Abstract
Background:
This study aimed to develop an efficient and easily calculable risk score that can be used to identify an individual’s risk of having been exposed to alcohol prenatally.
Methods:
Data for this study were collected as part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders, Phases 2 and 3. Two cohorts (ages 5–17 yrs.) completed a comprehensive neurobehavioral battery and a standard dysmorphology exam: a development cohort (DC; n =325) and a comparative cohort (CC; n = 523). Both cohorts included two groups: those with histories of heavy prenatal alcohol exposure (AE-DC, n = 121; AE-CC, n = 177) and a control group that included subjects with minimal or no prenatal alcohol exposure (CON-DC, n = 204; CON-CC, n = 346). Behavioral assessments and physical exam data were combined using regression techniques to derive a risk score indicating the likelihood of prenatal alcohol exposure. Subjects were then divided into two subgroups: 1) low-risk, and 2) high-risk. Chi-square (χ2) determined classification accuracy and an ROC was produced to assess the predictive accuracy. Correlations between risk scores and IQ and executive function (EF) scores were calculated.
Results:
Subjects were accurately classified in the DC (χ2= 78.61, p <.001) and CC (χ2 = 86.63, p <.001). The classification model also performed well in the DC (ROC = .835 [SE = 0.024, p <.001]) and CC (ROC = .786 [SE = .021, p <.001]. In the AE-CC and CON-CC, modest but significant associations between the risk score and EF (AE-CC: r = −.20, p = .034; CON-CC: r = −.28, p <.001) and IQ (AE-CC: r = −.20, p = .034; CON-CC: r = −.28, p <.001) were found.
Conclusion(s):
The risk score significantly distinguished alcohol-exposed from control subjects and correlated with important cognitive outcomes. It has significant clinical potential and could be easily deployed in clinical settings.
Keywords: Fetal alcohol spectrum disorders (FASD), prenatal alcohol exposure, risk score, behavior, diagnosis, identification
Introduction
Fetal alcohol syndrome (FAS), which requires the presence of distinct facial dysmorphology (Hoyme et al., 2016), was first recognized in 1973 (Jones and Smith), and is readily identified by trained clinicians (Jones et al., 2006). However, the majority of individuals affected by prenatal alcohol exposure do not present with all of these distinct facial features (i.e., a smooth philtrum, thin vermillion border, and small palpebral fissures) and are less likely to be identified despite having significant cognitive and behavioral impairments (Mattson et al., 2011, Mattson et al., 2019). Fetal alcohol spectrum disorder (FASD) refers to the range of disorders observed in association with prenatal alcohol exposure with and without the dysmorphology (Hoyme et al., 2016, Bertrand et al., 2005). The varying phenotypic presentations, combined with the overlapping clinical symptoms with other disorders, including attention-deficit/hyperactivity disorder (ADHD) and oppositional defiant disorder, results in many individuals going undiagnosed or being misdiagnosed (Chasnoff et al., 2015). When population-based studies of prevalence were carried out among first grade children in four regions of the United States, a conservative rate of FASD was reported as 1.1% to 5%, and the weighted prevalence was estimated to be 3.1% to 9.9%. (May et al., 2018). Less than 1% of the identified cases had been diagnosed previously, further underscoring the low rate of detection and diagnosis of children with an FASD. Therefore, the development of improved screening tools that can be used to identify children who may have been affected by prenatal alcohol exposure is essential.
Some clinical tools have shown promise in their ability to aid in the identification of individuals with prenatal alcohol exposure (Mattson and Riley, 2011, Mattson et al., 2010b, Mattson et al., 2013, Burd et al., 2010, Klug et al., 2021, Widder et al., 2021). However, these tools require a large amount of information which may not be readily available, including neurobehavior and intelligence quotient (IQ) data, maternal characteristics, and birth records. Consequently, there is a need to develop an efficient and easily calculable risk score to identify those that are at risk for FASD, especially when exposure history is unknown and/or suspected. Those identified as having an increased risk could then be referred for comprehensive evaluation. In this study, variables included in the risk score calculation included physical measurements as well as scores from parent questionnaires that assess adaptive functioning and behavioral concerns, all of which have previously been shown to be sensitive to the presence of prenatal alcohol exposure (Goh et al., 2016).
Adaptive Behavior
Adaptive functioning behaviors are those that are used in everyday activities and are required to successfully respond to daily demands (Sparrow et al., 1984). These behaviors translate to an individual’s ability to live independently, build relationships, and display social integration. A widely used measure of adaptive functioning is the Vineland Adaptive Behavior Scales (VABS; Sparrow et al., 2016), which breaks down adaptive behaviors into three main domains: 1) Communication, 2) Daily Living Skills, and 3) Socialization. Individuals with prenatal alcohol exposure display deficits in all three areas of the VABS (Crocker et al., 2009, Fagerlund et al., 2012, Jirikowic et al., 2008, Streissguth et al., 2004, Streissguth et al., 1991). Impairments in adaptive skills persist into adulthood and are greater than what would be predicted with IQ scores (Streissguth et al., 2004, Carr et al., 2010). As individuals age, there is a further decline observed in socialization (Crocker et al., 2009, Panczakiewicz et al., 2016, Whaley et al., 2001, Thomas et al., 1998, Fagerlund et al., 2012) and communication abilities (Crocker et al., 2009, Panczakiewicz et al., 2016) as individuals adapt to changing developmental expectations and environments. Previous studies comparing children with prenatal alcohol exposure to children with ADHD report that alcohol-exposed children display an arrest in the development of these skills, whereas children with ADHD exhibit a delay, with adaptive skills improving with age (Crocker et al., 2009). Alcohol-exposed children are also significantly more impaired in daily living skills compared to children with ADHD, suggesting that the use of measures of adaptive abilities could improve differential diagnosis between these two clinical groups.
Behavior Problems
The adaptive behavior impairments, as discussed above, are in part related to the challenges that alcohol-exposed individuals experience in home, school, and community settings (Streissguth et al., 2004, Rangmar et al., 2017). Parents indicate significant behavior problems for children with prenatal alcohol exposure (Mattson and Riley, 2000, Franklin et al., 2008, Vaurio et al., 2011), which are present for individuals with and without the facial dysmorphology (Mattson and Riley, 2000) and are not entirely explained by the diminished intellectual functioning commonly observed (Mattson and Riley, 2000, Vaurio et al., 2011). Increased externalizing problems (i.e., aggressive and delinquent behavior) are reported, compared to internalizing problems (i.e., withdrawn, somatic complaints, and anxious/depressed), for individuals with prenatal alcohol exposure (Mattson and Riley, 2000), although both are present. Behavioral difficulties continue into adulthood and often relate to a multitude of difficulties in mental health, social, and adaptive function (Moore and Riley, 2015, Spohr et al., 2007, Streissguth et al., 2004, Streissguth et al., 1996). Individuals with prenatal alcohol exposure are more likely to have issues in school, display inappropriate sexual behaviors, have increased rates of incarceration, and problems with alcohol/drugs. Other elements, including environmental or social factors, may play a role in the development of behavioral difficulties in affected children (Mattson et al., 2011). Early identification is critical as it allows for earlier intervention, which may serve as a protective factor against these continuing difficulties (Streissguth et al., 1996), further underlying the importance of developing a noninvasive risk score measure.
Study Aims
The overall goal of the current study was to develop and validate a screening tool to help clinicians identify children who may have been affected by prenatal alcohol exposure. To accomplish this goal, we created a numerical score, based on previously identified factors (Goh et al., 2016), that indicates an individual’s risk of prenatal alcohol exposure. Furthermore, we assessed the relationship between the risk score and important cognitive outcome variables (i.e., executive functioning abilities and an IQ score). We expected the risk score to be able to distinguish between alcohol-exposed and control subjects and to be correlated with the other cognitive variables.
Methods
Subjects
The current study collected data from subjects that were recruited for an ongoing multisite research study conducted as part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders, Phase 2 and 3 (CIFASD 2 & CIFASD 3). The total eligible sample for CIFASD 2 included 369 subjects. Subjects were excluded if they did not have all required data to create a risk score, which reduced the CIFASD 2 study sample to 325 subjects. Similarly, the total eligible sample for CIFASD 3 included 614 subjects, which was reduced to 523 subjects with complete risk score data. Both phases had subjects (5–17 years old) complete a standardized battery of neuropsychological tests as well as undergo a standardized dysmorphology exam (Mattson et al., 2010a, Jones et al., 2006). An FAS diagnosis was indicated if individuals had two of the three key facial features (short palpebral fissures, smooth philtrum, thin vermillion) and either microcephaly (head circumference ≤ 10th percentile) and/or growth deficiency (height or weight ≤ 10th percentile).
Histories of prenatal alcohol exposure were obtained through retrospective parental report, social service report, legal report, or medical records. Subjects included in the alcohol-exposed (AE) group had documented heavy prenatal alcohol exposure, defined as maternal ingestion of four or more drinks per occasion at least once per week or more than 13 drinks per week during pregnancy. When accurate exposure histories were unavailable, subjects were included in the study if there was suspected exposure and the subject met FAS criteria (defined above). Subjects were excluded from the AE group if there was evidence of other known causes of mental deficiency (e.g., congenital hypothyroidism, chromosomal abnormalities).
