric Administration. URL https://csl.noaa.gov/assessments/ozone/2010/. Accessed 10.27.21
- Pan L, Wang J, Wang X, Ji JS, Ye D, Shen J, Li L, Liu H, Zhang L, Shi X, Wang L. Prevention and control of coronavirus disease 2019 (COVID-19) in public places. Environ Pollut. 2022;292:118273. doi: 10.1016/j.envpol.2021.118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelaki I, Voutsa D. Formation of iodinated THMs during chlorination of water and wastewater in the presence of different iodine sources. Sci Total Environ. 2018;613–614:389–397. doi: 10.1016/j.scitotenv.2017.09.072. [DOI] [PubMed] [Google Scholar]
- Parveen N, Chowdhury S, Goel S. Probabilistic approach for health hazard assessment of trihalomethanes through successive showering events. Environ Sci Pollut Res. 2021 doi: 10.1007/s11356-021-17087-0. [DOI] [PubMed] [Google Scholar]
- Paul D, Kolar P, Hall SG. A review of the impact of environmental factors on the fate and transport of coronaviruses in aqueous environments. Npj Clean Water. 2021;4:7. doi: 10.1038/s41545-020-00096-w. [DOI] [Google Scholar]
- Phatthalung WN, Musikavong C. Emerging disinfection by-products’ formation potential in raw water, wastewater, and treated wastewater in Thailand. J. Environ. Sci. Health - Part ToxicHazardous Subst. Environ Eng. 2019;54:745–758. doi: 10.1080/10934529.2019.1592532. [DOI] [PubMed] [Google Scholar]
- Pignata C, Fea E, Rovere R, Degan R, Lorenzi E, de Ceglia M, Schilirò T, Gilli G. Chlorination in a wastewater treatment plant: acute toxicity effects of the effluent and of the recipient water body. Environ Monit Assess. 2012;184:2091–2103. doi: 10.1007/s10661-011-2102-y. [DOI] [PubMed] [Google Scholar]
- Probst LF, Guerrero ATG, de Queiroz Cardoso AI, Grande AJ, Croda MG, Venturini J, de Camargo Fonseca MC, Paniago AMM, Barreto JOM, do Vale Leone de Oliveira SM. Mask decontamination methods (model N95) for respiratory protection: a rapid review. Systematic Reviews. 2021;10:219. doi: 10.21203/rs.3.rs-38933/v3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachidi H, Hamdaoui S, Merimi I, Bengourram J, Latrache H. COVID-19: unbalanced management of occupational risks—case of the analysis of the chemical risk related to the use of disinfectants in the dairy industry in Morocco. Environ Sci Pollut Res. 2021 doi: 10.1007/s11356-021-13846-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redon P-O, Abdelouas A, Bastviken D, Cecchini S, Nicolas M, Thiry Y. Chloride and organic chlorine in forest soils: storage, residence times, and influence of ecological conditions. Environ Sci Technol. 2011;45:7202–7208. doi: 10.1021/es2011918. [DOI] [PubMed] [Google Scholar]
- Richardson SD, Plewa MJ, Wagner ED, Schoeny R, DeMarini DM. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat Res - Rev Mutat Res. 2007;636:178–242. doi: 10.1016/j.mrrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Romanucci V, Siciliano A, Galdiero E, Guida M, Luongo G, Liguori R, Di Fabio G, Previtera L, Zarrelli A. Disinfection by-products and ecotoxic risk associated with hypochlorite treatment of tramadol. Molecules. 2019;24(4):693. doi: 10.3390/molecules24040693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rúa-Gómez PC, Püttmann W. Occurrence and removal of lidocaine, tramadol, venlafaxine, and their metabolites in German wastewater treatment plants. Environ Sci Pollut Res. 2012;19:689–699. doi: 10.1007/s11356-011-0614-1. [DOI] [PubMed] [Google Scholar]
- Saiz-Lopez A, von Glasow R. Reactive halogen chemistry in the troposphere. Chem Soc Rev. 2012;41:6448. doi: 10.1039/c2cs35208g. [DOI] [PubMed] [Google Scholar]