Subjects in the control (CON) group were matched on age, race/ethnicity, sex, and socioeconomic status to the AE group at each site. Control subjects were screened for prenatal alcohol exposure and were excluded if there was evidence of greater than minimal alcohol exposure (defined as more than one drink per week on average or two or more drinks per occasion during pregnancy) by the biological mother during pregnancy) or if alcohol exposure was unknown. Both phases included control (i.e., CON) subjects with and without clinical concerns (e.g., parent concerns about behavior, ADHD diagnosis, or below average IQ score), however for the current study these subgroups were combined to form a larger, heterogeneous comparison group. Subjects were excluded from either phase of the CIFASD study if they had previously suffered a significant head injury with loss of consciousness greater than 30 minutes, their primary language was anything other than English, were a part of an international adoption after 5 years old or less than 2 years prior to assessment, and/or if there was evidence of a physical or psychiatric disability that would interfere with the completion of the assessments. Parents/primary caregivers completed questionnaires about the subject’s behavior. Informed consent was obtained prior to participation and financial incentive was provided upon completion. The Institutional Review Board at each CIFASD site approved the procedures of the study.
Development Cohort
The development cohort (DC) refers to subjects used to develop the risk score model. This cohort of subjects participated in the second phase of the CIFASD project (CIFASD 2) which took place between 2007–2012. Subjects were recruited from five sites across the United States: (1) the Center for Behavioral Teratology at San Diego State University, (2) The Marcus Institute at Emory University, (3) the Fetal Alcohol and Related Disorders Clinic at University of California, Los Angeles, (4) the Center on Alcohol, Substance Use and Addictions at the University of New Mexico, and (5) seven Northern Plains communities under the leadership of the Center on Alcohol, Substance Use, and Addictions at the University of New Mexico. See Mattson et al. (2010a) for more details.
Comparative Cohort
The comparative cohort (CC) refers to subjects used to provide evidence for the accuracy of the risk score model. This cohort of subjects participated in the third phase of the CIFASD project (CIFASD 3) which took place between 2012–2017. Subjects were recruited from four sites across the United States: 1) the Center for Behavioral Teratology at San Diego State University; 2) Emory Neurobehavior and Exposure Clinic; 3) University of Minnesota; and 4) the Children’s Hospital at the University of Southern California.
Risk Score Measures
The risk score was developed using measures that have previously been shown to successfully distinguish children affected by prenatal alcohol exposure from controls (Goh et al., 2016). All measures from this decision tree were included except for IQ as it was used in the comparative analyses (see Table 3).
Table 3.
Variables and Cut-Off Criteria for Each Measure in the Risk Score Model
| Risk Score Variables | Description | Included Measures | Variable Cut-Off Value* |
|---|---|---|---|
| Dysmorphology 1 | Physical measurements consistent with a diagnosis of FAS | Palpebral fissure length percentile ≤10 percentile, philtrum lipometer score >3, vermillion Border lipometer score >3 | >0 criteria |
| Dysmorphology 2 | Physical deficits associated with prenatal alcohol exposure | Incomplete extension of digits, Ptosis | >0 criteria |
| VABS | Parent-reported adaptive functioning skills | Socialization, Communication, Daily Living Skills | >1 domain with standard scores <86 |
| CBCL | Parent-reported behavior concerns | Somatic Complaints, Social Problems, Attention Problems, Rule Breaking Behavior, Aggressive Behavior | >0 subscales with a T-score >65 |
Subjects were assigned a score of 1 for each variable that exceeded the cutoff. Variables were weighed in the final risk score as follows: Risk Score = 1(Dysmorphology 1) + 1(Dysmorphology 2) + 2(VABS) + 1(CBCL).
FAS, Fetal Alcohol Syndrome; VABS, Vineland Adaptive Behavior Scale; CBCL, Child Behavior Checklist
Dysmorphology Variables
The risk score incorporated several physical measurements selected from the standardized dysmorphology exam that were grouped into two dysmorphology variables (based on Goh et al., 2016). Dysmorphology 1 included palpebral fissure length ≤10 percentile, vermillion border lipometer score >3, and philtrum lipometer score >3 (Astley, 2004). Dysmorphology 2 comprised ptosis and incomplete extension of one or more fingers. The selected measurements are those consistent with a diagnosis of FAS (Dysmorphology 1) as well as additional physical deficits associated with prenatal alcohol exposure (Dysmorphology 2; Jones et al., 2010, Del Campo and Jones, 2017). Although the phenotype of FAS has been well characterized, it is likely that prenatal alcohol exposure leads to a broader spectrum of structural deficits (for review see Del Campo and Jones, 2017). The Dysmorphology 2 variable included additional structural deficits that have been shown to be significantly related to the presence of FAS (Jones et al., 2010) and predictive of prenatal alcohol exposure in our previous study (Goh et al., 2016). This combined with evidence suggesting that prenatal alcohol exposure may lead to varying structural deficits based on age and racial background (Jones et al., 2010, Moore et al., 2007) lead us to the inclusion of the Dysmorphology 2 variable in the risk score.
Vineland Adaptive Behavior Scales – Second Edition (VABS-II)
The VABS-II is a questionnaire measuring adaptive functioning that is completed by the primary caregiver (Sparrow et al., 2005). Parents rate specific behaviors on a 3-point scale (0 = never/rarely performs behavior without help; 1 = sometimes performs the described behavior; 2 = usually performs the behavior without help). Standard scores from the following subscales were included in the current study: Communication, Socialization, and Daily Living Skills. Standard scores ≤ 85 indicated the presence of an adaptive functioning deficit, as used in the previous study (Goh et al., 2016).
Child Behavior Checklist
The Child Behavior Checklist (CBCL) is a widely used rating scale for assessing emotional and behavioral problems in children completed by a child’s parent/primary caregiver (Achenbach and Rescorla, 2001). Parents rate specific behaviors on a 3-point scale (0 = not true as far as you know; 1 = somewhat or sometimes true; 2 = very true or often true). T-scores from the following subscales were included in the calculation of the risk score (based on Goh et al., 2016): Somatic Complaints, Social Problems, Thought Problems, Attention Problems, Rule Breaking Behavior, Aggressive Behavior. Raw scores are transformed into age- and sex-adjusted T-scores (M = 50, SD = 10) for interpretation; T-scores ≥ 65 are considered clinically significant.
Comparative Measures
We also assessed the relationship between the risk score and measures of IQ and executive functioning, both of which are known deficits for individuals with prenatal alcohol exposure (Mattson et al., 2019).
IQ
The measure for IQ differed between cohorts. Subjects who participated in CIFASD 2 completed the Wechsler Intelligence Scale for Children – Fourth Edition (WISC-IV; Wechsler, 2003), which yields a full-scale IQ score (FSIQ). Subjects participating in CIFASD 3 completed the Differential Ability Scales – Second Edition (DAS-II; Elliott, 2007), and the General Conceptual Ability score (GCA) was utilized as a measure of intellectual functioning. Both tests have a population mean of 100 and a standard deviation of 15.
Executive Functioning
The Delis-Kaplan Executive Function System (D-KEFS; Delis et al., 2001) is a performance-based measure of executive functioning that comprises nine subtests that can be administered independently or together. Scores from six subtests determined to be the best measures of executive functioning abilities for this population were included in the current study to create an executive function (EF) composite variable: Trail Making, Verbal Fluency, Design Fluency, Color-word Interference, Twenty Questions, and Tower Test. See Table 1 for scores used and description of each. Principal Component Analysis (PCA) was used to create an EF composite score for each cohort separately.
Table 1.
Scores from D-KEFS Subtests Used to Create Executive Function Composite Variable
| D-KEFS Subtest | Scaled Score | Description |
|---|---|---|
| Trail Making | Number-Letter Switching Scaled Score | Time taken to accurately connect sequential numbers and letters, switching between each |
| Verbal Fluency | Total Correct Switching Scaled Score | Number of correct words produced alternating between two semantic categories |
| Design Fluency | Total Correct Switching Scaled Score | Number of correct designs made by alternating between filled and empty dots |
| Color-Word Interference | Inhibition/Switching Scaled Score | Time taken to accurately switch between reading ink colors and the actual printed word |
| Twenty Questions | Total Initial Abstraction Scaled Score | Number of objects eliminated by first question, summed across four items |
| Tower Test | Total Weighted Achievement Score | Sum of weighted achievement scores for all four items |
D-KEFS, Delis-Kaplan Executive Function System
In the DC, PCA with direct oblimin rotation was conducted using the DC cohort data to compute an EF composite variable from the 6 D-KEFS assessments. Factors with eigenvalues greater than one were retained for the composite variable. Kaiser-Meyer-Olkin Measure of Sampling Adequacy was .812, which was above the recommended value of .5, suggesting reasonable shared variance. The Bartlett’s Test of Sphericity was significant (χ2 = 384.17, p <.001) which indicated that the analysis could proceed. The communalities for each D-KEFS measure were all above .330 (see Table 2), which suggests that all measures are contributing to the solution. Examining the extraction sums of squared loadings, a one component solution accounted for 46.3% of the solutions variance.
Table 2.
Extraction and Component Loadings for the D-KEFS Executive Function Measures in Relation to the Executive Function Component Variable for Each Cohort
| D-KEFS Measure | Extraction | Component |
|---|---|---|
| Development Cohort | ||
| Tower Test | .448 | .669 |
| Twenty Questions | .330 | .574 |
| Trail Making | .607 | .779 |
| Verbal Fluency | .393 | .627 |
| Color-Word Interference | .414 | .643 |
| Design Fluency | .585 | .765 |
| Comparative Cohort | ||
| Trail Making | .683 | .826 |
| Twenty Questions | .683 | .826 |
D-KEFS, Delis-Kaplan Executive Function System
Due to differences in data collection for the EF variable in the CC cohort, only scores from Trail Making and Twenty Questions were available to create the EF composite variable. In order to be consistent with the methodology used in the DC as well as to reduce the number of analyses, another PCA using direct oblimin rotation was conducted with the available EF variables. Kaiser-Meyer-Olkin Measure of Sampling Adequacy was .500, indicating that there was shared variance between the factors. Therefore, Bartlett’s Test of Sphericity was examined. A significant value (χ2 = 49.94, p <.001) indicated that the analysis could proceed. The communalities for both D-KEFS measures were .683 (see Table 2), which suggests that both measures are contributing to the solution. Examining the extraction sums of squared loadings, a one component solution accounted for 68.29% of the solutions variance.
Data Analysis
Descriptive statistics were used to identify the sample characteristics in the AE/CON groups. Unadjusted analyses were conducted using independent sample t-tests for continuous data (e.g., age, IQ) and Chi-square (X2) tests for categorical data (e.g., sex, race, ethnicity, and handedness). For all analyses, two-sided tests with 95% confidence intervals (CIs) that exclude 1.0 or p-values < 0.05 were used to determine statistical significance. Subjects with missing data (e.g., executive functioning scores or IQ) were excluded from corresponding analyses. The statistical analyses were conducted using SAS 9.4 (SAS Institute Inc) and SPSS v.27 (IBM Corporation, 2020).
Risk Score Model Development
The risk score was developed following the methodology outlined by Rassi et al. (2006). Some adjustments were made in this analytic approach to account for differences in the research question. Rassi and colleagues (2006) used the Cox proportional-hazards model to develop a risk score for predicting death in association with Chagas’ heart disease, which identifies time after entry into the study as an indicator of death. However, as time is not a factor in the diagnosis of an FASD, we applied multivariable logistic regression to develop our risk factor model.
Risk factor variables in this analysis included data from the dysmorphology exam (i.e., Dysmorphology 1 and Dysmorphology 2), and the parent-completed behavior questionnaires (i.e., VABS, and CBCL). Table 3 includes a list of measures used in the model and the cut-off criteria for each. Multivariable logistic regression models were conducted to determine the associations between the Dysmorphology 1, Dysmorphology 2, VABS, and CBCL cut-off variables and the AE classification (AE/CON; yes/no) using standardized betas (β), standard errors (SE [β]), and the respective p-values. Each risk score factor was then assigned a point value by dividing each regression coefficient by the smallest regression coefficient in the model and rounding to the nearest whole number. Point values were then summed for each individual subject to calculate a risk score corresponding to the presence or absence of a risk factor.
Supportive Analyses
ROC analyses were used to identify the optimal cut-point in the risk score that maximized the trade-off between sensitivity (true positive rate), specificity (true negative rate), and the overall accuracy of the risk score model. Classification accuracies were tested using the X2 test and predictive accuracy was also quantified using sensitivity, specificity, and positive predictive values (probability that subjects with a high-risk score have AE) and negative predictive values (probability that subjects with a low-risk score are controls). Correlation analyses investigating the relationship between AE classification (yes/no), risk score, IQ, and an EF composite variable (described above) were also conducted.
To further corroborate the risk score model, the risk score was calculated for a second cohort of subjects, the CC. Demographics and the final risk score were assessed in those with and without AE. The cut-points identified in the DC were used in the CC. The ROC analysis was repeated to determine the predictive accuracy of the risk score in the CC. The analyses investigating the relationship between AE classification (yes/no), risk score, IQ, and the EF composite variable were repeated in the CC.
Results
Development Cohort Demographics
Demographic data are presented in Table 4. Subjects in the DC ranged in age from 6–17 years (M = 12.5; SD = 2.71) and comprised two groups: children with histories of heavy prenatal alcohol exposure (AE-DC; n = 121 [37.2% of DC]) and controls with or without clinical concerns (CON-DC; n = 204 [62.8% of DC]). Within the CON group 112 (54.9%) subjects were identified as having no clinical concerns. The AE-DC and CON-DC groups did not differ by sex (χ2= .16, p = .686 or age (t[294] = 1.95, p = .052). DC groups significantly differed on site (χ2 = 15.93, p = .003), race (χ2 = 5.01, p = .025), ethnicity (χ2 = 7.05, p = .029), and handedness (χ2 = 11.87, p = .003). As expected, groups also significantly differed on IQ (t[322] = −8.00, p <.001), FAS diagnosis (χ2 = 71.64, p <.001), and ADHD diagnosis (χ2 = 30.59, p <.001).
Table 4.
Demographic Data for Alcohol Exposed (AE) and Controls (CON) in the Development Cohort (DC) and the Comparative Cohort (CC)
| Development Cohort (N = 325) | Comparative Cohort (N = 523) | |||
|---|---|---|---|---|
| Variable | AE (n = 121) | CON (n = 204) | AE (n = 177) | CON (n = 346) |
| CIFASD Site [n (%)]a | ||||
| Atlanta | 30 (24.8) | 50 (24.5) | 51 (28.8) | 121 (35.0) |
| Los Angeles | 20 (16.5) | 10 (4.9) | 18 (10.2) | 20 (5.8) |
| San Diego | 51 (42.1) | 93 (45.6) | 37 (20.9) | 86 (24.9) |
| New Mexico | 5 (4.1) | 23 (11.3) | -- | -- |
| Northern Plains | 15 (12.4) | 28 (13.7) | -- | -- |
| Minnesota | -- | -- | 71 (40.1) | 119 (34.4) |
| Sex [n (% Females)]b | 52 (43.0) | 83 (40.7) | 93 (52.5) | 149 (43.1) |
| Age in years [M (SD)] | 12.5 (2.71) | 11.9 (2.55) | 10.7 (3.21) | 11.2 (3.54) |
| Race [n (% White)]a | 72 (59.5) | 146 (71.6) | 88 (49.7) | 186 (53.8) |
| Ethnicity [n (% Hispanic)]ab | 14 (11.6) | 48 (23.5) | 31 (17.5) | 42 (12.1) |
| Handedness [n (% Right)]a | 100 (82.6) | 192 (94.1) | 156 (88.6) | 299 (86.9) |
| FSIQ/GCA [M (SD)]ab | 84.6 (16.88) | 100.7 (17.75) | 89.0 (12.82) | 99.8 (16.96) |
| FAS Diagnosis [n (%)]ab | 33 (27.3) | 0 (0.0) | 24 (13.6) | 0 (0.0) |
| ADHD Diagnosisc [n (%)]ab | 67 (55.4) | 65 (31.9) | 120 (67.8) | 85 (24.6) |
CIFASD, Collaborative Initiative on Fetal Alcohol Spectrum Disorders; FAS, fetal alcohol syndrome; FSIQ, Full Scale IQ; GCA, General Conceptual Ability.
Significant differences between AE and CON groups in the DC
Significant differences between AE and CON groups in the CC
ADHD diagnosis based on the Computerized Diagnostic Interview Schedule for Children – Fourth Edition (C-DISC-4.0)
Development Cohort Risk Model Development
The multivariable logistic regression revealed that all components of the risk score (i.e., Dysmorphology 1, Dysmorphology 2, VABS, and CBCL) were significant predictors of group membership (DC-AE vs DC-CON) and were included in the risk score calculation (Table 4). The final risk score equation was: (Risk Score = 1[Dysmorphology 1] + 1[Dysmorphology 2] + 2 [VABS] + 1 [CBCL]), where the numbers in the brackets are 0 or 1 based on whether the child exceeded the cutoffs for each variable (see Table 3). The risk score outcome ranged from 0–5 points. See Table 6 for the distribution of risk score for both groups.
Table 6.