- Shah AD, Mitch WA. Halonitroalkanes, halonitriles, haloamides, and N-nitrosamines: a critical review of nitrogenous disinfection byproduct formation pathways. Environ Sci Technol. 2012;46:119–131. doi: 10.1021/es203312s. [DOI] [PubMed] [Google Scholar]
- Sharafi SM, Ebrahimpour K, Nafez A. Environmental disinfection against COVID-19 in different areas of health care facilities: a review. Rev Environ Health. 2020;63(2):193–198. doi: 10.1515/reveh-2020-0075. [DOI] [PubMed] [Google Scholar]
- Shimabukuro PMS, Duarte ML, Imoto AM, Atallah ÁN, Franco ESB, Peccin MS, Taminato M. Environmental cleaning to prevent COVID-19 infection. A rapid systematic review. Sao Paulo Med J. 2020;138:505–514. doi: 10.1590/1516-3180.2020.0417.09092020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson WR, Brown SS, Saiz-Lopez A, Thornton JA, von Glasow R. Tropospheric halogen chemistry: sources, cycling, and impacts. Chem Rev. 2015;115:4035–4062. doi: 10.1021/cr5006638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka PL, Oldfield S, Neece JD, Mullins CB, Allen DT. Anthropogenic sources of chlorine and ozone formation in urban atmospheres. Environ Sci Technol. 2000;34:4470–4473. doi: 10.1021/es991380v. [DOI] [Google Scholar]
- Teixidó E, Piqué E, Gonzalez-Linares J, Llobet JM, Gómez-Catalán J. Developmental effects and genotoxicity of 10 water disinfection by-products in zebrafish. J Water Health. 2015;13:54–66. doi: 10.2166/wh.2014.006. [DOI] [PubMed] [Google Scholar]
- Ternes TA, Hirsch R. Occurrence and behavior of X-ray contrast media in sewage facilities and the aquatic environment. Environ Sci Technol. 2000;34:2741–2748. doi: 10.1021/es991118m. [DOI] [Google Scholar]
- Thurman, E.M. (1985) Organic geochemistry of natural waters, Developments in Biogeochemistry. Springer, Netherlands. 10.1007/978-94-009-5095-5
- Ul’yanovskii NV, Kosyakov DS, Sypalov SA, Varsegov IS, Shavrina IS, Lebedev AT. Antiviral drug Umifenovir (Arbidol) in municipal wastewater during the COVID-19 pandemic: estimated levels and transformation. Sci Total Environ. 2022;805:150380. doi: 10.1016/j.scitotenv.2021.150380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong QT, Thang PQ, Ohura T, Choi S-D. Chlorinated and brominated polycyclic aromatic hydrocarbons in ambient air: seasonal variation, profiles, potential sources, and size distribution. Rev Environ Sci Biotechnol. 2020;19:259–273. doi: 10.1007/s11157-020-09535-z. [DOI] [Google Scholar]
- Wang H, Shi W, Ma D, Shang Y, Wang Y, Gao B. Formation of DBPs during chlorination of antibiotics and control with permanganate/bisulfite pretreatment. Chem Eng J. 2020;392:123701. doi: 10.1016/j.cej.2019.123701. [DOI] [Google Scholar]
- Wang L, Zhang X, Chen S, Meng F, Zhang D, Liu Y, Li M, Liu X, Huang X, Qu J. Spatial variation of dissolved organic nitrogen in Wuhan surface waters: correlation with the occurrence of disinfection byproducts during the COVID-19 pandemic. Water Res. 2021;198:117138. doi: 10.1016/j.watres.2021.117138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Jacob DJ, Fu X, Wang T, Breton ML, Hallquist M, Liu Z, McDuffie EE, Liao H. Effects of anthropogenic chlorine on PM2.5 and ozone air quality in China. Environ Sci Technol. 2020;54:9908–9916. doi: 10.1021/acs.est.0c02296. [DOI] [PubMed] [Google Scholar]
- White PJ, Broadley MR. Chloride in soils and its uptake and movement within the plant: a review. Ann Bot. 2001;88:967–988. doi: 10.1006/anbo.2001.1540. [DOI] [Google Scholar]