Distribution of Alcohol Exposed (AE) and Control (CON) Subjects on the Risk Score
| Development Cohort | Comparative Cohort | |||
|---|---|---|---|---|
| Risk Score [n (%)] | AE | CON | AE | CON |
| 0 points | 7 (5.8) | 81 (39.7) | 6 (3.4) | 98 (28.3) |
| 1 point | 21 (17.4) | 75 (36.8) | 24 (13.6) | 106 (30.6) |
| 2 points | 16 (13.2) | 23 (11.3) | 30 (16.9) | 64 (18.5) |
| 3 points | 19 (15.7) | 14 (6.9) | 34 (19.2) | 42 (12.1) |
| 4 points | 33 (27.3) | 10 (4.9) | 61 (34.5) | 28 (8.1) |
| 5 points | 25 (20.7) | 1 (0.5) | 22 (12.4) | 8 (2.3) |
Validation Analyses in the Development Cohort
To test the classification accuracy of the risk score in identifying subjects with prenatal alcohol exposure, we used an ROC analysis to identify the optimal cut-point that maximized the trade-off between sensitivity and specificity. The ROC analysis returned an area under the curve (AUC) of 0.835 (SE = 0.024, p < .001). Cut-points of 1.5 (sensitivity = 76.9%, specificity = 76.5%) and 2.5 (sensitivity = 63.6%, specificity = 87.7%) were identified to have the highest sensitivity and specificity and were considered in both the DC and CC analyses. Using these cut-points, subjects were divided into two subgroups (low- and high-risk). For analyses using the 1.5 cut-point, subjects with risk scores between 0–1 were labeled as low-risk, while subjects with scores between 2–5 were high-risk. For analyses using the 2.5 cut-point, subjects were identified as low-risk if they had a risk score between 0–2, while subjects with a score between 3–5 were identified as high-risk. Subgroup frequencies are presented in Table 7.
Table 7.
Number of Alcohol Exposed (AE) and Control (CON) Subjects for Each Risk Score Subgroup
| Development Cohort | Comparative Cohort | |||
|---|---|---|---|---|
| Risk Score Category [n (%)] | AE | CON | AE | CON |
| Cut-point of 1.5 a | ||||
| Low | 28 (23.1) | 156 (76.5) | 30 (16.9) | 204 (59.0) |
| High | 93 (76.9) | 48 (23.5) | 147 (83.1) | 142 (41.0) |
| Cut-point of 2.5 b | ||||
| Low | 44 (36.4) | 179 (87.7) | 60 (33.9) | 268 (77.5) |
| High | 77 (63.6) | 25 (12.3) | 117 (66.1) | 78 (22.5) |
Low-risk = 0–1 points; high-risk = 2–5 points
Low-risk = 0–2 points; high-risk = 3–5 points
1.5 cut-point
When utilizing the 1.5 cut-point, the majority of the sample were classified into the low-risk group (n=184 [56.6%]) and the remaining were in the high-risk group (n= 141 [45.5%]). Frequencies of DC-AE and DC-CON subjects in each subgroup were significantly different (χ2 = 76.79, p <.001) and are presented in Table 7 and Figure 1. Further analyses revealed that those who were classified in the high-risk group were more likely to be in the AE group compared to those who were classified in the low-risk group (OR = 10.79; 95% CI: 6.34–18.38, p <.001). The classification accuracy rate ([true positives + true negatives]/total subjects) for the score was 76.6%. The risk score yielded 76.9% sensitivity, 76.5% specificity, with positive and negative predictive values of 66.0% and 84.8%, respectively.
Figure 1.
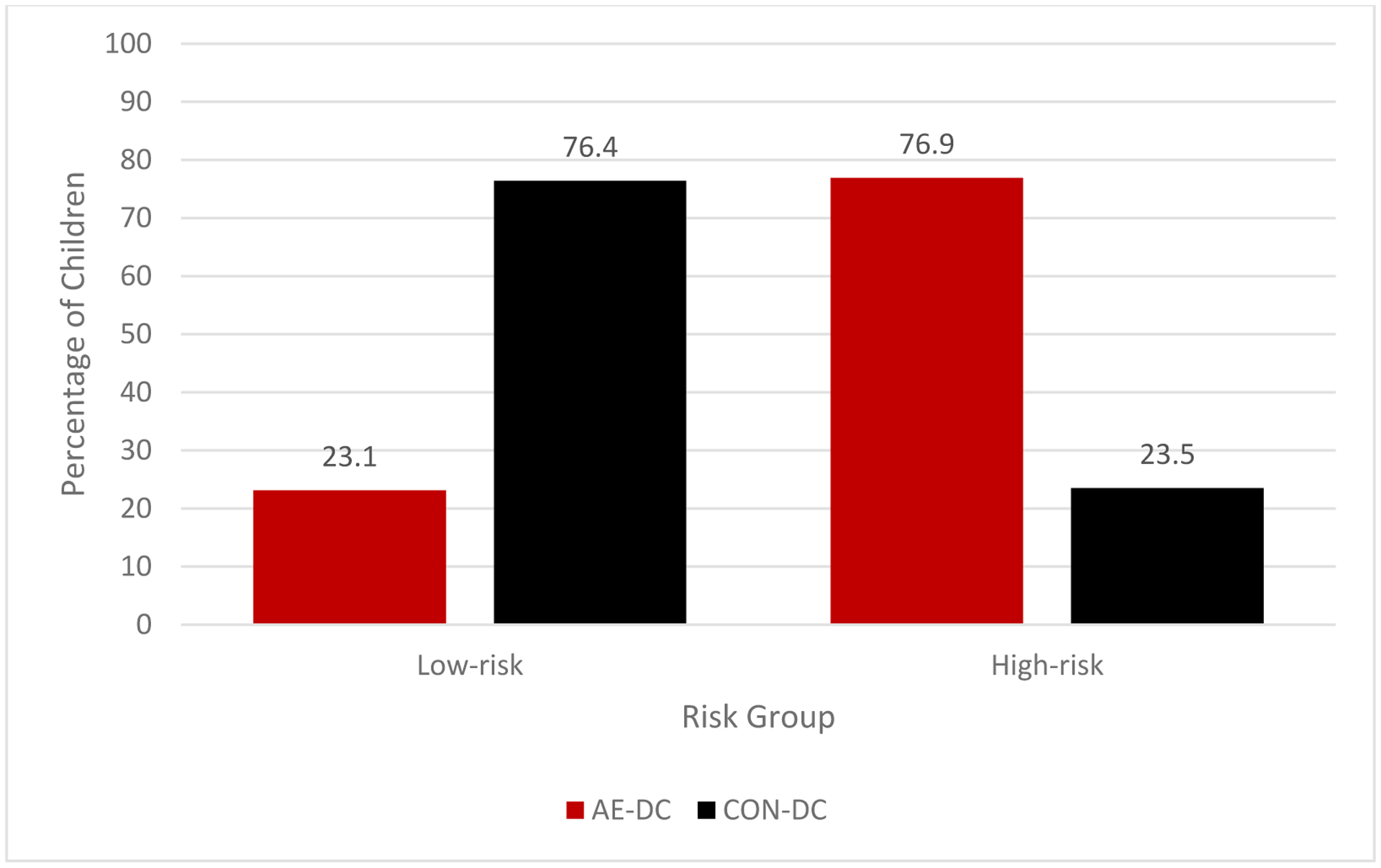
Percentage of alcohol-exposed (AE) and control (CON) subjects in each risk subgroup in the Development Cohort (DC) using the 1.5 cut-point.
2.5 cut-point
For analyses using the 2.5 cut-point, the majority of the sample was classified into the low-risk group (n=223 [68.6%]) and the remaining were in the high-risk group (n=102 [31.4%]). Frequencies of DC-AE and DC-CON subjects in each subgroup were significantly different (χ2 = 78.61, p <.001) and are presented in Table 7 and Figure 2. Further analyses revealed that those who were classified in the high-risk group were more likely to be in the AE group compared to those who were classified in the low-risk group (OR = 12.53; 95% CI: 7.17–21.91, p <.001). The classification accuracy rate for the risk score was 78.8%. The risk score yielded 63.6% sensitivity, 87.7% specificity, positive predictive values of 75.5%, and negative predictive values of 80.3%.
Figure 2.
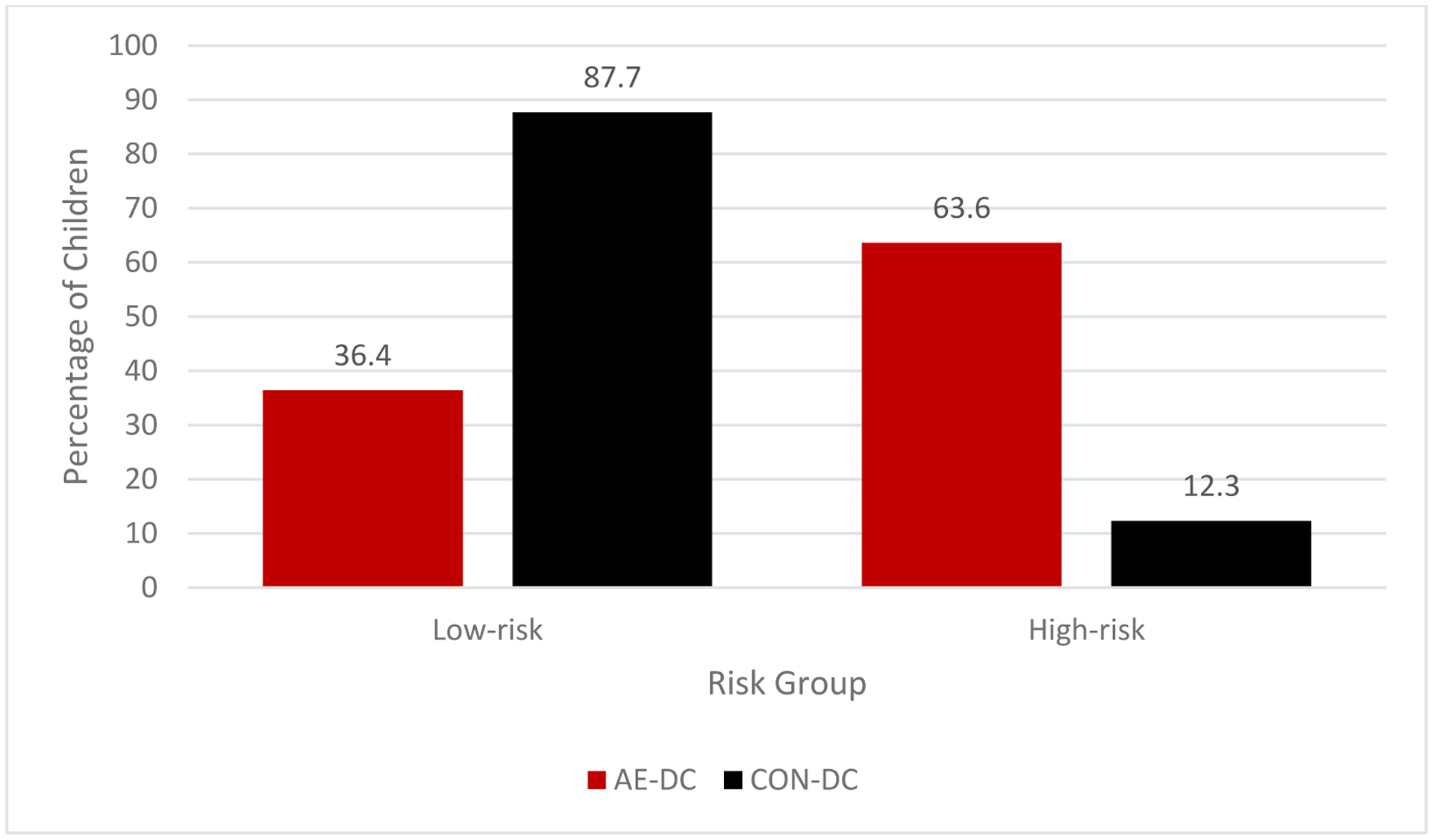
Percentage of alcohol-exposed (AE) and control (CON) subjects in each risk subgroup in the Development Cohort (DC) using the 2.5 cut-point.
Correlation of the Risk Score with IQ and Executive Function in the Development Cohort
Pearson correlations were run between the continuous risk score, IQ, and the EF composite variable. The risk score was significantly correlated with IQ for both groups (DC-AE: r = −.35, p <.001; DC-CON: r = −.31, p <.001) and the within-group correlations did not significantly differ from each other (z = .38, p = .35). Risk score was also correlated with the EF composite variable in the DC-AE group (r = −.30, p = .002) and DC-CON group (r = −.40, p <.001). The within-group correlations did not significantly differ from each other (z = .92, p =.177).
Comparative Cohort Demographics
Subjects in the CC ranged in age from 5–17 years (M = 11.1; SD = 3.40) and comprised the same two groups: children with histories of heavy prenatal AE (AE-CC; n = 177 [33.8%]) and controls with or without clinical concerns (CON-CC; n = 346 [66.2%]; Table 4). Within the CON group, 192 (55.5%) subjects were identified as having no clinical concerns. The AE-CC and CON-CC groups did not differ on age (t[473] = −.17, p = .087, site (χ2 =6.29, p = .098), race (χ2 = .766, p = .381), or handedness (χ2 = 1.88, p = .759). CC groups significantly differed on sex (χ2 = 4.23, p = .040) and ethnicity (χ2 = 9.36, p = .009). As expected, groups also differed on GCA scores (t[516] = −7.43, p = <.001), FAS status (χ2 = 70.78, p = <.001), and ADHD (χ2 = 104.75, p = <.001). See Table 6 for the distribution of AE and CON subjects across the risk score.
Validation Analyses in the Comparative Cohort
The ROC analysis of the risk score returned an AUC of 0.786 (SE = 0.021, p < .001). We tested the same cut-points (>1.5 and >2.5), as identified in the DC.
1.5 cut-point
When using the 1.5 cut-point, the majority of the sample was classified into the high-risk group (n = 289 [55.3%]) and the remaining were in the low-risk group (n = 234 [44.7%]). Frequencies of DC-AE and DC-CON subjects in each subgroup were significantly different (χ2 = 73.13, p <.001) and are presented in Table 7 and Figure 3. Further analyses revealed that those who were classified in the high-risk group were more likely to be in the AE group compared to those who were classified in the low-risk group (OR = 7.04; 95% CI: 4.50–11.01, p <.001). The classification accuracy rate was 67.1%. Using this cut-point, the risk score yielded 83.1% sensitivity, 59.0% specificity, and positive and negative predictive values of 50.8% and 87.2%, respectively.
Figure 3.
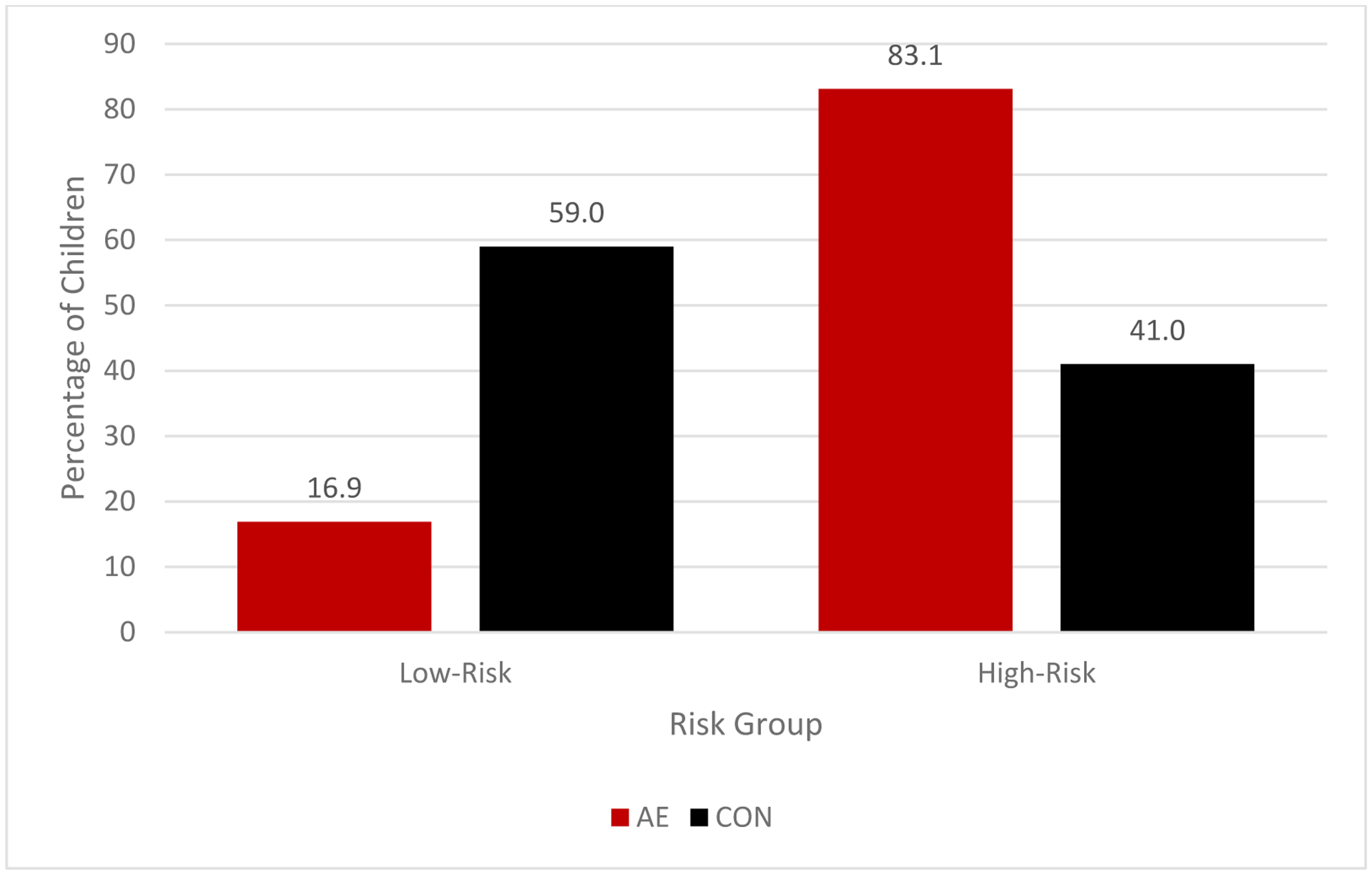
Percentage of alcohol-exposed (AE) and control (CON) subjects in each risk subgroup in the Comparative Cohort (CC) using the 1.5 cut point.