- Xie J, Tao L, Wu Q, Lei S, Lin T. Environmental profile, distributions and potential sources of halogenated polycyclic aromatic hydrocarbons. J Hazard Mater. 2021;419:126164. doi: 10.1016/j.jhazmat.2021.126164. [DOI] [PubMed] [Google Scholar]
- Xing Y-F, Xu Y-H, Shi M-H, Lian Y-X. The impact of PM2.5 on the human respiratory system. J Thorac Dis. 2016;8:E69–E74. doi: 10.3978/j.issn.2072-1439.2016.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, G., Magen, H., Tarchitzky, J., Kafkafi, U. (1999) Advances in chloride nutrition of plants, in: Sparks, D.L. (Ed.), Advances in agronomy. Academic Press, pp. 97–150. 10.1016/S0065-2113(08)60844-5
- Yang M, Zhang X. Comparative developmental toxicity of new aromatic halogenated DBPs in a chlorinated saline sewage effluent to the marine polychaete Platynereis dumerilii. Environ Sci Technol. 2013;47:10868–10876. doi: 10.1021/es401841t. [DOI] [PubMed] [Google Scholar]
- Yang M, Feng Y, Yuan L, Zhao H, Gao S, Li Z. High Concentration and frequent application of disinfection increase the detection of methicillin-resistant Staphylococcus aureus infections in psychiatric hospitals during the COVID-19 pandemic. Front Med. 2021;8:1918. doi: 10.3389/fmed.2021.722219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz M, Er C. The effect of sodium hypochlorite solutions on in vitro seedling growth and shoot regeneration of flax (Linum usitatissimum) Naturwissenschaften. 2002;89:259–261. doi: 10.1007/s00114-002-0310-6. [DOI] [PubMed] [Google Scholar]
- Yildiz M, FatihOzcan S, Kahramanogullari CT, Tuna E. The effect of sodium hypochlorite solutions on the viability and in vitro regeneration capacity of the tissue. Nat Prod J. 2012;2:328–331. doi: 10.2174/2210315511202040328. [DOI] [Google Scholar]
- Yoshino H, Urano K. Formation of chlorinated PAHs in exhaust gas from municipal waste incinerators, and their mutagenic activities. Toxicol Environ Chem. 1997;63:233–246. doi: 10.1080/02772249709358533. [DOI] [Google Scholar]
- Zhang D, Ling H, Huang X, Li J, Li W, Yi C, Zhang T, Jiang Y, He Y, Deng S, Zhang X, Wang X, Liu Y, Li G, Qu J. Potential spreading risks and disinfection challenges of medical wastewater by the presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of Fangcang Hospital. Sci Total Environ. 2020;741:140445. doi: 10.1016/j.scitotenv.2020.140445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Tang W, Chen Y, Yin W. Disinfection threatens aquatic ecosystems. Sci. 2020;368:146–147. doi: 10.1126/science.abb8905. [DOI] [PubMed] [Google Scholar]
- Zhang T, Xu Q, Shi Y-L, Chen Z, Lu Y, Yang H-W, Xie YF, Hou L. Study on the influence of operational and management processes of a water reclamation plant since COVID-19 situation. Environ Pollut. 2021;285:117257. doi: 10.1016/j.envpol.2021.117257. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu J, Tao W, Xiang S, Liu H, Yi K, Yang H, Xu J, Wang Y, Ma J, Wang X, Hu J, Wan Y, Wang X, Tao S. Impacts of chlorine emissions on secondary pollutants in China. Atmos Environ. 2021;246:118177. doi: 10.1016/j.atmosenv.2020.118177. [DOI] [Google Scholar]
- Zhang Z, Zhang Q, Lu T, Zhang J, Sun L, Hu B, Hu J, Peñuelas J, Zhu L, Qian H. Residual chlorine disrupts the microbial communities and spreads antibiotic resistance in freshwater. J Hazard Mater. 2022;423:127152. doi: 10.1016/j.jhazmat.2021.127152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhou Y, Han L, Guo X, Wu Z, Fang J, Hou B, Cai Y, Jiang J, Yang Z (2021) Impacts of COVID-19 pandemic on the aquatic environment associated with disinfection byproducts and pharmaceuticals. Sci Total Environ 151409. 10.1016/j.scitotenv.2021.151409 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.