2.5 cut-point
Utilizing the 2.5 cut-point, the most prevalent classification of the sample was the low-risk group (n = 328 [62.7%]) and the remaining were classified as high-risk (n = 195 [37.3%]). Frequencies of CC-AE and CC-CON subjects in each subgroup were significantly different (χ2 = 86.63, p <.001) and are presented in Table 7 and Figure 4. Further analyses revealed that those who were classified as high-risk were more likely to be in the AE group compared to those who were classified as low-risk (OR = 6.70; 95% CI: 4.49–10.0). The classification accuracy rate was 73.6%. The risk score performed well with 66.1% sensitivity, 77.5% specificity, and positive and negative predictive values of 60.0% and 81.7%, respectively.
Figure 4.
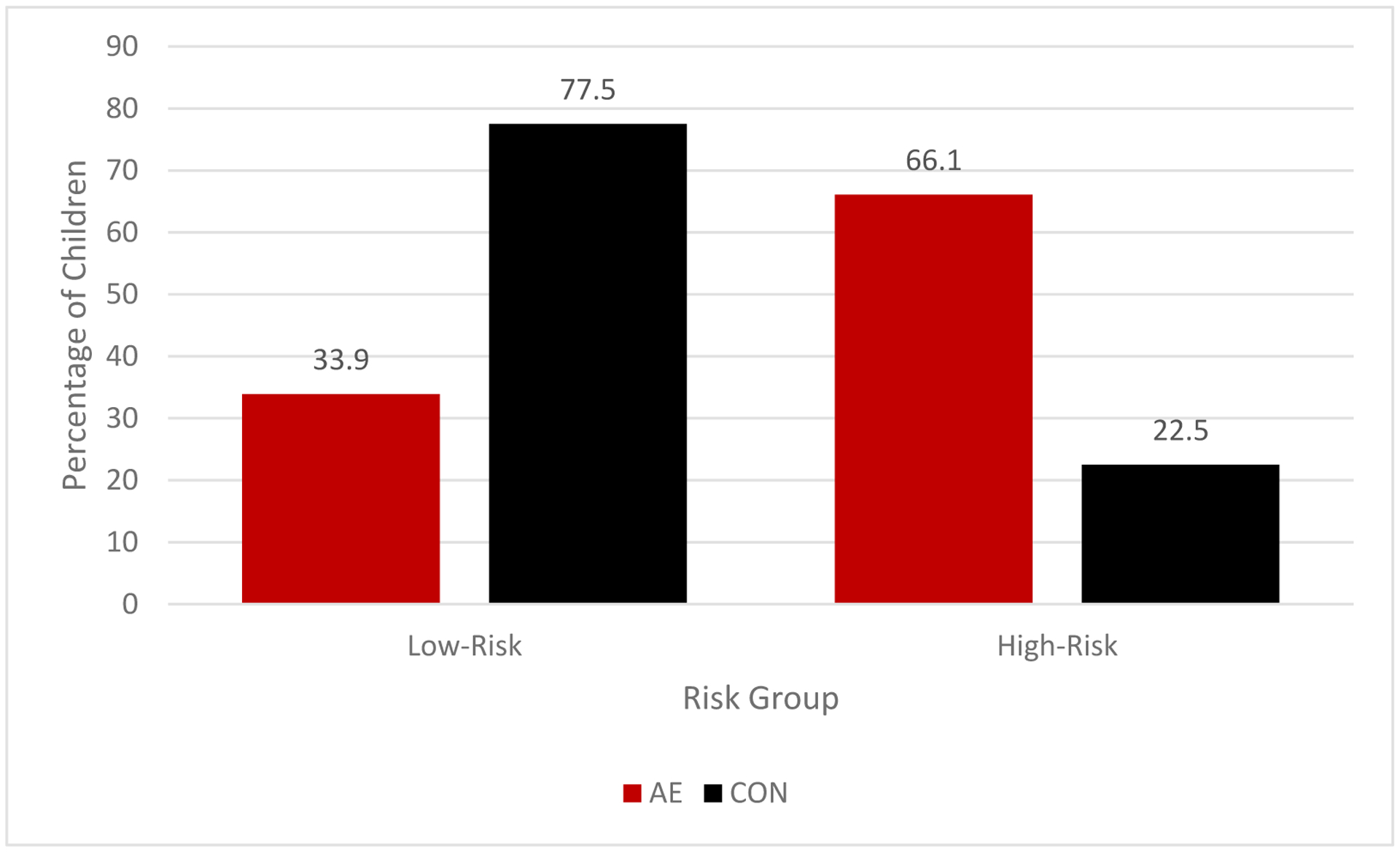
Percentage of alcohol-exposed (AE) and control (CON) subjects in each risk subgroup in the Comparative Cohort CC) using the 2.5 cut point.
Correlation of the Risk Score with IQ and Executive Function in the Comparative Cohort
Pearson correlations were run between the risk score, IQ, and the EF composite variable. The risk score was significantly correlated with IQ for both groups (CC-AE: r = −.29, p <.001; CC-CON: r = −.41, p <.001). There was no significant difference in the correlations between the AE and CON groups (z = 1.47, p = .071). Risk score was also correlated with the EF composite variable in the CC-AE group (r = −.20, p = .034) and CC-CON group (r = −.28, p <.001), which did not significantly differ from each other (z = 0.74, p = .23).
Post-Hoc Subgroup Analyses
To further examine the accuracy of the risk score in distinguishing children with histories of prenatal alcohol exposure from controls, we analyzed the risk score in 2 subgroups. First, we calculated the risk score for children who met criteria for FAS. The average risk score for this subgroup was 3.88 in the DC (vs. 3.03 for the entire DC alcohol-exposed group) and 3.58 in the CC (vs. 3.05 in the entire CC alcohol-exposed group). We also calculated the risk score for subjects in the control group that had histories of clinical concerns. The average score was 1.58 in the DC (vs. 1.02 for the entire DC control group) and 2.04 in the CC (vs. 1.48 in the entire CC control group).
Discussion
Principal Findings
This study aimed to develop and validate a risk score screening tool to help clinicians identify children who may have had prenatal alcohol exposure from those without exposure. Analyses demonstrated that the risk score accurately identified alcohol-exposed from control subjects. Follow-up analyses revealed that subjects classified in the high-risk group were more likely to have prenatal alcohol exposure compared to those classified in the low-risk group, using either cut-point. An individual’s risk score was also significantly correlated with their IQ and EF scores, providing supportive evidence for the score. Individuals with higher risk scores had lower IQ scores and decreased executive functioning abilities.
In this study we presented evidence for the use of the risk score using two different cut-points to distinguish between low- and high-risk subjects. Overall, the 2.5 cut-point performed best for subjects in the DC and CC. Across the two cohorts using the 2.5 cut-point, sensitivity ranged from 64%−66%, specificity was between 78%−88%, and the classification accuracy rate was from 74%−79%. However, findings from the study also provide supportive evidence of the use of the 1.5 cut-point; additional testing may provide further support for one of the cut-points. Further, the source of patients or subjects may be an important consideration. When we examined the risk score for the subgroup of controls with histories of clinical concerns, risk scores were slightly elevated compared to the control group as a whole. Thus, for the population of individuals who may be considered at higher risk for developmental conditions (e.g., patients in a developmental behavioral pediatrics clinic), the higher cut-point may have higher specificity and be more accurate overall. Conversely, when the comparison group is a community sample with low base rates of behavior disorders, the lower cut-point will have higher sensitivity.
Clinical Implications
Importantly, the risk score successfully differentiated between alcohol-exposed and those with minimal or no prenatal alcohol exposure with the use of physical and parent-report measures and was not dependent on a diagnosis of FAS. Alcohol-exposed children, regardless of FAS diagnosis, had higher risk scores than controls which is consistent with previous reports of significant cognitive and behavioral impairments in children with prenatal alcohol exposure with and without FAS (Mattson et al., 2019). The current study demonstrated that the risk score accurately identified more individuals with prenatal alcohol exposure as high-risk, while controls were more likely to be labeled as low-risk. Of note is that our risk score includes cut-off criteria that are more liberal than would be used to indicate impairment in clinical settings (typically −1.5 or −2.0 SD below the mean). The individual indicators used in the risk score are not in and of themselves indicators of impairment. But their combination in the risk score has been demonstrated herein to be sensitive to the effects of prenatal alcohol exposure. Patients who fall into the high-risk category but for whom alcohol exposure is suspected or unknown should be referred for additional evaluation including comprehensive psychoeducation/neuropsychological examination and appropriate intervention services. Early identification can help children receive services earlier during their development, which has previously been identified as a protective factor against the long-term consequences of prenatal alcohol exposure (Streissguth et al., 2004, Loock et al., 2005). Importantly, the use of the risk score reduces the number of children that should be referred for more detailed evaluation; using the 2.5 cut-point, 31% of the DC and 37% of the CC would require referral. This reduction lowers family and clinical burden as well as cost. Additionally, the risk score was shown to have high negative predictive values, meaning that for patients that receive a low-risk score, prenatal alcohol exposure is not likely.
Given the high rates of missed diagnoses and misdiagnosis for foster and adopted children with prenatal alcohol exposure that have been reported (Chasnoff et al., 2015) there is a need to improve upon current identification techniques. Overall accuracy rates of the risk score were 78.8% and 73.6% for the development and comparative samples, respectively. Studies aimed at developing a neurobehavioral profile for FASD have previously reported overall classification accuracy rates ranging between 71.5% and 84.7% (Mattson et al., 2010b, Mattson et al., 2013). Studies assessing the utility of other FASD screening tools have reported accuracy rates of 71.3%−89.6% (Klug et al., 2021) and 78.4%−82% (Burd et al., 2010), and sensitivity rates of 86% (Widder et al., 2021). Additionally, a hierarchical decision tree model using similar measures as described herein had accuracy rates of 79.5%−84.7% (Goh et al., 2016). Although some of these previous studies have higher accuracy and sensitivity rates compared to findings from the current study, the previous identification tools required subjects to complete a battery of neuropsychological assessments (Mattson et al., 2010b, Mattson et al., 2013, Klug et al., 2021), measures of intellectual functioning (Goh et al., 2016), required maternal information (Burd et al., 2010), or were created for use with adults only (Widder et al., 2021). In comparison, the risk score that we developed could be simply deployed in a clinical setting and therefore has significant clinical potential as an early screening tool. The variables used in the risk score model require a limited number of measures that can easily be obtained as part of standard clinical practice. Furthermore, the risk score may be useful for identifying children who have been exposed to alcohol if alcohol exposure is unknown. Many children who have an FASD diagnosis live in adoptive/foster family homes, where exposure is likely to be unknown (Astley, 2010). Clinicians, primary caregivers, and social workers can use this information in the initial stages of assessing a child.
Research Implications
The risk score that was developed in this study incorporates information related to dysmorphology and behavior. There are many other pre- and postnatal factors, such as genetic risk, maternal stress, and rearing environment that may influence a child’s outcome and were not assessed in this study. For instance, previous findings suggest that maternal psychopathology and other factors related to maternal drinking during pregnancy may be a better predictor of child internalizing behavior issues, whereas alcohol exposure is more directly related to externalizing behavior problems (Staroselsky et al., 2009, D’Onofrio et al., 2007). Future studies should examine other potential factors that could be added to the current risk score formula. This would potentially help the sensitivity and specificity of the risk score and aid clinicians in accurately identifying children at high-risk of prenatal alcohol exposure. Validation of the current risk score formula in additional samples, including those with other clinical groups, should be considered to establish reliability of the model. Future studies could also utilize the methodology of the current study (e.g., creating an EF composite variable) to validate other diagnostic tools. Additionally, studies should focus on how else to better integrate research tools into a clinical setting, such as finding behavioral measures that are quick and easy to administer.
Strengths and Limitations
This study is significantly strengthened by the large sample size and multiple subject cohorts that were used to separately develop and corroborate the risk score. Additionally, we analyzed the risk variables using a multifaceted approach not previously used in this population. In order to address the specificity of the risk score for children with prenatal alcohol exposure, we included a control group consisting of typically developing children and children with behavioral or cognitive concerns. The inclusion of a heterogeneous control group improved the specificity of the findings since children with prenatal alcohol exposure experience elevated levels of neurobehavioral deficits and other difficulties in mental health, social, and adaptive function (Olson and Sparrow, 2021) and can be misdiagnosed (Chasnoff et al., 2015, Kodituwakku, 2009). Additionally, data was collected from multiple sites across the United States providing evidence of generalizability of our results. Despite the many strengths of this study there were some limitations. Due to differing executive function measures used in each phase of the study, the EF composite variable was composed of only two measures in the comparative cohort versus the six scores used in the development cohort. Additionally, we utilized a heterogeneous control group. While we believe this to be a strength for our study, it may have resulted in weaker correlations and measures of classification accuracy. As such, our results may be viewed as conservative. Furthermore, recruitment procedures limited our understanding of the child’s environment which, as discussed above, can influence outcomes. Finally, although the risk score has improved clinical utility compared to those previously developed (e.g., Mattson et al., 2010b, Mattson et al., 2013, Klug et al., 2021), there are several limitations to the score that we hope to address in future studies. Currently, the tool requires information related to dysmorphology which necessitates a trained clinician. Secondly, the behavioral measures included require between 30 – 70 minutes to complete as well as reasonable literacy understanding, which may result in caregivers not willing/able to complete. Further, the measures have cross-cultural limitations. Thirdly, the VABS-II has an updated version (VABS-3) which has the same subdomains as the former. Preliminary data suggests that the VABS-3 results in similar outcomes as part of the risk score. Additionally, future studies should aim to assess the utility of the risk score in a broader health-care setting. Subjects were recruited primarily from FASD clinics and research projects and around 35% of our sample were alcohol-exposed. Further, 6.7% of the combined DC and CC samples (20% of the exposed sample) had FAS. While this diagnosis rate is higher than the general population, our study was designed to weight the presence of heavy exposure in our sample and should not be seen as a prevalence rate. The rates of prenatal alcohol exposure as well as the accuracy of the risk score in other high risk settings (e.g., a clinic specializing in developmental disabilities) or in settings where different diagnostic criteria are used are important topics for future research. Despite these limitations, the study has many strengths, and the results significantly add to the FASD literature.
Conclusion
The current study set out to identify a postnatal risk score model that could help distinguish children who may have been exposed to alcohol in utero from typically developing children as well as children with clinical behavioral or cognitive concerns. The validation of this risk score is important as it can help clinicians identify children who are at a high-risk of having been exposed to alcohol prenatally. Given the high rates of missed diagnoses and misdiagnosis (Chasnoff et al., 2015), the risk score model developed in the current study could prove greatly important in a clinical setting as an initial screening tool. Patients identified as high-risk may benefit from referral for further clinical evaluation and intervention.
Table 5.
Regression Coefficients for Multivariable Logistic Regression Risk Score Model in the Development Cohort
| Risk Factor | β | SE [β] | Wald | p |
|---|---|---|---|---|
| Dysmorphology 1 | 0.439 | 0.151 | 8.49 | 0.004 |
| Dysmorphology 2 | 0.417 | 0.157 | 7.00 | 0.008 |
| VABS | 0.925 | 0.172 | 28.83 | <.001 |
| CBCL | 0.501 | 0.167 | 9.03 | 0.003 |
VABS, Vineland Adaptive Behavior Scale; CBCL, Child Behavior Checklist
Acknowledgments
The authors thank the families who graciously participate in our studies. The authors have no financial or other conflicts of interest. All or part of this work was done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Additional information about CIFASD can be found at www.cifasd.org. Research described in this paper was supported by NIAAA grant number U01 AA014834. Additional support was provided by U24 AA014811, U24 AA014815, T32 AA013525.
References
- Achenbach TM, Rescorla LA (2001) Manual for the ASEBA School-Age Forms & Profiles, University of Vermont, Research Center for Children, Youth, & Families, Burlington, VT. [Google Scholar]
- Astley SJ (2004) Diagnostic Guide for Fetal Alcohol Spectrum Disorders: The 4-Digit Diagnostic Code. 3rd ed., University of Washington, Seattle. [Google Scholar]
- Astley SJ (2010) Profile of the first 1,400 patients receiving diagnostic evaluations for fetal alcohol spectrum disorder at the Washington State Fetal Alcohol Syndrome Diagnostic & Prevention Network. The Canadian Journal of Clinical Pharmacology 17:e132–164. [PubMed] [Google Scholar]
- Bertrand J, Floyd RL, Weber MK (2005) Guidelines for identifying and referring persons with fetal alcohol syndrome. MMWR Recomm Rep 54:1–14. [PubMed] [Google Scholar]
- Burd L, Klug MG, Li Q, Kerbeshian J, Martsolf JT (2010) Diagnosis of fetal alcohol spectrum disorders: A validity study of the fetal alcohol syndrome checklist. Alcohol 44:605–614. [DOI] [PubMed] [Google Scholar]
- Carr JL, Agnihotri S, Keightley M (2010) Sensory processing and adaptive behavior deficits of children across the fetal alcohol spectrum disorder continuum. Alcohol Clin Exp Res 34:1022–1032. [DOI] [PubMed] [Google Scholar]
- Chasnoff IJ, Wells AM, King L (2015) Misdiagnosis and Missed Diagnoses in Foster and Adopted Children with Prenatal Alcohol Exposure. Pediatrics 135:264–270. [DOI] [PubMed] [Google Scholar]
- Crocker N, Vaurio L, Riley EP, Mattson SN (2009) Comparison of adaptive behavior in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Alcohol Clin Exp Res 33:2015–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Van Hulle CA, Waldman ID, Rodgers JL, Rathouz PJ, Lahey BB (2007) Causal inferences regarding prenatal alcohol exposure and childhood externalizing problems. Arch Gen Psychiatry 64:1296–1304. [DOI] [PubMed] [Google Scholar]
- Del Campo M, Jones KL (2017) A review of the physical features of the fetal alcohol spectrum disorders. Eur J Med Genet 60:55–64. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH (2001) The Delis-Kaplan Executive Function System: Examiner’s Manual, The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Elliott CD (2007) Differential Ability Scales – Second Edition (DAS-II), Harcourt Assessment, San Antonio, TX. [Google Scholar]
- Fagerlund A, Ase F, Autti-Ramo I, Ilona A-R, Kalland M, Mirjam K, Santtila P, Pekka S, Hoyme HE, Eugene HH, Mattson SN, Sarah MN, Korkman M, Marit K (2012) Adaptive behaviour in children and adolescents with foetal alcohol spectrum disorders: a comparison with specific learning disability and typical development. Eur Child Adolesc Psychiatry 21:221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin L, Deitz J, Jirikowic T, Astley S (2008) Children with fetal alcohol spectrum disorders: Problem behaviors and sensory processing. Am J Occup Ther 62:265–273. [DOI] [PubMed] [Google Scholar]
- Goh PK, Doyle LR, Glass L, Jones KL, Riley EP, Coles CD, Hoyme HE, Kable JA, May PA, Kalberg WO, Sowell ER, Wozniak JR, Mattson SN (2016) A Decision Tree to Identify Children Affected by Prenatal Alcohol Exposure. J Pediatr 177:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley D, Marais AS, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Jewett T, Coles CD, Chambers C, Jones KL, Adnams CM, Shah PE, Riley EP, Charness ME, Warren KR, May PA (2016) Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM SPSS Statistics for Macintosh, Version 27.0. [computer program]. Armonk, NY: IBM Corp; 2020. [Google Scholar]
- Jirikowic T, Olson HC, Kartin D (2008) Sensory processing, school performance, and adaptive behavior of young school-age children with fetal alcohol spectrum disorders. Phys Occup Ther Pediatr 28:117–136. [DOI] [PubMed] [Google Scholar]
- Jones KL, Hoyme HE, Robinson LK, del Campo M, Manning MA, Prewitt LM, Chambers CD (2010) Fetal Alcohol Spectrum Disorders: Extending the Range of Structural Defects. Am J Med Genet A 152A:2731–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Robinson LK, Bakhireva LN, Marintcheva G, Storojev V, Strahova A, Sergeevskaya S, Budantseva S, Mattson SN, Riley EP, Chambers CD (2006) Accuracy of the diagnosis of physical features of fetal alcohol syndrome by pediatricians after specialized training. Pediatrics 118:E1734–E1738. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW (1973) Recognition of the fetal alcohol syndrome in early infancy. Lancet 302:999–1001. [DOI] [PubMed] [Google Scholar]
- Klug MG, O’Connell AM, Palme A, Kobrinsky N, Burd L (2021) A Validation Study of the Alcohol Related Neurodevelopmental Disorders Behavioral Checklist. Alcoholism: Clinical and Experimental Research 45:765–772. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW (2009) Neurocognitive profile in children with fetal alcohol spectrum disorders. Dev Disabil Res Rev 15:218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loock C, Conry J, Cook JL, Chudley AE, Rosales T (2005) Identifying fetal alcohol spectrum disorder in primary care. CMAJ 172:628–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Bernes GA, Doyle LR (2019) Fetal Alcohol Spectrum Disorders: A Review of the Neurobehavioral Deficits Associated With Prenatal Alcohol Exposure. Alcoholism, clinical and experimental research 43:1046–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT (2011) Fetal Alcohol Spectrum Disorders: Neuropsychological and behavioral features. Neuropsychology review 21:81–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Foroud T, Sowell ER, Jones KL, Coles CD, Fagerlund A, Autti-Rämö I, May PA, Adnams CM, Konovalova V, Wetherill L, Arenson AD, Barnett WK, Riley EP (2010a) Collaborative initiative on fetal alcohol spectrum disorders: methodology of clinical projects. Alcohol 44:635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP (2000) Parent ratings of behavior in children with heavy prenatal alcohol exposure and IQ-matched controls. Alcoholism, clinical and experimental research 24:226–231. [PubMed] [Google Scholar]
- Mattson SN, Riley EP (2011) The quest for a neurobehavioral profile of heavy prenatal alcohol exposure. Alcohol Research & Health 34:51–55. [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Fagerlund A, Autti-Ramo I, Jones KL, May PA, Adnams CM, Konovalova V, Riley EP, Collaborative Initiative on Fetal Alcohol Spectrum D (2010b) Toward a neurobehavioral profile of fetal alcohol spectrum disorders. Alcoholism, clinical and experimental research 34:1640–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Glass L, Deweese BN, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Adnams CM, Jones KL, Riley EP (2013) Further development of a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol Clin Exp Res 37:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, Kopald D, Hasken JM, Xu R, Honerkamp-Smith G, Taras H, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Vaux K, Jewett T, Elliott AJ, Kable JA, Akshoomoff N, Falk D, Arroyo JA, Hereld D, Riley EP, Charness ME, Coles CD, Warren KR, Jones KL, Hoyme HE (2018) Prevalence of Fetal Alcohol Spectrum Disorders in 4 US Communities. Jama 319:474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Riley EP (2015) What Happens When Children with Fetal Alcohol Spectrum Disorders Become Adults? Curr Dev Disord Rep 2:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore ES, Ward RE, Wetherill LF, Rogers JL, Autti-Raemoe I, Fagerlund A, Jacobson SW, Robinson LK, Hoyme HE, Mattson SN, Foroud T (2007) Unique facial features distinguish fetal alcohol syndrome patients and controls in diverse ethnic populations. Alcoholism-Clinical and Experimental Research 31:1707–1713. [DOI] [PubMed] [Google Scholar]
- Olson HC, Sparrow J (2021) A shift in perspective on secondary disabilities in fetal alcohol spectrum disorders. Alcohol Clin Exp Res 45:916–921. [DOI] [PubMed] [Google Scholar]
- Panczakiewicz AL, Glass L, Coles CD, Kable JA, Sowell ER, Wozniak JR, Jones KL, Riley EP, Mattson SN (2016) Neurobehavioral Deficits Consistent Across Age and Sex in Youth with Prenatal Alcohol Exposure. Alcoholism, clinical and experimental research 40:1971–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangmar J, Dahlgren Sandberg A, Aronson M, Fahlke C (2017) Self-reported health, use of alcohol and illicit drugs, and criminality among adults with foetal alcohol syndrome. Nordic Studies on Alcohol and Drugs 34:255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassi A, Little WC, Xavier SS, Rassi SG, Rassi AG, Rassi GG, Hasslocher-Moreno A, Sousa AS, Scanavacca MI (2006) Development and validation of a risk score for predicting death in Chagas’ heart disease. N Engl J Med 355:799–808. [DOI] [PubMed] [Google Scholar]
- SAS Version 9.4 [computer program]. Cary, North Carolina. [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV (1984) Vineland Adaptive Behavior Scales: Survey Form Manual. Interview ed., American Guidance Service, Circle Pines, MN. [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA (2005) Vineland Adaptive Behavior Scales, Second Edition: Survey forms manual. Second ed., AGS, Circle Pines, MN. [Google Scholar]
- Sparrow SS, Cicchetti DV, Saulnier CA (2016) Vineland Adaptive Behavior Scales, Third Edition (Vineland-3), Pearson, Bloomington, MN. [Google Scholar]
- Spohr HL, Willms J, Steinhausen HC (2007) Fetal alcohol spectrum disorders in young adulthood. J Pediatr 150:175–179, 179 e171. [DOI] [PubMed] [Google Scholar]
- Staroselsky A, Fantus E, Sussman R, Sandor P, Koren G, Nulman I (2009) Both parental psychopathology and prenatal maternal alcohol dependency can predict the behavioral phenotype in children. Pediatr Drugs 11:22–25. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF (1991) Fetal alcohol syndrome in adolescents and adults. J Am Med Assoc 265:1961–1967. [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Kogan J, Bookstein FL (1996) Final report: Understanding the occurrence of secondary disabilities in clients with fetal alcohol syndrome (FAS) and fetal alcohol effects (FAE), University of Washington Publication Services, Seattle, WA. [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK (2004) Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr 25:228–238. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Kelly SJ, Mattson SN, Riley EP (1998) Comparison of social abilities of children with fetal alcohol syndrome to those of children with similar IQ scores and normal controls. Alcohol Clin Exp Res 22:528–533. [PubMed] [Google Scholar]
- Vaurio L, Riley EP, Mattson SN (2011) Neuropsychological comparison of children with heavy prenatal alcohol exposure and an IQ-matched comparison group. Journal of the International Neuropsychological Society : JINS 17:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2003) Wechsler intelligence scale for children–Fourth Edition (WISC-IV). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Whaley SE, O’Connor MJ, Gunderson B (2001) Comparison of the adaptive functioning of children prenatally exposed to alcohol to a nonexposed clinical sample. Alcohol Clin Exp Res 25:1018–1024. [PubMed] [Google Scholar]
- Widder M, Mierzwa L, Schwerg L, Schecke H, Kornhuber J, Bouna-Pyrrou P, Bumb JM, Richter-Schmidinger T, Lenz B (2021) Evaluation of the German biographic screening interview for fetal alcohol spectrum disorder (BSI-FASD). Scientific Reports 11:5233. [DOI] [PMC free article] [PubMed] [Google Scholar]


